Abstract
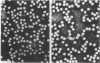
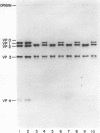
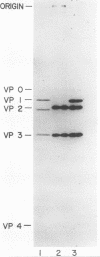
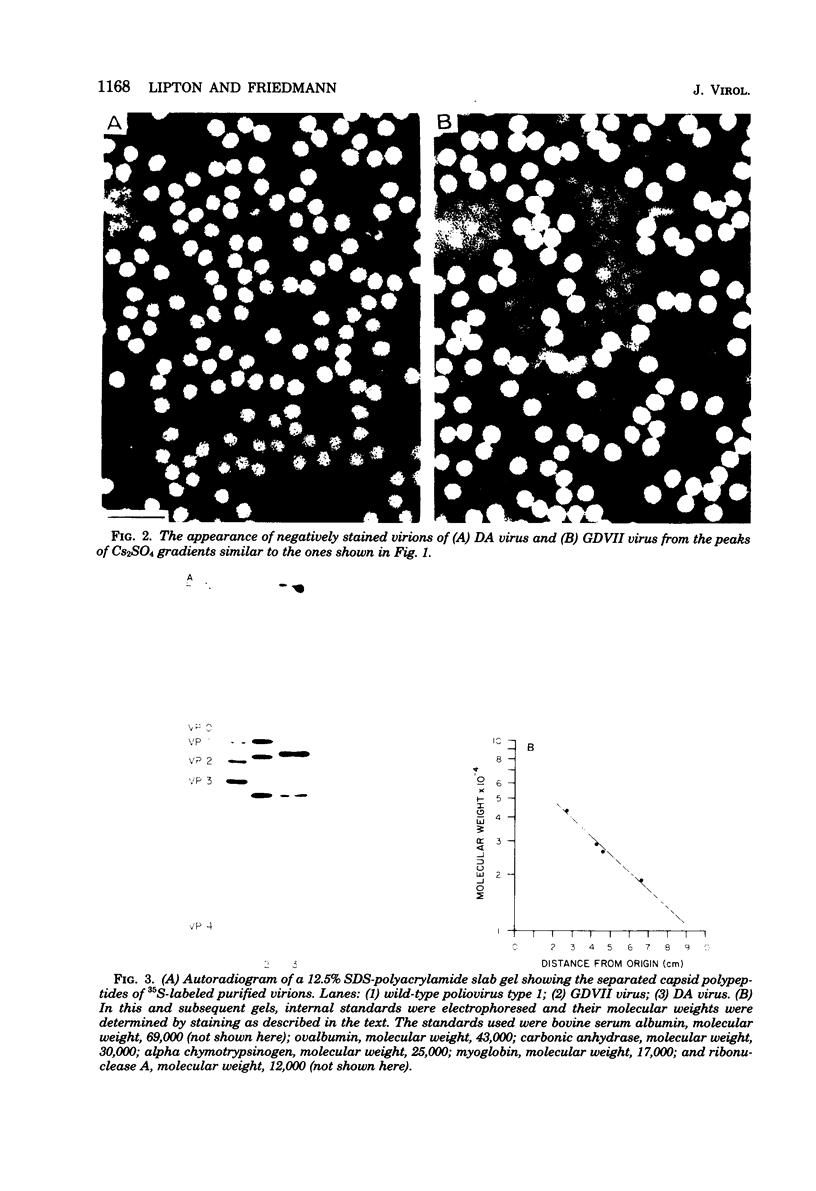
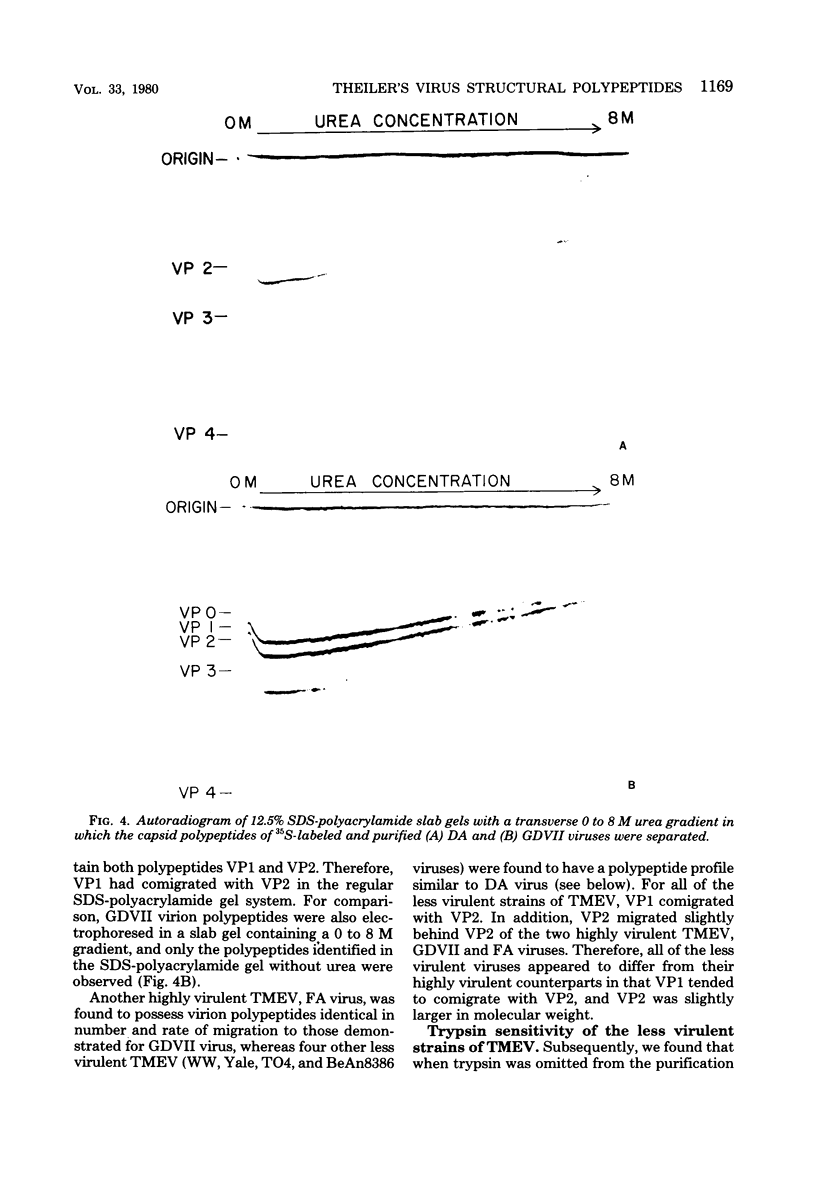
Theiler's murine encephalomyelitis viruses (TMEV) are separable into two groups based on their biological behavior: those highly virulent isolates which are unable to cause persistent infection and the less virulent isolates which regularly produce persistent central nervous system infection in mice. Two highly virulent and five less virulent TMEV were found to have the same buoyant density (1.34 g/ml) on isopycnic centrifugation and virion structure by electron microscopy. Negatively stained virus particles purified in Cs2SO4 gradients appeared to have icosahedral symmetry and measured 28 nm in diameter. Mature virions were found to possess three major structural polypeptides, VP1, VP2 and VP3, in the range of 25,000 to 35,000 daltons, and a smaller fourth major polypeptide, VP4, of 6,000 daltons on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The precursor of VP2 and VP4, VP0, which is a minor polypeptide of mature picornavirus particles, was also identified. However, a slight but consistent difference in several of the capsid polypeptides between the highly virulent and less virulent TMEV was found. VP1 was slightly larger (34,000 versus 33,500 daltons) and VP2 was slightly smaller (31,000 versus 32,000 daltons) for the highly virulent strains compared to the same polypeptide species in the less virulent viruses. VP0 was also slightly smaller (35,500 versus 36,000 daltons) for the highly virulent isolates compared to their less virulent counterparts. Finally, trypsin which was used initially in our purification procedure resulted in preferential cleavage of a 2,000-molecular-weight fragment or fragments from VP1 of only the less virulent isolates.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman L. D., Caliguiri L. A., Lilly L. S. Cleavage site alterations in poliovirus-specific precursor proteins. Virology. 1976 Aug;73(1):216–227. doi: 10.1016/0042-6822(76)90076-3. [DOI] [PubMed] [Google Scholar]
- Bernard S., Wantyghem J., Grosclaude J., Laporte J. Chromatographic preparation of purified structural proteins from foot-and-mouth disease virus. Biochem Biophys Res Commun. 1974 Jun 4;58(3):624–632. doi: 10.1016/s0006-291x(74)80464-x. [DOI] [PubMed] [Google Scholar]
- Carthew P., Martin S. J. The iodination of bovine enterovirus particles. J Gen Virol. 1974 Sep;24(3):525–534. doi: 10.1099/0022-1317-24-3-525. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Primary demyelination in Theiler's virus infection. An ultrastructural study. Lab Invest. 1975 Dec;33(6):626–637. [PubMed] [Google Scholar]
- FRANKLIN R. M., WECKER E., HENRY C. C Some properties of an infectious ribonucleic acid from mouse encephalomyelitis virus. Virology. 1959 Feb;7(2):220–235. doi: 10.1016/0042-6822(59)90188-6. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lipton H. L. Characterization of the TO strains of Theiler's mouse encephalomyelitis viruses. Infect Immun. 1978 Jun;20(3):869–872. doi: 10.1128/iai.20.3.869-872.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonberg-Holm K., Butterworth B. E. Investigation of the structure of polio- and human rhinovirions through the use of selective chemical reactivity. Virology. 1976 May;71(1):207–216. doi: 10.1016/0042-6822(76)90106-9. [DOI] [PubMed] [Google Scholar]
- McAllister R. M., Melnyk J., Finkelstein J. Z., Adams E. C., Jr, Gardner M. B. Cultivation in vitro of cells derived from a human rhabdomyosarcoma. Cancer. 1969 Sep;24(3):520–526. doi: 10.1002/1097-0142(196909)24:3<520::aid-cncr2820240313>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Milstien J. B., Walker J. R., Eron L. J. Correlation of virus polypeptide structure with attenuation of poliovirus type 1. J Virol. 1977 Sep;23(3):811–815. doi: 10.1128/jvi.23.3.811-815.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips B. A., Fennell R. Polypeptide composition of poliovirions, naturally occurring empty capsids, and 14S precursor particles. J Virol. 1973 Aug;12(2):291–299. doi: 10.1128/jvi.12.2.291-299.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picornaviridae: second report. Intervirology. 1978;10(3):165–180. doi: 10.1159/000148981. [DOI] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]