Abstract
Biliary complications after liver transplantation remain common. Resources for livers are limited, and these individuals are often ill, making nonoperative treatment and management attractive options. The endoscopic route for evaluation (endoscopic retrograde cholangiopancreatography) remains preferable, due to its safety profile, as opposed to the percutaneous route (percutaneous transhepatic cholangiography with percutaneous transhepatic biliary drainage), though the endoscopic route may not be possible in patients with a Roux-en-Y reconstruction. The two most common early complications include leaks from the anastomosis or cystic duct stump (of the donor or native duct) and obstruction at the surgical anastomosis. Nonoperative treatment is often successful in early complications. Late complications presenting with leaks and obstruction are often more difficult to treat nonoperatively and frequently require surgical treatment or retransplantation, though both endoscopic and percutaneous methods can be useful in the management of these complications or as a bridge to definitive surgical therapy.
Keywords: Liver transplantation, anastomotic leak, anastomotic stricture, hepatic artery thrombosis, split liver
Complications of the biliary tract can occur in the immediate post—liver transplant period, as well as years after the transplant. Often, in the immediate post-transplant period, technical aspects of the operation and vascular insufficiency are leading causes of complications. In late-appearing complications, recurrent disease (ie, the reason for the transplant in the first place), rejection of the graft,1 secondary neoplasms, and stone formation, as well as vascular insufficiency, are most culpable for causing complications. A particularly serious complication is that of leaks occurring in a living donor.2,3
Transplant patients involve special circumstances, as most have benign disease and the potential for prolonged survival. The damaged organ cannot be easily replaced, as resources (both the liver itself and financial means) are limited; thus, it is necessary to make every effort to salvage the transplanted organ. Lastly, the immunosuppression used in these patients alters their response to infection and the healing process following the intervention, as well as their response to any complications of the intervention.4-7
Anatomy of the Surgical Construction Determines the Type and Management of Therapy
The surgical construction of the biliary tree determines the intervention required when a complication occurs. With rare exceptions, duct-to-duct reconstruction (choledochocholedochostomy [CDCD]; Figure 1) is best managed endoscopically via endoscopic retrograde cholangiography (ERCP), whereas duct-to-bowel reconstruction (Roux-en-Y choledochojejunostomy; Figure 2) is managed via a percutaneous route (percutaneous transhepatic cholangiography [PTC] with percutaneous transhepatic biliary drainage [PTBD]). Endoscopic management has been shown to be possible with the use of a double-balloon endoscope or variable-stiffness pediatric colonoscope.8,9
Figure 1.
Normal duct-to-duct anastomosis between the donor and native livers. Often, the entire length is longer than a native duct, as “extra” duct is present to prevent too much traction. This can impact the length of the stent.
Figure 2.
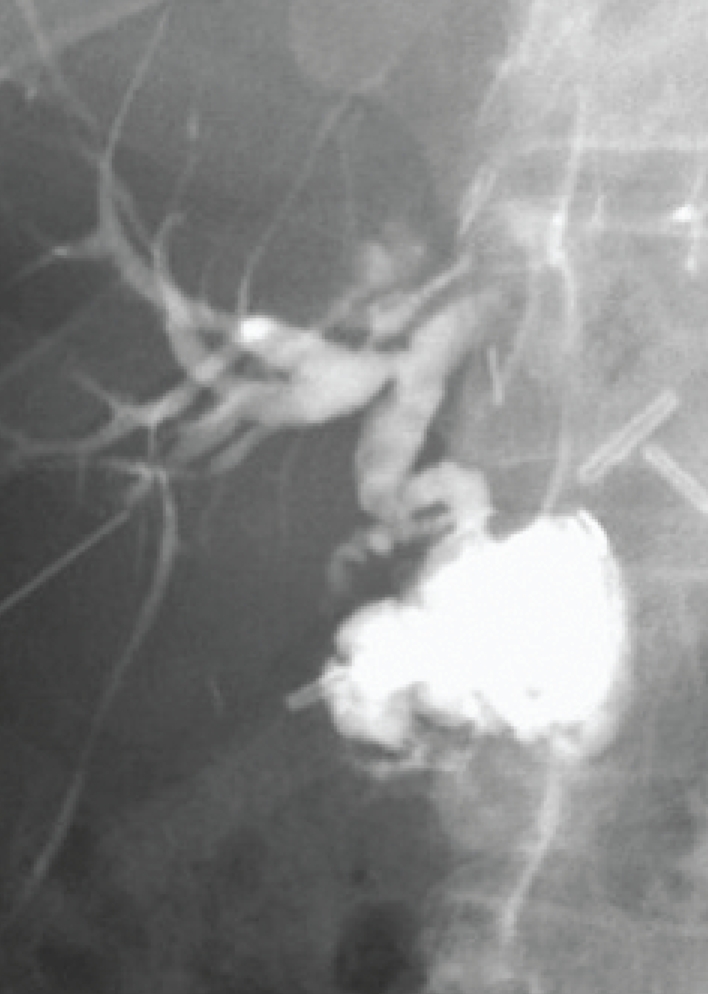
Normal hepaticojejunostomy, which is often the preferred reconstruction in abnormal common bile ducts, cases of biliary atresia, small children, and other instances where there is a mismatch between the native and donor ducts.
Endoscopy, while safer due to its use of the body's natural openings and conduits, is usually not feasible with a Roux-en-Y construction of a hepaticojejunostomy in a transplanted liver. To make matters worse, the transplanted liver is usually a normal liver, but when there is a leak, the intrahepatic ducts may be smaller than normal, making percutaneous drainage more difficult.10-12
Clearly, CDCD is the preferred anastomosis, as it is anatomic and preserves the sphincter mechanism while allowing retrograde access and avoidance of bowel surgery. Unfortunately, CDCD cannot always be performed, particularly in cases of sclerosing cholangitis, biliary atresia, duct size mismatch, and in the repair of a problematic CDCD anastomosis.13,14
Having to decide between cadaveric and living donor transplants is becoming more common. The technology used for harvesting the lobe of a living person has improved, and the availability of cadaveric livers remains limited. Complications rarely occur in the donor. Beyond problems related to general medical and surgical care, the most frequent complication is related to the leakage of bile from the cut edge (Figure 3) or a bile duct.15-18
Figure 3.
Cut-edge leak in a recipient of a left lobe from a living donor.
It is possible that post-transplantation complications are underreported, particularly as the symptoms are variable. It may also be the case that many complications are not noticed if an intervention is not performed. In addition, if no intervention is needed, it may be thought that the aberration is merely a normal component of the healing process. Despite these possibilities, it is generally thought that complications associated with liver transplantation are actually decreasing, as is the overall complication rate, possibly secondary to the standardization of technique.
Complications can be seen in patients presenting with a biliary leak, patients in whom obstruction is the main cause, or both. The factors that impact complications are often vascular, in the form of hepatic artery thrombosis (HAT) or low flow. Preservation injury, ABO blood group incompatibility, and surgical technique also contribute significantly to complications.
Presentation and Early Evaluation
Patients usually present with sustained abnormal blood tests, as well as pain, fever, bilious ascites, and sustained output through drain sites. Noninvasive radiologic imaging begins with extracorporeal ultrasound examination, which is often used in combination with Doppler examination of the flow characteristics of the hepatic artery and portal vein. Computed tomography (CT), CT cholangiography, and magnetic resonance cholangiopancreatography (MRCP; Figures 4 and 5) often follow, and, more recently, endoscopic ultrasound (EUS) is performed to access the liver, biliary tract, and vascular patency. Doppler examination is possible with EUS, but requires sedation, and many of the ill postoperative patients would require endotracheal intubation (with general anesthesia) or sedation monitored by an anesthesiologist.
Figure 4.
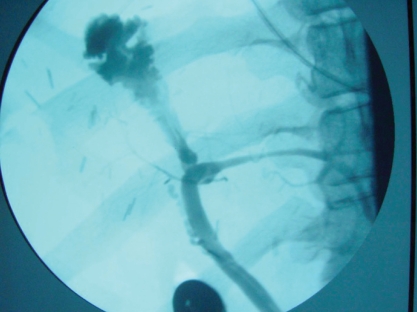
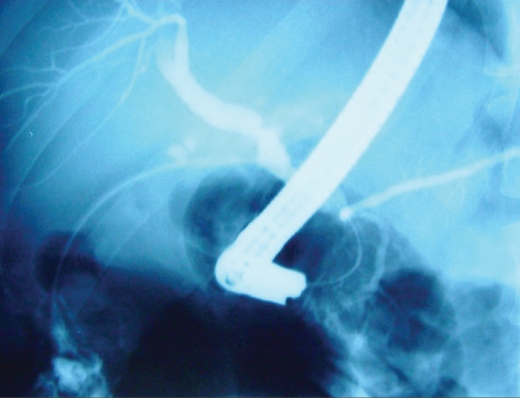
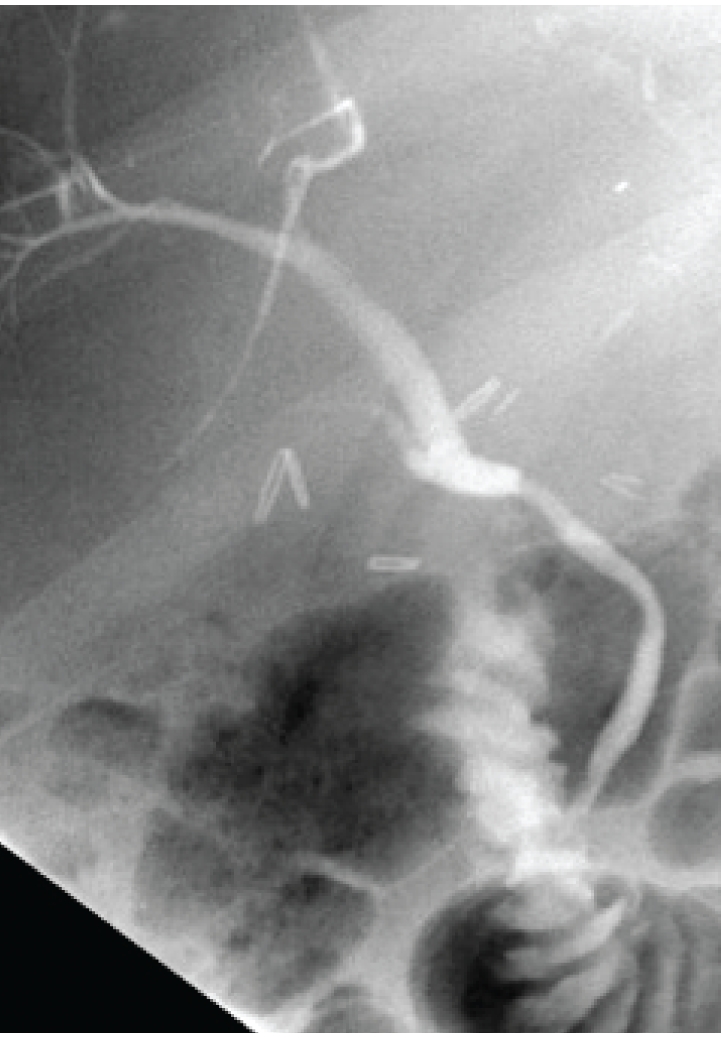
Endoscopic retrograde cholangiopancreatography showing a normal native duct in the lower portion of the image. Behind the endoscope and near the clips is a high-grade anastomotic stricture with massive dilation of the donor duct.
Figure 5.
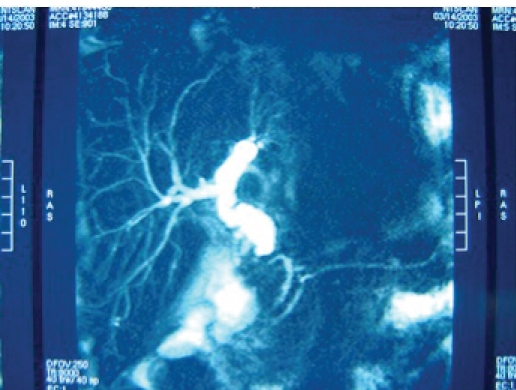
Magnetic resonance cholangiopancreatography (MRCP) of the patient in Figure 4. Note the high resolution and detail. MRCP lends itself very well as a screening test for anastomotic abnormalities.
The next step in the evaluation of these patients depends upon the initial findings. If a stricture or fluid collection is noted, a cholangiogram is performed. This is the next step unless the surgical connection precludes ERCP. PTCs and PTBDs are reserved for failures with ERCP or anatomies that preclude it (eg, Roux-en-Y hepaticojejunostomy or gastric outlet obstruction). As suggested earlier, PTC is more hazardous, as the ducts are frequently of a normal size (due to the normal liver transplanted) or even diminutive (due to a leak). With a high-grade anastomotic stricture, the ducts in the donor liver can be dilated, making PTC easier and safer. Naturally, a severe coagulopathy again makes PTC more dangerous than ERCP and is best avoided. Thus, the determination of whether to perform ERCP or PTC depends upon anatomic considerations, the diameter of the donor biliary tree, and the overall morbidity related to issues of sedation and coagulation status.19,20
Liver biopsies are often performed by protocol to access the graft in the postoperative period and can detect aspects of rejection, ischemia, recurrent disease, or obstruction (Table 1).
Table 1.
Early Evaluation of Liver Transplant Complications
Noninvasive
|
Early Complications (<30 Days Post-Transplant)
Early complications may be caused by the handling and harvesting of the graft, unappreciated disease of the graft, as well as preservation injuries. Often, these complications are recognized prior to the placement of the liver.
More common complications involve obstructions and leaks resulting from surgical technique or vascular insufficiency. Complications in living donors also appear within this early time period.
Leaks
Leaks most frequently involve the two cystic duct remnants (donor or native; Figure 6) through inadequate ligation and are possibly associated with a distal obstruction such as a stone, stricture, neoplasm, ampullary blockage, surgical anastomosis resulting from surgical technique or ischemia, or a T-tube site or tract after being removed (Figure 7). The last possibility is mainly of historical interest, as T-tubes are rarely employed any longer in CDCD reconstructions. Leaks can also occur at a hepaticojejunostomy and require intervention.18-22
Figure 6.
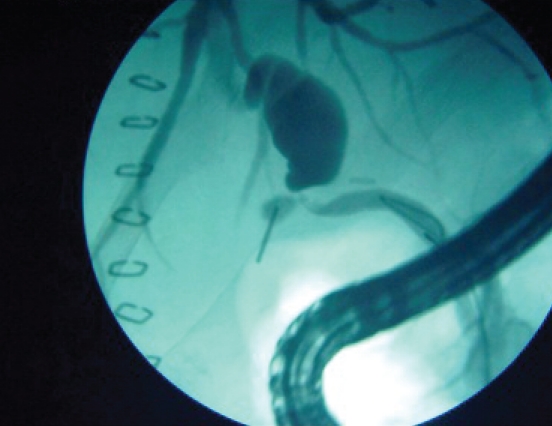
A leak from the donor cystic duct. Note that there is a leak from the cystic duct remnant proximal to the surgical anastomosis.
Figure 7.
T-tube track leak after the T-tube is removed due to the lack of maturation of the T-tube track. A leak can also occur at the T-tube insertion site. These leaks used to be very frequent (>20%) but have disappeared with the retirement of the T-tube in duct-to-duct anastomosis.
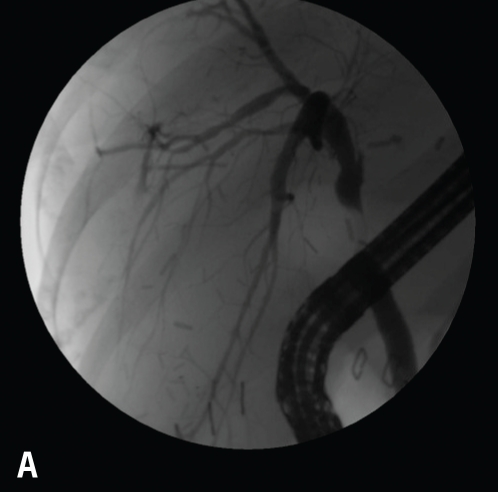
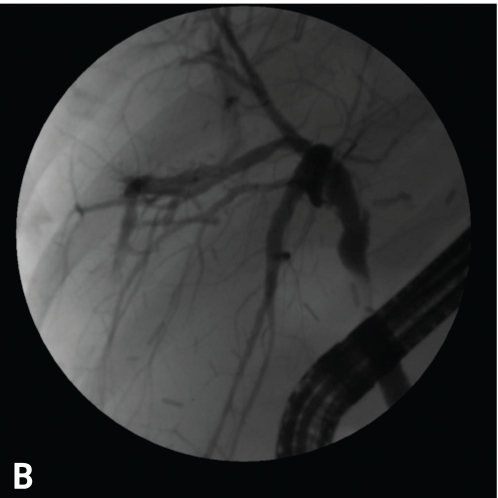
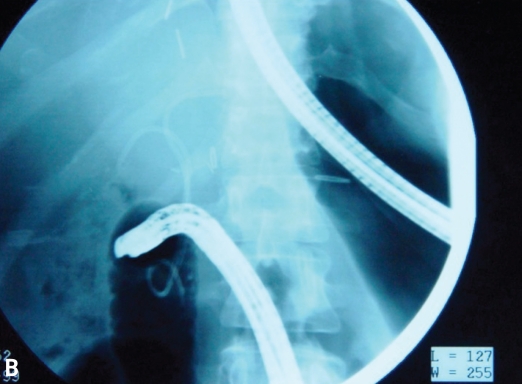
In addition, leaks can occur along a liver edge after a liver biopsy (Figure 8) associated with a more distal blockage, as a result of an injury to the liver surface during surgery, or along a cut edge in either the recipient or donor of a split liver (Table 2).
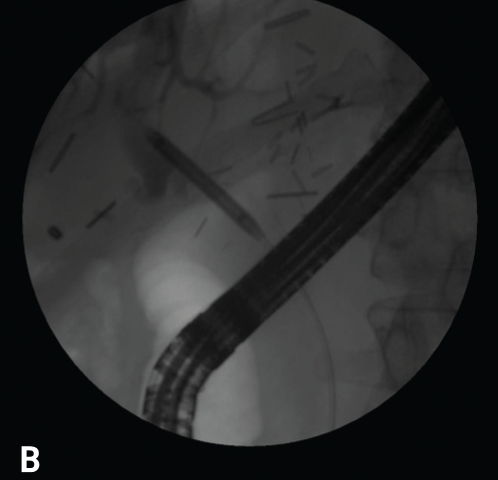
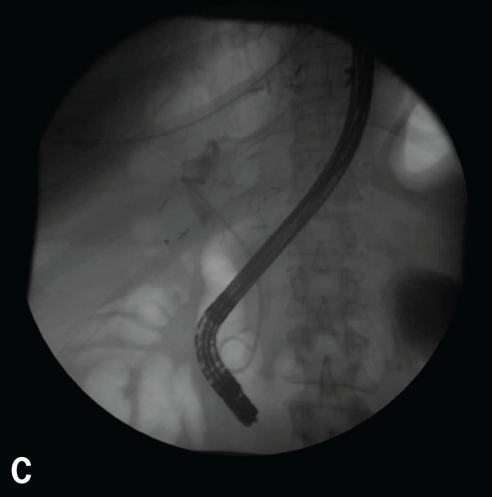
Figure 8.
Note the early blush at 10 o'clock (A). The blush of contrast has increased, and the anastomotic stricture that is present likely predisposed this individual to the leak after the biopsy (B). This case was managed with a stent and endoscopic dilation with a 4-mm balloon at l0 atm (150 psi).
Table 2.
Causes and Locations of Leaks Following Liver Transplantation
|
Leaks are generally treated via diversion of bile through endoscopic stenting. Occasionally, an endoscopic sphincterotomy is performed to remove a stone, relieve the obstruction contributing to the leak such as a distal choledochocoele, or place a second removable stent to facilitate drainage. In cases where ERCP cannot be performed, PTBD is used for diversion. However, as mentioned earlier, this scenario can have increased morbidity, as the ducts are often small, due to the leak. In all instances of a leak associated with a major collection, the collection must also be drained to prevent secondary infections and late complications of adhesions associated with bile (Figure 9). Reoperation is always considered for an early leak.
Figure 9.
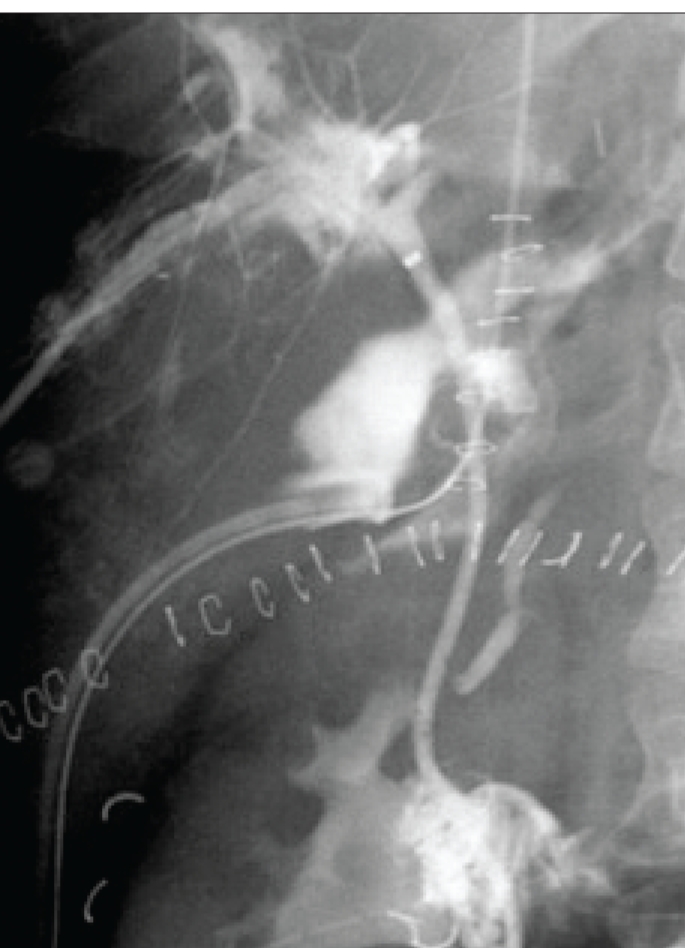
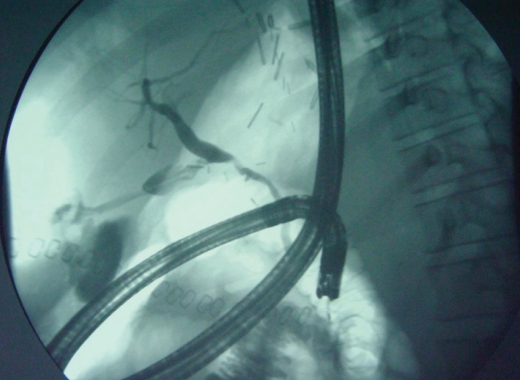
Anastomotic leak with a stent through the surgical anastomosis and percutaneous drain in a major collection. Anastomotic leaks often heal with structuring.
If bile is diverted outside of the body with either a percutaneous drainage catheter or nasobiliary catheter, the levels of the immunosuppressive agent must be carefully monitored; for example, cyclosporine may not be recirculated via the normal enterohepatic circuit, and the levels may decline.
At the University of California, San Francisco, we initially placed nasobiliary drains and monitored the leaks almost daily to determine the rate of closure. We determined that simple leaks such as T-tube tract or site leaks, cystic duct leaks, liver biopsy site leaks, and minor capsular leaks all closed in 2—3 weeks. This slight delay in closure was likely secondary to the immunosuppression that slows scar formation. The nasobiliary catheter permitted endoscopists to evaluate closure and pull out the catheter over a wire as soon as closure was seen without needing a second endoscopy for stent removal.
The nasobiliary catheter has the following disadvantages: patients often could not tolerate the catheter for 2 or 3 weeks; it often delayed their discharge, as patients would frequently choose not to go home with the nasobiliary catheter; the catheter could be dislodged, necessitating an urgent replacement; and, as noted earlier, immunosuppressives such as cyclosporine would have to be refed through a second (nasogastric) tube to maintain its levels.21
Leaks caused by a cystic duct remnant typically close in 2—3 weeks. Generally, one 7 Fr stent (double pigtail) placed above the leak solves the problem. Occasionally, bilateral stenting with an endoscopic sphincterotomy is needed. Cut-edge leaks often take longer to close (up to 8 weeks), and anastomotic leaks often heal with a stricture.
Obstruction
Endoscopic therapy, if feasible, is the least morbid short-term solution for all causes of obstruction. On the other hand, if the obstruction occurs in the common duct, hepatic duct, at the surgical anastomosis, or at the ampulla, proximal dilation will occur, and a PTBD may be possible to perform, except with noncorrectable coagulopathy. Alternately, reoperation can sometimes be a reasonable alternative in both late and early complications.
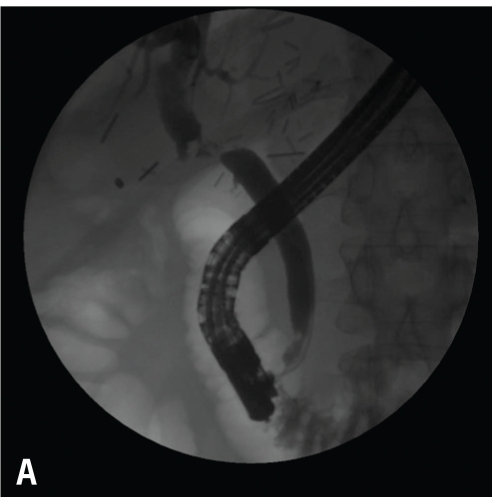
The most common early obstruction occurs at the CDCD surgical anastomosis. The causes can be surgical technique, edema, torsion, redundancy, vascular insufficiency, or, less likely, a stone or mucocele. These options generally lend themselves to endoscopic stent placement with resolution of the obstructive symptoms and liver tests. If the obstruction occurs very early in the course of management (<14 days), endoscopists may be hesitant to perform endoscopic balloon dilation for fear of disrupting the surgical anastomosis, and may place a stent. After several weeks, the stent is removed, and dilatation is performed for strictures. The stent is replaced if there is no or sluggish drainage, and the time between dilations increases until the stricture has resolved or the anastomosis is reconstructed (Figure 10). With a hepati-cojejunal anastomosis, the same guidelines apply, though the approach is percutaneous, and an external drainage catheter is needed, with possible bile refeeding.23-29 After the stents are removed, liver tests are followed to access any early obstruction.
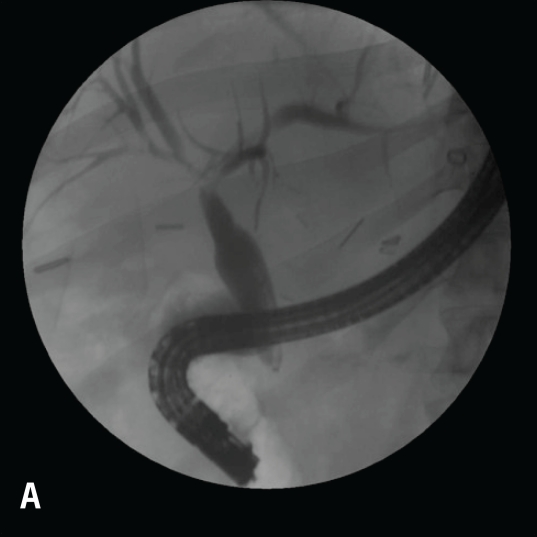
Figure 10.
Anastomotic stricture (A). Balloon dilation (4 mm at 10 atm; B). 7 Fr 10 double pigtail stent in place through the surgical anastomosis (C).
There has been recent interest in placing multiple stents in much the same way that multiple stents are placed for other benign strictures postdilation in the hope of keeping the anastomotic strictures open. If a mucocele is causing the obstruction, a stent can relieve the obstruction temporarily. Ultimately, the mucus building up in the entrapped cystic duct requires surgical treatment. Ampullary obstruction from a choledochocoele, stone, or papillary stenosis usually responds to an endoscopic sphincterotomy for a durable solution. If the bile duct filling defects are a consequence of vascular insufficiency and sloughing of the biliary mucosa, the solution of endoscopic sphincterotomy is temporary (Figure 11). Stricturing of the entire bile duct may follow as a long-term sequelae of vascular insufficiency. In the future, vascular insufficiency, ductal reconstruction, retransplan-tation, and chronic stenting are issues that may have to be addressed (Table 3).
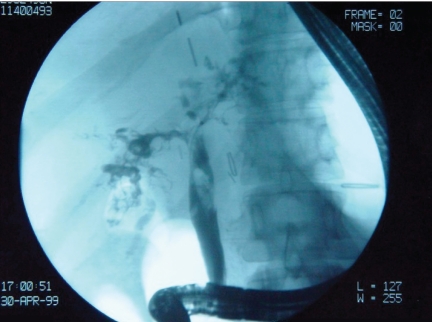
Figure 11.
Multiple stones in the bile duct. Note the multiple bile duct filling defects, which are usually related to a vascular insult and evolve into stricturing.
Table 3.
Common Causes of Obstructions
|
Primary intrahepatic disease can cause stricturing and obstruction. Stenting or biliary diversion can help obstruction at the bifurcation, but multiple strictures in the liver do not lend themselves to minimally invasive interventions. Although the removal of stones and debris can usually be achieved, recurrence is likely if arterial insufficiency is the underlying cause. Ductal abnormalities secondary to rejection can improve with antirejection treatment, and prolonged preservation injuries can improve over time.30,31
The development of a cholangiopathy associated with biliary cast syndrome is a difficult problem. Factors such as biliary infection, bile duct damage, ischemia, and possibly hemolysis may be involved. Although casts can often be removed with endoscopic management, surgery is often required.32
In recent years, the use of livers donated after cardiac death has been demonstrated to have a higher short-term graft failure, a higher relisting risk, and a higher risk of retransplantation, primarily due to an increased risk of biliary complications associated with ischemic cholangiography.33-36
Early complications appear to be more responsive to nonsurgical therapy and have a greater likelihood of avoiding operation.
Late Complications (>90 Days Post-Transplantation)
Biliary complications that occur after several months often have more serious causes. Liver tests are studied on a regular basis in post-transplant patients and are often the first indication that there is a problem. An ultrasound and Doppler examination of the hepatic vasculature (hepatic artery and portal vein), accompanied by a percutaneous liver biopsy, often comprise the next step. When coagulopathy is present, a transjugular biopsy is often performed. If the cause is suspected to be leakage or ductal obstruction, ERCP or PTC is performed.
Leaks are less common as late complications unless associated with severe vascular insufficiency and stricturing.37-39 Anastomotic strictures that occur late are usually vascular in origin. Other causes of obstruction that can occur later, particularly when nonanastomotic, are related to HAT (with intrahepatic strictures), ABO incompatibility, prolonged preservation, opportunistic infections, recurrent hepatitis B or C, ductopenic rejection, recurrent primary sclerosing cholangitis, stones or casts, post-transplant lymphoproliferative disorder or other tumors, and sphincter dysfunction or stenosis.31,40
Ductopenic rejection, post-transplant lymphoproliferative disorder, and opportunistic infections can often respond to medical management, though these patients may need transient endoscopic or percutaneous intervention to relieve large duct obstruction. Recurrent hepatitis B and C can occasionally be managed by medical treatment, though endoscopic or percutaneous interventions usually have little effect here.
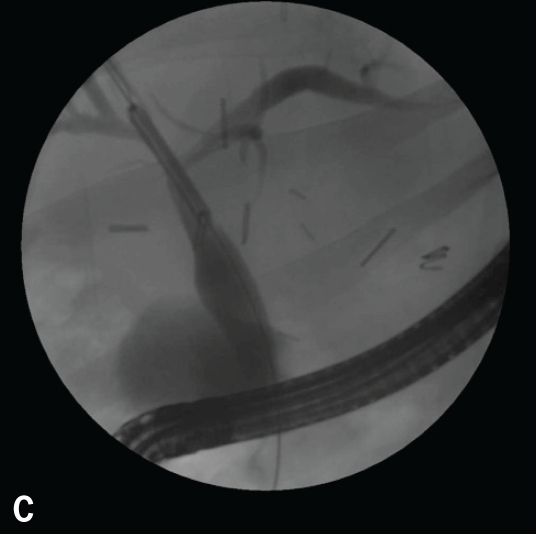
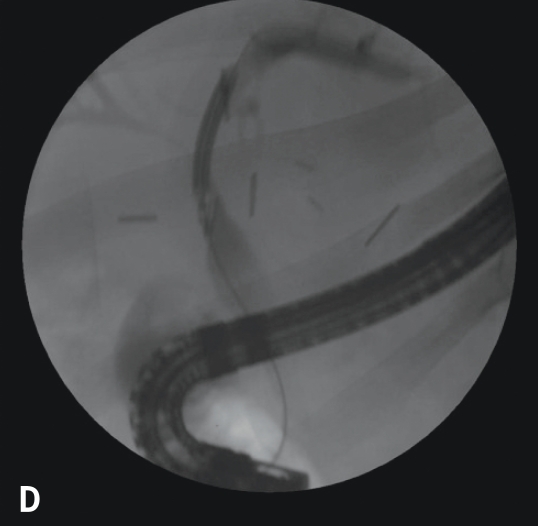
Recurrent primary sclerosing cholangitis can often respond to endoscopic management with stricture dilation and, occasionally, stent placement. However, this disease appears to be different from the original disease, in that there are usually associated casts and stones. In this sense, the disease is likely related to vascular insufficiency and resembles recurrent pyogenic cholangitis. This situation can be managed with endoscopic and percutaneous therapy, though a durable cure is unlikely. The disadvantage to stent placement is that if the stent occludes, there is a risk of cholangitis and hepatic micro-abscesses (behind strictures) in an immunocompromised host, making cure difficult and retransplantation dangerous (Figure 12).
Figure 12.
Vascular insult resulting in bilateral strictures at the bifurcation (A). Initially, the jaundice was treated with bilateral stenting (two 7 Fr 10-cm double pigtail stents; B). The patient was maintained with periodic balloon dilation of the right duct (C). Dilation of the left duct was performed with 4-mm balloons at 10 atm (150 psi; D).
Late strictures are usually not self-limiting with endoscopic or percutaneous therapy; they usually persist unless medically reversible. With this assumption in mind, a reasonable algorithm for late-appearing nonanastomotic strictures is not to perform endoscopic or percutaneous therapy on patients with minimal symptoms and preserved synthetic function. Balloon dilation should be the primary treatment for symptomatic patients, and stents should be reserved for failures of endoscopic or percutaneous dilations and used as a rebridge to retransplantation (Figure 13).
Figure 13.
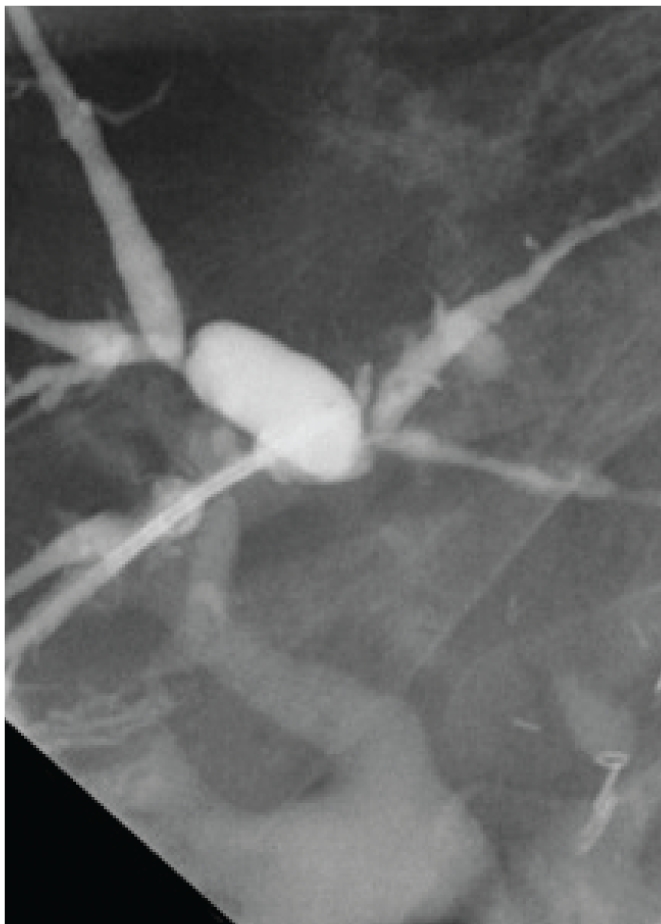
Diffuse intrahepatic stricturing
From a practical standpoint, as the practitioner wonders about the causes of late complications, it would be naive to ignore the possibility of compliance with antirejection drugs and the return of a patient to alcohol use. It is also necessary to accept endoscopic or percutaneous long-term management as a successful outcome because resources for performing repeat transplantation are markedly limited, surgery may be too hazardous, and portal vein or hepatic artery thrombosis may make retransplantation impossible.41-43
It is likely that endoscopic and percutaneous therapy have minimized the need for post-transplant biliary surgery. According to a 10-year experience at the University of California, San Francisco, there were 1,061 cases of adult liver transplants, of which 959 were orthotopic liver transplants and 102 were living donor procedures. Of these patients, 947 underwent CDCD reconstruction and 114 had a hepaticojejunostomy. The study also noted that 232 (22%) of the adults experienced biliary complications: 140 bile duct strictures, 59 bile duct leaks, 18 cases of papillary stenosis, 16 cases of diffuse bile duct injury (usually ischemic), 7 cases of choledocholithiasis, and 2 cases of extrinsic compression from post-transplant lymphoproliferative disorder.44
The study also noted a trend toward increased complications in patients undergoing a surgical take-back procedure for hemorrhage, sepsis, and obstruction, patients with a choledochoenteric anastomosis, and those undergoing a living donor transplant (Table 4).
Table 4.
Frequency of Various Adult Biliary Complications Over 10 Years at the University of California, San Francisco
|
Of the 114 patients who had choledochoenteric anastomosis, 33 underwent percutaneous management for a complication and 21 of the 33 (64%) required surgical revision.
In addition, 166 individuals underwent ERCP with a CDCD anastomosis and 101 of 132 (77%) had endo-scopically manageable pathology and required no surgical revision. This figure included 64 of 100 cases (64%) of strictures, 16 of 31 cases (52%) of leaks, 16 of 16 cases (100%) of papillary stenosis or choledochocoele, and 4 of 14 cases (29%) of diffuse biliary injuries.
It is thus fair to conclude that endoscopic management and therapy can minimize the need for post-transplant biliary surgery in the cases of biliary tract complications of strictures and papillary stenosis. These techniques are generally safe and nearly always effective in the short term and can serve as a bridge to surgical therapy. Further endoscopic therapy may be the preferred therapy for chronic management of strictures and stones if definitive curative surgery (retransplant or reconstruction) is not possible.
References
- 1.Ward EM, Kiely MJ, Maus TP, Wiesner RH, Krom RA. Hilar biliary strictures after liver transplantation: cholangiography and percutaneous treatment. Radiology. 1990;177:259–263. doi: 10.1148/radiology.177.1.2399328. [DOI] [PubMed] [Google Scholar]
- 2.Chespak LW, Ring EJ, Shapiro HA, Gordon RL, Ostroff JW. Multidisciplinary approach to complex endoscopic biliary intervention. Radiology. 1989;170:995–997. doi: 10.1148/radiology.170.3.2916069. [DOI] [PubMed] [Google Scholar]
- 3.Ostroff JW. The use of ERCP in pancreatic and biliary tract disease. In: Jacobson IR, editor. ERCP and Its Applications. Philadelphia, Pennsylvania: Lippincott-Raven; 1998. p. 3. [Google Scholar]
- 4.Sharma S, Gurakar A, Camci C, Jabbour N. Avoiding pitfalls: what an endoscopist should know in liver transplantation–part II. Dig Dis Sci. 2009;54:1386–1402. doi: 10.1007/s10620-008-0520-7. [DOI] [PubMed] [Google Scholar]
- 5.Londono MC, Balderramo D, Cardenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol. 2008;14:493–497. doi: 10.3748/wjg.14.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroff JW. Post-transplant biliary problems. Gastrointest Endosc Clin N Am. 2001;11:163–183. [PubMed] [Google Scholar]
- 7.Johnson LB, Al-Kawas FH. The bile duct—the Achilles' heel of living donor liver transplantation. Am J Gastroenterol. 2004;99:1296–1297. doi: 10.1111/j.1572-0241.2004.70775.x. [DOI] [PubMed] [Google Scholar]
- 8.Chahal P, Baron TH, Poterucha JJ, Rosen CB. Endoscopic retrograde cholangiography in post-orthotopic liver transplant population with Roux-en-Y biliary reconstruction. Liver Transpl. 2007;13:1168–1173. doi: 10.1002/lt.21198. [DOI] [PubMed] [Google Scholar]
- 9.Emmett D, Mallat D. Double-balloon ERCP in patients who have undergone Roux-en-Y surgery: a case series. Gastrointest Endosc. 2009;66:1038–1041. doi: 10.1016/j.gie.2007.06.056. [DOI] [PubMed] [Google Scholar]
- 10.Pascher A, Neuhaus P. Bile duct complications after liver transplantation. Transpl Int. 2005;18:627–642. doi: 10.1111/j.1432-2277.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 11.Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245–257. doi: 10.1159/000144653. [DOI] [PubMed] [Google Scholar]
- 12.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006:89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 13.Hampe T, Dogan A, Encke J, Mehrabi A, Schemmer P, et al. Biliary complications after liver transplantation. Clin Transplant. 2006;20(17):93–96. doi: 10.1111/j.1399-0012.2006.00607.x. [DOI] [PubMed] [Google Scholar]
- 14.Chahal P, Baron T, Rose CB, Poterucha JJ. Endoscopic retrograde cholangiopancreatography in post-orthotopic liver transplant patients with Roux-en-Y biliary reconstruction: six year single institution experience. Gastrointest Endosc. 2005;61 Ab200(Tl239) [Google Scholar]
- 15.Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, et al. Endoscopic management of biliary complications after adult living donor living transplantation. Am J Gastroenterol. 2006;101:2230–2236. doi: 10.1111/j.1572-0241.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 16.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limanond P, Raman SS, Ghobrial RM, Busuttil RW, Lu DS. The utility of MRCP in preoperative mapping of biliary anatomy in adult-to-adult living related liver transplant donors. J Magn Reson Imaging. 2004;19:209–215. doi: 10.1002/jmri.10446. [DOI] [PubMed] [Google Scholar]
- 18.Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110–117. doi: 10.1053/jlts.2002.31315. [DOI] [PubMed] [Google Scholar]
- 19.Park JS, Kim MH, Lee SK, Seo DW, Lee SS, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78–85. doi: 10.1067/mge.2003.11. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Lee BJ, Won JY, Choi JY, Lee DK. Percutaneous transhepatic biliary drainage may serve as a successful rescue procedure in failed cases of endoscopic therapy for a post-living donor liver transplantation biliary stricture. Gastrointest Endosc. 2009;69:38–46. doi: 10.1016/j.gie.2008.03.1113. [DOI] [PubMed] [Google Scholar]
- 21.Ostroff JW, Roberts JP, Gordon RL, Ring EJ, Ascher NL. The management of T tube leaks in orthotopic liver transplant recipients with endoscopically placed nasobiliary catheters. Transplantation. 1990;49:922–924. doi: 10.1097/00007890-199005000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Sung RS, Campbell DA, Jr, Rudich SM, Punch JD, Shieck VL, et al. Long-term follow-up of percutaneous transhepatic balloon cholangioplasty in the management of biliary strictures after liver transplantation. Transplantation. 2004;77:110–115. doi: 10.1097/01.TP.0000101518.19849.C8. [DOI] [PubMed] [Google Scholar]
- 23.Osorio RW, Freise CE, Stock PG, Lake JR, Laberge JM, et al. Nonoperative management of biliary leaks after orthotopic liver transplantation. Transplantation. 1993;55:1074–1077. doi: 10.1097/00007890-199305000-00026. [DOI] [PubMed] [Google Scholar]
- 24.Icoz G, Kilic M, Zeytunlu M, Celebi A, Ersoz G, et al. Biliary reconstructions and complications encountered in 50 consecutive right-lobe living donor liver transplantations. Liver Transpl. 2003;9:575–580. doi: 10.1053/jlts.2003.50129. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759–769. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 26.Williams E, Draganov PV. Endoscopic management of biliary strictures after liver transplantation. World J Gastroenterol. 2009;15:3725–3733. doi: 10.3748/wjg.15.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88–94. doi: 10.1002/lt.20548. [DOI] [PubMed] [Google Scholar]
- 28.Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–379. doi: 10.1067/s0016-5107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 29.Thuluvath PJ, Atassi T, Lee J. An endoscopic approach to biliary complications following orthotopic liver transplantation. Liver Int. 2003;23:156–162. doi: 10.1034/j.1600-0676.2003.00823.x. [DOI] [PubMed] [Google Scholar]
- 30.Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 2: management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725–732. doi: 10.1002/lt.21165. [DOI] [PubMed] [Google Scholar]
- 31.Nishida S, Nakamura N, Kadono J, Komokata T, Sakata R, et al. Intrahepatic biliary strictures after liver transplantation. J. Hepatobiliary Pancreat Surg. 2006;13:511–516. doi: 10.1007/s00534-005-1081-1. [DOI] [PubMed] [Google Scholar]
- 32.Parry SD, Muiesan P. Cholangiopathy and the biliary cast syndrome. Eur J Gastroenterol Hepatol. 2003;15:341–343. doi: 10.1097/00042737-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Pascher A, Neuhaus P. Biliary complications after deceased-do nor orthotopic liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:487–496. doi: 10.1007/s00534-005-1083-z. [DOI] [PubMed] [Google Scholar]
- 34.Skaro AI, Jay CL, Baker TB, Wang E, Pasricha S, et al. The impact of ischemic cholangiopathy in liver transplantation using donors after cardiac death: the untold story. Surgery. 2009;146:543–552. doi: 10.1016/j.surg.2009.06.052. discussion 552-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maheshwari A, Maley W, Li Z, Thuluvath PJ. Biliary complications and outcomes of liver transplantation from donors after cardiac death. Liver Transpl. 2007;13:1645–1653. doi: 10.1002/lt.21212. [DOI] [PubMed] [Google Scholar]
- 36.Selck FW, Grossman EB, Ratner LE, Renz JF. Utilization, outcomes, and retransplantation of liver allografts from donation after cardiac death: implications for further expansion of the deceased-donor pool. Ann Surg. 2008;248:599–607. doi: 10.1097/SLA.0b013e31818a080e. [DOI] [PubMed] [Google Scholar]
- 37.Gunsar F, Rolando N, Pastacaldi S, Patch D, Raimondo ML, et al. Late hepatic artery thrombosis after orthotopic liver transplantation. Liver Transpl. 2003;9:605–611. doi: 10.1053/jlts.2003.50057. [DOI] [PubMed] [Google Scholar]
- 38.Stange BJ, Glanemann M, Nuessler NC, Settmacher U, Steinmüller T, Neuhaus P. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9:612–620. doi: 10.1053/jlts.2003.50098. [DOI] [PubMed] [Google Scholar]
- 39.Vivarelli M, Cucchetti A, La Barba G, Bellusci R, De Vivo A, et al. Ischemic arterial complications after liver transplantation in the adult: multivariate analysis of risk factors. Arch Surg. 2004;139:1069–1074. doi: 10.1001/archsurg.139.10.1069. [DOI] [PubMed] [Google Scholar]
- 40.Alazmi WM, Fogel EL, Watkins JL, McHenry L, Tector JA, et al. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy. 2006;38:571–574. doi: 10.1055/s-2006-925027. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa K, Yazumi S, Egawa H, Tamaki H, Asada M, et al. Endoscopic management of postoperative biliary complications in donors for living donor liver transplantation. Clin Gastroenterol Hepatol. 2003;1:183–188. doi: 10.1053/cgh.2003.50027. [DOI] [PubMed] [Google Scholar]
- 42.Young L, Arya S, Harolds JA, Cassidy FP, Jr, Nour B, Parker M. Sonographic evaluation of complications of liver transplantation. J. Diagnostic Med Sonography. 2003;19:145–154. [Google Scholar]
- 43.Kulaksiz H, Weiss KH, Gotthardt D, Adler G, Stremmel W, et al. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy. 2008;40:746–751. doi: 10.1055/s-2008-1077489. [DOI] [PubMed] [Google Scholar]
- 44.Buxbaum JL, Bagatelos KC, Biggins SW, Roberts J, Ostroff JW. ERCP minimizes the need for post-liver transplant biliary surgery: report of a ten years experience. Presented at Digestive Disease Week; May 30—June 4, 2009; Chicago, Illinois.