Abstract
Obscure gastrointestinal bleeding (OGIB) is defined as bleeding from the gastrointestinal tract that persists or recurs after a negative initial evaluation using bidirectional endoscopy and radiologic imaging with small-bowel radiograph. The main challenges related to evaluation of OGIB include the high miss rate for lesions on initial evaluation with standard endoscopy and the limited capacity of older diagnostic modalities to effectively examine the small bowel. The introduction of capsule endoscopy, balloon-assisted enteroscopy, spiral enteroscopy, and computed tomography (CT) enterography have served to overcome the limitations of older diagnostic tests. Capsule endoscopy is currently recommended as the third test of choice in the evaluation of patients with OGIB, after a negative bidirectional endoscopy. Balloon-assisted enteroscopy is useful for both the diagnosis and endoscopic management of OGIB. CT enterography is superior to small-bowel radiograph for luminal and extraluminal small-bowel examination. These advances in small-bowel diagnostics and the capacity to successfully perform endoscopic therapeutics have largely replaced surgical procedures and resulted in a trend toward noninvasive evaluation and endoscopic management of OGIB.
Keywords: Obscure gastrointestinal bleeding, video capsule endoscopy, balloon-assisted enteroscopy, double-balloon enteroscopy, single-balloon enteroscopy, spiral enteroscopy, computed tomography enterography
In patients who present with gastrointestinal bleeding, the underlying etiology may not be evident on initial evaluation in 10–20% of cases. Recurrent or persistent bleeding occurs in approximately half of these patients (5%) and can pose a significant challenge to both diagnosis and management. The underlying etiology often remains elusive despite extensive evaluations, thereby resulting in recurrent hospitalizations and multiple transfusions.1,2 Obscure gastrointestinal bleeding (OGIB) has, thus, been defined historically as bleeding from the gastrointestinal tract that persists or recurs after a negative initial evaluation, using bidirectional endoscopy and radiologic imaging with small bowel follow-through (SBFT) or enteroclysis.3
The main challenges related to the evaluation of OGIB include the high miss rate for lesions on initial endoscopic evaluation with standard endoscopy (esophagogastroduodenoscopy [EGD] and colonoscopy), as well as the limited capacity of older diagnostic modalities to effectively examine the small bowel (SB), particularly for mucosal disease. The mainstay in the management of these patients has, hence, traditionally involved the use of invasive procedures such as intra-operative enteroscopy (IOE) and exploratory laparotomy. The introduction of video capsule endoscopy (CE), balloon-assisted enteroscopy (BAE; single- and double-balloon enteroscopy [SBE and DBE]), spiral enteroscopy, and computed tomography enterography (CTE) represent significant technological advances that have overcome the limitations of older diagnostic tests. These novel modalities have largely replaced invasive surgical procedures, thereby resulting in a major change in the approach to diagnosis and management of OGIB. This paper is a comprehensive outline of OGIB, with a description and comparison of traditional and novel examinations and their respective roles in OGIB.
Classification of Obscure Gastrointestinal Bleeding
OGIB may be categorized according to clinical presentation of the patient and location of the bleeding source. Based upon presentation, OGIB may be classified as overt or occult bleeding. Overt OGIB is defined as clinically perceptible bleeding that recurs or persists after a negative initial endoscopic evaluation (EGD and colonoscopy) and radiologic evaluation (SBFT or enteroclysis). In comparison, occult OGIB is defined as iron-deficiency anemia, with or without a positive fecal occult blood test.3,4
Prior to the introduction of CE and BAE, gastrointestinal bleeding was classified as originating proximal or distal to the ligament of Treitz. Following the introduction of novel SB imaging techniques, it has been proposed that gastrointestinal bleeding be reclassified as upper (proximal to the ampulla of Vater), mid (ampulla of Vater to ileocecal valve), or lower (colonic sources) gastrointestinal bleeding.3,5
Etiologies of Obscure Gastrointestinal Bleeding
Although it is common practice to use the terms OGIB and SB bleeding interchangeably, lesions that manifest as OGIB include both missed lesions located within reach of standard endoscopy, as well as SB lesions. The importance of these missed lesions can be ascertained by the high reported yield of second-look endoscopy: 35–75% in patients undergoing repeat EGD and 6% on repeat colonoscopy.6-9 The main reasons for a negative initial evaluation include slow or intermittent bleeding; failure to detect vascular lesions due to anemia, dehydration, or sedatives; compromised visualization due to the presence of blood or poor colon preparation; failure to visualize the ampulla; failure to perform a careful examination by the endoscopist; and delay in the performance of endoscopic evaluation for more than 48 hours after initial presentation.2,4
SB lesions account for the majority of the etiologies of OGIB (∼75%) and predominantly include vascular lesions (∼70%) in the Western population and ulcerations (∼45%) in the Asian population.5,10-13 In addition to intraluminal etiologies, extraluminal sources, including aortoenteric fistulae, hemobilia, and hemosuccus pancreaticus, can also present as OGIB. Failure to maintain a high index of suspicion for these causes can lead to a significant delay in their diagnosis, as well as unnecessary interventions being performed for incidental lesions detected on endoscopy.14,15 The main etiologies of OGIB are outlined in Table 1.
Table 1.
Etiologies of Obscure Gastrointestinal Bleeding
| Vascular |
| Inflammatory |
|
| Neoplastic |
|
| Extraluminal |
|
| Rare causes |
|
- GAVE=
gastric antral vascular ectasia;
- GIST=
gastrointestinal stromal tumor;
- NSAID=
nonsteroidal anti-inflammatory drug.
Figure 2.
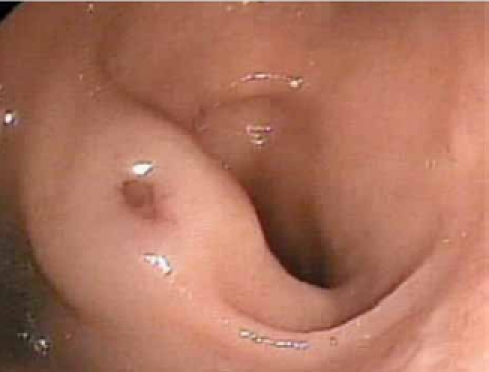
Dieulafoy lesion in the proximal ileum detected on retrograde double-balloon enteroscopy in a patient with overt obscure gastrointestinal bleeding. The location was tattooed with Spot injection. Segmental resection of the small bowel was performed.
Figure 3.
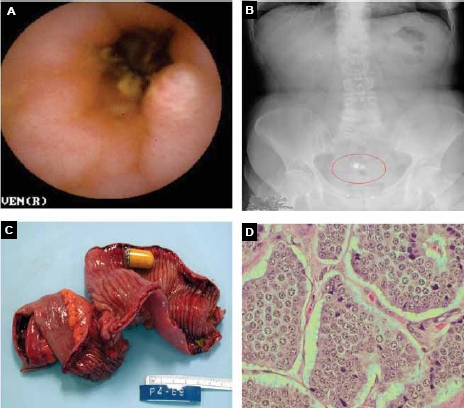
Submucosal tumor with overlying ulceration detected on capsule endoscopy in a 46-year-old man with occult obscure gastrointestinal bleeding (A). Retained capsule seen on abdominal radiograph (B). Gross specimen of resected ileum with carcinoid tumor and retained capsule endoscope (C). Surgical pathology was consistent with nests of neuroendocrine cells, which corresponds to a carcinoid tumor (D).
Figure 4.
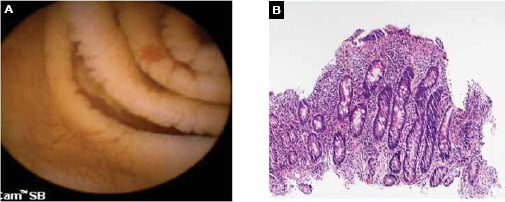
Angioectasia and mucosal scalloping of the small bowel detected on capsule endoscopy in a patient with overt obscure gastrointestinal bleeding and iron-deficiency anemia. Argon plasma coagulation of multiple angioectasias was performed, and small-bowel biopsies were obtained on double-balloon enteroscopy (A). Small-bowel biopsies were consistent with villous atrophy secondary to celiac sprue (B).
Figure 5.
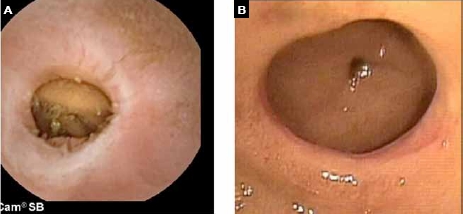
Diaphragm disease related to nonsteroidal anti-inflammatory drug use seen on video capsule endoscopy (A) and confirmed on double-balloon enteroscopy (B) in a patient with occult obscure gastrointestinal bleeding.
Evaluation of Obscure Gastrointestinal Bleeding
A detailed history and physical examination can provide important clues to the underlying etiology, but endoscopic evaluation remains the cornerstone of the diagnosis and management of OGIB. In patients with occult OGIB, it is important to exclude malabsorption and hematologic causes of anemia, and document objective evidence of gastrointestinal bleeding. A thorough SB examination is important, as 2–10% of these patients have been reported to have underlying tumors, of which the majority appear to be malignant.16-19 The main limitations of SB evaluation in the past were related to its length (>6 m) and the limited intubation depth with conventional endoscopy, as well as the low sensitivity of traditional radiologic tests for detection of flat mucosal lesions such as angioectasias.20,21
These shortcomings have been overcome by recent developments in both endoscopic (video CE, SBE, and DBE) and radiologic techniques (CTE). These advances in SB diagnostics, as well as the capacity to successfully perform endoscopic therapeutic interventions, have largely replaced surgical procedures (intra-operative enteroscopy [IOE], laparoscopy, and exploratory laparotomy), resulting in a trend toward noninvasive evaluation and management of OGIB.22-25
Details pertaining to clinical presentation (eg, presence or absence of overt bleeding), nature of bleeding (eg, hematemesis, hematochezia, or melena), bleeding diathesis (eg, von Willebrand disease), medication use (eg, aspirin or nonsteroidal anti-inflammatory drugs), comorbidities (eg, valvular heart disease, vasculitis, or amyloidosis), prior procedures/surgeries (eg, liver biopsy, liver transplantation, abdominal aortic aneurysm repair, or bowel resection), prior radiation therapy, and family history (eg, inflammatory bowel disease or polyposis syndromes) may provide important clues to the underlying etiology of OGIB. Physical examination, including a detailed dermatologic evaluation, may also be useful in the diagnosis of systemic syndromes (eg, hereditary hemorrhagic telangiectasias, amyloidosis, and blue-rubber bleb nevus syndrome).
Traditional Endoscopic Tests
Push Enteroscopy. Push enteroscopy (PE) allows only a limited evaluation of the proximal SB, approximately 50–100 cm distal to the ligament of Treitz. The diagnostic yield of PE is reported to be between 3–70%, with the majority of SB findings being vascular lesions.3,26-28 Interestingly, most of the lesions diagnosed on PE have been found in locations accessible to standard EGD and may account for the lesions missed on initial endoscopy.6,29 Although the use of an overtube may allow for deeper SB intubation (up to 150 cm), it does not appear to increase the diagnostic yield of the test.30
The main disadvantages of PE are related to the looping of the enteroscope and patient discomfort. This technique has been largely replaced by CE for diagnostic evaluation and BAE for endoscopic treatment in the SB. Its role is currently limited to endoscopic therapeutics in patients who have only very proximal SB lesions detected on CE.31
Sonde Enteroscopy. Sonde enteroscopy is an endoscopic technique that is dependent on peristaltic propagation of a flexible enteroscope through the SB. Examination of the SB is then performed during withdrawal of the enteroscope. This modality is no longer utilized in clinical practice due to patient discomfort and long procedure duration.32,33
Intra-operative Enteroscopy. IOE involves evaluation of the SB on laparotomy and may be performed orally, rectally, or via enterotomy, wherein the scope is inserted through a surgical incision in the SB. Although the diagnostic yield of IOE has been reported to be between 58–88%, rebleeding may occur in up to 60% of patients.34-37 Complication rates have been reported to be between 0–52%, and major complications include serosal tears, avulsion of mesenteric vessels, prolonged ileus, and perforation.35,37-39 In addition, earlier reports indicated a high mortality rate of 11% with this procedure.38,40 Due to these reasons, IOE should be reserved only for patients who present with recurrent bleeds requiring multiple transfusions or hospitalizations after a comprehensive negative evaluation.4
New Endoscopic Tests
Video Capsule Endoscopy. Video capsule endoscopes measure 22 mm × 11 mm and have the capacity to capture images at the rate of 2 frames/second over an 8-hour period. Images are transmitted to a recording device and can be downloaded and viewed on a computer station with appropriate software. CE allows noninvasive evaluation of the entire SB in 79–90% of patients, with a diagnostic yield of 38–83% in OGIB.41 The main utility of this test lies in its high positive (94–97%) and negative (83–100%) predictive value in the evaluation of OGIB.42-44 Findings on CE may lead to endoscopic or surgical intervention, or a change in medical management in 37–87% of patients.42,45 After undergoing CE-directed interventions, 50–66% of patients have been reported to remain transfusion-free without recurrent bleeding at follow-up.43,46 Two studies with a mean follow-up period of 17 and 19 months reported a low rebleeding rate of 11% and 5.6%, respectively, in patients with a negative CE.44,47
The yield of CE may be influenced by multiple factors, with a higher likelihood of positive findings in patients with a hemoglobin level of less than 10 g/dL, longer duration of bleeding (>6 months), more than 1 episode of bleeding, overt (rather than occult) bleeding (60% vs 46%), and use of CE within 2 weeks of the bleeding episode (91% vs 34%).48-51
The main limitations of the test include a lack of therapeutic capabilities, inability to control its movement through the gastrointestinal tract, and the high rate of incidental findings in up to 23% of healthy controls.52 Another important disadvantage is the potential for missing solitary lesions in the SB. A pooled analysis of 32 trials that included 691 capsule examinations found a false-negative rate of 11% for all SB findings and 19% for neoplasms with CE.53 CE may also be complicated by retention (1–13%), disintegration, and perforation, which precludes its use in patients with a suspected obstruction or stricture.54-56
Balloon-Assisted Enteroscopy. BAE utilizes the principle of push-and-pull enteroscopy and is comprised of DBE and SBE.57 DBE consists of an enteroscope and an overtube, both of which have balloons at their distal ends, as its name suggests. In comparison, SBE consists of an enteroscope and an overtube, with a balloon on only the overtube. The balloons on the double-balloon enteroscope and overtube are composed of latex, whereas the balloon on the single-balloon overtube is made of silicon. The enteroscope in both systems has a working length of 200 cm, and the overtube is 140 cm in length. The outer diameter is 9.4 mm on the double-balloon enteroscope and 9.2 mm on the single-balloon enteroscope.
The technique of BAE involves a series of steps called an advancement cycle: the enteroscope and overtube are introduced into the SB, and the balloon on the overtube is inflated. The enteroscope is advanced further into the SB. The balloon on the double-balloon enteroscope is then inflated, and the overtube is subsequently advanced over the enteroscope. Both the overtube and enteroscope are then drawn back (with both balloons inflated on DBE, and the overtube balloon inflated and the distal end of the enteroscope hooked over a fold with SBE). This allows the SB to plicate over the enteroscope. By repeating this series of steps, a longer SB distance can be traversed compared to conventional endoscopy. BAE can be performed via the oral (antegrade) or aboral (retrograde) approaches.
Double-Balloon Enteroscopy. DBE allows deeper intubation of the SB (240–360 cm with the antegrade approach and 102–140 cm with the retrograde approach), as compared to PE (90–150 cm) and ileoscopy (50–80 cm).58-61 DBE has the additional advantage over CE of both diagnostic and therapeutic capabilities, including biopsies, tattoo, hemostasis, polypectomy, balloon dilation, and foreign body retrieval (eg, for retained capsules).61-63 The diagnostic yield of DBE is between 60–80% in patients with OGIB and other SB disorders. Successful use of endoscopic therapeutic interventions has been reported in 40–73% of patients.12,58,60,64
Total enteroscopy with DBE is defined as complete evaluation of the SB either with a single approach or a combined antegrade and retrograde approach. The decision to perform total enteroscopy is usually dependent upon the discretion of the endoscopist, degree of clinical suspicion for a SB lesion, and inability to detect the lesion using a single approach. However, despite the best attempts of the endoscopist, total enteroscopy may not be feasible in all patients, as it has a reported success rate ranging from 16% to 86%.5,59,60,65 A higher success rate with total enteroscopy has been reported in the Asian population, as compared to the Western population, and it is unclear whether this is a reflection of the difference in body habitus or technique employed by the endoscopists.
The main limitations of DBE include its invasive nature, prolonged duration, and need for additional personnel. The complication rate for diagnostic procedures is 0.8% and up to 4% if therapeutics such as electrocoagulation, polypectomy, or dilation are performed. The main complications reported with this technique are ileus, pancreatitis, and perforation.58,61,66
Single-Balloon Enteroscopy. SBE is the latest balloon-assisted endoscopic technique that has been introduced for the evaluation and management of SB disorders. A preliminary report of 78 SBE procedures performed in 41 patients, of whom 12 had OGIB, found that SBE allowed evaluation of the SB in a safe and effective manner, including performance of total enteroscopy (25%; 6/24). The diagnostic yield in OGIB was 66% (4/12 patients), and therapeutics such as argon plasma coagulation were successfully performed.24 Another study evaluated 20 patients with suspected SB disorders and found a diagnostic yield of 60% using SBE.67 Similar to DBE, there is a risk of perforation in patients with SB lesions who undergo SBE.24,68
Additional studies are necessary to determine the true efficacy and safety of SBE in the evaluation of OGIB.
Spiral Enteroscopy. The Discovery SB overtube is a spiral-shaped overtube with a working length of 130 cm and a raised helix at the distal end. The technique of spiral enteroscopy allows for advancement and withdrawal of the enteroscope through the SB by using clockwise and counterclockwise movements, respectively. The distal end of the overtube is positioned 25 cm from the tip of the enteroscope and locked into place. The system is then advanced to the ligament of Treitz with gentle rotation. The collar is subsequently unlocked, and the enteroscope is advanced past the ligament of Treitz. The overtube is then advanced using clockwise rotation until pleating of the SB no longer occurs over the enteroscope. The enteroscope is then unlocked and advanced to facilitate further advancement into the SB. In order to ease withdrawal of the enteroscope, the overtube is rotated in a counterclockwise direction.69 A preliminary study of 27 adult patients reported a diagnostic yield of 33% using this technique and an average depth of insertion of 176 cm from the ligament of Treitz.69 Another study of 90 procedures reported a mean depth of insertion of 262±57 cm and a total procedure time of 33.6±8 min.70 This endoscopic modality also allows the use of therapeutics, including biopsies, hemostasis, and polypectomies. Only minor complications of sore throat and minimal mucosal trauma have been reported thus far and no perforations.69
Additional studies are necessary to determine how this technique compares to BAE in the evaluation and management of OGIB.
Comparison of Endoscopic Modalities in Obscure Gastrointestinal Bleeding
Multiple retrospective and prospective studies have found CE to be superior to both PE and SBFT in the evaluation of OGIB. A meta-analysis of studies that compared CE and PE showed that CE had an incremental yield of 30% (yield, 56% vs 26%) for clinically significant findings in patients with OGIB. Similarly, CE had an incremental yield of 36% over SBFT (yield, 42% vs 6%).71 In order to establish 1 additional diagnosis, the number needed to test with CE was 3. Based upon a subanalysis of the data, CE had a higher yield for both vascular and inflammatory lesions. CE has, therefore, largely replaced PE and SBFT in the evaluation of the SB and is currently recommended as the third test of choice in patients with OGIB who have had a negative EGD and colonoscopy.
A study by May and colleagues that compared DBE to PE in 52 patients with OGIB found that DBE not only allowed a greater depth of intubation (230 cm vs 80 cm), but also had a higher yield for SB findings (73% vs 44%).72 Furthermore, DBE facilitated detection of additional lesions in the distal SB in patients who had positive findings on PE.
Several studies have compared the yield of CE to DBE but have shown inconsistent results due to their small sample size. A meta-analysis of 11 studies that compared these modalities in patients with SB disease (the majority with OGIB) showed a comparable diagnostic yield (60% vs 57%; IYw 3%) for all SB findings. The yield for these tests was also similar for vascular, inflammatory, and neoplastic lesions.73 Another meta-analysis of 8 studies also found no difference in diagnostic yield between the two tests for the evaluation of SB disease (odds ratio [OR], 1.21 [95% confidence interval {CI}, 0.64–2.29]). In patients with OGIB, CE had a higher yield compared to DBE using a single approach (OR, 1.61 [95% CI, 1.07–2.43]) but a significantly lower yield compared to DBE using a combined antegrade and retrograde approach (OR, 0.12 [95% CI, 0.03–0.52]).74 This finding reinforces the importance of total enteroscopy with DBE in patients with a high clinical suspicion for a SB lesion.
CE may be useful as a screening tool prior to DBE in patients with OGIB. This approach of a targeted DBE has been shown to increase both the diagnostic (73–93%) and therapeutic (57–73%) yields of the test. Furthermore, CE transit times appear to be useful in guiding the optimal route of DBE. Due to deeper intubation of the SB and a higher success rate with the antegrade approach, this is the preferred route for lesions suspected of being within the proximal 75% of the SB based upon transit time, whereas the retrograde route is used for more distal lesions. Due to the high negative predictive value of CE, the approach of CE-guided DBE allows avoidance of DBE in patients with a low pretest probability for SB findings.75-77
However, the concept of CE-guided DBE may not be applicable in all patients. A study evaluated the outcomes of 51 patients with OGIB who underwent both CE and DBE. The overall yield with both tests was similar. Nevertheless, CE detected 31 lesions not found on DBE, and, similarly, DBE detected 21 lesions missed on CE.78 CE has been found to have a false-negative rate of 11% for all SB findings and, more importantly, 19% for neoplasms. A study that evaluated the role of repeat CE in patients with OGIB found additional findings on the second examination in up to 75% of patients with OGIB, leading to a change in management in 62% of patients.79 There have also been several reports of neoplasms missed on CE and subsequently diagnosed on DBE.80,81 Hence, in patients with a negative CE in whom there is a high clinical suspicion for a SB lesion, DBE should still be pursued, including consideration for total enteroscopy.82
The indications for DBE in OGIB are manifold and include patients who have a positive CE, both for tissue diagnosis and therapeutics; patients in whom CE is contraindicated; patients with a negative CE, but high clinical suspicion for SB lesions; and in patients with active bleeding.
A cost-effectiveness analysis that compared various diagnostic modalities (PE, DBE, CE-guided DBE, angiography, and IOE) found that DBE was not only the most cost-effective approach in the evaluation of overt OGIB, but also had the highest success rate for bleeding cessation. However, the investigators concluded that CE-guided DBE may be associated with better long-term outcomes compared to the initial DBE approach due to decreased risk of complications and appropriate utilization of endoscopic resources.83
Radiologic Evaluation
The primary objective of traditional radiologic tests, including tagged red blood cell scans and angiography, is localization of the bleeding source, with the intent to perform therapeutic embolization of the feeding blood vessel. Novel imaging studies such as CTE and computed tomography angiography (CTA) not only allow localization of the bleeding source and diagnosis of the underlying etiology of OGIB, but also facilitate both luminal and extraluminal evaluation of the SB.
Older Radiologic Tests
Technetium 99m-labeled Red Blood Cell Nuclear Scan. Technetium 99m-labeled red blood cell nuclear scan can detect gastrointestinal bleeding at a rate of 0.1–0.4 mL/min. Due to its noninvasive nature and higher sensitivity, as compared to angiography, this test has been considered useful for screening and localization of bleeding, prior to the use of selective angiography. However, studies have shown that red blood cell scans have a low accuracy for localization of bleeding source and may, hence, be of limited utility in the evaluation of OGIB.84 Moreover, delay in scanning may lead to misinterpretation of findings, with pooling of blood in dependent sites being mistaken for the bleeding source.85
Technetium 99m Pertechnetate Scintigraphy (Meckel Scan). Technetium 99m pertechnetate scintigraphy (Meckel scan) is useful in the diagnosis of Meckel diverticulum. This test relies on the uptake of the pertechnetate anion by ectopic gastric mucosa and has a sensitivity of 64–100%.86-89 False-negative results may be due to a recent barium radiograph, the small size of the diverticulum, an impaired vascular supply, or washout of the isotope in the setting of active gastrointestinal bleeding.89,90
Angiography. Angiography is useful for both localization of active bleeding and diagnosis of nonbleeding vascular lesions and tumors. Angiography has the potential to detect bleeding at a rate of more than 0.5 mL/min. Its yield has been reported to be dependent upon the rate of bleeding, with successful localization of the bleeding source in 50–75% of patients with active bleeding and less than 50% in patients with slow or cessation of bleed-ing.91 The classic angiographic finding of an angioectasia is a slow filling vein. Other findings include the presence of a vascular tuft and an early filling vein.92 The main benefit of angiography is the ability to perform therapeutic embolization with the use of Gelfoam or coils.93 The procedure may be associated with complications of pseudoaneurysm, arterial thrombosis, dissection, and bowel infarction.94
Provocative testing with the use of anticoagulants (heparin) and antifibrinolytics (streptokinase [Streptase, Aventis Behring] and urokinase [Kinlytic, Microbix Bio-systems]) prior to angiography has been found to be of limited value and, therefore, may not be a safe or cost-effective approach in the evaluation of OGIB.4
New Radiologic Tests
Advances in computed tomography imaging, particularly of the SB, have increased the use of this technique in the evaluation of gastrointestinal bleeding. Several computed tomography protocols have been described, including CTA, CTE, and computed tomography enteroclysis (CTentero). These techniques can differ in the amount and route of oral contrast administration and the number of imaging phases related to intravenous contrast administration (precontrast, arterial, venous, and delayed).
Computed Tomography Enterography and Computed Tomography Enteroclysis. CTE and CT-entero are dedicated examinations of the SB that allow the detection of both vascular lesions and tumors. The technique optimizes luminal distension by administering larger volumes of neutral oral contrast via a peroral (CTE) or nasojejunal intubation (CT-entero) approach, thereby allowing optimal visualization of mucosal details and vasculature.
Unlike the evaluation of inflammatory bowel disease, which acquires images only during a single phase, evaluation of gastrointestinal bleeding usually involves multiphasic imaging (arterial, enteric, and delayed imaging, with or without precontrast images).25,95 A study that evaluated 22 patients with OGIB using multiphasic CTE reported a diagnostic yield of 45%.96
Typical features of angiodysplasias on computed tomography include the presence of a vascular tuft in the arterial phase and an early draining mesenteric vein. Active bleeding may also be identified on multiphasic imaging by the increasing accumulation of contrast in the SB lumen.25 CTE has the additional advantage of identification of SB strictures/obstruction prior to CE and provides important information on luminal and extraluminal findings that cannot be detected on CE.97
The technique may be limited by inadequate bowel distention with oral contrast due to patient intolerance, bowel obstruction, or gastrointestinal dysmotility, and contraindication to the use of intravenous contrast in patients with renal insufficiency or contrast allergy.
Computed Tomography Angiography. CTA is a technique that uses less neutral oral contrast material than CTE and therefore has less luminal distention. Vascular contrast is most typically administered intravenously but occasionally has been described intra-arterially via mesenteric angiography or aortography, followed by computed tomography imaging.98 The computed tomography appearance of vascular lesions will generally be identical to the appearance on CTE/CT-entero using intravenous contrast, with more intense enhancement if an intra-arterial approach is used. CTA may be preferred over CTE/CT-entero if an emergent examination is required (massive gastrointestinal hemorrhage) or the patient is intolerant to oral contrast.
Management of Obscure Gastrointestinal Bleeding
The management of patients with OGIB should be individualized based upon several important factors, including clinical presentation (obscure versus occult gastrointestinal bleeding), type of bleeding (melena or hematochezia), duration and frequency of bleeding, severity and acuity of bleeding, need for packed red blood cell (PRBC) transfusions, presence or absence of iron-deficiency anemia, and associated clinical symptoms (abdominal pain and/or weight loss). As medical management has not been shown to be effective in the long-term management of patients with OGIB, definitive treatment with endoscopic interventions, angiographic embolization, or surgical resection continues to remain the mainstay in the initial management of these patients. Supportive management with iron therapy and/or PRBC transfusions is a reasonable option in the subset of patients who have undergone a comprehensive negative diagnostic evaluation; those with recurrent bleeding (without hemodynamic instability) after undergoing endoscopic/radiologic treatment or surgery; and/or those with contraindications for endoscopic/radiologic management or surgery.
We propose the following algorithm in the evaluation of patients with OGIB. The diagnostic approach is, in part, determined by the clinical presentation of the patient. A second-look EGD and colonoscopy should be considered in all patients with occult or recurrent overt bleeding due to the high rate of missed lesions. If no bleeding source is identified on conventional endoscopy, SB evaluation with CE should be pursued. Therapeutics can then be performed using PE or BAE, as warranted, based upon the type and location of the finding. If the lesion is not amenable to endoscopic treatment, appropriate medical or surgical management should be pursued. In those patients in whom CE is contraindicated due to suspected/known obstruction or stricture, and in patients in whom a tumor is suspected, CTE may be the preferred initial test for SB evaluation. A Meckel scan may also be performed in younger patients presenting with OGIB.
In patients with active overt bleeding, management should be individualized according to the clinical situation. If the patient is hemodynamically stable and endoscopic resources are available, the endoscopist may either perform CE-guided BAE or proceed directly to BAE after a negative evaluation using bidirectional endoscopy. In the setting of brisk or massive bleeding or hemodynamic instability, it would be prudent to proceed with a radioisotope bleeding scan and/or angiography to localize and treat the bleeding source. IOE should be reserved for patients with severe recurrent bleeding and transfusion dependency and those with a SB lesion not accessible with BAE.
Although medical management with hormonal therapy (estrogen with/without progesterone),99,100 somatostatin analogues,101 thalidomide,102 erythropoietin, and von Willebrand factor103 have all been utilized, these modalities do not appear to be useful in the long-term management of most patients with ongoing gastrointestinal bleeding. Rebleeding can occur in up to 46% of patients after medical management.104 Hormonal therapy may lead to improved vascular integrity and decreased vascular angiogenesis by inhibiting endothelial growth factor.105,106 The use of ethinyl estradiol (0.035 mg) with or without norethisterone (1 mg) has been found to be useful in patients with hereditary hemorrhagic telangiectasia, von Willebrand disease, and renal failure.107,108 Its role in the management of patients with sporadic angioectasias remains a matter of controversy.99,100 A study evaluated the role of hormonal therapy in 40 patients with OGIB and found that patients remained transfusion-free without rebleeding as long as they continued treatment.109 However, a larger randomized trial of 72 patients with sporadic angioectasias found no benefit with the use of hormonal therapy.100 Small case series evaluating the utility of somatostatin analogues, thalidomide, von Willebrand factor, and erythropoietin have all shown conflicting results regarding the benefits of the medications in these patients. Hence, medical management should be reserved for patients with a negative comprehensive evaluation and those who fail endoscopic, radiologic, and/or surgical management.
An approach to the evaluation and management of OGIB is presented in Figure 1.
Figure 1.
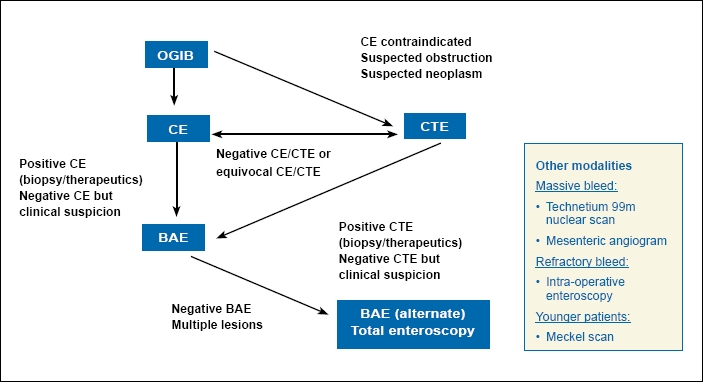
Evaluation and management of obscure gastrointestinal bleeding (OGIB).
BAE=balloon-assisted enteroscopy; CE=capsule endoscopy; CTE=computed tomography enterography.
Summary
OGIB represents one of the most challenging disorders faced by gastroenterologists due to its evasive nature and relative dearth of endoscopic and radiologic tools to facilitate an adequate evaluation of the gastrointestinal tract. However, the introduction of new SB imaging and endoscopic modalities has served to largely overcome these obstacles. With rapidly evolving technology, our ability to diagnose and treat patients with OGIB has improved enormously, resulting in a significant change in the paradigm of the management of this disorder.
Contributor Information
Shabana F. Pasha, Dr. Pasha serves as Assistant Professor of Medicine.
Amy K. Hara, Dr. Hara is Associate Professor of Radiology in the Department of Radiology at the Mayo Clinic in Scottsdale, Arizona.
Jonathan A. Leighton, Dr. Leighton is Professor of Medicine and Chair of the Division of Gastroenterology at the Mayo Clinic in Scottsdale, Arizona.
References
- 1.Thompson JN, Salem RR, Hemingway AP, Rees HC, Hodgson HJ, et al. Specialist investigation of obscure gastrointestinal bleeding. Gut. 1987;28:47–51. doi: 10.1136/gut.28.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S, Rockey DC. Obscure gastrointestinal bleeding. Gastroenterol Clin North Am. 2005;34:679–698. doi: 10.1016/j.gtc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697–1717. doi: 10.1053/j.gastro.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Leighton JA, Goldstein J, Hirota W, Jacobson BC, Johanson JF, et al. Obscure gastrointestinal bleeding. Gastrointest Endosc. 2003;58:650–655. doi: 10.1016/s0016-5107(03)01995-3. [DOI] [PubMed] [Google Scholar]
- 5.Ell C, May A. Mid-gastrointestinal bleeding: capsule endoscopy and push-and-pull enteroscopy give rise to a new medical term. Endoscopy. 2006;38:73–75. doi: 10.1055/s-2005-921131. [DOI] [PubMed] [Google Scholar]
- 6.Zaman A, Katon RM. Push enteroscopy for obscure gastrointestinal bleeding yields a high incidence of proximal lesions within reach of a standard endoscope. Gastrointest Endosc. 1998;47:372–376. doi: 10.1016/s0016-5107(98)70221-4. [DOI] [PubMed] [Google Scholar]
- 7.Descamps C, Schmit A, Van Gossum A. “Missed” upper gastrointestinal tract lesions may explain “occult” bleeding. Endoscopy. 1999;31:452–455. doi: 10.1055/s-1999-151. [DOI] [PubMed] [Google Scholar]
- 8.Spiller RC, Parkins RA. Recurrent gastrointestinal bleeding of obscure origin: report of 17 cases and a guide to logical management. Br J Surg. 1983;70:489–493. doi: 10.1002/bjs.1800700812. [DOI] [PubMed] [Google Scholar]
- 9.Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499–503. doi: 10.1055/s-2004-814399. [DOI] [PubMed] [Google Scholar]
- 10.Cellier C. Obscure gastrointestinal bleeding: role of videocapsule and double-balloon enteroscopy. Best Pract Res Clin Gastroenterol. 2008;22:329–340. doi: 10.1016/j.bpg.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Thompson JN, Hemingway AP, McPherson GA, Rees HC, Allison DJ, Spencer J. Obscure gastrointestinal haemorrhage of small-bowel origin. Br Med J (Clin Res Ed) 1984;288:1663–1665. doi: 10.1136/bmj.288.6431.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto H, Kita H, Sunada K, Hayashi Y, Sato H, et al. Clinical outcomes of double-balloon endoscopy for the diagnosis and treatment of small-intestinal diseases. Clin Gastroenterol Hepatol. 2004;2:1010–1016. doi: 10.1016/s1542-3565(04)00453-7. [DOI] [PubMed] [Google Scholar]
- 13.Fry LC, Bellutti M, Neumann H, Malfertheiner P, Monkemuller K. Incidence of bleeding lesions within reach of conventional upper and lower endoscopes in patients undergoing double-balloon enteroscopy for obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2009;29:342–349. doi: 10.1111/j.1365-2036.2008.03888.x. [DOI] [PubMed] [Google Scholar]
- 14.Toyoki Y, Hakamada K, Narumi S, Nara M, Ishido K, Sasaki M. Hemosuccus pancreaticus: problems and pitfalls in diagnosis and treatment. World J Gastroenterol. 2008;14:2776–2779. doi: 10.3748/wjg.14.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasha SF, Blair JE, Garvey PB, Gray RJ, Mulligan DC, et al. Hemosuccus pancreaticus in the era of capsule endoscopy and double balloon enteroscopy complicated by multifocal mycobacterium chelonae/abscessus infection. Case Rep Gastroenterol. 2007;1:38–47. doi: 10.1159/000104977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rondonotti E, Pennazio M, Toth E, Menchen P, Riccioni ME, et al. Small-bowel neoplasms in patients undergoing video capsule endoscopy: a multicenter European study. Endoscopy. 2008;40:488–495. doi: 10.1055/s-2007-995783. [DOI] [PubMed] [Google Scholar]
- 17.Bailey AA, Debinski HS, Appleyard MN, Remedios ML, Hooper JE, et al. Diagnosis and outcome of small bowel tumors found by capsule endoscopy: a three-center Australian experience. Am J Gastroenterol. 2006;101:2237–2243. doi: 10.1111/j.1572-0241.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 18.Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer. 2006;107:22–27. doi: 10.1002/cncr.21975. [DOI] [PubMed] [Google Scholar]
- 19.Pasha SF, Sharma VK, Carey EJ, Shiff AD, Heigh RI, et al. Utility of video capsule endoscopy in the detection of small bowel tumors–a single center experience of 1000 consecutive patients. 2007. Presented at ICCE; Madrid, Spain. Abstract 45.
- 20.Upchurch BR, Vargo JJ. Small bowel enteroscopy. Rev Gastroenterol Disord. 2008;8:169–177. [PubMed] [Google Scholar]
- 21.Lewis BS.Small intestinal bleeding Gastroenterol Clin North Am 20002967–95. vi [PubMed] [Google Scholar]
- 22.Buchman AL, Wallin A. Videocapsule endoscopy renders obscure gastrointestinal bleeding no longer obscure. J Clin Gastroenterol. 2003;37:303–306. doi: 10.1097/00004836-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto H, Yano T, Kita H, Sunada K, Ido K, Sugano K.New system of double-balloon enteroscopy for diagnosis and treatment of small intestinal disorders Gastroenterology 20031251556 author reply 1556-1557 [DOI] [PubMed] [Google Scholar]
- 24.Tsujikawa T, Saitoh Y, Andoh A, Imaeda H, Hata K, et al. Novel single-balloon enteroscopy for diagnosis and treatment of the small intestine: preliminary experiences. Endoscopy. 2008;40:11–15. doi: 10.1055/s-2007-966976. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen SR, Huprich JE, Hara AK. CT enterography: noninvasive evaluation of Crohn’s disease and obscure gastrointestinal bleed. Radiol Clin North Am. 2007;45:303–315. doi: 10.1016/j.rcl.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Chak A, Koehler MK, Sundaram SN, Cooper GS, Canto MI, Sivak MV., Jr Diagnostic and therapeutic impact of push enteroscopy: analysis of factors associated with positive findings. Gastrointest Endosc. 1998;47:18–22. doi: 10.1016/s0016-5107(98)70293-7. [DOI] [PubMed] [Google Scholar]
- 27.Lin S, Branch MS, Shetzline M. The importance of indication in the diagnostic value of push enteroscopy. Endoscopy. 2003;35:315–321. doi: 10.1055/s-2003-38144. [DOI] [PubMed] [Google Scholar]
- 28.Chak A, Cooper GS, Canto MI, Pollack BJ, Sivak MV., Jr Enteroscopy for the initial evaluation of iron deficiency. Gastrointest Endosc. 1998;47:144–148. doi: 10.1016/s0016-5107(98)70347-5. [DOI] [PubMed] [Google Scholar]
- 29.Linder J, Cheruvattath R, Truss C, Wilcox CM. Diagnostic yield and clinical implications of push enteroscopy: results from a nonspecialized center. J Clin Gastroenterol. 2002;35:383–386. doi: 10.1097/00004836-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 30.Taylor AC, Chen RY, Desmond PV. Use of an overtube for enteroscopy--does it increase depth of insertion? A prospective study of enteroscopy with and without an overtube. Endoscopy. 2001;33:227–230. doi: 10.1055/s-2001-12799. [DOI] [PubMed] [Google Scholar]
- 31.Sidhu R, McAlindon ME, Kapur K, Hurlstone DP, Wheeldon MC, Sanders DS. Push enteroscopy in the era of capsule endoscopy. J Clin Gastroenterol. 2008;42:54–58. doi: 10.1097/01.mcg.0000225655.85060.74. [DOI] [PubMed] [Google Scholar]
- 32.Gostout CJ. Sonde enteroscopy. Technique, depth of insertion, and yield of lesions. Gastrointest Endosc Clin N Am. 1996;6:777–792. [PubMed] [Google Scholar]
- 33.Waye JD. Small-intestinal endoscopy. Endoscopy. 2001;33:24–30. doi: 10.1055/s-2001-11184. [DOI] [PubMed] [Google Scholar]
- 34.Zaman A, Sheppard B, Katon RM. Total peroral intraoperative enteroscopy for obscure GI bleeding using a dedicated push enteroscope: diagnostic yield and patient outcome. Gastrointest Endosc. 1999;50:506–510. doi: 10.1016/s0016-5107(99)70073-8. [DOI] [PubMed] [Google Scholar]
- 35.Lopez MJ, Cooley JS, Petros JG, Sullivan JG, Cave DR. Complete intraoperative small-bowel endoscopy in the evaluation of occult gastrointestinal bleeding using the sonde enteroscope. Arch Surg. 1996;131:272–277. doi: 10.1001/archsurg.1996.01430150050010. [DOI] [PubMed] [Google Scholar]
- 36.Cave DR, Cooley JS. Intraoperative enteroscopy. Indications and techniques. Gastrointest Endosc Clin N Am. 1996;6:793–802. [PubMed] [Google Scholar]
- 37.Ress AM, Benacci JC, Sarr MG. Efficacy of intraoperative enteroscopy in diagnosis and prevention of recurrent, occult gastrointestinal bleeding. Am J Surg. 1992;163:94–98. doi: 10.1016/0002-9610(92)90259-t. discussion 98-99. [DOI] [PubMed] [Google Scholar]
- 38.Desa LA, Ohri SK, Hutton KA, Lee H, Spencer J. Role of intraoperative enteroscopy in obscure gastrointestinal bleeding of small bowel origin. Br J Surg. 1991;78:192–195. doi: 10.1002/bjs.1800780219. [DOI] [PubMed] [Google Scholar]
- 39.Whelan RL, Buls JG, Goldberg SM, Rothenberger DA. Intra-operative endoscopy. University of Minnesota experience. Am Surg. 1989;55:281–286. [PubMed] [Google Scholar]
- 40.Lewis BS, Wenger JS, Waye JD. Small bowel enteroscopy and intraoperative enteroscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 1991;86:171–174. [PubMed] [Google Scholar]
- 41.Rondonotti E, Villa F, Mulder CJ, Jacobs MA, de Franchis R. Small bowel capsule endoscopy in 2007: indications, risks and limitations. World J Gastroenterol. 2007;13:6140–6149. doi: 10.3748/wjg.v13.i46.6140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 43.Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy. 2004;36:1067–1073. doi: 10.1055/s-2004-826034. [DOI] [PubMed] [Google Scholar]
- 44.Lai LH, Wong GL, Chow DK, Lau JY, Sung JJ, Leung WK. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol. 2006;101:1224–1228. doi: 10.1111/j.1572-0241.2006.00565.x. [DOI] [PubMed] [Google Scholar]
- 45.Ben Soussan E, Antonietti M, Herve S, Savoye G, Ramirez S, et al. Diagnostic yield and therapeutic implications of capsule endoscopy in obscure gastrointestinal bleeding. Gastroenterol Clin Biol. 2004;28:1068–1073. doi: 10.1016/s0399-8320(04)95183-4. [DOI] [PubMed] [Google Scholar]
- 46.Estevez E, Gonzalez-Conde B, Vazquez-Iglesias JL, de Los Angeles Vazquez-Millan M, Pertega S, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol. 2006;18:881–888. doi: 10.1097/00042737-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc. 2008;68:1122–1127. doi: 10.1016/j.gie.2008.06.054. [DOI] [PubMed] [Google Scholar]
- 48.Bresci G, Parisi G, Bertoni M, Tumino E, Capria A. The role of video capsule endoscopy for evaluating obscure gastrointestinal bleeding: usefulness of early use. J Gastroenterol. 2005;40:256–259. doi: 10.1007/s00535-004-1532-5. [DOI] [PubMed] [Google Scholar]
- 49.Carey EJ, Leighton JA, Heigh RI, Shiff AD, Sharma VK, et al. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol. 2007;102:89–95. doi: 10.1111/j.1572-0241.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- 50.May A, Wardak A, Nachbar L, Remke S, Ell C. Influence of patient selection on the outcome of capsule endoscopy in patients with chronic gastrointestinal bleeding. J Clin Gastroenterol. 2005;39:684–688. doi: 10.1097/01.mcg.0000173857.22933.3b. [DOI] [PubMed] [Google Scholar]
- 51.Mishkin DS, Chuttani R, Croffie J, Disario J, Liu J, et al. ASGE Technology Status Evaluation Report: wireless capsule endoscopy. Gastrointest Endosc. 2006;63:539–545. doi: 10.1016/j.gie.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein JL, Eisen GM, Lewis B, Gralnek IM, Zlotnick S, Fort JG. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib naproxen plus omeprazole and placebo. Clin Gastroenterol Hepatol. 2005;3:133–141. doi: 10.1016/s1542-3565(04)00619-6. [DOI] [PubMed] [Google Scholar]
- 53.Lewis BS, Eisen GM, Friedman S. A pooled analysis to evaluate results of capsule endoscopy trials. Endoscopy. 2005;37:960–965. doi: 10.1055/s-2005-870353. [DOI] [PubMed] [Google Scholar]
- 54.Fry LC, De Petris G, Swain JM, Fleischer DE. Impaction and fracture of a video capsule in the small bowel requiring laparotomy for removal of the capsule fragments. Endoscopy. 2005;37:674–676. doi: 10.1055/s-2005-870252. [DOI] [PubMed] [Google Scholar]
- 55.Cheifetz AS, Kornbluth AA, Legnani P, Schmelkin I, Brown A, et al. The risk of retention of the capsule endoscope in patients with known or suspected Crohn’s disease. Am J Gastroenterol. 2006;101:2218–2222. doi: 10.1111/j.1572-0241.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 56.Um S, Poblete H, Zavotsky J.Small bowel perforation caused by an impacted endocapsule Endoscopy 200840 (suppl 2) E122–E123. [DOI] [PubMed] [Google Scholar]
- 57.Monkemuller K, Fry LC, Bellutti M, Malfertheiner P.Balloon-assisted enteroscopy: unifying double-balloon and single-balloon enteroscopy Endoscopy 200840537 author reply 539 [DOI] [PubMed] [Google Scholar]
- 58.May A, Nachbar L, Ell C. Double-balloon enteroscopy (push-and-pull enteroscopy) of the small bowel: feasibility and diagnostic and therapeutic yield in patients with suspected small bowel disease. Gastrointest Endosc. 2005;62:62–70. doi: 10.1016/s0016-5107(05)01586-5. [DOI] [PubMed] [Google Scholar]
- 59.Mehdizadeh S, Ross A, Gerson L, Leighton J, Chen A, et al. What is the learning curve associated with double-balloon enteroscopy? Technical details and early experience in 6 U.S. tertiary care centers. Gastrointest Endosc. 2006;64:740–750. doi: 10.1016/j.gie.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Gross SA, Stark ME. Initial experience with double-balloon enteroscopy at a U.S. center. Gastrointest Endosc. 2008;67:890–897. doi: 10.1016/j.gie.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 61.Heine GD, Hadithi M, Groenen MJ, Kuipers EJ, Jacobs MA, Mulder CJ. Double-balloon enteroscopy: indications, diagnostic yield and complications in a series of 275 patients with suspected small-bowel disease. Endoscopy. 2006;38:42–48. doi: 10.1055/s-2005-921188. [DOI] [PubMed] [Google Scholar]
- 62.Nakamura M, Niwa Y, Ohmiya N, Miyahara R, Ohashi A, et al. Preliminary comparison of capsule endoscopy and double-balloon enteroscopy in patients with suspected small-bowel bleeding. Endoscopy. 2006;38:59–66. doi: 10.1055/s-2005-870446. [DOI] [PubMed] [Google Scholar]
- 63.May A, Ell C. Push-and-pull enteroscopy using the double-balloon technique/ double-balloon enteroscopy. Dig Liver Dis. 2006;38:932–938. doi: 10.1016/j.dld.2006.07.101. [DOI] [PubMed] [Google Scholar]
- 64.Zhong J, Ma T, Zhang C, Sun B, Chen S, et al. A retrospective study of the application on double-balloon enteroscopy in 378 patients with suspected small-bowel diseases. Endoscopy. 2007;39:208–215. doi: 10.1055/s-2007-966190. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto H, Sekine Y, Sato Y, Higashizawa T, Miyata T, et al. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest Endosc. 2001;53:216–220. doi: 10.1067/mge.2001.112181. [DOI] [PubMed] [Google Scholar]
- 66.Mensink PB, Haringsma J, Kucharzik T, Cellier C, Perez-Cuadrado E, et al. Complications of double balloon enteroscopy: a multicenter survey. Endoscopy. 2007;39:613–615. doi: 10.1055/s-2007-966444. [DOI] [PubMed] [Google Scholar]
- 67.Vargo JJ, Upchurch BR, Dumot J, Zuccaro G, Stevens T, Santisi J.Clinical utility of the Olympus single balloon enteroscope: the initial U.S. experience Gastrointest Endosc 200765AB9017536233 [Google Scholar]
- 68.Tominaga K, Iida T, Nakamura Y, Nagao J, Yokouchi Y, Maetani I.Small intestinal perforation of endoscopically unrecognized lesions during peroral single-balloon enteroscopy Endoscopy. 200840 (suppl 2) E213–E214. [DOI] [PubMed] [Google Scholar]
- 69.Akerman PA, Agrawal D, Chen W, Cantero D, Avila J, Pangtay J. Spiral enteroscopy: a novel method of enteroscopy by using the Endo-Ease Discovery SB overtube and a pediatric colonoscope. Gastrointest Endosc. 2009;69:327–332. doi: 10.1016/j.gie.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 70.Buscaglia JM, Dunbar KB, Okolo PI 3rd, Judah J, Akerman PA, et al. The spiral enteroscopy training initiative: results of a prospective study evaluating the Discovery SB overtube device during small bowel enteroscopy (with video) Endoscopy. 2009;41:194–199. doi: 10.1055/s-0028-1119602. [DOI] [PubMed] [Google Scholar]
- 71.Triester SL, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:2407–2418. doi: 10.1111/j.1572-0241.2005.00274.x. [DOI] [PubMed] [Google Scholar]
- 72.May A, Nachbar L, Schneider M, Ell C. Prospective comparison of push enteroscopy and push-and-pull enteroscopy in patients with suspected small-bowel bleeding. Am J Gastroenterol. 2006;101:2016–2024. doi: 10.1111/j.1572-0241.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 73.Pasha SF, Leighton JA, Das A, Harrison ME, Decker GA, et al. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol. 2008;6:671–676. doi: 10.1016/j.cgh.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol. 2007;13:4372–4378. doi: 10.3748/wjg.v13.i32.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gay G, Delvaux M, Fassler I. Outcome of capsule endoscopy in determining indication and route for push-and-pull enteroscopy. Endoscopy. 2006;38:49–58. doi: 10.1055/s-2005-921176. [DOI] [PubMed] [Google Scholar]
- 76.Kaffes AJ, Siah C, Koo JH. Clinical outcomes after double-balloon enteroscopy in patients with obscure GI bleeding and a positive capsule endoscopy. Gastrointest Endosc. 2007;66:304–309. doi: 10.1016/j.gie.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 77.Hendel JW, Vilmann P, Jensen T. Double-balloon endoscopy: who needs it? Scand J Gastroenterol. 2008;43:363–367. doi: 10.1080/00365520701799468. [DOI] [PubMed] [Google Scholar]
- 78.Kamalaporn P, Cho S, Basset N, Cirocco M, May G, et al. Double-balloon enteroscopy following capsule endoscopy in the management of obscure gastrointestinal bleeding: outcome of a combined approach. Can J Gastroenterol. 2008;22:491–495. doi: 10.1155/2008/942731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jones BH, Fleischer DE, Sharma VK, Heigh RI, Shiff AD, et al. Yield of repeat wireless video capsule endoscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol. 2005;100:1058–1064. doi: 10.1111/j.1572-0241.2005.40722.x. [DOI] [PubMed] [Google Scholar]
- 80.Ross A, Mehdizadeh S, Tokar J, Leighton JA, Kamal A, et al. Double balloon enteroscopy detects small bowel mass lesions missed by capsule endoscopy. Dig Dis Sci. 2008;53:2140–2143. doi: 10.1007/s10620-007-0110-0. [DOI] [PubMed] [Google Scholar]
- 81.Postgate A, Despott E, Burling D, Gupta A, Phillips R, et al. Significant small-bowel lesions detected by alternative diagnostic modalities after negative capsule endoscopy. Gastrointest Endosc. 2008;68:1209–1214. doi: 10.1016/j.gie.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 82.Pasha SF, Leighton JA. How useful is capsule endoscopy for the selection of patients for double-balloon enteroscopy? Nat Clin Pract Gastroenterol Hepatol. 2008;5:490–491. doi: 10.1038/ncpgasthep1201. [DOI] [PubMed] [Google Scholar]
- 83.Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc. 2008;68:920–936. doi: 10.1016/j.gie.2008.01.035. [DOI] [PubMed] [Google Scholar]
- 84.Voeller GR, Bunch G, Britt LG. Use of technetium-labeled red blood cell scintigraphy in the detection and management of gastrointestinal hemorrhage. Surgery. 1991;110:799–804. [PubMed] [Google Scholar]
- 85.Emslie JT, Zarnegar K, Siegel ME, Beart RW., Jr Technetium-99m-labeled red blood cell scans in the investigation of gastrointestinal bleeding. Dis Colon Rectum. 1996;39:750–754. doi: 10.1007/BF02054439. [DOI] [PubMed] [Google Scholar]
- 86.Sfakianakis GN, Conway JJ. Detection of ectopic gastric mucosa in Meckel’s diverticulum and in other aberrations by scintigraphy: ii. indications and methods—a 10-year experience. J Nucl Med. 1981;22:732–738. [PubMed] [Google Scholar]
- 87.Brown CK, Olshaker JS. Meckel’s diverticulum. Am J Emerg Med. 1988;6:157–164. doi: 10.1016/0735-6757(88)90055-1. [DOI] [PubMed] [Google Scholar]
- 88.Schwartz MJ, Lewis JH. Meckel’s diverticulum: pitfalls in scintigraphic detection in the adult. Am J Gastroenterol. 1984;79:611–618. [PubMed] [Google Scholar]
- 89.Garretson DC, Frederich ME. Meckel’s diverticulum. Am Fam Physician. 1990;42:115–119. [PubMed] [Google Scholar]
- 90.DiGiacomo JC, Cottone FJ. Surgical treatment of Meckel’s diverticulum. South Med J. 1993;86:671–675. doi: 10.1097/00007611-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 91.Browder W, Cerise EJ, Litwin MS. Impact of emergency angiography in massive lower gastrointestinal bleeding. Ann Surg. 1986;204:530–536. doi: 10.1097/00000658-198611000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lewis B, Goldfarb N. Review article: The advent of capsule endoscopy--a not-so-futuristic approach to obscure gastrointestinal bleeding. Aliment Pharmacol Ther. 2003;17:1085–1096. doi: 10.1046/j.1365-2036.2003.01556.x. [DOI] [PubMed] [Google Scholar]
- 93.Rahn NH 3rd, Tishler JM, Han SY, Russinovich NA. Diagnostic and interventional angiography in acute gastrointestinal hemorrhage. Radiology. 1982;143:361–366. doi: 10.1148/radiology.143.2.6978500. [DOI] [PubMed] [Google Scholar]
- 94.Silverstein FE, Gilbert DA, Tedesco FJ, Buenger NK, Persing J. The national ASGE survey on upper gastrointestinal bleeding. I. Study design and baseline data. Gastrointest Endosc. 1981;27:73–79. doi: 10.1016/s0016-5107(81)73155-9. [DOI] [PubMed] [Google Scholar]
- 95.Maglinte DD, Sandrasegaran K, Lappas JC, Chiorean M. CT enteroclysis. Radiology. 2007;245:661–671. doi: 10.1148/radiol.2453060798. [DOI] [PubMed] [Google Scholar]
- 96.Huprich JE, Fletcher JG, Alexander JA, Fidler JL, Burton SS, McCullough CH. Obscure gastrointestinal bleeding: evaluation with 64-section multiphase CT enterography--initial experience. Radiology. 2008;246:562–571. doi: 10.1148/radiol.2462061920. [DOI] [PubMed] [Google Scholar]
- 97.Filippone A, Cianci R, Milano A, Valeriano S, Di Mizio V, Storto ML. Obscure gastrointestinal bleeding and small bowel pathology: comparison between wireless capsule endoscopy and multidetector-row CT enteroclysis. Abdom Imaging. 2008;33:398–406. doi: 10.1007/s00261-007-9271-8. [DOI] [PubMed] [Google Scholar]
- 98.Ettorre GC, Francioso G, Garribba AP, Fracella MR, Greco A, Farchi G. Helical CT angiography in gastrointestinal bleeding of obscure origin. AJR Am J Roentgenol. 1997;168:727–731. doi: 10.2214/ajr.168.3.9057524. [DOI] [PubMed] [Google Scholar]
- 99.Lewis BS, Salomon P, Rivera-MacMurray S, Kornbluth AA, Wenger J, Waye JD. Does hormonal therapy have any benefit for bleeding angiodysplasia? J Clin Gastroenterol. 1992;15:99–103. doi: 10.1097/00004836-199209000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Junquera F, Feu F, Papo M, Videla S, Armengol JR, et al. A multicenter randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001;121:1073–1079. doi: 10.1053/gast.2001.28650. [DOI] [PubMed] [Google Scholar]
- 101.Blich M, Fruchter O, Edelstein S, Edoute Y. Somatostatin therapy ameliorates chronic and refractory gastrointestinal bleeding caused by diffuse angiodysplasia in a patient on anticoagulation therapy. Scand J Gastroenterol. 2003;38:801–803. doi: 10.1080/00365520310001969. [DOI] [PubMed] [Google Scholar]
- 102.Shurafa M, Kamboj G. Thalidomide for the treatment of bleeding angiodysplasias. Am J Gastroenterol. 2003;98:221–222. doi: 10.1111/j.1572-0241.2003.07201.x. [DOI] [PubMed] [Google Scholar]
- 103.Morris ES, Hampton KK, Nesbitt IM, Preston FE, Thomas EG, Makris M. The management of von Willebrand’s disease-associated gastrointestinal angiodysplasia. Blood Coagul Fibrinolysis. 2001;12:143–148. doi: 10.1097/00001721-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Richter JM, Christensen MR, Colditz GA, Nishioka NS. Angiodysplasia. Natural history and efficacy of therapeutic interventions. Dig Dis Sci. 1989;34:1542–1546. doi: 10.1007/BF01537107. [DOI] [PubMed] [Google Scholar]
- 105.Menefee MG, Flessa HC, Glueck HI, Hogg SP. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease). An electron microscopic study of the vascular lesions before and after therapy with hormones. Arch Otolaryngol. 1975;101:246–251. doi: 10.1001/archotol.1975.00780330042011. [DOI] [PubMed] [Google Scholar]
- 106.Fujita H, Momoi M, Chuganji Y, Tomiyama J. Increased plasma vascular endothelial growth factor levels in patients with angiodysplasia. J Intern Med. 2000;248:268–269. doi: 10.1046/j.1365-2796.2000.00717-2.x. [DOI] [PubMed] [Google Scholar]
- 107.McGee RR. Estrogen-progestogen therapy for gastrointestinal bleeding in hereditary hemorrhagic telangiectasia. South Med J. 1979;72:1503. doi: 10.1097/00007611-197911000-00055. [DOI] [PubMed] [Google Scholar]
- 108.Bronner MH, Pate MB, Cunningham JT, Marsh WH. Estrogen-progester-one therapy for bleeding gastrointestinal telangiectasias in chronic renal failure. An uncontrolled trial. Ann Intern Med. 1986;105:371–374. doi: 10.7326/0003-4819-105-3-371. [DOI] [PubMed] [Google Scholar]
- 109.Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998;93:1250–1254. doi: 10.1111/j.1572-0241.1998.404_i.x. [DOI] [PubMed] [Google Scholar]


