Giant-cell hepatitis is most commonly found in neonates and rarely seen in the adult population. Diagnosis in adult cases is often difficult to make, as the presentation and etiologies vary. The disease can progress to fulminant hepatitis, necessitating liver transplantation, in some cases.
Case Report
A 56-year-old man presented to the emergency room with bilateral knee pain and jaundice and reported having dark orange urine for 3 days along with dark, hard stools and abdominal pain. His past medical history was significant for autoimmune hemolytic anemia, idiopathic thrombocytopenic purpura (ITP), and splenectomy. His medications included prednisone 40 mg daily, fexofenadine, and insulin. His physical examination was negative for chronic liver disease stigmata except for scleral and sublingual icterus and hives over the trunk and extremities.
Initial laboratory tests showed a white blood cell count of 19.0 × 103/UL, total bilirubin of 13.2 mg/dL, direct bilirubin of 9.5 mg/dL, albumin of 3.0 g/dL, alkaline phosphatase of 246 U/L, aspartate aminotransferase/alanine aminotransferase levels of 435/758 U/L, international normalized ratio of 1.66, and gammaglutamyl transpeptidase of 103 U/L. Ultrasound, computed tomography scan of the abdomen, and magnetic resonance cholangiopancreatography were all normal.
Further laboratory evaluation was negative for hepatitis B virus (HBV), hepatitis C virus (HCV), HIV, lyme disease, antineutrophilic cytoplasmic antibodies, antinuclear antibody, parvovirus, cytomegalovirus, antismooth muscle, and liver/kidney antibodies. Only hepatitis A virus immunoglobulin (Ig)G antibodies and Epstein-Barr virus (EBV) IgG antibodies were positive. A liver biopsy obtained 2 months prior to admission was significant for plasmocytosis and macrovesicular steatosis. However, the current biopsy revealed periportal and lobular grade III hepatitis with lymphoplasmacytosis and rosetting with increasing cholestasis and cholangitis consistent with an autoimmune hepatitis. Interestingly, EBV was negative on tissue evaluation.
The patient’s total bilirubin continued to rapidly rise, and mycophenolate mofetil 1 g twice-daily dosing was started in addition to the prednisone 40 mg daily. The patient developed acute renal failure, requiring hemodialysis. One week after admission, the quantitative EBV titer came back as 323,000 copies/mL, and at this time, mycophenolate was discontinued and the patient was placed on acyclovir for presumed EBV-induced hepatitis. The patient had also developed a Streptococcus pneumoniae bronchitis and was treated with levofloxacin (Levaquin, Ortho McNeil Janssen). Shortly after this time, the patient developed fulminant hepatic failure, with an international normalized ratio that rose to 2.95 and total bilirubin that rose to 67.4 mg/dL and he thus underwent liver transplantation. The patient’s immunosuppressive medications included a taper of intravenous basiliximab (Simulect, Novartis) 20 mg, methylprednisolone sodium succinate (Solu-Medrol, Pharmacia and Upjohn), tacrolimus (Protopic, Astellas) 2 mg oral, twice daily, and mycophenolate mofetil 500 mg every 12 hours.
The explanted liver showed severe giant-cell hepatitis suggestive of a giant-cell hepatitis variant of autoimmune hepatitis (Figure 1). The patient’s renal function and liver function tests continued to improve, with a total bilirubin of 13.3 mg/dL and aspartate aminotransferase/alanine aminotransferase levels of 40/103 U/L at the time of discharge (Table 1). The patient was discharged from the hospital on tacrolimus 4 mg twice daily, mycophenolate 1 g twice daily, and acyclovir 200 mg twice daily.
Figure 1.
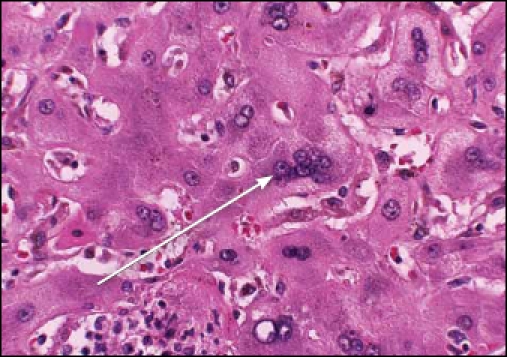
High magnification microphotograph showing giant-cell hepatitis with multinucleated hepatocytes and focal necrosis with inflammation (arrow; hematoxylin and eosin stain; ×400 magnification).
Table 1.
Significant Laboratory Findings over Patient Hospital Course
| Laboratory values | At admission | Pre-transplant | Post-transplant |
|---|---|---|---|
| Total bilirubin (mg/dL) | 13.2 | 67.4 | 13.3 |
| Aspartate aminotransferase (U/L) | 435 | 231 | 40 |
| Alanine amino-transferase (U/L) | 758 | 645 | 103 |
| International normalized ratio | 1.66 | 2.95 | 0.92 |
| Creatinine (mg/dL) | 0.9 | 5.3 | 1.5 |
Discussion
Giant-cell hepatitis is a condition characterized by inflammation and large multinucleated hepatocytes in the parenchyma. Although the formation of giant hepatocytes has been shown to be a common response of the newborn liver to a variety of attacks, its occurrence in adults is very rare (0.1–0.25% of all hepatic diseases).1-2 There have been approximately 100 reported cases of adult giant-cell hepatitis within the last two decades.3 The combination of hepatitis and extensive giant-cell transformation in the adult liver has been referred to as postinfantile giant-cell hepatitis or syncytial giantcell hepatitis.4 Cases are variable in both their clinical presentation and etiology. Adult giant-cell hepatitis has been associated with autoimmune disorders, and multiple patients have been found to have antismooth muscle and antinuclear antibody–positive serology.5
Viral infections (eg, EBV, paramyxovirus, and viral hepatitis), medications, and cholestatic disorders have also been seen as causes of this disease.1,4,6,7 In fact, there have been some reports suggesting a novel paramyxo-like virus as the cause of some cases of adult giant-cell hepatitis.4,8 In one of the largest studies to date with 78 patients, the various conditions found to be associated with adult giant-cell hepatitis were as follows: autoimmune hepatitis in 29 (37.2%) patients, HCV infection in 6 (7.7%) patients, HBV infection in 4 (5.1%) patients, HBV and HCV plus HIV infection in 1 (1.3%) patient, EBV infection in 1 (1.3%) patient, and drug-induced hepatitis in 1 (1.3%) patient. No associated possible etiology was found in 36 of the 78 patients (46.2%).9 In our case, the patient had a history of ITP as well as a positive serology for EBV.
Adult giant-cell hepatitis has been shown to be a progressive and often fatal disease process, with a survival rate of only approximately 50% without orthotopic liver transplantation (OLT). The high mortality rate is often due to severe liver failure, anemia that is difficult to control, or sepsis in the setting of aggressive use of immunosuppressants.8 Indeed, our patient was found to have fulminant hepatic failure with uncontrollable anemia and was diagnosed with a Streptococcus pneumoniae bronchitis in the setting of corticosteroid and mycophenolate mofetil immunosuppression.
Corticosteroids have been found to definitively improve the clinical picture in those individuals who are seropositive for autoimmune markers.1-2 It has also been shown that in many autoimmune seropositive cases of giant-cell hepatitis, disease recurrence was seen after OLT, and in these cases, transplant is no longer considered appropriate treatment.10 These patients should continue to be monitored indefinitely for any signs of recurrence via frequent liver function tests and autoimmune panels as well as serial liver biopsies.
This case of adult giant-cell hepatitis is unique in that the initial liver biopsy did not provide the appropriate diagnosis. It was not apparent until evaluation of the gross explanted liver specimen that the etiology of the patient’s liver failure might be giant-cell hepatitis. A complicating feature of this patient’s case was his EBV-positive status. This finding directed his treatment toward a likely EBVinduced hepatitis. When his clinical status continued to deteriorate despite treatment, other more rare causes of hepatitis were investigated. Interestingly, as mentioned above, it has been shown that EBV is a possible etiology of giant-cell hepatitis resulting in fulminant hepatic failure.9 It is unclear what role EBV played in this patient’s hepatic failure, but this case demonstrates the importance of a thorough immunologic evaluation of possible etiologies for patients with hepatic failure. The diagnosis of giantcell hepatitis must be considered in cases of apparent idiopathic hepatic failure.
This patient was seen recently for follow-up evaluation in the liver transplantation clinic and was still doing well 3 months post-transplant.
References
- 1.Johnson SJ, Mathew J, MacSween RN, Bennett MK, Burt AD. Post-infantile giant cell hepatitis: histological and immunohistochemical study. J Clin Pathol. 1994;47:1022–1027. doi: 10.1136/jcp.47.11.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devaney K, Goodman ZD, Ishak KG. Postinfantile giant-cell transformation in hepatitis. Hepatology. 1992;16:327–333. doi: 10.1002/hep.1840160208. [DOI] [PubMed] [Google Scholar]
- 3.Kryczka W, Walewska-Zielecka B, Dutkiewicz E. Acute seronegative hepatitis C manifesting itself as adult giant cell hepatitis: a case report and review of literature. Med Sci Monit. 2003;9(suppl 3):29–31. [PubMed] [Google Scholar]
- 4.Phillips MJ, Blendis LM, Poucell S, Offterson J, Petric M, et al. Syncytial giant cell hepatitis. Sporadic hepatitis with distinctive pathological features, a severe clinical course, and paramyxoviral features. N Engl J Med. 1991;324:455–460. doi: 10.1056/NEJM199102143240705. [DOI] [PubMed] [Google Scholar]
- 5.Gorelik M, Debski R, Frangoul H. Autoimmune hemolytic anemia with giant cell hepatitis: case report and review of the literature. J Pediatr Hematol Oncol. 2004;26:837–839. [PubMed] [Google Scholar]
- 6.Lau JY, Koukoulis G, Mieli-Vergani G, Portmann BC, Williams R. Syncytial giant-cell hepatitis: a specific disease entity? J Hepatol. 1992;15:216–219. doi: 10.1016/0168-8278(92)90039-r. [DOI] [PubMed] [Google Scholar]
- 7.Fimmel CJ, Guo L, Compans RW, Brunt EM, Hickman S, et al. A case of syncytial giant cell hepatitis with features of a paramyxoviral infection. Am J Gastroenterol. 1998;93:1931–1937. doi: 10.1111/j.1572-0241.1998.00548.x. [DOI] [PubMed] [Google Scholar]
- 8.Vajro P, Migliaro F, Ruggeri C, Di Cosmo N, Crispino G, et al. Life saving cyclophosphamide treatment in a girl with giant cell hepatitis and autoimmune hemolytic anemia: case report and up-to-date on therapeutical options. Dig Liver Dis. 2006;38:846–850. doi: 10.1016/j.dld.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Thaler H. Post-infantile giant cell hepatitis. Liver. 1982;2:393–403. doi: 10.1111/j.1600-0676.1982.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 10.Pappo O, Yunis E, Jordan JA, Jaffe R, Mateo R, et al. Recurrent and de novo giant cell hepatitis after orthotopic liver transplantation. Am J Surg Pathol. 1994;18:804–813. doi: 10.1097/00000478-199408000-00007. [DOI] [PubMed] [Google Scholar]


