Abstract
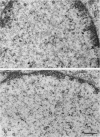
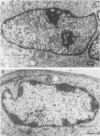
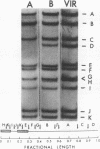
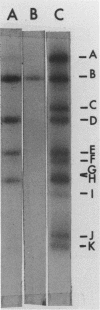
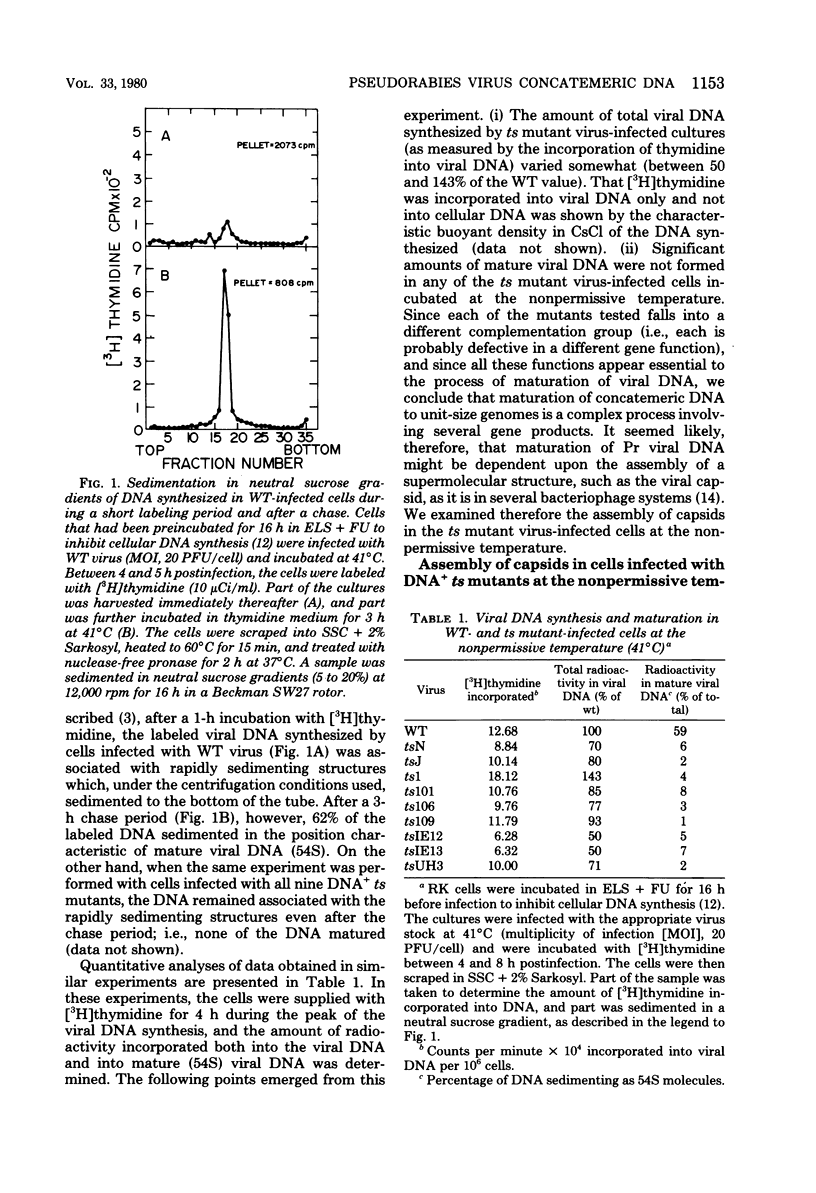
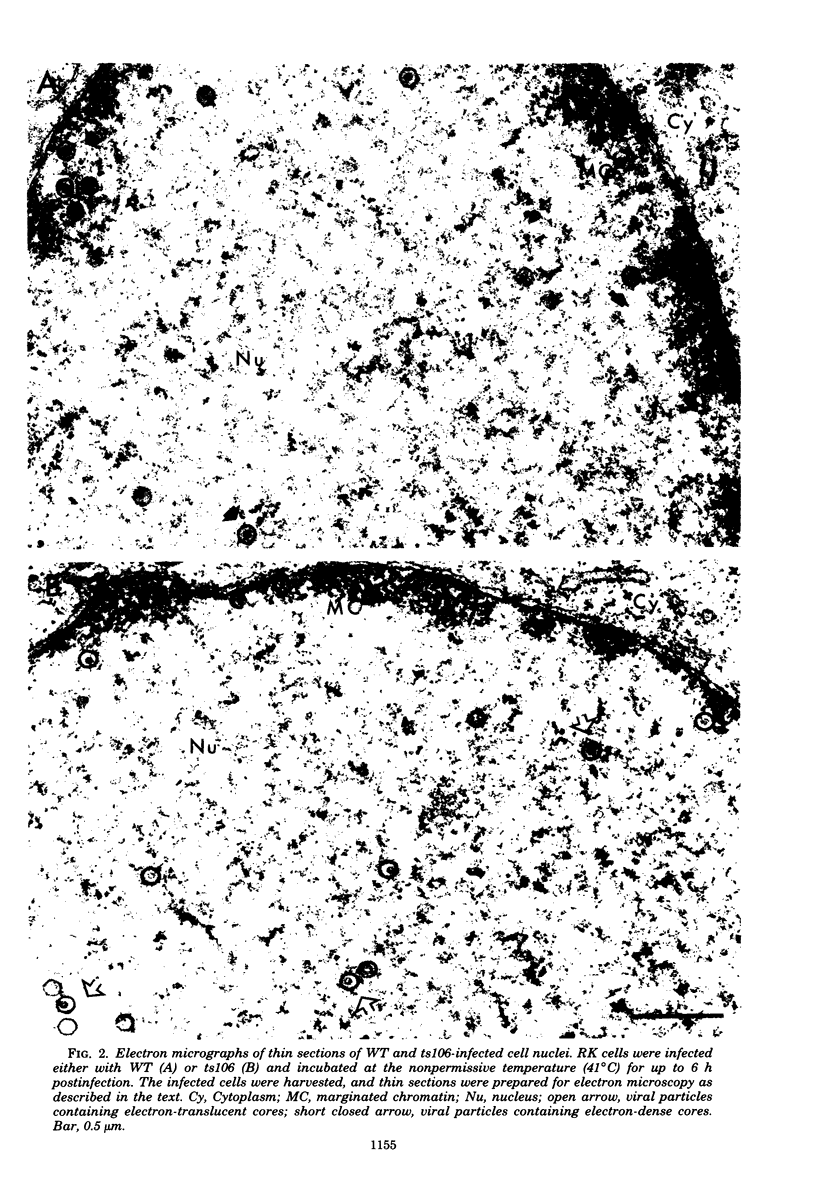
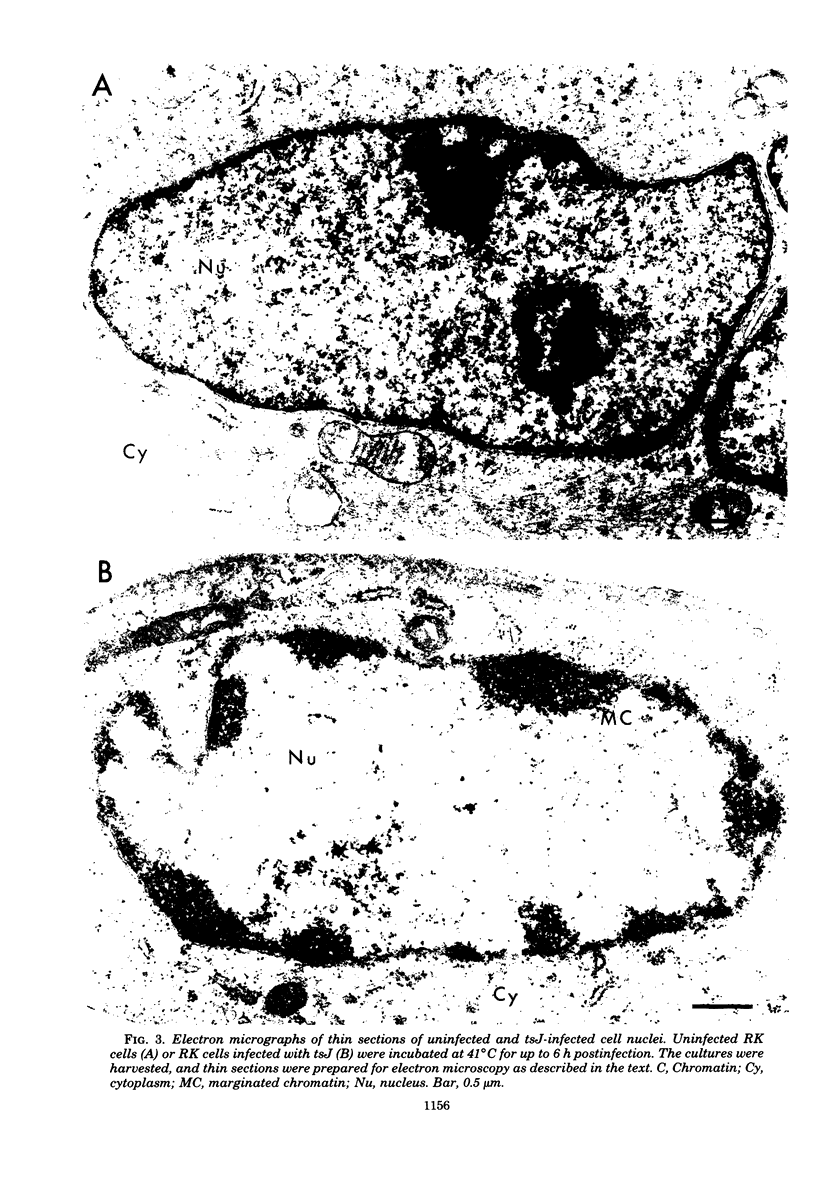
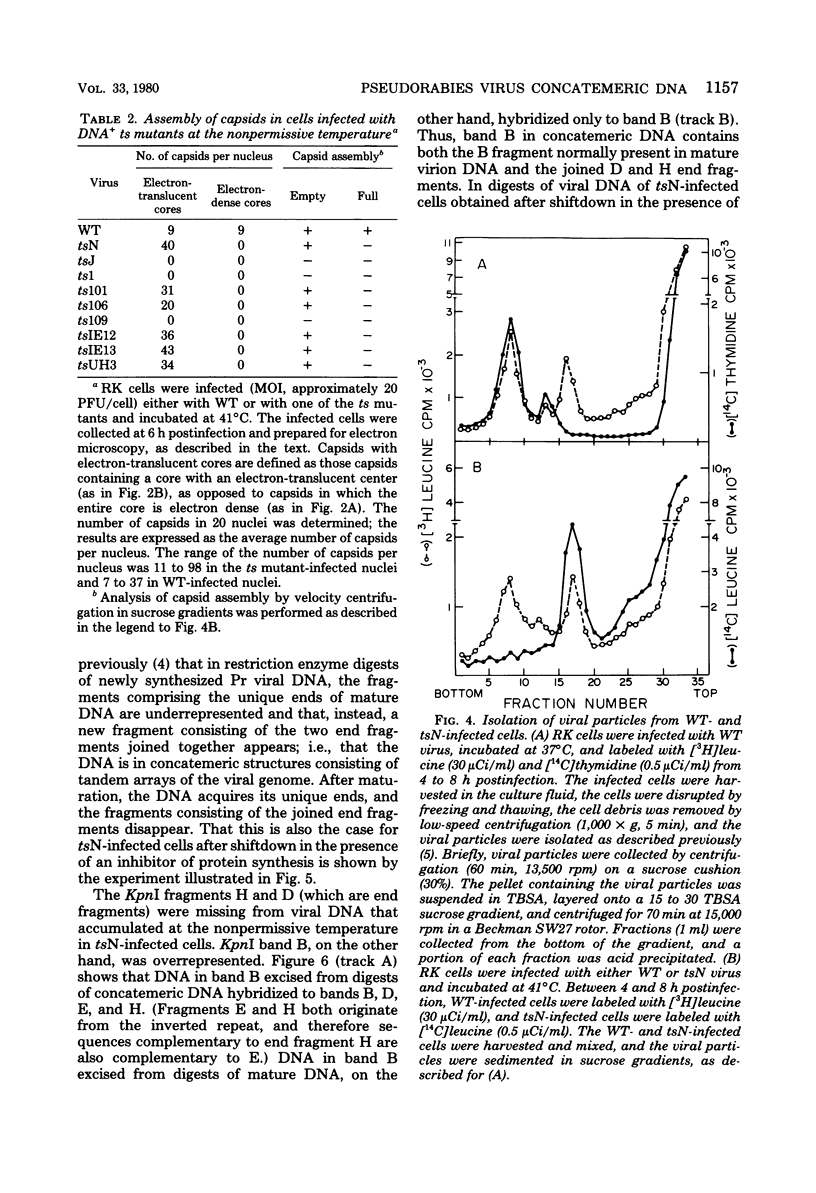
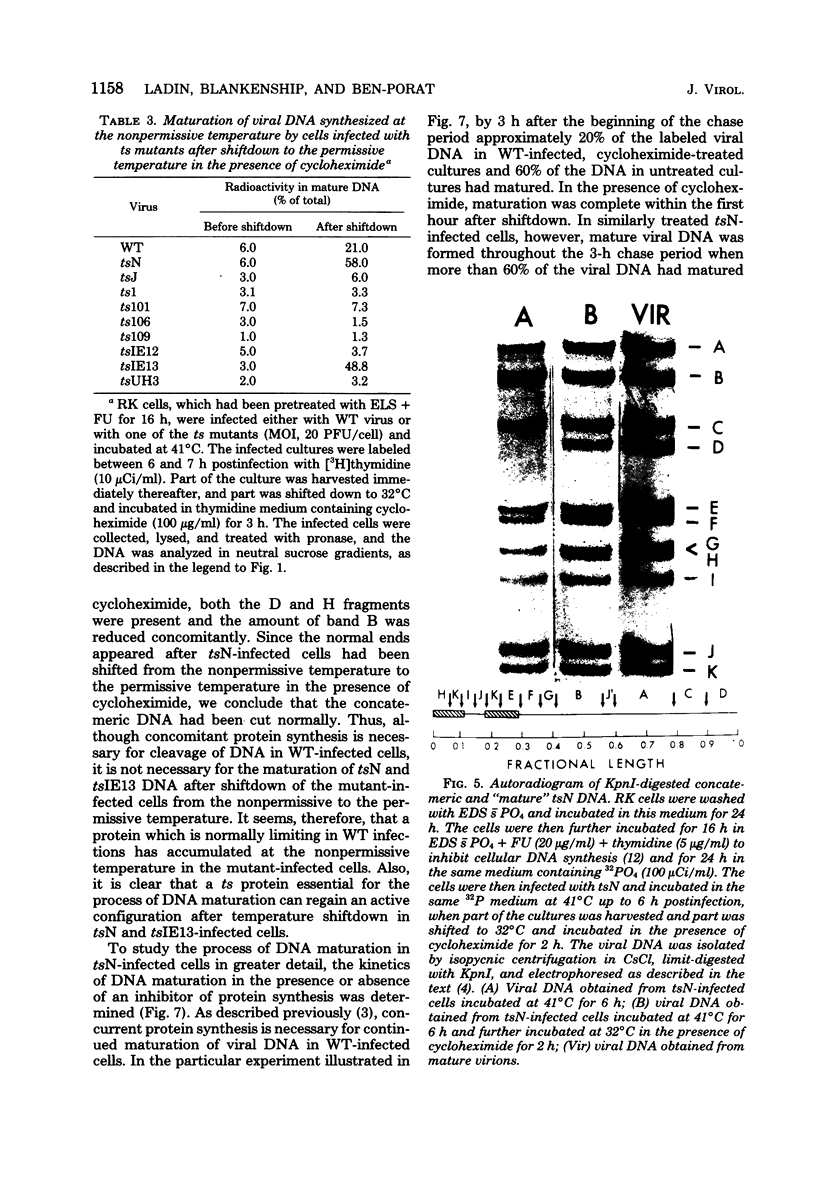
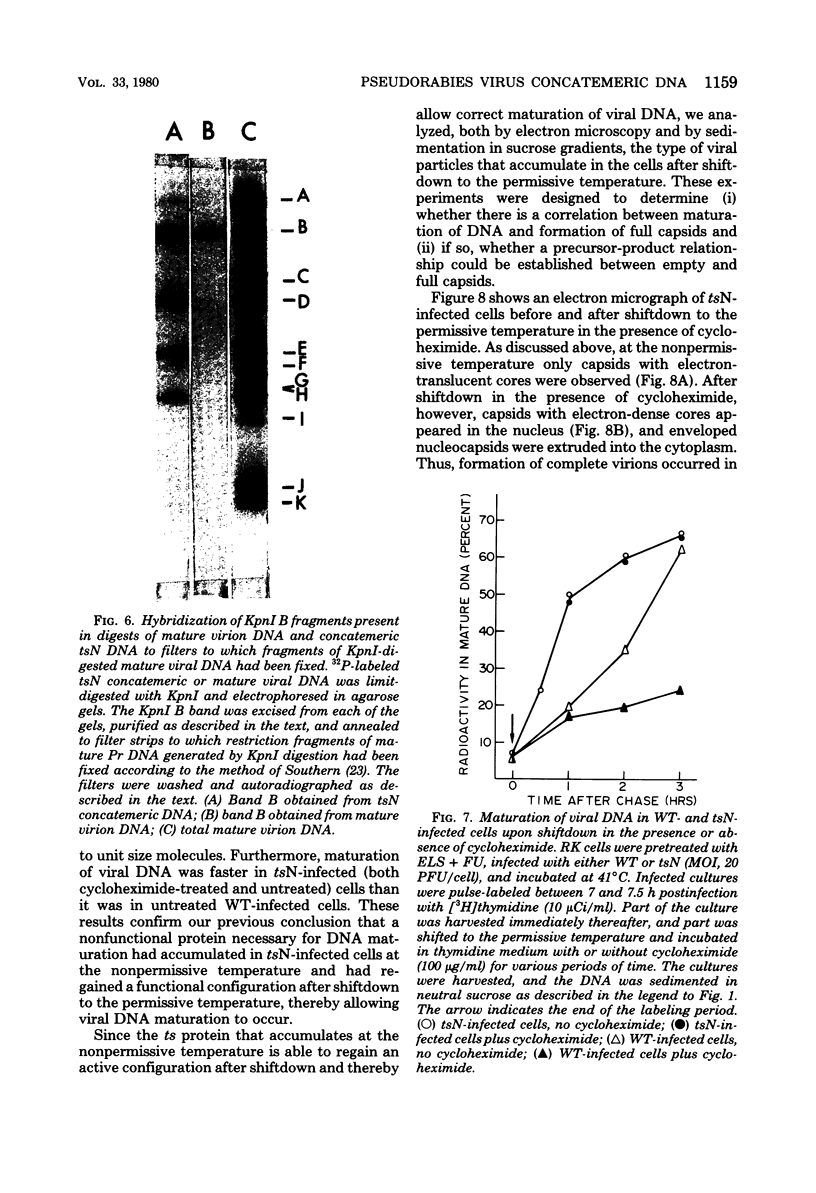
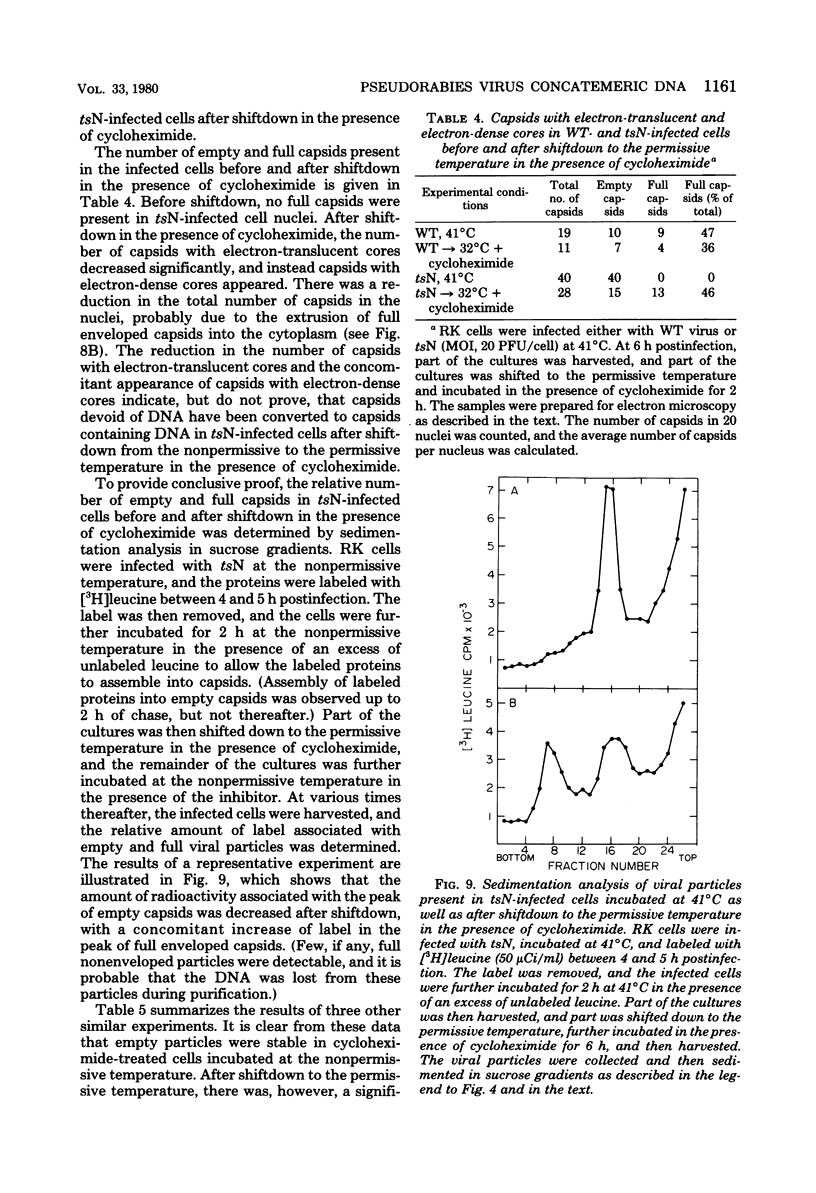
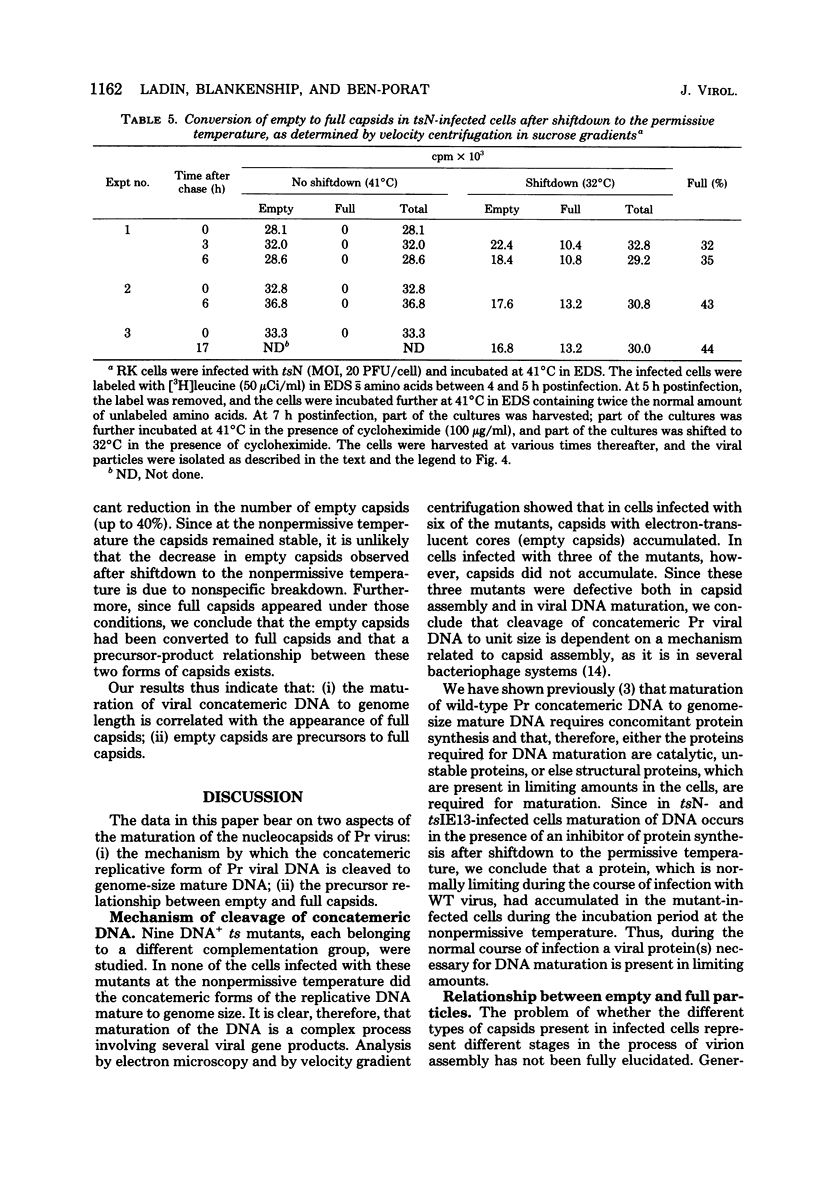
The maturation of pseudorabies virus DNA from the replicative concatemeric form to molecules of genome length was examined using nine DNA+ temperature-sensitive mutants of pseudorabies virus, each belonging to a different complementation group. At the nonpermissive temperature, cells infected with each of the mutants synthesized concatemeric DNA. Cleavage of the concatemeric DNA to genome-length viral DNA was defective in all the DNA+ ts mutants tested, indicating that several viral gene products are involved in the DNA maturation process. In none of the ts mutant-infected cells were capsids with electron-dense cores (containing DNA) formed. Empty capsids with electron-translucent cores were, however, formed in cells infected with six of the nine temperature-sensitive mutants; in cells infected with three of the mutants, no capsid assembly occurred. Because these three mutants are deficient both in maturation of DNA and in the assembly of viral capsids, we conclude that maturation of viral DNA is dependent upon the assembly of capsids. In cells infected with two of the mutants (tsN and tsIE13), normal maturation of viral DNA occurred after shiftdown of the cells to the permissive temperature in the presence of cycloheximide, indicating that the temperature-sensitive proteins involved in DNA maturation became functional after shiftdown. Furthermore, because cycloheximide reduces maturation of DNA in wild-type-infected cells but not in cells infected with these two mutants, we conclude that a protein(s) necessary for the maturation of concatemeric DNA, which is present in limiting amounts during the normal course of infection, accumulated in the mutant-infected cells at the nonpermissive temperature. Concomitant with cleavage of concatemeric DNA, full capsids with electron-dense cores appeared after shiftdown of tsN-infected cells to the permissive temperature, indicating that there may be a correlation between maturation of DNA and formation of full capsids. The number of empty and full capsids (containing electron-dense cores) present in tsN-infected cells incubated at the nonpermissive temperature, as well as after shiftdown to the permissive temperature in the presence of cycloheximide, was determined by electron microscopy and by sedimentation analysis in sucrose gradients. After shiftdown to the permissive temperature in the presence of cycloheximide, the number of empty capsids present in tsN-infected cells decreased with a concomitant accumulation of full capsids, indicating that empty capsids are precursors to full capsids.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The chemical composition of herpes simplex and pseudorabies viruses. Virology. 1962 Mar;16:261–266. doi: 10.1016/0042-6822(62)90246-5. [DOI] [PubMed] [Google Scholar]
- BEN-PORAT T., KAPLAN A. S. The synthesis and fate of pseudorabies virus DNA in infected mammalian cells in the stationary phase of growth. Virology. 1963 Jun;20:310–317. doi: 10.1016/0042-6822(63)90120-x. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S., Stehn B., Rubenstein A. S. Concatemeric forms of intracellular herpesvirus DNA. Virology. 1976 Feb;69(2):547–560. doi: 10.1016/0042-6822(76)90484-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Rixon F. J. Replication of herpesvirus DNA. IV: analysis of concatemers. Virology. 1979 Apr 15;94(1):61–70. doi: 10.1016/0042-6822(79)90438-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Tokazewski S. A. Replication of herpesvirus DNA. II. Sedimentation characteristics of newly synthesized DNA. Virology. 1977 Jun 15;79(2):292–301. doi: 10.1016/0042-6822(77)90356-7. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Veach R. A. Origin of replication of the DNA of a herpesvirus (pseudorabies). Proc Natl Acad Sci U S A. 1980 Jan;77(1):172–175. doi: 10.1073/pnas.77.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral G. A., Schaffer P. A. Electron microscope studies of temperature-sensitive mutants of herpes simplex virus type 2. J Virol. 1976 May;18(2):727–737. doi: 10.1128/jvi.18.2.727-737.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Friedmann A., Coward J. E., Rosenkranz H. S., Morgan C. Electron microscopic studies on assembly of herpes simplex virus upon removal of hydroxyurea block. J Gen Virol. 1975 Feb;26(2):171–181. doi: 10.1099/0022-1317-26-2-171. [DOI] [PubMed] [Google Scholar]
- Jean J. H., Ben-Porat T. Appearance in vivo of single-stranded complementary ends on parental herpesvirus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2674–2678. doi: 10.1073/pnas.73.8.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. MODE OF REPLICATION OF PSEUDORABIES VIRUS DNA. Virology. 1964 May;23:90–95. doi: 10.1016/s0042-6822(64)80011-8. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. The action of 5-fluorouracil on the nucleic acid metabolism of pseudorabies virus-infected and noninfected rabbit kidney cells. Virology. 1961 Jan;13:78–92. doi: 10.1016/0042-6822(61)90034-4. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nii S., Morgan C., Rose H. M. Electron microscopy of herpes simplex virus. II. Sequence of development. J Virol. 1968 May;2(5):517–536. doi: 10.1128/jvi.2.5.517-536.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D. J., Randall C. C. Molecular anatomy of herpesviruses: recent studies. Prog Med Virol. 1976;22:152–210. [PubMed] [Google Scholar]
- Perdue M. L., Cohen J. C., Kemp M. C., Randall C. C., O'Callaghan D. J. Characterization of three species of nucleocapsids of equine herpesvirus type-1 (EHV-1). Virology. 1975 Mar;64(1):187–204. doi: 10.1016/0042-6822(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Ben-Porat T. Structural evolution of the DNA of pseudorabies-defective viral particles. Virology. 1979 Aug;97(1):151–163. doi: 10.1016/0042-6822(79)90381-7. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. S., Kaplan A. S. Electron microscopic studies of the DNA of defective and standard pseudorabies virions. Virology. 1975 Aug;66(2):385–392. doi: 10.1016/0042-6822(75)90211-1. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Brunschwig J. P., McCombs R. M., Benyesh-Melnick M. Electron microscopic studies of temperature-sensitive mutants of herpes simplex virus type 1. Virology. 1974 Dec;62(2):444–457. doi: 10.1016/0042-6822(74)90406-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]