Current Research on Nitazoxanide in the Treatment of Chronic Hepatitis C
G&H What was the therapeutic focus in the original development of nitazoxanide?
EK Nitazoxanide (NTZ, Alinia®, Romark Laboratories, L.C.) is the first in a new class of small molecules, the thiazolides. NTZ was originally developed as an antiparasitic drug and ultimately licensed by the US Food and Drug Administration (FDA) in 2002 to treat diarrhea caused by Cryptosporidium parvum or Giardia lamblia in immunocompetent adults and children. The mechanism of action of NTZ against parasites appears to be interference with anaerobic energy metabolism via noncompetitive inhibition of the pyruvate:ferridoxin oxidoreductase (PFOR) enzyme-dependent electron transfer reaction.
G&H How was the possible application of NTZ in treatment of hepatitis C first discovered?
EK Initial studies of NTZ were conducted in patients with Cryptosporidium parvum, some of whom had AIDS and hepatitis B or C co-infection. When the manufacturer submitted its package for initial regulatory approval to the FDA, it was noted that the mean aminotransferase levels in these patients had decreased significantly. Dr. Brent Korba of Georgetown University subsequently conducted laboratory studies using standard antiviral assays and showed that NTZ was a potent inhibitor of hepatitis B virus and hepatitis C virus (HCV) replication in vitro. After the antiviral activity of NTZ was established in the laboratory, the manufacturer made the strategic decision to focus initial research on the treatment of chronic hepatitis C because of the unmet medical need in this disease state. This decision led to phase II studies to treat chronic hepatitis C with NTZ, initially as monotherapy and subsequently in combination with peginterferon, with or without ribavirin (RBV).
G&H Could you describe the mechanism of NTZ in the setting of antiviral therapy?
EK NTZ has been shown to have antiviral activity against a number of viruses, and the mechanism of action against viruses appears to be different from its activity against parasites. Studies are currently underway in several laboratories to determine the specific antiviral mechanism of action, which may be different for individual viruses.
As background regarding the antiviral effect of NTZ against HCV, initial studies by Dr. Korba had shown that NTZ was a potent inhibitor of HCV genotype 1a and 1b in replicon models and pretreatment of HCV replicon-containing cells with NTZ potentiated the effect of subsequent treatment with NTZ plus interferon. In addition, pilot clinical studies showed that NTZ can augment the antiviral effect of interferon. The current hypothesis regarding the mechanism of action of NTZ against HCV comes from studies conducted by Dr. Menashe Elazar and colleagues in the laboratory of Dr. Jeffrey Glenn at Stanford University, and a manuscript summarizing their studies is currently in press in Gastroenterology. These investigators have demonstrated that NTZ increases the phosphorylation of eukaryotic initiation factor 2-alpha (eIF2a), which plays an important role in mediating host antiviral defenses. In addition, in the setting of cotreatment with interferon, NTZ-induced eIF2α phosphorylation was enhanced, which may explain the increased effect in the laboratory and clinically when NTZ is used in combination with interferon. NTZ was also shown to increase protein kinase activated by double-stranded RNA (PKR) autophosphorylation, which induces eIF2a phosphorylation and ultimately inhibits translation of viral RNA (Figure). Thus, NTZ appears to ramp up natural innate host cell antiviral defense mechanisms.
Figure 1.
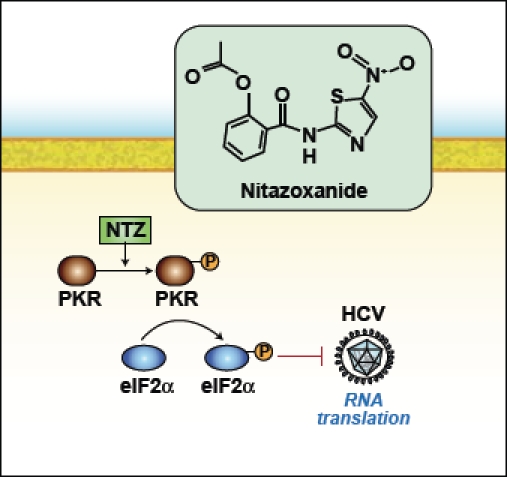
Nitazoxanide (NTZ) increases phosphoryation of protein kinase activated by RNA (PKR) and induces eukaryotic initiation factor 2-apha (eIF2α), which ultimately inhibits translation of viral RNA.
Adapted from Darling JM, Fried MW. Nitazoxanide: beyond parasites toward a novel agent for hepatitis C. Gastroenterology. 2009;136: 760-763.
-
NTZ is also active against other viruses, including influenza, rotavirus, herpes simplex, and hepatitis B, as mentioned above. In the case of these other viruses, the antiviral mechanism of action of NTZ may be different from its activity against HCV based on preliminary studies that are underway. In the case of the influenza virus, NTZ inhibits the formation of specific viral glycoproteins in their final assembly in the endoplasmic reticulum. This action of NTZ prevents the viral particles from exiting the cell through the cell membrane and thus their avail-ability to infect a new cell. Whether or not this alternative mechanism of action will provide an additional explanation of the activity of NTZ against HCV is unknown but currently under study.
Thus, thiazolides have been demonstrated to have activity against both RNA and DNA viruses, which is a unique property of this class of drugs. This wide spectrum of antiviral activity supports a cell-mediated effect, rather than a mechanism that specifically targets the virus.
-
G&H Has NTZ been associated with any adverse events or contraindications to use?
EK The safety profile of NTZ has been well established since its initial approval to treat diarrhea due to Cryptosporidium and Giardia in adults and children in 2002. There have been no serious toxicities associated with short-term and long-term use of NTZ. The side effects that have been noted include mild gastrointestinal symptoms, typically abdominal pain, diarrhea, nausea, and vomiting. Abdominal pain occurs in less than 10% of users of NTZ and is generally mild, and the other side effects have been noted in less than 5% of patients. There are no contraindications to NTZ, though its safety in patients with renal failure has yet to be determined. There does not appear to be any interaction of NTZ with cytochrome P450 enzymes.
The results of laboratory studies conducted by Dr. Korba have demonstrated that NTZ also appears to carry no risk of the development of antiviral drug resistance, which was expected based on its cell-mediated mechanism of action. This characteristic distinguishes NTZ from direct, targeted antiviral agents that suppress wild-type HCV, which can lead to the emergence of drug-resistant mutant strains.
-
G&H How do the interferon-boosting properties of NTZ differ from those of RBV?
EK Clinically, NTZ appears to have a similar effect to RBV in the treatment of chronic hepatitis C, in that it enhances the antiviral response of interferon at least in part by reducing the relapse rate after a standard course of treatment. In the phase II clinical trials that have been completed to date in 140 naïve patients with chronic hepatitis C, mostly with genotype 4, the relapse rates in the NTZ arms were 10% (peginterferon plus NTZ, using a 12-week NTZ lead-in), 7% (peginterferon plus NTZ, using a 4-week NTZ lead-in), and 4% (peginterferon plus RBV plus NTZ, using a 12-week NTZ lead-in) versus 25% in control patients treated with the standard-of-care therapy using peginterferon plus RBV.
The mechanism of action of RBV remains unknown, and there are currently four competing theories of how this drug works, all based on good studies. What is known with certainty is that RBV increases the sustained virologic response when used in combination with interferon or peginterferon, compared to interferon or peginterferon monotherapy. NTZ appears to have a similar effect in reducing the relapse rate, based on the results of studies summarized above.
-
G&H How might NTZ be utilized in future therapeutic regimens for hepatitis C?
EK The manufacturer of NTZ is planning to conduct phase III studies of peginterferon and RBV plus NTZ versus the standard of care with peginterferon and RBV, as well as additional studies to evaluate the potential replacement of RBV with NTZ in combination with peginterferon. These studies will be conducted in patients with chronic hepatitis C genotype 1 infection. If peginterferon and NTZ without RBV can achieve sustained virologic response rates similar to those with peginterferon and RBV, as suggested by the completed phase II studies in patients with genotype 4 infection, this combination would represent a major advance in the treatment of chronic hepatitis C. RBV, although an important and useful drug, is associated with significant side effects, particularly anemia. If RBV can be substituted out for a drug like NTZ with only minimal gastrointestinal toxicity, it would represent a significant improvement in the tolerability of standard interferon-based regimens.
Currently, there are two phase II studies underway in the United States in patients with chronic hepatitis C and genotype 1 infection. One study has enrolled 112 naive patients and the other study involves 64 patients who are well-characterized nonresponders to prior peginterferon and RBV. All patients are being randomized to standard-of-care versus triple therapy with peginterferon, RBV, and NTZ. New direct-acting antiviral agents, such as telaprevir and boceprevir, are also being studied in a similar manner, as a third add-on drug to standard therapy.
-
G&H How is the NTZ formulation being modified to optimize dosing for hepatitis C treatment?
EK A 675-mg, controlled-release tablet of NTZ has been developed and is currently under study. Because the standard NTZ tablet was initially developed as an antiparasitic, no formal dose-finding studies were undertaken in the initial studies of its potential role in the treatment of chronic hepatitis C. A phase I study evaluating pharmacokinetics and side effects of two dosages of the controlled-release tablet (675 mg and 1,350 mg, both bid) has been completed and showed improved pharmacokinetics and viral kinetics against HCV when compared with the standard tablet of 500 mg bid, with slightly increased gastrointestinal side effects that were not limiting.
A study is currently underway evaluating regimens using either one 675 mg, controlled-release tablet twice daily or two 675 mg tablets twice daily, in combinations with peginterferon alfa-2a and RBV, for the treatment of chronic hepatitis C. This study is being conducted in Egypt in patients with genotype 4 infection. Interim results, which were presented at the Asian Pacific Association for the Study of the Liver in early 2009, are very encouraging. Results showed that 88% of patients receiving the 675 mg bid dose and 100% of those receiving the 1,350 mg bid dose, in combination with peginterferon and RBV, achieved an early virologic response.
-
G&H Is there a possibility of future use of both NTZ and the direct antivirals as a new combination regimen?
EK The holy grail of future treatment for hepatitis C is the development of a safe and effective all-oral regimen that avoids interferon and its contraindications and multiple side effects. Theoretically, NTZ, with a mechanism of action that is interferon-like, could be used in combination with a protease inhibitor and a third drug, such as a polymerase inhibitor, as an effective combination regimen. In addition, some of the protease inhibitors are associated with an increased incidence of anemia, which might be avoided in an RBV-free regimen using NTZ as an alternative. As mentioned above, NTZ does not appear to have a risk of direct antiviral drug resistance, and the development of resistance associated with the use of protease and polymerase inhibitors is blunted by combination therapy, which is likely to be also true with NTZ. These potential combinations are highly speculative and obviously require many additional studies to determine if these hypotheses might in fact be true.
Suggested Reading
- Korba BE, Montero AB, Farrar K, Gaye K, Mukerjee S, et al. Nitazoxanide, tizoxanide and other thiazolides are potent inhibitors of hepatitis B virus and hepatitis C virus replication. Antiviral Res. 2008;77:56–63. doi: 10.1016/j.antiviral.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Korba BE, Elazar M, Lui P, Rossignol JF, Glenn JS, et al. Potential for hepatitis C virus resistance to nitazoxanide or tizoxanide. Antimicrob Agents Chemother. 2008;52:4069–4071. doi: 10.1128/AAC.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elazar M, Liu M, McKenna S, Liu P, Gehrig EA, et al. The anti-hepatitis C agent nitazoxanide induces phosphorylation of eIF2a via PKR activation. Gastroenterology. doi: 10.1053/j.gastro.2009.07.056. In press. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at post-translational level. J Biol Chem. doi: 10.1074/jbc.M109.029470. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol J-F, Elfert A, El-Gohary Y, Keeffe EB. Improved virologic response in chronic hepatitis C genotype 4 treated with nitazoxanide, peginterferon, and ribavirin. Gastroenterology. 2009;136:856–862. doi: 10.1053/j.gastro.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Darling JM, Fried MW. Nitazoxanide: beyond parasites toward a novel agent for hepatitis C. Gastroenterology. 2009;136:760–763. doi: 10.1053/j.gastro.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, Elfert A, Keeffe EB. Evaluation of a 4 week lead-in phase with nitazoxanide (NTZ) prior to peginterferon (PegIFN) plus NTZ for treatment of chronic hepatitis C: final report [abstract] Hepatology. 2008;48(suppl):1132A–1133A. [Google Scholar]
- Keeffe EB, Rossignol JF, Elfert A, Abdelatif S, Cravens L, Phuong TLT. Controlled release tablet improves pharmacokinetics, viral kinetics and tolerability of nitazoxanide for treatment of chronic hepatitis C [abstract] Hepatol Int. 2009;3:49. [Google Scholar]


