Abstract
Hepatocellular carcinoma (HCC) is the most common form of liver cancer worldwide. The impact of this disease is great, as it is the third-leading cause of global cancer-related mortality. Traditionally, patients with HCC did not present until they were in late stages of the disease, limiting their therapeutic options. In recent years, improvements in disease awareness, as well as in surveillance and screening techniques, have led to earlier diagnosis and the potential for improved prognosis and patient survival. Some current treatments rely on surgical or locoregional techniques, many of which were not suitable for patients with advanced stage disease. In addition to surgical resection, advances in radiofrequency ablation and tran-sarterial chemoembolization procedures have increased survival. However, these improvements are short-lived, requiring alternative therapies for patients with recurrent or advanced-stage HCC. Although conventional chemotherapeutic agents have traditionally been administered in this setting, their role in HCC is decreasing as advances in targeted therapies have proven successful in this disease. Notably, treatment with the multi-targeted tyrosine kinase inhibitor sorafenib led to significant improvements in survival in phase III clinical studies, resulting in its approval for unresectable HCC. This clinical roundtable provides an overview of HCC, first focusing on the recognition of the disease. This overview is followed by an in-depth discussion of successful management of HCC using a multimodality approach. Techniques in surgical resection and locoregional therapy are described, as are the safety and efficacy of new systemic and targeted agents. Upon completion of this activity, physicians will have an improved understanding of the occurrence, diagnosis, and treatment of HCC.
Overview of Hepatocellular Carcinoma
Liver cancer is a broad term that includes both primary hepatic malignancies and metastatic lesions that have spread from a separate primary site to the liver. A number of primary liver cancers have been identified; these malignancies are named for the region of the liver from which they arise. For example, hepatocellular carcinomas (HCC) arise from liver hepatocytes. A second liver cancer type, cholangiocarcinoma, arises from the bile ducts that transport bile to the gallbladder and intestine. Hepatic angiosarcomas and hemangiosarcomas are more rare, arising from the liver vasculature. Finally, hepatoblastoma is a type of liver cancer that typically occurs in very young (<4 years) children and arises from liver progenitor cells. Of these liver cancers, HCC is the most common form, accounting for approximately 75% of cases.1
Epidemiology
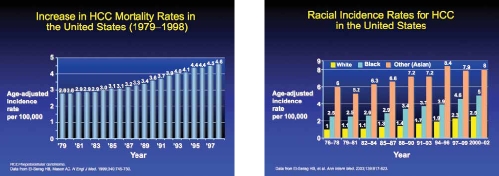
HCC is a global disease. The impact of HCC is significant; it is the third most common cause of cancer-related mortality in the world.2 HCC-related mortality is affected by ethnicity, with different mortality rates apparent among different ethnicities (Table 1). For example, in the United States, patients of Vietnamese ancestry have been found to have a particularly high mortality rate compared with other ethnic groups.3 However, it is unclear why particular ethnicities have a greater likelihood of dying from HCC than others.
Table 1.
HCC Among Men in the United States: Effect of Immigration
| 1999–2001 | 1989–1991 | 1979–1981 | ||||
|---|---|---|---|---|---|---|
| Race/ethnicity | SMR | RR | SMR | RR | SMR | RR |
| Hispanic Native | 13.4 | 2.7 | 8.1 | 2.3 | 5.8 | 1.8 |
| Hispanic Immigrant | 6.9 | 1.4 | 4.7 | 1.3 | 4.7 | 1.4 |
| Asian Native | 6.7 | 1.3 | 4.9 | 1.4 | 6.1 | 1.9 |
| Asian Immigrant | 18.2 | 3.7 | 17.8 | 5.1 | 13.8 | 4.3 |
Data from El-Serag HB et al. Epidemiology of hepatocellular carcinoma among Hispanics in the United States. Arch Intern Med. 2007;167:1983-1989.
- HCC=
hepatocellular carcinoma
- RR=
rate ratio
- SMR=
standardized mortality rate.
In regions of the world where the incidence of HCC is particularly high, the majority of cases are due to hepatitis B and hepatitis C, the primary risk factors for HCC.4 Additionally, the rising incidence in developed countries is also due to a high rate of alcohol consumption or the occurrence of non-alcoholic fatty liver disease. In fact, the rising incidence of non-alcoholic fatty liver disease predicts that the number of HCC cases will also increase in the coming years.
The worldwide incidence of liver cancers was 564,000 in the year 2000, with at least 20,000 cases seen in 2009.2 Notably, the incidence of HCC varies geographically, with approximately 81% of the tumors occurring in the devel-oping world.2 Of these, the highest incidences occur in western and central Africa as well as in East and Southeast Asia, including China. Compared to these regions, the incidence of HCC in the developed world (except Japan) is significantly less. However, recent studies have shown a rising incidence in developed countries.5–8 In the United States, the incidence of HCC doubled from 1.4 to 2.4 cases per 100,000 persons between 1976 to 1980 and 1991 to 1995, with even more impressive increases as of 2009.2 These dra-matic increases were likely due to the spread of hepatitis C virus contracted from unscreened blood transfusions and intravenous drug use, an increased number of immigrants with hepatitis B virus (HBV) infection, and the epidemic of obesity and fatty liver. Many of the current cases of HCC arose in patients who first contracted the hepatitis C virus several decades ago and who subsequently developed liver injury, cirrhosis, and, ultimately, HCC. Another explanation for the rising incidence of HCC in the developed world is a rising awareness and diagnosis, coupled with improved screening for HBV and HCC with subsequent improved surveillance. Finally, increasing HCC incidence may also be due to a concomitant increase in the obesity epidemic, as obesity has been found to be a risk factor for HCC and is associated with higher mortality rates once cancer is diagnosed. The American Cancer Society estimated that in 2009, the number of newly diagnosed liver cancers would be 22,620,9 and that liver cancer would be the sixth most common cause of cancer death in men and the ninth most common in women in the United States.
One major route by which HCC develops is a sequence of events initiated by genetic and epigenetic changes in the hepatocyte or stem cells within the liver. Often, these genetic changes arise from damage triggered by a virus (such as hepatitis B virus or hepatitis C virus), inflammation, or a toxin. This inflammatory activity leads to fibrosis and, eventually, liver cirrhosis, at which point regenerative nodules may begin to develop that can then evolve to dysplasia and subsequently to cancer. This damaged tissue produces, in sequence, well differentiated tumor cells, followed by moderately differentiated tumor cells, and then poorly differentiated tumor cells. Anaplasia, or the complete dedifferentiation of hepatocytes, is another final pathway of HCC pathogenesis.
Presentation and Concurrent Liver Dysfunction
Up to a decade ago, HCC patients would typically present when they were in a very late stage of the disease, primarily because there was very little knowledge and awareness of HCC among the medical community and lack of screening and surveillance.
In 2005, guidelines from the American Association for the Study of Liver Diseases stated that screening and surveillance was the standard of care and highlighted the data that surveillance would improve survival.10 As these surveillance strategies have since become standard of care in the community, patients have begun to present in earlier stages of HCC. These patients have a lower number of tumors, and the tumors that they do have are much smaller at the time of diagnosis. When patients do present with late-stage HCC, they often have evidence of metastatic disease. HCC typically metastasizes locally to the portal vein and local lymph nodes, as well as to more distant organs, including the brain, bone, and lung. Local sites of metastasis also include the peritoneum.
Liver function is typically assessed using a liver panel. These liver panels can include levels of albumin and bilirubin and calculation of the international normalized ratio, a measure of blood clotting. A physical examination is used to assess for visible signs of liver dysfunction, such as jaundice, hepatic encephalopathy, and signs of fluid retention.
Tumor Characterization
Tumor characterization is first performed using several imaging modalities. The standard for HCC screening and surveillance is ultrasound imaging. However, some practi-tioners also rely on computed tomography (CT) at regular intervals of 6 or 12 months. However, the radiation exposure associated with CT scans mitigates the usefulness for surveillance. These high levels of radiation can trigger other malignancies, and the risk should be discussed with the patient when deciding on the type of screening to perform.
Alternatively, magnetic resonance imaging (MRI) scans represent a growing standard for advanced imaging follow-up when an abnormal lesion is identified on ultrasound. The best results with MRI scans occur with multiphase imaging, comprised of a non-contrast phase, an arterial contrast phase, and 2 venous contrast phases. This multiphase imaging allows for the clear and accurate determination of rapid arterial concentration or enhancement. A rapid venous washout can help to differentiate HCC from dysplastic nodules. Because of the possibility of an adverse reaction to the gadolinium contrast agent used in MRI when a patient has renal insufficiency, it is important to obtain informed consent for this procedure from the patient.
A new contrast agent, gadoxetate disodium, is a novel gadolinium-based contrast agent with liver organ-specificity. Because of its ability to target the liver, this agent can increase the specificity and sensitivity of MRI to screen for HCC. Additionally, the high rate of biliary excretion of this agent makes it safer to use in patients with renal insufficiency.
Several types of information can be gleaned from a combination of imaging studies. For example, the tumor location (or locations) will be documented. A radiologist can provide specific information on the segments, the number and size of the lesion(s), the vascular proximity, and vascular involvement, as well as the degree of vascular invasion. The presence or absence of metastatic disease can also be clarified.
Although historically, liver biopsy was the primary strategy by which HCC was diagnosed, advances in the imaging modalities discussed here have allowed patients to be diagnosed without tissue assessment. Currently, fewer than 5% of individuals have their HCC diagnosed by a liver biopsy. However, liver biopsy is useful because it helps to grade the tumor and determine if there is neurovascular or lymphatic involvement. The biopsy may add more detailed information to help determine the optimal management of the patient when gene array, proteomic, and other methodologies are available. This additional information may allow the practitioner to determine the best intervention for the patient as well as help with prognosis. In the near future, liver biopsies may be useful to help guide oral multikinase therapy as well as tumor-targeted therapy. Furthermore, in the future, liver biopsies may prove to be especially beneficial as more information is gained regarding the other gene profiles of these tumors to guide patient management.
Biomarkers are another useful tool to help characterize HCC. Three biomarker tests are currently approved by the US Food and Drug Administration (FDA) in this setting. Throughout the medical community, a-fetoprotein (AFP) remains one of the tests that is a component of the standard of care for surveillance, but serves as a supplement to ultrasound. It is important to emphasize that the American Association for the Study of Liver Diseases (AASLD) guidelines removed AFP as a primary screening and surveillance modality for HCC due to the lack of sensitivity and specificity. In addition to AFP, des-carboxy-prothrombin and AFP-L3, a glycoform of AFP, are used by some practitioners to add in diagnosis and management of HCC. In some cases, imaging studies may be negative for HCC, but a biomarker panel may support the diagnosis of a liver malignancy. In these patients, the physician may request additional review of the imaging studies, to ensure that they were sufficient to document the absence or presence of HCC. Biomarkers may also be useful in identifying patients at risk of overall recurrence or early recurrence, and therefore they may be helpful in surveillance planning. However, before biomarkers become a standard for determining prognosis, more information is needed about their use and accuracy in this setting.
The Barcelona Clinic Liver Cancer (BCLC) criteria for staging patients have been very useful. The criteria have been validated for use in determining which patients would benefit from certain treatments, such as resection, liver transplant, ablation treatment, radiation therapies, targeted and systemic therapies, or symptomatic care.11 The BCLC criteria should be used in conjunction with the Child-Pugh score and the Eastern Cooperative Oncology Group (ECOG) score in order to appropriately categorize the patient and consider whether transjugular portal pressure measurements might be necessary to help stage the presence and/or severity of portal hypertension.
Defining the Roles of HCC Caregivers
For optimal care, patients with HCC should be managed by a multidisciplinary team consisting of several specialists. Because imaging studies are critical to diagnose HCC and to determine the course of treatment, interventional radiologists can provide a great deal of information to direct patient care. Oncologic-oriented hepatologists can provide expert opinion on the best choices for therapy. Because of their experience, these physicians can also provide invaluable opinions as to whether a patient should be treated with systemic therapy or chemotherapy. Hepatologists can also guide the use of newer systemic agents and help manage their associated side effects. Radiation oncologists are more frequently becoming a member of the management team for the subset of HCC patients who require external beam irradiation. With the use of biomarkers, pathologists are needed to provide analysis of tissue samples. Gastroenterologists often are also members of this care team; however, if their practice does not frequently care for HCC patients, they will require extensive education to remain current in HCC disease management. As patients present with earlier stages of HCC, surgical oncologists can help to recognize those who are candidates for resection. Oncology nursing staff members are a critical component of patient care, as these providers can help to ensure patient compliance to treatment. A regimen including systemic oral therapies may be difficult for some patients to follow, and nursing staff may be especially helpful in these cases.
In addition to the HCC care team, primary care physicians have an important role, as they are often the physician who screens and performs surveillance for the disease. In conjunction with primary care providers, hospitalists and intensivists should remain up-to-date with current HCC management. These providers offer an important point of contact for patients. They can discuss the many interventions that are available for HCC and suggest ways to pursue these treatments, with the goal of improving patient quality of life and prolonging survival.
References
- 1.American Cancer Society. What is liver cancer? [Accessed February 25, 2010]. Available at: http://www.cancer.org/docroot/cri/content/cri_2_2_1x_what_is_liver_cancer_25.asp.
- 2.Shariff MI, Cox IJ, Gomaa AI, Khan SA, Gedroyc W, Taylor-Robinson SD. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeutics. Expert Rev Gastroenterol Hepatol. 2009;3:353–367. doi: 10.1586/egh.09.35. [DOI] [PubMed] [Google Scholar]
- 3.McCracken M, Olsen M, Chen MS, Jr, et al. Cancer incidence, mortality, and associated risk factors among Asian Americans of Chinese, Filipino, Vietnamese, Korean, and Japanese ethnicities. CA Cancer J Clin. 2007;57:190–205. doi: 10.3322/canjclin.57.4.190. [DOI] [PubMed] [Google Scholar]
- 4.Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma—epidemio-logical trends and risk factors. Dig Dis. 2009;27:80–92. doi: 10.1159/000218339. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5 suppl 1):S27–S34. doi: 10.1053/j.gastro.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37(2):S88–S94. doi: 10.1111/j.1872-034X.2007.00168.x. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–813. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 9.American Cancer Society. Cancer Facts and Figures 2009. [Accessed February 24, 2010]. Available at: http://www.cancer.org/downloads/STT/500809web.pdf.
- 10.Bruix J, Sherman M, for the Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 11.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
Applications and Limitations of Surgical and Regional Therapeutic Techniques
Surgical options for patients with HCC are largely categorized as either tumor resections or liver transplantation. Three criteria are critical when evaluating a patient as a candidate for surgical therapy: performance status, liver function, and overall tumor burden. Establishing whether the patient has developed HCC in the setting of chronic liver disease is essential, as it will have a great impact on determining whether resection can be performed. Liver synthetic function can be evaluated using total bilirubin, creatinine, prothrombin time, and albumin. It is also necessary to determine the degree of portal hypertension, which may ultimately prove to be a contraindication in some patients. Multiple studies have shown direct portal vein or hepatic venous measurements to be effective for identifying patients as surgical candidates.1 However, many physicians, especially in the United States, find these measurements to be quite burdensome. As an alternative, physicians may use platelet count (greater than or less than 100), endoscopy (to show an absence of obvious varices), or CT or MRI scans to determine if there is evidence of portal hypertension.
The BCLC staging classification, first proposed in 1999, was specifically designed to identify the best candidates for the best therapies based on tumor burden, performance status, and hepatic function.2 One recent study validated the BCLC system as the best classification for determining patient prognosis with surgical therapy.3 The BCLC consists of 4 stages. Early stage (A) describes patients with asymptomatic early tumors, who are often candidates for more radical types of therapy, such as resection, transplantation, or percutaneous treatment. Intermediate stage (B) includes patients with asymptomatic multifocal HCC; these patients are candidates to receive local therapy such as transarterial chemoembolization. Advanced stage (C) describes patients with symptomatic tumors and/or an invasive tumor pattern that is either a vascular invasion or an extrahepatic spread; sorafenib has been shown to improve survival for these patients. Patients with end-stage disease (D) are patients with hepatic failure, who have an extremely grim prognosis; the goal of treatment for these patients is limited to symptom palliation. A prospective validation of the BCLC classification scheme found it to be an independent predictor for patient survival.4
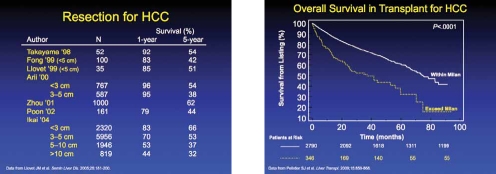
The largest experience with surgical resection for HCC comes from the Japanese Liver Cancer Study Group. This study analyzed about 13,000 cases of surgical resections from multiple centers. Tumor size was found to be a primary determinant of surgical outcome and overall survival (OS).5 For example, patients with a single lesion of <3 cm had a 5-year OS rate of over 50%. However, the 5-year survival decreased to approximately 30% in patients with a tumor lesion between 3–5 cm.
Recently, in the United States, a group investigated data from the Surveillance of Epidemiology and End Results database. This study showed that in 788 patients with early-stage HCC (median size 3.2 cm), the 5-year rate of OS was 39%.6 Although surgical resection can be successfully performed in patients with HCC in the setting of a cirrhotic liver, the 5-year survival rates are between 30–50%. Thus, the application of resection for HCC in the cirrhotic liver should be performed in highly selected patients without portal hypertension. Among patients who lack evidence of cirrhosis or portal hypertension, the degree of resection can be more liberal, and patients with larger tumors can be included.
The other main surgical intervention for HCC patients is liver transplantation. In the seminal publication from Mazzaferro and colleagues, it was shown that a 4-year survival of 75% was associated with patients who had received a transplantation for HCC adhering to the following criteria: a single lesion <5 cm or up to 3 lesions that were each <3 cm, without microvascular or extrahepatic involvement (ie, the Milan criteria).7
After this publication, the United Network for Organ Sharing (UNOS) adopted the Milan criteria to determine candidates for transplantation. A study reviewing liver transplant since the implementation of the Milan criteria found a 5-year OS of 62%.8 Importantly, this study showed that patients who did not meet the Milan criteria had a much worse survival, indicating the importance of adhering to this criteria in order to maximize the use of scarce resource. Patients with HCC who meet the Milan criteria are then given high priority using the model of end-stage liver disease (MELD) organ allocation system.
Radiofrequency Ablation
Radiofrequency ablation (RFA) is a technique in which an electromagnetic energy deposition is used to thermally ablate the hepatic tumor tissue, resulting in coagulated necrosis. RFA is the most widely used ablation technique for HCC.9 As such, it is the standard nonsurgical treatment for patients with early-stage HCC (ie, Barcelona stage A). Depending on the type of electrode used, ablation diameters up to between 5 and 7 cm are possible.
RFA is most often compared with another local ablation modality, percutaneous ethanol injection (PEI), for the treatment of early HCC. In 4 randomized trials, the rates of complete response are higher with RFA (90–96%) compared with PEI (80–88%; Table 1).10-13 Typically, the complete response associated with RFA is achieved in fewer sessions than those required by PEI. More importantly, RFA was associated with an increased OS. Based on these results, RFA has become the preferred ablation method for local HCC, and it is the treatment modality recommended by the AASLD.14 Because of the potential risk of thermal injury to critical structures such as the biliary ducts and gastrointestinal tract, tumor location is a major limitation of RFA therapy.15
Table 1.
Ablation for Early Stage HCC
| CR% | Sessions | Survival | |||||
|---|---|---|---|---|---|---|---|
| Author | N | Tumor Size | PEI | RFA | PEI | RFA | Difference |
| Livraghi10 | 86 | <3 cm | 80 | 90 | 4.8 | 1.2 | No |
| Lencioni12 | 102 | Milan | 82 | 91 | 5.4 | 1.1 | Yes Recur-free |
| Lin13 | 157 | <4 cm | 88 | 96 | 6.5 | 1.6 | Yes |
| Shiina11 | 232 | Milan | NA | 6.4 | 2.1 | Yes | |
- CR=
complete response
- HCC=
hepatocellular carcinoma
- NA=
not available
- PEI=
percutaneous ethanol injection
- RFA=
radiofrequency ablation
Transarterial Chemoembolization
Characteristically, hepatic cancers are highly vascular and depend on this extensive arterial blood flow tumor proliferation. Transarterial chemoembolization (TACE) is a local therapy in which chemotherapy is delivered directly to the tumor followed by embolization of the arterial blood vessels (using either a gel foam or microparticles).16 A catheter is guided into either the right or left hepatic artery, depending on the tumor location, and advanced to selectively determine the tumor feeding artery.17
A systematic review of clinical studies that evaluated the TACE procedure reported that doxorubicin was the most frequently used single-agent chemotherapeutic drug (used in 36% of the reviewed procedures).18 Other agents included cisplatin (used in 31% of the procedures), mitoxantrone, and mitomycin C. Double therapy has also been evaluated in other studies. However, a statistically significant benefit of single therapy versus combinations of agents has not been shown.
TACE was associated with improved survival in a large meta-analysis of HCC patients.19 Patients included in these trials had BCLC stage B. In one meta-analysis, compared with nonactive treatment, TACE was shown to decrease the 2-year mortality rate (odds ratio [OR], 0.54; 95% confidence interval [CI], 0.33–0.89; P=.015). More recently, another meta-analysis found that TACE improved survival (OR, 0.53; 95% CI, 0.32–0.89; P=.017;Figure 1).21 Although this meta-analysis also included non-chemotherapy embolization, further sensitivity analysis found a significant benefit with TACE (OR, 0.42; 95% CI, 0.20–0.88, P=.021).
Figure 1.
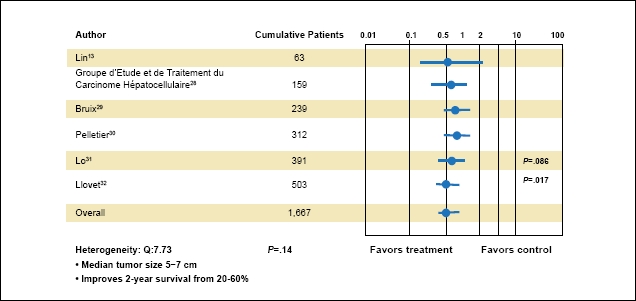
Transarterial chemoembolization (TACE) in hepatocellular carcinoma. This meta-analysis found that TACE improved survival.
Adapted from Llovet JM et al. Hepatology. 2003;37:429-442.
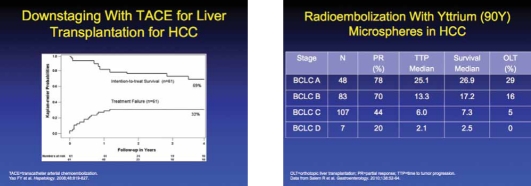
Emerging data have also suggested that TACE can be performed for patients with HCC that exceed the Milan criteria in order to downstage their tumor burden so that liver transplantation can be performed. A clinical study reported that tumor downstaging successfully occurred in 70.5% of 61 patients with a UNOS stage above T2.22 The 4-year OS after downstaging was 69.3%, and the 4-year post-transplantation survival rate was 92.1%. The authors indicated that before liver transplantation was allowed, the tumor had to have a complete response for at least 4 months.
Some centers have begun using doxorubicin-loaded drug-eluting beads in an effort to improve upon the TACE procedure. The advantage is the delivery of a constant dose of chemotherapy directly to the liver without systemic effects, and to also be an embolic agent at the same time.23 However, the benefit of the use of these beads over traditional TACE techniques has yet to be established in a large-scale, well-designed clinical trial.
Radioembolization
During radioembolization, a radioactive isotope is delivered intra-arterially to the HCC. The most common procedure is the use of glass microspheres labeled with radioactive yttrium-90.24 Intra-arterial delivery of these microspheres provides a high dose of radiation locally to HCC. Radioembolization with yttrium-90 is generally performed as a single procedure and often does not require hospitalization. However, its expense may limit its role in the treatment of HCC.
Recently, one study of 71 patients with unresectable HCC compared the effectiveness of TACE versus yttrium-90 radioembolization and reported that both techniques were equally beneficial and produced similar rates of OS.25 In another trial, radioembolization with yttrium-90 resulted in an improved rate of OS compared with TACE (41.6 vs 19.2 months; P=.008), and a greater proportion of patients who were successfully downstaged to Milan criteria (58% vs 31%).26 Radioembolization may benefit patients with Child-Pugh A disease more than those with Child-Pugh B disease.27 Even though these data are encouraging, the last 2 studies come from a single center, and it is unclear if they are generalizable. At the moment, it appears that TACE's effectiveness has been determined by randomized trials, and it should be the first-line treatment for patients with HCC that are unresectable.
References
- 1.Bruix J, Castells A, Bosch J, et al. Surgical resection of hepatocellular carcinoma in cirrhotic patients: prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–10122. doi: 10.1016/s0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 3.Vitale A, Saracino E, Boccagni P, et al. Validation of the BCLC prognostic system in surgical hepatocellular cancer patients. Transplant Proc. 2009;41:1260–1263. doi: 10.1016/j.transproceed.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 4.Cillo U, Vitale A, Grigoletto F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 6.Nathan H, Schulick RD, Choti MA, Pawlik TM. Predictors of survival after resection of early hepatocellular carcinoma. Ann Surg. 2009;249:799–805. doi: 10.1097/SLA.0b013e3181a38eb5. [DOI] [PubMed] [Google Scholar]
- 7.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier SJ, Fu S, Thyagarajan V, et al. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- 9.Callstrom MR, Charboneau JW. Technologies for ablation of hepatocellular carcinoma. Gastroenterology. 2008;134:1831–1835. doi: 10.1053/j.gastro.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655–661. doi: 10.1148/radiology.210.3.r99fe40655. [DOI] [PubMed] [Google Scholar]
- 11.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Lencioni RA, Allgaier HP, Cioni D, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 13.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M, for the Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 15.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38–46. doi: 10.1053/j.tvir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Gunvén P. Liver embolizations in oncology: a review. Part I. Arterial (chemo) embolizations. Med Oncol. 2008;25:1–11. doi: 10.1007/s12032-007-0039-3. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4:424–432. doi: 10.1038/ncponc0844. [DOI] [PubMed] [Google Scholar]
- 18.Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6–25. doi: 10.1007/s00270-006-0062-3. [DOI] [PubMed] [Google Scholar]
- 19.Georgiades CS, Hong K, Geschwind JF. Radiofrequency ablation and chemoembolization for hepatocellular carcinoma. Cancer J. 2008;14:117–122. doi: 10.1097/PPO.0b013e31816a0fac. [DOI] [PubMed] [Google Scholar]
- 20.Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 21.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 22.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varela M, Real MI, Burrel M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Amélie D, Patrick F, Alain H. State of the art: radiolabeled microspheres treatment for liver malignancies. Expert Opin Pharmacother. 2010;11:579–586. doi: 10.1517/14656560903520916. [DOI] [PubMed] [Google Scholar]
- 25.Kooby DA, Egnatashvili V, Srinivasan S, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. doi: 10.1111/j.1600-6143.2009.02695.x. [DOI] [PubMed] [Google Scholar]
- 27.Salem R, Lewandowski RJ, Mulcahy MF, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 28.A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma. Groupe d'Etude et de Traitement du Carcinome Hépatocellulaire. N Engl J Med. 1995;332:1256–1261. doi: 10.1056/NEJM199505113321903. [DOI] [PubMed] [Google Scholar]
- 29.Bruix J, Llovet JM, Castells A, et al. Transarterial embolization versus symptomatic treatment in patients with advanced hepatocellular carcinoma: results of a randomized, controlled trial in a single institution. Hepatology. 1998;27:1578–1583. doi: 10.1002/hep.510270617. [DOI] [PubMed] [Google Scholar]
- 30.Pelletier G, Ducreux M, Gay F, et al. Treatment of unresectable hepatocellular carcinoma with lipiodol chemoembolization: a multicenter randomized trial. Groupe CHC. J Hepatol. 1998;29:129–134. doi: 10.1016/s0168-8278(98)80187-6. [DOI] [PubMed] [Google Scholar]
- 31.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 32.Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
Systemic Therapy for Advanced Stage Hepatocellular Carcinoma
Systemic therapy is an important treatment option for many patients with advanced stage HCC. Often, patients considered for systemic therapy are not optimal candidates for other interventions, including surgical resection of the tumor or local regional therapy, or for liver transplantation. Historically, systemic chemotherapy had only a modest effect on the disease, with little impact on either response rate or OS. However, it is important to note that many of the clinical studies evaluating either single-agent or combination chemotherapy in HCC contained only a small number of patients, thus limiting the ability to calculate survival changes with a high degree of confidence.
The traditional reference single agent in HCC, doxorubicin, is clearly a suboptimal therapeutic option, with only marginal antitumor activity and no effect on survival.1 Despite these drawbacks, doxorubicin is still often administered in conventional clinical practice. Its use as a reference single agent in clinical studies should be strongly discouraged, as it acts effectively as a placebo but can produce significant side effects.
One approach to the development of new therapeutic agents has focused on the molecular pathogenesis of HCC. There are 2 major mechanisms implicated in HCC carcinogenesis, including liver cirrhosis following tissue damage and the occurrence of genetic mutations in one or more oncogenic or tumor suppressor genes. Multiple cellular signaling pathways have been investigated for the development of novel targeted agents. Angiogenic pathways represent a particular vehicle for new targets, as a number of angiogenic factors have been implicated in HCC, reflecting the highly vascular nature of the tumor.2 These include vascular endothelial growth factor (VEGF), platelet-derived growth factor, transforming growth factor, epidermal growth factor (EGF), and hepatocyte growth factor. For example, increased levels of VEGF have been shown to occur in HCC compared with the adjacent normal liver tissue.3 In addition, elevated VEGF expression has been associated with a poorer patient prognosis.4,5 There is also some evidence suggesting that increased levels of VEGF are associated with early relapse.6
Sorafenib
Multi-targeted kinase inhibitors represent one of the most important new classes of agents introduced for the treatment of HCC. Of these, sorafenib was the first to gain approval from the FDA. Sorafenib targets both tumor cell proliferation and angiogenesis pathways, and for these reasons, it was thought it could be useful in patients with HCC. Sorafenib was subsequently investigated in clinical trials (Figure 1), and promising efficacy results led to its evaluation in 2 phase III trials.
Figure 1.
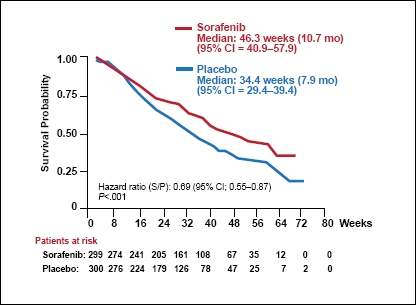
Overall survival.
CI=confidence interval.
Data from Llovet JM, et al. N Engl J Med. 2008;359: 378-390
The Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol (SHARP) trial was a multicenter, 2-arm, double-blind phase III clinical study that compared a standard dose of sorafenib (400 mg twice daily) administered orally versus placebo.7 It was a large trial, which randomized 602 patients with measurable, unresectable, advanced HCC in a 1:1 ratio. Patients continued to receive therapy until observation of both radiologic and symptomatic effects, unacceptable adverse events, or death. The baseline characteristics were well balanced between the 2 treatment groups. Most of the study subjects were men (87% of patients in each arm). The majority of patients were European, and the etiology of liver disease was distributed among hepatitis B virus, hepatitis C virus, and alcohol-induced liver disease.
Patients in the sorafenib group experienced a significantly longer OS (1 of 2 primary endpoints) compared with the placebo group (10.7 vs 7.9 months, hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.55–0.87; P<.001). The 1-year rate of OS was also higher among patients who received sorafenib compared with placebo (44% vs 33%). The 4-month progression-free survival was 62% among patients who received sorafenib and 42% in the placebo group. However, the improvement in the second primary endpoint, median time to symptomatic progression, did not reach statistical significance. In contrast, the secondary endpoint, time to radiologic progression, was significantly longer among patients who received sorafenib therapy (5.5 vs 2.8 months, HR, 0.58; 95% CI, 0.45–0.74; P<.001). Sorafenib patients also experienced a significantly improved disease control rate, which was primarily due to a higher incidence of stable disease, as the rate of response was relatively modest (the overall response was only 2.3% among sorafenib-treated patients).
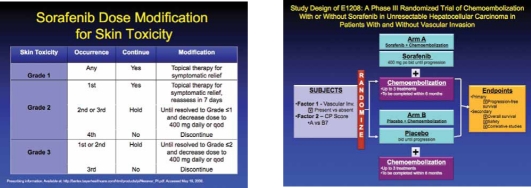
Overall, sorafenib was generally well-tolerated, although drug-related adverse events did occur at a higher frequency in the treatment group compared with placebo (80% vs 52%); most of these were grade 1 or 2 in severity. Grade 3 adverse events that occurred significantly more in the sorafenib group compared with placebo included diarrhea (8% vs 2%; P<.001), hand-foot skin reaction (8% vs <1%; P<.001), and weight loss (2% vs 0%; P=.03). More patients who received sorafenib had to undergo a dose reduction due to adverse events (26% vs 7%). Because of the potential for sorafenib-related adverse events, dose modification schedules and guidelines have been developed.8 Dose reduction strategies associated with sorafenib include both a delay in dose administration and a decrease in dosage. Cardiac toxicity, a particularly concerning adverse event associated with the use of tyrosine kinase inhibitors, was not a significant issue in this trial.
The second major phase III trial that evaluated sorafenib in HCC was the Asia-Pacific, a randomized, double-blind, placebo-controlled study.9 Compared with the SHARP study, this trial was smaller, randomizing a total of 226 patients with advanced HCC to receive either oral sorafenib (400 mg twice daily) or placebo. Randomization occurred in a 2:1 ratio. Enrolled patients were from China, South Korea, and Taiwan. Patients had not previously received systemic therapy, and all were categorized as Child-Pugh liver function class A. Due to the location of this trial, the hepatitis B virus accounted for the majority of disease etiology.
As in the SHARP trial, sorafenib was found to be significantly superior to placebo. Median OS was significantly prolonged among patients who received sorafenib compared with placebo (6.5 vs 4.2 months, HR, 0.68; 95% CI, 0.50–0.93; P=.014). Median time to progression was also improved with sorafenib (2.8 vs 1.4 months, HR, 0.57; 95% CI, 0.42–0.79; P=.0005). As was seen in other studies, sorafenib did not produce a significant response rate.
The safety profile of sorafenib was similar to that reported in the SHARP trial. Among 149 assessable patients, the most frequently reported grade 3 or 4 adverse events were hand-foot skin reaction, diarrhea, and fatigue; of these, hand-foot skin reaction and diarrhea were the most common causes of dose reduction.
Although the overall efficacy results of the Asia-Pacific study were similar to those in the SHARP trial, they were less robust. This difference may reflect the study population, which had more advanced disease.
To improve upon the efficacy associated with sorafenib, biomarkers with the potential to predict response to sorafenib have been investigated. One biomarker currently under investigation is the phosphorylated form of extracelluar signal-regulated kinase (p-ERK), which is a downstream molecule in the RAF/MEK/ERK signaling pathway, a target of sorafenib.10 Constitutive activation of this pathway leads to high levels of p-ERK, and possibly indicates that the tumor cell relies on this pathway for survival. Using immunohistochemistry, patients whose tumor cells had a greater staining intensity for p-ERK experienced a longer time to progression,11,12 which suggests an improved response to sorafenib, perhaps due to pathway inhibition. Other possible biomarkers under investigation to predict response to sorafenib include HGF and c-Kit. It is possible that these markers are related to OS in HCC patients.
Sorafenib-based Combinations
Results from a phase II trial that evaluated the combination of sorafenib with doxorubicin were recently reported.13 This small study of 96 patients suggested that progression-free survival was superior among patients who received the combination regimen. Based on these promising results, a larger randomized phase II trial is planned. Because of the danger of cardiotoxicity associated with both drugs, this adverse event will be carefully monitored.
There is also increasing interest in evaluating sorafenib in combination with biologic therapies, including brivanib and bevacizumab, and evaluating the addition of sorafenib to liver-directed therapies such as in the randomized phase III ECOG 1208 trial comparing transarterial chemoembolization with or without sorafenib.
Other Agents Under Investigation in HCC
Sunitinib, another multi-targeted tyrosine kinase inhibitor, has also been evaluated as a new agent in HCC. Recently, 2 phase II trials of this agent in advanced HCC were reported. In the first, sunitinib exhibited modest antitumor activity and a relatively acceptable safety profile.14 The second study produced only a low response rate with significant toxicity.15
Therapies targeting the EGF receptor are another possible new treatment in HCC. In a phase II trial of the EGF receptor inhibitor cetuximab, no antitumor activity was evident.16 However, a separate study found that cetuximab given in combination with gemcitabine plus oxaliplatin may be beneficial for patients with progressive, advanced HCC.17
In addition to these investigational studies, there is a need to develop better parameters to assess efficacy in HCC. Several randomized studies have prospectively evaluated alternative imaging modality methods. Recently, a focus on molecular imaging has emerged as a research endeavor with potential advantages over anatomical imaging methods, including measurement of tumor biology and molecular features rather than simply density, size, and shape. Another imaging strategy to better assess efficacy is the shape-constrained region growing algorithm. The use of this processing system allows improved demarcation of the tumor and also makes possible post-treatment evaluation of tumor necrosis.
References
- 1.Bruix J, Sherman M, for the Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 2.Pang R, Poon RT. Angiogenesis and antiangiogenic therapy in hepatocellular carcinoma. Cancer Lett. 2006;242:151–167. doi: 10.1016/j.canlet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061–1065. doi: 10.5858/2000-124-1061-IEOVEG. [DOI] [PubMed] [Google Scholar]
- 4.Jia JB, Zhuang PY, Sun HC, et al. Protein expression profiling of vascular endothelial growth factor and its receptors identifies subclasses of hepatocellular carcinoma and predicts survival. J Cancer Res Clin Oncol. 2009;135:847–854. doi: 10.1007/s00432-008-0521-0. [DOI] [PubMed] [Google Scholar]
- 5.Stroescu C, Dragnea A, Ivanov B, et al. Expression of p Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointestin Liver Dis. 2008;17:411–417. [PubMed] [Google Scholar]
- 6.Amaoka N, Osada S, Kanematsu M, et al. Clinicopathological features of hepatocellular carcinoma evaluated by vascular endothelial growth factor expression. J Gastroenterol Hepatol. 2007;22:2202–2207. doi: 10.1111/j.1440-1746.2006.04790.x. [DOI] [PubMed] [Google Scholar]
- 7.Rimassa L, Santoro A. Sorafenib therapy in advanced hepatocellular carcinoma: the SHARP trial. Expert Rev Anticancer Ther. 2009;9:739–745. doi: 10.1586/era.09.41. [DOI] [PubMed] [Google Scholar]
- 8.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Cao Y, Chen C, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–11858. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 11.Zhu AX. Predicting the response to sorafenib in hepatocellular carcinoma: where is the evidence for phosphorylated extracellular signaling-regulated kinase (pERK)? BMC Med. 2009;7:42. doi: 10.1186/1741-7015-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, Zhou X, Shen H, Wang D, Wang Y. Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med. 2009;7:41. doi: 10.1186/1741-7015-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abou-Alfa GK, Johnson P, Knox J, et al. Final results from a phase II (PhII), randomized, double-blind study of sorafenib plus doxorubicin (S+D) versus placebo plus doxorubicin (P+D) in patients (pts) with advanced hepatocellular carcinoma. Abstract presented at: American Society of Clinical Oncology Gastrointestinal Cancers Symposium; January 25-27, 2008; Orlando, FL.
- 14.Zhu AX, Sahani DV, Duda DG, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faivre S, Raymond E, Boucher E, et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: an open-label, multicentre, phase II study. Lancet Oncol. 2009;10:794–800. doi: 10.1016/S1470-2045(09)70171-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhu AX, Stuart K, Blaszkowsky LS, et al. Phase 2 study of cetuximab in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:581–589. doi: 10.1002/cncr.22829. [DOI] [PubMed] [Google Scholar]
- 17.Asnacios A, Fartoux L, Romano O, et al. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: results of a multicenter phase 2 study. Cancer. 2008;112:2733–2739. doi: 10.1002/cncr.23489. [DOI] [PubMed] [Google Scholar]
Biographies



Slide Library
For a free electronic download of these slides, please direct your browser to the following web address: http://www.clinicaladvances.com/index.php/our_publications/gastro_hep-issue/gh_March_2010/
A Multidisciplinary Approach to the Management of Hepatocellular Carcinoma
CME Post-Test: Circle the correct answer for each question below.
-
In addition to infection with the hepatitis b or C virus,which of the following is considered a predominant riskfactor for the development of HCC?
Autoimmune disease
Intravenous drug use
Non-alcoholic fatty liver disease
Tobacco smoking
-
Which of the following is considered the standardimaging modality for characterization of HCC tumors?
Ultrasound
CT
MRI
PET
-
True or False? the majority of cases of HCC arediagnosed using liver biopsy.
True
False
-
Which of the following is not a major criteria whenevaluating a patient as a candidate for surgicaltherapy?
Performance status
Liver function
Biomarker expression
Overall tumor burden
-
Which of the following is not a major limitation of RFA treatment of HCC?
Availability of the procedure
Tumor location
High cost
Adverse events
-
True or False? A statistically significant benefit of doublet therapy versus single therapy for TACE has not been conclusively shown.
True
False
-
Increased expression of which of the following signaling molecules has been associated with poor patient prognosis?
VEGF
TGF
HGF
EGF
-
Which of the following has been the traditional reference agent used in the evaluation of new agents for HCC?
Sorafenib
Cisplatin
Mitomycin C
Doxorubicin
-
In the SHARP trial, what was the approximate rate of 1-year survival associated with sorafenib?
15%
35%
44%
60%
-
Which of the following was not one of the results of the Asia-pacific study?
Sorafenib produced a significant improvement in median overall survival compared with placebo
Sorafenib produced a significant improvement in median time to progression compared with placebo
Sorafenib produced a significant improvement in the rate of tumor response compared with placebo
The safety profile of sorafenib was found to be poorly tolerated
Evaluation Form A Multidisciplinary Approach to the Management of Hepatocellular Carcinoma
PIM is committed to excellence in continuing education, and your opinions are critical to us in this effort. To assist us in evaluating the effectiveness of this activity and to make recommendations for future educational offerings, please take a few minutes to complete this evaluation form. You must complete this evaluation form to receive acknowledgment for completing this activity.
Please rate your level of agreement by circling the appropriate rating:
1 = Strongly Disagree 2 = Disagree 3 = Neutral 4 = Agree 5 = Strongly Agree
Learning Objectives
After participating this activity, I am now better able to:
| 1. Describe the importance of new study findings from recent abstracts, posters, and clinical presentations in the natural history of HCC. | 1 | 2 | 3 | 4 | 5 |
| 2. Assess the results of these new study findings, including current clinical trials evaluating optimal medical treatment regimens and the effect on extending survival in HCC. | 1 | 2 | 3 | 4 | 5 |
| 3. Integrate into clinical practice the latest knowledge and methods for treating patients with HCC in an effort to improve current prognosis statistics, with a clear understanding of what the roles of the oncologist, hepatologist, and gastroenterologist are in treating HCC patients, as well as how each role impacts care. | 1 | 2 | 3 | 4 | 5 |
| 4. Identify future research directions for all therapies in HCC in light of recent clinical data. | 1 | 2 | 3 | 4 | 5 |
Based upon your participation in this activity, choose the statement(s) that apply:
 I gained new strategies/skills/information that I can apply to my area of practice.
I gained new strategies/skills/information that I can apply to my area of practice.
 I plan to implement new strategies/skills/information into my practice.
I plan to implement new strategies/skills/information into my practice.
What strategies/changes do you plan to implement into your practice? ________________________________________
______________________________________________________________________________________
What barriers do you see to making a change in your practice? ______________________________________________________
____________________________________________________________________________________________
Which of the following best describes the impact of this activity on your performance?
 I will implement the information in my area of practice.
I will implement the information in my area of practice.
 I need more information before I can change my practice behavior.
I need more information before I can change my practice behavior.
 This activity will not change my practice, as my current practice is consistent with the information presented.
This activity will not change my practice, as my current practice is consistent with the information presented.
 This activity will not change my practice, as I do not agree with the information presented.
This activity will not change my practice, as I do not agree with the information presented.
Please rate your level of agreement by circling the appropriate rating:
1 = Strongly Disagree 2 = Disagree 3 = Neutral 4 = Agree 5 = Strongly Agree
The content presented:
| Enhanced my current knowledge base | 1 | 2 | 3 | 4 | 5 |
| Addressed my most pressing questions | 1 | 2 | 3 | 4 | 5 |
| Promoted improvements or quality in health care | 1 | 2 | 3 | 4 | 5 |
| Was scientifically rigorous and evidence-based | 1 | 2 | 3 | 4 | 5 |
| Avoided commercial bias or influence | 1 | 2 | 3 | 4 | 5 |
Would you be willing to participate in a post-activity follow-up survey?
 Yes
Yes  No
No
Please list any topics you would like to see addressed in future educational activities:_________________________________
________________________________________________________________________________________
If you wish to receive acknowledgment for completing for this activity, please complete the post-test by selecting the best answer to each question, complete this evaluation verification of participation, and fax to: (303) 790-4876.
Post-test Answer Key
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Request for Credit
Name __________________________________ Degree _____________________________________
Organization _____________________________________ Specialty _____________________________________
Address _____________________________________________________________________________
City, State, Zip _________________________________________________________________________
Telephone _________________________ Fax __________________________ E-mail _____________________________________
Signature _____________________________________ Date ______________________
For Physicians Only: I certify my actual time spent to complete this educational activity to be: ______
 I participated in the entire activity and claim 1.0 credits.
I participated in the entire activity and claim 1.0 credits.
 I participated in only part of the activity and claim _____ credits.
I participated in only part of the activity and claim _____ credits.
Project ID: 6977
Footnotes
Supported through an educational grant from Bayer Healthcare Pharmaceuticals and Onyx Pharmaceuticals, Inc.
Sponsored by

Disclosure of Conflicts of Interest:Postgraduate Institute for Medicine (PIM) assesses conflict of interest with its instructors, planners, managers, and other individuals who are in a position to control the content of CME activities. All relevant conflicts of interest that are identified are thoroughly vetted by PIM for fair balance, scientific objectivity of studies utilized in this activity, and patient care recommendations. PIM is committed to providing its learners with high-quality CME activities and related materials that promote improvements or quality in healthcare and not a specific proprietary business interest of a commercial interest.
The faculty reported the following financial relationships or relationships to products or devices they or their spouse/life partner have with commercial interests related to the content of this CME activity:
Robert A. Gish, MD— Consulting fees, fees for non-CME services, and contracted research: Bayer/Onyx
Jorge A. Marrero, MD, MS— Advisory board: Bayer; Contracted research: Bayer and BMS
Al B. Benson III, MD— Consulting fees: Bayer/Onyx
The following PIM planners and managers, Jan Hixon, RN, BSN, MA, Trace Hutchison, PharmD, Julia Kimball, RN, BSN, Samantha Mattiucci, PharmD, and Jan Schultz, RN, MSN, CCMEP, hereby state that they or their spouse/life partner do not have any financial relationships or relationships to products or devices with any commercial interest related to the content of this activity of any amount during the past 12 months.
Jacquelyn Matos: No real or apparent conflicts of interest.
Lisa Cockrell: No real or apparent conflicts of interest.
Disclosure of Unlabeled Use:This educational activity may contain discussion of published and/or investigational uses of agents that are not indicated by the FDA. Postgraduate Institute for Medicine (PIM), Gastroenterology & Hepatology, Clinical Advances in Hematology & Oncology, Bayer Healthcare Pharmaceuticals, and Onyx Pharmaceuticals do not recommend the use of any agent outside of the labeled indications.
The opinions expressed in the educational activity are those of the faculty and do not necessarily represent the views of PIM, Gastro-Hep Communica-tions, Millennium Medical Publishing, Bayer Healthcare Pharmaceuticals, or Onyx Pharmaceuticals. Please refer to the official prescribing information for each product for discussion of approved indications, contraindications, and warnings.
Disclaimer:Participants have an implied responsibility to use the newly acquired information to enhance patient outcomes and their own professional development. The information presented in this activity is not meant to serve as a guideline for patient management. Any procedures, medications, or other courses of diagnosis or treatment discussed or suggested in this activity should not be used by clinicians without evaluation of their patient's conditions and possible contraindications or dangers in use, review of any applicable manufacturer's product information, and comparison with recommendations of other authorities.
This supplement was authored by an independent medical writer based on presentations and discussions by Robert G. Gish, MD, Jorge A. Marrero, MD, MS, andAl B. Benson III, MD.
Funding for this Clinical Roundtable Monograph has been provided through an educational grant from Bayer Healthcare Pharmaceuticals and Onyx Pharmaceuticals. Support of this monograph does not imply the supporter's agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., Millennium Medical Publishing, Inc., the supporter, and the participants shall not be held responsible for errors or for any conse-quences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorse-ments are made for any drug or compound at present under clinical investigation.
Contributor Information
Robert G Gish, Medical Director, Liver Transplant Program Chief, Division of Complex GI, California Pacific Medical Center, San Francisco, California.
Jorge A Marrero, Keith S. Henley, MD, Collegiate Professor of Gastroenterology Director, Multidisciplinary Liver Tumor Program, University of Michigan, Ann Arbor, Michigan.
Al B. Benson, III, Associate Director for Clinical Investigations, Professor of Medicine, Northwestern University, Feinberg School of Medicine, Division of Hematology/Oncology, Robert H. Lurie Comprehensive Cancer Center, Chicago, Illinois.