Chronic hepatitis C virus (HCV) and autoimmune hepatitis (AIH) overlap syndrome is an uncommon but well-documented condition. There are no standard guidelines for treating this complex disease. Generally, interferon is contraindicated in autoimmune diseases.1 There are a few case reports and case series in which interferon alfa three times per week or pegylated interferon with ribavirin have been used with immunosuppressive medications such as prednisone and azathioprine, with variable response.2,3 We report a case of HCV-AIH overlap syndrome that was first treated aggressively with immunosuppressive therapy, with partial response, and subsequently treated with consensus interferon (Infergen; interferon alfacon-1, Three Rivers Pharmaceuticals), achieving sustained virologic response (SVR). To our knowledge, the use of consensus interferon has not been reported in the literature for the treatment of chronic HCV in the presence of AIH.
Case Report
A 40-year-old white woman with a body mass index of 27.1 kg/m2 was referred to our medical center for treatment of chronic HCV. Her only complaints consisted of generalized fatigue and joint aches and pain. Her chronic HCV infection was allegedly acquired via blood transfusion in the early 1990s. The patient denied a history of intravenous drug use, tattoos, or high-risk sexual activity, and her past medical history was only significant for mild depression. No stigmatas of advanced liver disease were seen on physical examination. Her initial laboratory data revealed an alanine aminotransferase (ALT) level of 313 U/L (normal, 9-52 U/L), aspartate aminotransferase (AST) of 380 U/L (normal, 14-36 U/L), total bilirubin of 0.4 mg/dL (normal, 0.2-1.3 mg/dL), albumin of 4.6 g/dL (normal, 3.5-5 g/dL), international normalized ratio of 1.1, and HCV RNA viral load of 7,290,000 IU/mL (6.8 logs). Genotype analysis revealed that the patient had HCV genotype 3A, and serologic markers of AIH were positive. Antinuclear antibody (ANA) measured 1:80, antismooth muscle antibody (ASMA) measured 1:160, and immunoglobulin G (IgG) measured 2,369 mg/dL (normal, 694-1,618 mg/dL); thus, AIH type 1 was suspected. Ultrasound of the liver demonstrated fatty liver, and liver biopsy findings revealed chronic hepatitis, with grade 3 necroinflammatory activity with a mixture of plasma cells and portal lymphocytes focally invading the bile ducts. Steatosis with ballooning and steatohepatitis were also noted (Figures 1,2, and 3). Fibrosis was graded as stage 3. The biopsy findings were consistent with both AIH and chronic HCV, with predominant features of viral hepatitis. Co-infection with hepatitis B and other liver diseases such as hemochromatosis, Wilson disease, alpha-1 antitrypsin deficiency, and primary biliary cirrhosis were excluded by the appropriate laboratory tests.
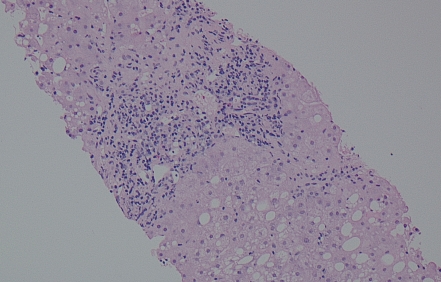
Figure 1.
Hematoxylin and eosin stain of liver biopsy revealing steatosis, portal inflammation, and piecemeal necrosis (interface hepatitis). l0x magnification. Image courtesy of Manjula Balasubramanian, MD, FCAP, Chief of Clinical Pathology and Laboratory Medicine, Albert Einstein Healthcare Network, Philadelphia, Pennsylvania.
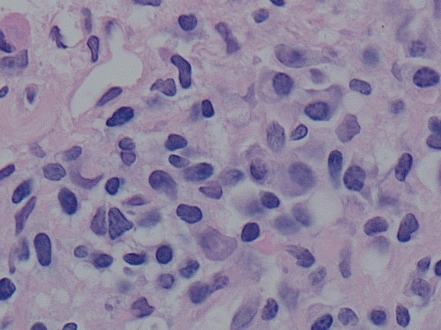
Figure 2.
Hematoxylin and eosin stain of liver biopsy revealing plasma cells and lymphocytes invading bile ducts. 60x magnification. Image courtesy of Manjula Balasubramanian, MD, FCAP, Chief of Clinical Pathology and Laboratory Medicine, Albert Einstein Healthcare Network, Philadelphia, Pennsylvania.
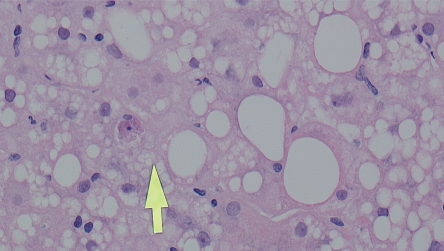
Figure 3.
Hematoxylin and eosin stain of liver biopsy revealing steatosis, balloon cells, and apoptosis (arrow). 40x magnification. Image courtesy of Manjula Balasubramanian, MD, FCAP, Chief of Clinical Pathology and Laboratory Medicine, Albert Einstein Healthcare Network, Philadelphia, Pennsylvania.
The patient was started on oral prednisone 30 mg and azathioprine 50 mg daily. After 4 weeks of therapy, her AST and ALT levels improved to 75 U/L and 125 U/L, respectively. Her azathioprine dose was increased to 100 mg (1.5 mg/kg body weight) daily, and her prednisone dose was decreased to 25 mg daily. The patient was monitored for the next 3 months on the same regimen, without any further improvement in transaminase levels. Due to the suboptimal response to first-line therapy, alternative treatment with oral prednisone 25 mg and mycophenolate mofetil (Cellcept, Roche Palo) 500 mg twice daily was started. The mycophenolate mofetil was gradually increased to 1 g twice daily (total, 2 g daily). This regimen was continued for the next 4 months, but no improvement in transaminase levels was observed. The prednisone was tapered and then discontinued over a 1-month period while the mycophenolate mofetil was continued. The AST and ALT levels remained elevated at 99 U/L and 115 U/L, respectively.
As a result of the suboptimal response to prednisone and mycophenolate mofetil, treatment for HCV was started. The risks and benefits were discussed with the patient in great detail, including the possibility of AIH flare-ups while on interferon. At the start of treatment, the patient's viral load was 9,650,000 IU/mL. After clearance from the ophthalmology and psychiatry departments (the latter of which started the patient on a stable dose of oral duloxetine [Cymbalta, Eli Lilly]), consensus interferon 15 μg three times per week, subcutaneously, with ribavirin 800 mg/day orally were started. As treatment was well tolerated without any significant side effects, consensus interferon was increased to a daily dose after 4 weeks. The patient responded very well to the therapy and achieved rapid virologic response (defined as an undetectable viral load at Week 4 of treatment), with normalization of liver transaminase levels. The patient successfully completed 24 weeks of treatment with consensus interferon and achieved end-of-treatment response (defined as an undetectable viral load at the completion of treatment) and SVR (defined as an undetectable viral load after 24 weeks following completion of treatment). The patient also took growth factors such as filgrastim (Neupogen, Amgen) and epoetin alfa (Procrit, Amgen). Although mycophenolate mofetil was continued throughout the interferon treatment without any evidence of AIH flare-ups, secondary to neutropenia, the dose of mycophenolate mofetil was decreased from 2 g/day to 1.5 g/day. After the successful completion of consensus interferon therapy, the mycophenolate mofetil dose was gradually decreased over 6 months to 750 mg/day.
Discussion
There are 3 types of AIH.4 Type 1, which our patient had, is seen more frequently in middle-aged to elderly women, has higher levels of IgG, shows a better response to steroid therapy, and progresses less frequently to cirrhosis compared to the other types.4 The concurrent presence of AIH in chronic HCV-infected patients constitutes HCV-AIH overlap syndrome. These patients manifest clinical, histologic, biochemical, and immunologic features of both HCV and AIH. Serologic markers of AIH such as ANA (10-33%), ASMA (13-66%), or anti-liver and kidney microsomal (LKM) antibody-1 (0-3%) have been reported in chronic HCV-infected patients.5-7
This concurrence of viral infection and autoantibodies not only confounds the diagnosis but also complicates the treatment strategy. Generally, interferon is contraindi-cated in autoimmune diseases. Almost all patients treated with pegylated interferon and ribavirin experience 1 or more adverse events during the course of therapy. The most common of these adverse events are influenza-like symptoms, psychiatric symptoms (depression, suicidal thoughts, irritability, and insomnia), and bone marrow suppression. Less common adverse events include weight loss, hair loss, thyroid dysfunction, pulmonary toxicity, colitis, vision loss, and hypersensitivity reaction.1,8 Administration of interferon in patients with chronic HCV-AIH overlap syndrome can lead to an exacerbation of the underlying AIH.9,10 This situation is well described by papers such as those by Vento and associates and Papo and colleagues.11,12 Treatment with immunosuppressive therapy rarely leads to complete normalization of liver biochemical profile and is frequently accompanied by an increase in HCV RNA level.13
To date, there are no standard guidelines on how to approach patients with HCV-AIH overlap syndrome. One management strategy is to determine the predominant entity in order to select the appropriate type of therapy.2,14 Chronic HCV-AIH overlap syndrome can be divided into autoimmune- or viral-predominant disease. Patients with autoimmune-predominant disease have ASMA or ANA titers of equal to or more than 1:320 or have ASMA and ANA titers of equal to or more than 1:40, as well as histology findings that include piecemeal necrosis (interface hepatitis), lobular hepatitis, and portal plasma cell infiltrates.14-16
Patients with viral-predominant disease have ASMA or ANA titers of less than 1:320 or have antibodies to LKM type-1 and hepatitis C viremia, as well as histology findings that include portal lymphoid aggregates, steatosis, or bile duct injury. Tissue damage is more focal in HCV and more diffuse in AIH liver histology.14-16 An immunosuppression trial with corticosteroids (CS) monotherapy or reduced-dose CS combined with azathioprine is an option for patients with autoimmune-predominant disease. Cyclosporine may be effective as short-term front-line therapy, and calcineurin inhibitors may salvage patients who are refractory to CS regimens.2,14,17,18 Interferon therapy with close monitoring is an option in viral-predominant disease.14
Conclusion
We present a case of HCV-AIH overlap syndrome. Due to the discordant treatment options of HCV and AIH, it is challenging to determine therapy when the diseases coexist. In our case, consensus interferon was preferred due to its strong potency and short half-life19 compared to pegylated interferon and natural interferon alfa. If the patient had developed a flare of AIH, treatment would have been stopped and rapid washout of the system could have been achieved. We realize that our patient had a very favorable genotype (genotype 3A), which responds better to treatment than genotype 1 and has a very high response rate to interferon. However, we suggest that this approach can be attempted in selected patients with other genotypes as well, under close observation and with regular follow-ups, particularly with a hepatologist. For the future, however, further studies and larger case series are required to establish appropriate guidelines.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seeff LB. AASLD practice guidelines: diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiano TD, Te HS, Thomas RM, Hussain H, Bond K, Black M. Results of steroid-based therapy for the hepatitis C—autoimmune hepatitis overlap syndrome. Am J Gastroenterol. 2001;96:2984–2991. doi: 10.1111/j.1572-0241.2001.04672.x. [DOI] [PubMed] [Google Scholar]
- 3.Petersen-Benz C, Kasper HU, Dries V, Goeser T. Differential efficacy of corticosteroids and interferon in a patient with chronic hepatitis C—autoimmune hepatitis overlap syndrome. Clin Gastroenterol Hepatol. 2004;2:440–443. doi: 10.1016/s1542-3565(04)00130-2. [DOI] [PubMed] [Google Scholar]
- 4.Czaja AJ. Autoimmune hepatitis. In: Feldman M, Scharschmidt BF, Sleisenger MH, Fordtran JS, editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 6th ed. Philadelphia, Pennsylvania: WB Saunders Company; 1998. pp. 1265–1274. [Google Scholar]
- 5.Clifford BD, Donahue D, Smith L, Cable E, Luttig B, et al. High prevalence of serological markers of autoimmunity in patients with chronic hepatitis C. Hepatology. 1995;21:613–619. [PubMed] [Google Scholar]
- 6.Lenzi M, Bellentani S, Saccoccio G, Muratori P, Masutti F, et al. Prevalence of non-organ-specific autoantibodies and chronic liver disease in the general population: a nested case-control study of the Dionysos cohort. Gut. 1999;45:435–441. doi: 10.1136/gut.45.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams MJ, Lawson A, Neal KR, Ryder SD, Irving WL Trent HCV Group. Autoantibodies in chronic hepatitis C virus infection and their association with disease profile. J Viral Hepat. 2009;16:325–331. doi: 10.1111/j.1365-2893.2008.01035.x. [DOI] [PubMed] [Google Scholar]
- 8.Pegasys (PegInterferon alfa-2a) [package insert]. Hoffman-La Roche, Lab, Inc.
- 9.Garcia-Buey L, Garcia-Monzon C, Rodriguez S, Borque MJ, García-Sánchez A, et al. Latent autoimmune hepatitis triggered during interferon therapy in patients with chronic hepatitis C. Gastroenterology. 1995;108:1770–1777. doi: 10.1016/0016-5085(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 10.Shindo M, Di Bisceglie AM, Hoofnagle JH. Acute exacerbation of liver disease during interferon alfa therapy for chronic hepatitis C. Gastroenterology. 1992;102((4 pt 1)):l406–1408. [PubMed] [Google Scholar]
- 11.Vento S, Di Perri G, Garofano T, Cosco L, Concia E, et al. Hazards of interferon therapy for HBV-seronegative chronic hepatitis. Lancet. 1989;2:926. doi: 10.1016/s0140-6736(89)91595-x. [DOI] [PubMed] [Google Scholar]
- 12.Papo T, Marcellin P, Bernuau J, Durand F, Poynard T, Benhamou JP. Auto-immune chronic hepatitis exacerbated by alpha-interferon. Ann Intern Med. 1992;116:51–53. doi: 10.7326/0003-4819-116-1-51. [DOI] [PubMed] [Google Scholar]
- 13.Magy N, Cribier B, Schmitt C, Ellero B, Jaeck D, et al. Effects of corticosteroids on HCV infection. Int J Immunopharmacol. 1999;21:253–261. doi: 10.1016/s0192-0561(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 14.Czaja AJ. Autoimmune hepatitis and viral infection. Gastroenterol Clin North Am. 1994;23:547–566. [PubMed] [Google Scholar]
- 15.Czaja AJ. The variant forms of autoimmune hepatitis. Ann Intern Med. 1996;125:588–598. doi: 10.7326/0003-4819-125-7-199610010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Bach N, Thung SN, Schaffner F. The histological features of chronic hepatitis C and autoimmune chronic hepatitis: a comparative analysis. Hepatology. 1992;15:572–577. doi: 10.1002/hep.1840150403. [DOI] [PubMed] [Google Scholar]
- 17.Bellary S, Schiano T, Hartman G, Black M. Chronic hepatitis with combined features of autoimmune chronic hepatitis and chronic hepatitis C: favorable response to prednisone and azathioprine. Ann Intern Med. 1995;123:32–34. doi: 10.7326/0003-4819-123-1-199507010-00004. [DOI] [PubMed] [Google Scholar]
- 18.Montano Loza AJ, Czaja AJ. Current therapy for autoimmune hepatitis. Nat Clin Pract Gastroenterol Hepatol. 2007;4:202–214. doi: 10.1038/ncpgasthep0768. [DOI] [PubMed] [Google Scholar]
- 19.Infergen (Interferon alfacon-1) [package insert] Three Rivers Pharmaceuticals, LLC. Revised August 2009.