Abstract
Because Crohn's disease (CD) is a chronic, incurable condition, patients require life-long therapeutic approaches to initiate and maintain symptom control, improve quality of life, avoid hospitalizations and surgery, and minimize short- and long-term toxicity and complications such as stricturing, fistulae, osteoporosis and associated bony fractures, and linear growth failure in pediatric patients. Many physicians use symptom-based classifications, such as those published in the American College of Gastroenterology Practice Guidelines, to classify disease severity. However, all current classifications for CD focus predominantly on treating the present symptoms and not on the long-term treatment goal of altering the natural history of the disease. If physicians were able to identify disease phenotypes; at diagnosis, they could advise a course of robust intervention or more. conservative therapeutic modalities with a lower risk of toxicity, as appropriate. Over the past several years, much interest has developed in the role of genetic and serologic markers as prognostic tools in CD. With these genetic and serologic data in mind, clinicians have a growing ability to harness laboratory and genetic testing information in order to stratify patients relative to their risk of disease progression from the time of diagnosis, allowing for a more individualized treatment plan for each patient.
Keywords: Crohn's disease, serology, genetics
The natural history of Crohn's disease (CD) is characterized by a spectrum of clinical and pathologic patterns. CD can involve any part of the gastrointestinal tract from the oropharynx to the perianal area. The most common location is the ileocecal region, followed by the terminal ileum alone, diffuse small bowel, and then isolated colonic disease. The asymmetric inflammation seen with CD can be transmural, often extending through to the serosa, resulting in sinus tracts or fistula formation. Although Keywords CD is primarily a disease of the gastrointestinal tract, patients Crohn's disease, serology, genetics can exhibit extraintestinal manifestations, including fever, weight loss, arthralgias, arthritis, renal disease, mucocutaneous lesions, or ophthalmologic complications.1 The incidence and prevalence of CD in the United States are estimated at 5 per 100,000 and 50 per 100,000, respectively.2,3
Because CD is neither medically nor surgically curable, patients require life-long therapeutic approaches to maintain symptomatic control, improve quality of life, avoid hospitalizations and surgery, and minimize short- and long-term toxicity and complications.4 Although the disease can affect any age group, its onset is typically within the second and fourth decades of life. Because of the life-long need for disease management, the financial burden associated with CD is disproportionately large. In 2008, the mean annual direct treatment costs for CD in the United States were estimated to be $7,189 per patient, or about $3.6 billion total. Approximately 31% of the total annual costs were attributable to hospitalization, 33% to outpatient care, and 35% to pharmaceutical prescription.5
Beyond the financial burden, CD is also associated with significantly reduced health-related quality of life (HRQL) when compared with the mean for healthy controls.6 As might be expected, HRQL in CD is directly correlated with disease activity, with patients experiencing active disease having a lower mean HRQL than those experiencing fluctuating disease or those in remission.7 A study by Lix and colleagues found that patients with CD have higher levels of perceived stress, health anxiety, pain anxiety, and pain catastrophizing over time than patients with ulcerative colitis (UC).8 This effect remains even after controlling for disease activity. The authors suggest that CD is likely associated with more pain, or less predictable pain, than is UC. As individuals progress through the disease course, pain may become a more pronounced factor and maladaptive responses may be more likely to emerge.
The Natural History of CD
CD is a dynamic and progressive disease. Although the majority of patients have uncomplicated, nonstricturing, nonpenetrating disease at diagnosis, most eventually develop complications. In their study of 297 CD patients over 25 years, Louis and colleagues found that 46% of patients had a change in disease behavior in the first 10 years of follow-up.9 The most prominent change was from nonstricturing, nonpenetrating disease to either stricturing (27%) or penetrating (29%) disease. The authors further found that the rate of change from uncomplicated disease to either the stricturing or penetrating phenotype remained stable during the 25 years of the study, with approximately 25–33% of the cases of uncomplicated disease changing to stricturing or penetrating disease every 5 years. They concluded that virtually all CD patients in their population would develop either stricturing or penetrating disease if followed sufficiently.
Because of the progressive nature of the disease, patients with CD are likely to require hospitalization at some point during their disease course. From 1990 to 2003, the hospitalization rates for patients with a primary diagnosis of CD ranged from 9.3 to 17.1 per 100,000 people.10 Patients with CD also have a high likelihood of requiring surgery. Cosnes and colleagues11 followed 565 patients with CD and found that the annual rate of intestinal resection was between 3.3% and 7.5% over 26 years. Similarly, Jess and colleagues followed 58 Danish patients with CD diagnosed between 1991 and 1993 and found a 10-year cumulative intestinal resection probability of 65%.12 The risk of repeat surgery is also high for patients with CD, about 5% each year after the first surgery.13 Ultimately, upwards of 80% of CD patients will require surgery in their lifetime.14
Special Considerations in Pediatric CD
In general, inflammatory bowel disease (IBD) has an early age of onset. Approximately 20% of all patients with IBD develop symptoms during childhood,15 with about 5% being diagnosed before 10 years of age.16 Typical complications of CD in the pediatric population include failure to reach puberty, osteopenia, and linear growth failure.
Active or relapsing disease may slow or even arrest the progression of puberty once it has begun. Unlike with healthy children, delayed or shortened puberty in children with CD may result in permanent growth impairment. Thus, inducing disease remission before the onset of puberty and maintenance of remission during the pubertal years is crucial in order to avoid the consequences of a missed pubertal growth spurt, such as unacceptably reduced adult stature, abnormal bone mineralization, and the maintenance of prepubertal levels of sex hormones.17
Osteopenia is another important potential complication of pediatric IBD because more than 90% of peak bone mass is attained during childhood and adolescence. A failure to attain peak bone mass during this stage in development increases future fracture potential. In one study, the total body bone mass density (BMD) Z-score (mean±SD) was - 0.78±1.02 for 58 children with CD and - 0.17±0.95 for 49 healthy control children (P<.01).18 Of additional concern, the BMD Z-scores of the children with CD did not increase significantly after 2 years, indicating that the rate of bone mineral accrual did not accelerate.
Growth failure is another common complication of pediatric CD. Severe linear growth retardation occurs in up to 46% of patients; only about 12% have a normal height velocity at the time of diagnosis. Indeed, Kanof and colleagues found that linear growth impairment in pediatric CD may precede weight loss and can be the earliest indicator of disease.19 The etiology of growth failure in these children is poorly understood; however, inflammation, poor nutrition, and steroid therapy appear to be its principal determinants.20 Whereas nutritional supplementation can improve the linear growth of children with CD,21 current practice guidelines for managing growth failure emphasize that the effective and sustained cessation of active inflammation leading to a growth-promoting remission should be the major treatment goal.22
Assessment and Prognosis in CD
The management of patients with CD depends strongly upon the physician's assessment of the severity of disease. This assessment forms the basis for all therapeutic decision-making. In an ideal world, the physician would have a set of validated tools with which to accurately evaluate the severity of the disease at any given point and, similarly, would have a set of tools with which to make an accurate prognosis, thus allowing the alteration of the natural history of the disease by formulating a strategy to avoid hospitalizations and surgery, while minimizing short- and long-term toxicity and complications from therapy. In today's world of increasing knowledge and treatment options, altering the natural history of CD is the ultimate goal.23
In regard to prognostic factors in CD, extensive disease, stricturing disease, young age, and positive smoking history are well-known negative prognostic factors for hospitalization and surgery24; however, these are not highly accurate predictors for use on the individual patient level. In regard to measuring disease activity, no gold standard measure of clinical disease activity has been established.4 In current practice, disease severity is established on the basis of clinical parameters, systemic manifestations, and the global impact of the disease on the individual's quality of life. Composite indices of disease activity such as the Crohn's Disease Activity Index (CDAI)25 have been used in controlled clinical trials to provide reliable and reproducible correlates to clinicians' and patients' global assessment of well-being.26 Although the CDAI is in wide use in research, it has many limitations that often preclude its use in clinical practice. First, the calculation of the CDAI is based on a diary filled out by the patient for 7 days before evaluation. Second, several of the items such as “general well-being” and “intensity of abdominal pain” are subjective and depend wholly on the patient's perception. Third, the CDAI is not accurate in patients with fistulizing and stenosing behavior, nor is it useful in patients with previous extensive ileo-colonic resections or stoma.27 Lastly, the CDAI is subject to considerable interobserver variability. Even among experienced researchers in academic clinical practices or in industry-sponsored drug studies, much variation exists in the administration and implementation of the CDAI score. For example, Sands and colleagues found much disagreement among 100 such researchers on the definition of “liquid or very soft stools,” the recording of the number of stools, the recording of pain ratings, and the scoring of extraintestinal manifestations and fistulae. The recording of fever, the scoring for the use of opiates, and the standard for the weight variable also varied widely among respondents.28
Because of these limitations, many physicians use symptom-based classifications such as those published in the American College of Gastroenterology Practice Guidelines. Such classifications would describe, for example, mild-moderate CD as thus: “applies to ambulatory patients able to tolerate oral alimentation without manifestations of dehydration, toxicity (high fevers, rigors, prostration), abdominal tenderness, painful mass, obstruction, or greater than 10% weight loss.”29 Both the CDAI and other validated disease activity assessment scales, as well as symptom-based classifications, are useful in some ways for providing the physician a snapshot of disease activity. However, it has been argued that all current classifications for CD focus predominantly on treating the present symptoms and not on the long-term treatment goal of altering the natural history of the disease.23
Laboratory Values for Assessment and Prognosis in CD
In the past 10 years, much interest has been generated in the use of laboratory values for the assessment of disease severity as well as for prognostic purposes. Currently, the two most commonly used parameters are the erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) level.
A study by Fagan and colleagues showed that both CRP and ESR correlate well with disease activity, although the correlation was better for CRP.30 However, a wide range of CRP values was observed and overlap existed between mild to moderate (10–50 mg/L), moderate to severe (50–80 mg/L), and severe disease (>80 mg/L).
With regard to prognosis, the GETAID group prospectively followed 71 CD patients with medically induced remission and measured laboratory markers (full blood count, CRP, ESR, α1 antitrypsin, orosomucoid) every 6 weeks.31 In total, 38 patients relapsed (defined as a CDAI score of > 150 with an increase of > 100 points from baseline) after a median of 31 weeks. Only 2 laboratory markers were predictive of relapse: CRP (>20 mg/L) and ESR (>15 mm). Patients positive for both markers had an 8-fold increased risk for relapse, with a negative predictive value of 97%, suggesting that normal CRP and ESR could almost certainly rule out relapse in the next 6 weeks. In contrast, the positive predictive value of having both markers positive was only 15%, meaning that a double-positive result is not very useful for the prediction of relapse in the next 6 weeks. Therefore, the data indicate that CRP and ESR values can be helpful in predicting short-term incidence of relapse and can be integrated into the overall management of the patient. However, their level of accuracy and wide variation based on the patient's current level of inflammation limit their use as stand-alone prognostic tools and as predictors of long-term disease activity.
Imaging Modalities for Assessment and Prognosis in CD
There are a number of imaging choices to evaluate disease severity and location in CD, each with advantages and drawbacks. These modalities include upper gastrointesti-nal (GI) small-bowel follow-through, push enteroscopy, capsule endoscopy, computed tomography (CT) scanning, nuclear medicine imagining, and magnetic resonance imaging (MRI). Push enteroscopy has utility in evaluating patients with proximal small-bowel disease32; however, there are a number of drawbacks to this technique. It can only access about a third of the small bowel, it is invasive, it typically requires sedation and analgesia, and it carries a danger of perforation.33 Capsule endoscopy provides a novel alternative. Several studies in adults have suggested that capsule endoscopy has a superior diagnostic yield and sensitivity when compared with colonoscopy/ileoscopy, barium small-bowel follow-through, CT enterography, or MRI enterography.34-36 Drawbacks, however, include the inability of some patients to swallow the capsule, a risk that the capsule might become trapped within the GI tract and require surgical removal,37 and expense.
In comparison, CT scanning is widely available and allows complete evaluation of the colon, but is, of course, associated with increased exposure to radiation. Radiolabeled white blood cell scintigraphy allows detection of inflammatory disease and can distinguish between CD and UC; however, it is unreliable as a screening test for proximal small-bowel disease.38 MRI enterography, on the other hand, requires no ionizing radiation and yields excellent soft tissue contrast. It is useful for identifying fistulae and stenosis and in localizing affected bowel segments, especially in patients with more proximal bowel involvement.39 The cost of repeated MRI, however, may limit its use in clinical practice.
Although these imaging modalities are necessary and useful for the evaluation of disease activity and location in patients with CD, they carry significant disadvantages, including inconvenience of repeated imaging for the patient, radiation exposure, and expense. In addition, they offer very little in the way of prognostic value.
Improved Prognosis and Patient Outcomes
Although the clinical features of a CD patient's disease, combined with ESR and CRP data, offer the physician a certain amount of prognostic information, it is clear that the tools available at present are inadequate for the task of altering the natural history of the disease. CD is a chronic relapsing disorder with great heterogeneity in the course of the disease over time. Jess and colleagues found that, in the first 5 years after diagnosis, about 18% of patients experience an indolent disease course; 57% experience moderate disease activity; and 25% experience aggressive disease activity.12 If, instead of measuring disease severity at single time points, the physician could actually predict a patient's future disease severity with high accuracy, overall strategies for CD management might change markedly. If the physician were able to identify aggressive disease phenotypes at diagnosis, he or she could advise a course of robust intervention and fast-acting, aggressive therapy. Similarly, when the physician is able to predict an indo-lent disease course at diagnosis, he or she can concentrate on prescribing slower-acting therapeutic modalities with a low risk of toxicity or side effects. The ultimate tool, of course, would not only predict a patient's disease course; it would also predict that patient's response to an array of therapeutic options, allowing the physician to individually tailor each patient's therapy to optimize outcomes and maximize therapeutic efficacy.
Current Overview of Genetic and Serologic Predictive Markers
Over the past several years, much interest has developed in the role of genetic and serologic markers as prognostic tools in CD. As stable markers that can be tested non-invasively, genetic polymorphisms and serologic proteins represent an appealing target for prognostic examination.
The Predictive Role of Genetic Markers in CD
Data from human genetic studies have uncovered several genes that appear to be associated with CD (so-called “susceptibility genes”). Of these, the most well-characterized is the innate immune gene NOD2.40-42 NOD2 is a member of a family of intracellular cytosolic proteins that plays a role in the secretion of antibacterial substances in response to the presence of bacterial antigens.43,44 It is found in epithelial cells of the small and large intestine, as well as monocytes, macrophages, T and B cells, Paneth cells, and dendritic cells.45-47 At least 27 mutations of the NOD2 gene have been described, but the majority of susceptibility has been attributed to 3 common mutations: R702W and G908R, which are missense mutations, and 1007fs, a frameshift mutation.42,48,49
Studies have shown that the presence of certain mutations at the NOD2 locus is prognostic of a more aggressive and complicated disease course. In 2002, Abreu and colleagues found that CD patients carrying NOD2 mutations were nearly 3-fold more likely to have fibrostenosing disease than were similar CD patients without NOD2.50
In another study, Lesage and colleagues found that carrying more than 1 mutation in the NOD2 gene was particularly indicative of an aggressive disease course. The authors analyzed NOD2 sequence data from 453 patients with CD, 159 patients with UC, and 103 healthy control subjects.51 No NOD2 sequence mutations were found to be associated with UC, but 50% of patients with CD carried at least 1 mutation in the NOD2 gene. Of the 67 sequence variations that the authors identified from the CD patients' NOD2 genes, the variants R702W, G908R, and 1007fs represented 32%, 18%, and 31%, respectively, of the total. Interestingly, the authors found that 17% of CD patients had a double mutation in the NOD2 gene and these patients were characterized by a younger age at onset (16.9 years vs 19.8 years; P=.01), a more frequent stricturing phenol-type (53% vs 28%; P=.00003), and less frequent colonic involvement (43% vs 62%; P=.003) than were seen in those patients who had no mutation. The proportion of familial and sporadic cases and the proportion of patients with smoking habits were similar in the groups of patients with CD with or without mutations. Based on the data from these studies, it is clear that certain mutations in the NOD2 gene confer a significantly elevated risk for an aggressive disease course, particularly in patients with double mutations.
The Role of Serologic Markers in Determining CD Disease Behavior
Serologic testing in IBD was first initiated in an effort to differentiate disease states in patients with indeterminate colitis. Early studies from the 1990s suggested that anti-Saccharomyces cerevisiae antibody (ASCA) was associated with CD and that perinuclear antineutrophil cytoplasmic antibody (pANCA) was associated with UC.52,53 Eventually investigators realized that ASCA and pANCA screening were useful for differentiating disease states only in some patients—between 30% and 50% of the IBD population could not be evaluated accurately with these markers because they did not screen positively for either of them. This is particularly true for patients with indeterminate colitis (IC).54
Research into the role of ASCA and pANCA testing in IBD was not, however, abandoned. More recently, their role as prognostic factors has been fruitfully explored. Several studies from the past 5 years indicate that, among patients who already have a diagnosis of CD, ASCA and pANCA testing can help to identify those who are at high risk for early complications and the need for surgery. Forcione and colleagues conducted a case-control study in 2004 and showed that positivity for ASCA seems to define a subgroup of CD patients that are at risk for early surgery.55 The study enrolled 35 newly diagnosed adult patients with CD who had surgery within 3 years of diagnosis (cases) and compared these patients with 35 control patients with CD who did not undergo major surgery for CD within 3 years of diagnosis. Control patients were matched for age, sex, disease location, and smoking status. The authors found that ASCA immmunoglobulin (Ig)A positivity was associated with an over 8-fold increased risk of early surgery and that ASCA IgG positivity was associated with a 5-fold increased risk.
Because of these promising data, researchers considered other markers that could be added to ASCA and pANCA in order to improve the utility of serologic testing for prognostic purposes. Early data from Landers and colleagues demonstrated that there are subsets of CD patients with differing immune responses to several microbial antigens, including Escherichia coli outer-membrane porin C (OmpC) and Pseudomonas fluorescens–related protein (I2).56 They found that the level of immune response to these antigens, as well as the level of ASCA and pANCA, was stable over time for each patient and did not significantly vary even with changes in disease activity. The authors concluded that, rather than a global, characteristic “Crohn's” loss of tolerance to these antigens, CD patients make up a heterogeneous population in which there are various patient subsets that have differing responses to microbial and autoantigens.
Subsequent studies were able to demonstrate that the number of immune responses to the different microbial antigens and the magnitude of these immune responses correlated with the presence of complicated CD phenotypes. In one such study by Mow and colleagues, sera from 303 CD patients were tested for antibodies to I2, OmpC, ASCA, and pANCA, as well as for 3 CD-associated variants of the NOD2 gene (R702W, G908R, and 1007fs) and the results were compared with clinical data.57 The investigators found that patients with antibodies against I2 were more likely to have fibrostenosing CD (64.4% vs 40.7%; P<.001) and to require small-bowel surgery (62.2% vs 37.4%;P<.001). Patients positive for anti-OmpC were more likely to have internal perforating disease (50.0% vs 30.7%; P=.001) and to require small bowel surgery (61.4% vs 44.2%; P=.003). Patients triply positive for ASCA and antibodies to I2 and OmpC were the most likely to have undergone small-bowel surgery compared with patients without reactivity (72.0% vs 23.0%; odds ratio [OR]=8.6; P<.001).
A second study from this group focused on CBir1 (flagellin), a microbial antigen that produces strong adaptive immune responses in mouse models of colitis.58 Targan and colleagues tested sera from 484 CD patients that had been previously typed for ASCA, pANCA, and antibodies to I2 and OmpC. The investigators found that, as with antibodies to I2 and OmpC, there were a subset of CD patients who tested positive for anti-CBir1 and that this positivity stayed relatively constant regardless of disease activity (Figure 1). The data also showed that the expression of anti-CBir1 was associated independently with small-bowel disease (OR=2.16; P=.009), internalpenetrating disease (OR=2.01; P=.006), and fibrostenosing disease (OR=1.71; P=.03).
Figure 1.
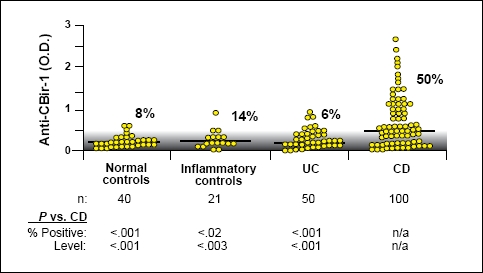
Fifty percent of patients with CD have antibodies to CBir1. The gray area indicates the negative range as defined by less than 2 standard deviations above the mean of the normal controls; lines indicate the median level for each group. The percentage of positive samples for each group is shown.
Reproduced with permission from Targan, et al.58
These results were extended into the pediatric population by Dubinsky and colleagues in their prospective multicenter study published in October of 2008.59 Sera from 796 pediatric CD patients were tested for anti-CBir1, anti-OmpC, ASCA, and pANCA, and the patients were followed over time. After a median follow-up of 32 months, 32% of the patients had developed at least 1 disease complication, and 18% underwent surgery. As in the adult population, children with antibody positivity were more likely to develop a complication: 9% of the seropositive patients had internal penetrat-ing/stricturing disease versus 2.9% in the seronegative group (P=.01). Twelve percent of the seropositive group underwent surgery versus 2% in the seronegative group (P=.0001). In addition, the frequency of internal penetration, stricturing, and surgery significantly increased with increasing antibody levels in this cohort, as did the rate of disease progression. The authors also looked at the correlation between antibody positivity and disease location, and they found that ASCA positivity was associated with small-bowel disease (OR=2.9, 95% confidence interval [CI]: 2.1–4.0;; P<.0001) and pANCA positivity was associated with large-bowel disease (OR=4.0, 95% CI: 1.8–8.8; P<.0001). See Figure 2.
Figure 2.
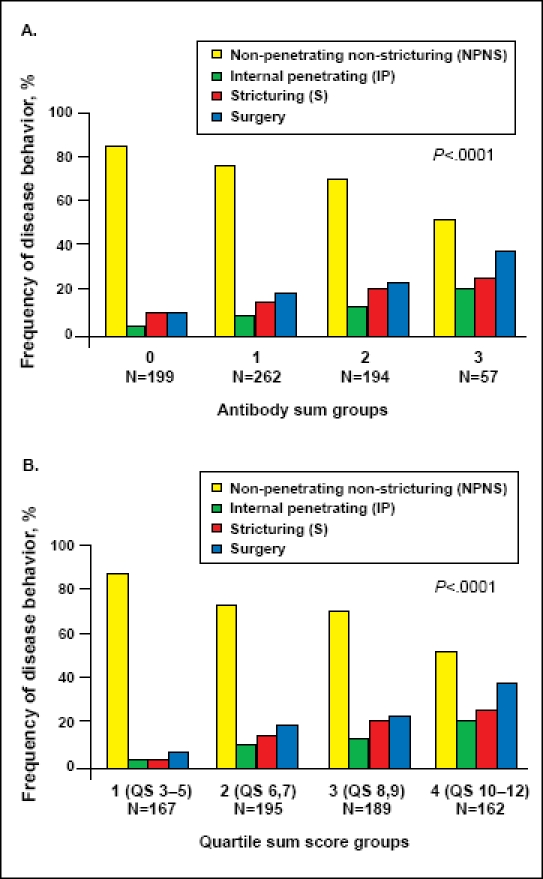
(A) Frequency of NPNS, IP, S, and surgery among the different antibody sum groups. Sum calculated based on the presence or absence of anti-CBir1, anti-OmpC, ASCA, and pANCA. P trend <.0001. (B) Frequency of NPNS, IP, S, and surgery among the different quartile sum score groups; P trend <.0001.
Reproduced with permission from Dubinsky et al.59
The Relationship Between Genetic and Serological Markers and Prognosis in CD
With the accrual of these genetic and serological data, it became clear that there could be a relationship between the genetic susceptibility conferred by NOD2 mutations and the immune reactivity to microbial antigens that seemed to define various subsets of patients with CD. Beckwith and colleagues first studied the question of the mechanism by which NOD2 mutations confer susceptibility to the development of CD through animal model studies. In mice, the “cytokine-deficiency-induced colitis susceptibility 1” gene (Cdcs1) is a major modifier of murine IBD. In the C3Bir interleukin–10–deficient mouse model, the presence of a mutation in the Cdcs1 gene has been associated with an impairment of innate responsiveness to bacterial antigens, including CBir1. Interestingly, in this mouse model, a hyperresponsive increase in the adaptive CD4+ T-cell response to bacterial antigens has been demonstrated. This hyperresponsiveness on the part of the adaptive immune system overcompensates for the weakness in the innate immune system and leads to chronic intestinal inflammation.60
Devlin and colleagues then explored the idea that a similar situation may occur in human patients with CD.61 The authors hypothesized that loss-of-function mutations of the NOD2 gene could conceivably result in an overly compensatory adaptive immunologic response to microbial antigens and lead to the development of chronic intestinal inflammation. To test this idea, they enrolled a cohort of 732 unrelated CD patients, 220 unaffected relatives of the patients with CD, and 200 healthy controls in their study. Sera from the study participants were tested for ASCA and antibodies to I2, OmpC, and CBir1. DNA from the same subjects was tested for 3 of the CD-associated variants of the NOD2 gene (R702W, G908R, and 1007fs). The authors found that NOD2 mutations were indeed associated with a hyperresponsive adaptive immunologic response. In those patients carrying any NOD2 variant, the mean number of positive antibody tests was higher than in those carrying no variant (2.24±1.21 vs. 1.92±1.24, respectively; P=.0008). Moreover, patients carrying any NOD2 variant had a significantly elevated cumulative serologic response to all 4 antigens when compared with patients carrying no variant. Interestingly, the same results were found among the unaffected relatives of the CD patients. Those relatives that carried NOD2 variants had a significantly elevated cumulative serologic response to all 4 antigens when compared with relatives carrying no variant.
Shortly thereafter, Papadakis and colleagues reported similar results that tied together NOD2 mutations, reactivity to CBir1, and complicated disease.62 In their study, sera were collected from 731 patients with CD and tested for anti-CBir1, and the patients' genomic DNA was tested for CD-associated NOD2 variants. In this cohort, there was a statistically significant association between the presence of NOD2 variants and the mean serum level of CBir1 antibodies in patients who did test positive for anti-CBir1. The mean anti-CBir1 level was 28.36(range, 3.01–257) in anti-CBir1-positive patients with no NOD2 variants and 33.83 (range, 0–280) in anti-CBir1-positive patients with at least one NOD2 variant (P=.01). With regard to the association between anti-CBir1 and complicated disease, a multivariate logistic regression analysis showed that anti-CBir1 was independently associated with fibrostenosis and a com-plicated/penetrating disease phenotype, irrespective of patients' positivity for ASCA, anti-I2, and anti-OmpC. However, the magnitude of anti-CBir1 reactivity, when added to the quantitative response toward the other 3 CD-associated antigens, did enhance the discrimination of fibrostenosis, internal penetration, UC-like CD, and small-bowel involvement (Figure 3).
Figure 3.
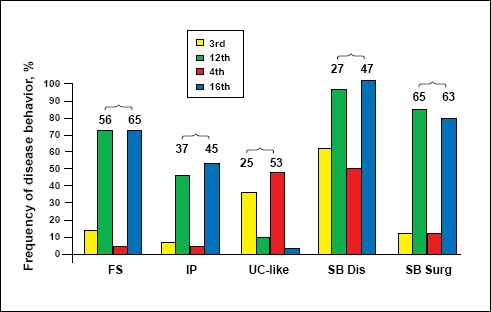
Quantitative antibody responses to 3 versus 4 antigens enhances the discrimination of fibrostenosis (FS), internal penetrating (IP), ulcerative colitis (UC)-like, and small bowel (SB) disease, but not surgery. The numbers represent the differences in the frequency of the indicated phenotype between the lowest versus highest quartile sum scores for 3 (3rd vs 12th) or 4 antibodies (4th vs 16th).
Reproduced with permission from Papadakis, et al.62
In sum, the current data indicate that, for many patients with CD, the presence of mutations in the innate immunity gene NOD2 result in an overcompensatory response by the adaptive immune system. This hyperresponse seems to lead to the formation of antibodies to microbial antigens such as oligomannan (ASCA), CBir1, I2, and OmpC. These antibodies appear to play a major role in the development of chronic intestinal inflammation, such that both increasing numbers of positive antibody tests as well as the strength of the response to each antigen are associated with a complicated and aggressive disease course.
Individualizing Therapy Based on Genetic and Serologic Prognosis
With these genetic and serologic data in mind, clinicians have the ability to use laboratory and genetic testing information in order to stratify patients relative to their risk of disease progression from the time of diagnosis. This approach permits physicians to develop a more individualized treatment plan for each patient and will empower them to explain the risk/benefit ratio of these treatments to patients and families in a manner that is backed by concrete data.
This has become particularly important over the past decade with the increasing use of biologic therapies. Although these therapies have been proven effective in clinical trials, they also carry a small but real risk of lymphoma and opportunistic infection.63 Because of the risks and expense associated with biologic therapies, there has been much discussion regarding their optimal role in the treatment of CD. Should these agents be saved as a last resort for the patient who has failed therapy with corti-costeroids or immunomodulators, or should they be used early and aggressively in order to arrest the disease course before it becomes complicated?64
This so-called “step-up versus top-down” debate has proponents and critics on both sides, and it is outside the scope of this review to discuss all of the available clinical trial data. Nonetheless, the identification of those patients at greatest risk for rapid disease progression would be of great value in stratifying patients at the time of diagnosis into a more or less aggressive treatment paradigmbe it surgical, medical, or biologic. The most current data suggest that genetic markers and serological markers of immune reactivity can identify those patients most likely to benefit from the early use of aggressive therapies, and that physicians can use this information to begin to meet the goal of altering the natural history of disease.
Acknowledgments
Dr. Lichtenstein serves as a consultant to Prometheus Laboratories and acknowledges the support of Prometheus Laboratories for development of this manuscript.
References
- 1.Hendrickson BA, Gokhale R, Cho JH. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev. 2002;15:79–94. doi: 10.1128/CMR.15.1.79-94.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loftus EV, Jr, et al. Crohn's disease in Olmsted County, Minnesota, 1940–1993: Incidence, prevalence, and survival. Gastroenterology. 1998;114:1161–1168. doi: 10.1016/s0016-5085(98)70421-4. [DOI] [PubMed] [Google Scholar]
- 3.Logan RF. Inflammatory bowel disease incidence: Up, down or unchanged? Gut. 1998;42:309–311. doi: 10.1136/gut.42.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanauer SB. Review articles: Drug therapy, inflammatory bowel disease. N Engl J Med. 1996;334:841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 5.Kappelman MD, Rifas-Shiman SL, Porter CQ, et al. Direct health care costs of Crohn's disease and ulcerative colitis in US children and adults. Gastroenterology. 2008;135:1907–1913. doi: 10.1053/j.gastro.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Love JR, Irvine EJ, Fedorak RN. Quality of life in inflammatory bowel disease. J Clin Gastroenterol. 1992;14:15–19. doi: 10.1097/00004836-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 7.Porcelli P, Zaka S, Centonze S, et al. Psychological distress and levels of disease activity in inflammatory bowel disease. Ital J Gastroenterol. 1994;26:111–115. [PubMed] [Google Scholar]
- 8.Lix LM, Graff LA, Walker JR, et al. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1575–1584. doi: 10.1002/ibd.20511. [DOI] [PubMed] [Google Scholar]
- 9.Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49:777–782. doi: 10.1136/gut.49.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bewtra M, Su C, Lewis JD. Trends in hospitalization rates for inflammatory bowel disease in the United States. Clin Gastroenterol Hepatol. 2007;5:597–601. doi: 10.1016/j.cgh.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Cosnes J, Nion-Larmurier I, Beaugerie L, et al. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jess T, Riis L, Vind I, et al. Changes in clinical characteristics, course, and prognosis of inflammatory bowel disease during the last 5 decades: a populationbased study from Copenhagen, Denmark. Inflamm Bowel Dis. 2007;13:481–489. doi: 10.1002/ibd.20036. [DOI] [PubMed] [Google Scholar]
- 13.Aeberhard P, Berchtold W, Riedtmann HJ, Stadelmann G. Surgical recurrence of perforating and nonperforating Crohn's disease. A study of 101 surgically treated patients. Dis Colon Rectum. 1996;39:80–87. doi: 10.1007/BF02048274. [DOI] [PubMed] [Google Scholar]
- 14.Munkholm P, Langholz E, Davidsen M, Binder V. Intestinal cancer risk and mortality in patients with Crohn's disease. Gastroenterology. 1993;105:1716–1723. doi: 10.1016/0016-5085(93)91068-s. [DOI] [PubMed] [Google Scholar]
- 15.Rogers BMG, Clark LM, Kirsner JB. The epidemiologic and demographic characteristics of inflammatory bowel disease: an analysis of a computerized file of 1400 patients. J Chronic Dis. 1971;24:743–773. doi: 10.1016/0021-9681(71)90087-7. [DOI] [PubMed] [Google Scholar]
- 16.Mir-Madjlessi SH, Michener WM, Farmer RG. Course and prognosis of idiopathic ulcerative proctosigmoiditis in young patients. J Pediatr Gastroenterol Nutr. 1986;5:570–576. [PubMed] [Google Scholar]
- 17.Kelsen J, Baldassano RN. Inflammatory bowel disease: the difference between children and adults. Inflamm Bowel Dis. 2008;14(2):S9–11. doi: 10.1002/ibd.20560. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester FA, Wyzga N, Hyams JS, et al. Natural history of bone metabolism and bone mineral density in children with inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:42–50. doi: 10.1002/ibd.20006. [DOI] [PubMed] [Google Scholar]
- 19.Kanof ME, Lake AM, Bayless TM. Decreased height velocity in children and adolescents before the diagnosis of Crohn's disease. Gastroenterology. 1988;95:1523–1527. doi: 10.1016/s0016-5085(88)80072-6. [DOI] [PubMed] [Google Scholar]
- 20.Shamir R, Phillip M, Levine A. Growth retardation in pediatric Crohn's disease: pathogenesis and interventions. Inflamm Bowel Dis. 2007;13:620–628. doi: 10.1002/ibd.20115. [DOI] [PubMed] [Google Scholar]
- 21.Aiges H, Markowitz J, Rosa J, et al. Home nocturnal supplemental nasogastric feedings in growth-retarded adolescents with Crohn's disease. Gastroenterology. 1989;97:905–910. doi: 10.1016/0016-5085(89)91496-0. [DOI] [PubMed] [Google Scholar]
- 22.Heuschkel R, Salvestrini C, Beattie RM, et al. Guidelines for the management of growth failure in childhood inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:839–849. doi: 10.1002/ibd.20378. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, van Assche G, Rutgeerts P. Review article: Altering the natural history of Crohn's disease--evidence for and against current therapies. Aliment Pharmacol Ther. 2007;25:3–12. doi: 10.1111/j.1365-2036.2006.03134.x. [DOI] [PubMed] [Google Scholar]
- 24.Romberg-Camps MJ, Dagnelie PC, Kester AD, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009;104:371–383. doi: 10.1038/ajg.2008.38. [DOI] [PubMed] [Google Scholar]
- 25.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70:439–444. [PubMed] [Google Scholar]
- 26.Feagan BG, McDonald JWD, Koval JJ. Therapeutics and inflammatory bowel disease: A guide to the interpretation of randomized controlled trials. Gastroenterology. 1996;110:275–283. doi: 10.1053/gast.1996.v110.pm8536868. [DOI] [PubMed] [Google Scholar]
- 27.Sostegni R, Daperno M, Scaglione N, et al. Review article: Crohn's disease: monitoring disease activity. Aliment Pharmacol Ther. 2003;17(2):11–7. doi: 10.1046/j.1365-2036.17.s2.17.x. [DOI] [PubMed] [Google Scholar]
- 28.Sands BE, Ooi CJ. A survey of methodological variation in the Crohn's disease activity index. Inflamm Bowel Dis. 2005;11:133–138. doi: 10.1097/00054725-200502000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hanauer S, Sandborn W. The Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn's disease in adults. Am J Gastroenterol. 2001;96:635–643. doi: 10.1111/j.1572-0241.2001.3671_c.x. [DOI] [PubMed] [Google Scholar]
- 30.Fagan EA, Dyck RR, Maton PN, et al. Serum levels of C-reactive protein in Crohn's disease and ulcerative colitis. Eur J Clin Invest. 1982;12:351–359. doi: 10.1111/j.1365-2362.1982.tb02244.x. [DOI] [PubMed] [Google Scholar]
- 31.Consigny Y, Modigliani R, Colombel JF, et al. A simple biological score for predicting low risk of short-term relapse in Crohn's disease. Inflamm Bowel Dis. 2006;12:551–557. doi: 10.1097/01.ibd.0000225334.60990.5b. [DOI] [PubMed] [Google Scholar]
- 32.Darbari A, Kalloo AN, Cuffari C. Diagnostic yield, safety, and efficacy of push enteroscopy in pediatrics. Gastrointest Endosc. 2006;64:224–228. doi: 10.1016/j.gie.2006.02.039. [DOI] [PubMed] [Google Scholar]
- 33.Fireman Z, Mahajna E, Broide E, et al. Diagnosing small bowel Crohn's disease with wireless capsule endoscopy. Gut. 2003;52:390–392. doi: 10.1136/gut.52.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hara AK, Leighton JA, Heigh RI, et al. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128–34. doi: 10.1148/radiol.2381050296. [DOI] [PubMed] [Google Scholar]
- 35.Marmo R, Rotondano G, Piscopo R, et al. Capsule endoscopy versus enteroclysis in the detection of small-bowel involvement in Crohn's disease: a prospective trial. Clin Gastroenterol Hepatol. 2005;3:772–776. doi: 10.1016/s1542-3565(05)00483-0. [DOI] [PubMed] [Google Scholar]
- 36.Albert JG, Martiny F, Krummenerl A, et al. Diagnosis of small bowel Crohn's disease: a prospective comparison of capsule endoscopy with magnetic resonance imaging and fluoroscopic enteroclysis. Gut. 2005;54:1721–1727. doi: 10.1136/gut.2005.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin OS, Brandabur JJ, Schembre DB, et al. Acute symptomatic small bowel obstruction due to capsule impaction. Gastrointest Endosc. 2007;65:725–728. doi: 10.1016/j.gie.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 38.Peacock K, Porn U, Howman-Giles R, et al. 99mTc-stannous colloid white cell scintigraphy in childhood inflammatory bowel disease. J Nucl Med. 2004;45:261–265. [PubMed] [Google Scholar]
- 39.Pilleul F, Godefroy C, Yzebe-Beziat D, et al. Magnetic resonance imaging in Crohn's disease. Gastroenterol Clin Biol. 2005;29:803–808. doi: 10.1016/s0399-8320(05)86351-1. [DOI] [PubMed] [Google Scholar]
- 40.Hugot JP, Laurent-Puig P, Gower-Rousseau C, et al. Mapping of a susceptibility locus for Crohn's disease on chromosome 16. Nature. 1996;379:821–823. doi: 10.1038/379821a0. [DOI] [PubMed] [Google Scholar]
- 41.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 42.Ogura Y, Bonen DK, Inohara N, et al. A frameshift mutation in NOD2 associ-ated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 43.Inohara N, Ogura Y, Nunez G. Nods: a family of cytosolic proteins that regu-late the host response to pathogens. Curr Opin Microbiol. 2002;5:76–80. doi: 10.1016/s1369-5274(02)00289-8. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 45.Gutierrez O, Pipaon C, Inohara N, et al. Induction of Nod2 in myelomono-cytic and intestinal epithelial cells via nuclear factor-kappa B activation. J Biol Chem. 2002;277:41701–41705. doi: 10.1074/jbc.M206473200. [DOI] [PubMed] [Google Scholar]
- 46.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–1009. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 47.Lala S, Ogura Y, Osborne C, et al. Crohn's disease and the NOD2 gene: a role for Paneth cells. Gastroenterology. 2003;125:47–57. doi: 10.1016/s0016-5085(03)00661-9. [DOI] [PubMed] [Google Scholar]
- 48.Shaw SH, Hampe J, White R, et al. Stratification by CARD15 variant geno-type in a genome-wide search for inflammatory bowel disease susceptibility loci. Hum Genet. 2003;113:514–521. doi: 10.1007/s00439-003-1020-7. [DOI] [PubMed] [Google Scholar]
- 49.Sugimura K, Taylor KD, Lin YC, et al. A novel NOD2/CARD15 haplo-type conferring risk for Crohn disease in Ashkenazi Jews. Am J Hum Genet. 2003;72:509–518. doi: 10.1086/367848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abreu MT, Taylor KD, Lin YC, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679–88. doi: 10.1053/gast.2002.35393. [DOI] [PubMed] [Google Scholar]
- 51.Lesage S, Zouali H, Cezard JP, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. Am J Hum Genet. 2002;70:845–857. doi: 10.1086/339432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinton JF, Sendid B, Reumaux D, et al. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut. 1998;42:788–791. doi: 10.1136/gut.42.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffenberg EJ, Fidanza S, Sauaia A. Serologic testing for inflammatory bowel disease. J Pediatr. 1999;134:447–452. doi: 10.1016/s0022-3476(99)70202-7. [DOI] [PubMed] [Google Scholar]
- 54.Sandborn WJ, Loftus EV Jr, Colombel JF, et al. Evaluation of serologic disease markers in a population-based cohort of patients with ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 2001;7:192–201. doi: 10.1097/00054725-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Forcione DG, Rosen MJ, Kisiel JB, et al. Anti-Saccharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for early surgery in Crohn's disease. Gut. 2004;53:1117–1122. doi: 10.1136/gut.2003.030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn's disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–699. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 57.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Targan SR, Landers CJ, Yang H, et al. Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn's disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 59.Dubinsky MC, Kugathasan S, Mei L, et al. Increased immune reactivity predicts aggressive complicating Crohn's disease in children. Clin Gastroenterol Hepatol. 2008;6:1105–1111. doi: 10.1016/j.cgh.2008.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beckwith J, Cong Y, Sundberg JP, et al. Cdcs1, a major colitogenic locus in mice, regulates innate and adaptive immune response to enteric bacterial antigens. Gastroenterology. 2005;129:1473–1484. doi: 10.1053/j.gastro.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 61.Devlin S, Yang H, Ippoliti A, et al. NOD2 variants and antibody response to microbial antigens in Crohn's disease patients and their unaffected relatives. Gastroenterology. 2007;132:576–586. doi: 10.1053/j.gastro.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 62.Papadakis KA, Yang H, Ippoliti A, et al. Anti-flagellin (CBir1) phenotypic and genetic Crohn's disease associations. Inflamm Bowel Dis. 2007;13:524–530. doi: 10.1002/ibd.20106. [DOI] [PubMed] [Google Scholar]
- 63.Mackey AC, Green L, Liang LC, et al. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease. J Pediatr Gastroenterol. 2007;44:265–267. doi: 10.1097/MPG.0b013e31802f6424. [DOI] [PubMed] [Google Scholar]
- 64.Baert F, Caprilli R, Angelucci E. Medical therapy for Crohn's disease: topdown or step-up? Dig Dis. 2007;25:260–266. doi: 10.1159/000103897. [DOI] [PubMed] [Google Scholar]


