Abstract
The ability of an environmental factor or toxicant to promote a phenotype or disease state not only in the individual exposed, but also in subsequent progeny for multiple generations, is termed transgenerational inheritance. The majority of environmental agents do not promote genetic mutations or alterations in DNA sequence, but do have the capacity to alter the epigenome. Although most environmental exposures will influence somatic cells and not allow the transgenerational transmission of a phenotype, the ability of an environmental factor to reprogram the germline epigenome can promote a transgenerational inheritance of phenotypes and disease states. A limited number of critical developmental periods exist when environmental signals can produce a significant epigenetic reprogramming of the germline. In this review, the ability of environmental factors or toxicants to promote epigenetic transgenerational phenotypes is reviewed.
Keywords: adult onset, disease environment, epigenetics, transgenerational
The majority of environmental factors and toxicants do not have the ability to alter DNA sequence or promote genetic mutations [1,2], which is due in large part to the stable nature of the genome. Environmental agents that promote abnormal phenotypes or disease often act in early-life exposures that lead later in life to adult-onset disease [3]. The environmental effects do not appear to be mediated through genetic mechanisms, but instead altered molecular processes such as epigenetics [1,4,5]. In the event these environmental factors promote a heritable or familial transmission of the disease phenotype, it often involves non-Mendelian inheritance.
Changes in the epigenome have been described in response to environmental stimuli in several organisms (e.g., rodents and humans). Examples range from nutritional factors such as folate methyl donors [6,7], inorganic contaminants such as arsenic [8,9], drugs such as cocaine [10], endocrine disruptors such as bisphenol A (BPA) [11-13], phytoestrogens [14,15] and chemicals used in agriculture, such as vinclozolin [16]. Other studies have focused more on relating behavior to DNA methylation through examining maternal behavior [17] or depression [18]. Metabolic disease associated with nutrient restriction also involves epigenetics [19]. In addition, nutrition has been shown to effect epigenetics and development in honey bees [20].
The heritable transmission of environmentally induced phenotypes is referred to as transgenerational inheritance [1,21]. In order to distinguish those transgenerational processes that require a persistent multigenerational action of the environment from those requiring only a one-time single generation exposure, we proposed a clarification of the term transgenerational epigenetics, separating them into intrinsic and extrinsic categories [22]. The vast majority of epigenetic changes derived from environmental exposures will involve somatic cells and not promote transgenerational phenotypes. However, these somatic cell effects will be critical for the individual exposed in regards to potential adult-onset disease or other alterations in phenotype [1]. In contrast to germline development, somatic cell-sensitive periods in which environmental signals produce epigenetic changes are numerous [23-26]. An extrinsic transgenerational phenotype requires a continued multigenerational exposure to the factor (often at only a specific period of development) triggering an epigenetic change. For example, the ability of good maternal behavior (e.g., early postnatal pup licking in rodents) can program the same good maternal behavior in the female adults that then pass this on to their offspring in a similar manner [27,28]. Without the continued generational maternal behavior and epigenetic programming of the brain and behavior in the female offspring, the transgenerational phenotype would be lost [27,28]. In the event a germline epigenetic alteration is involved, the exposure has the potential to promote an intrinsic transgenerational phenomena, which will promote a transgenerational phenotype independent of continued environmental exposures [16](Table 1). Examples of intrinsic trangenerational phenotypes are described.
Table 1.
Sites of action and phenotypes of environmental factors and toxicants.
| Site action | Biological response and toxicology |
|---|---|
| Somatic cells | Allows tissue-specific toxicology, and critical for adult-onset disease in the individual exposed, but not capable of transmitting a transgenerational phenotype |
| Germ cells | Allows transmission between generations, and in the absence of direct exposure promotes a transgenerational phenotype |
Epigenetics
Epigenetics is defined as 'molecular factors and processes around DNA that regulate genome activity independent of DNA sequence and are mitotically stable'. Regulatory processes such as DNA methylation, histone modifications, chromatin structure and noncoding RNA are all examples of epigenetic factors (Table 2). Epigenetic regulation of genome activity is critical in the development and maintenance of cellular function and differentiation. The term epigenetics was originally coined by Conrad Waddington in his discussion of gene-environ ment interactions [29]. The first molecular factor identified was DNA methylation in the 1970s [30] followed by histone modifications in the 1990s [31]. Therefore, the molecular elements of epigenetic regulatory processes have only recently been elucidated [1]. These epigenetic processes are likely to be equally important in regulating genome activity (i.e., gene expression) as the DNA sequence (i.e., genetics).
Table 2.
Examples of epigenetic processes.
| Process | Molecular modification |
|---|---|
| DNA methylations | Methyl cytosine at CpG sites |
| Histone modifications | Methylation, acetylation phosphorylation, ubiquitination |
| Chromatin structure | Loop and bend structures and nuclear matrix associations |
| Noncoding RNA | Small RNA influencing RNA stability and gene expression |
The ability of environmental factors or toxicants to influence epigenetic processes associated with altered phenotypes has been shown in a number of different model systems [1,3,4]. One of the first observations of how the environ ment could influence epigenetics and phenotype was observed in plants [32]. A classic mouse model with a metastable epiallele developed is the Agouti line [33,34]. An epigenetic modification of the Agouti locus alters coat color and influences metabolic disease (e.g., obesity) [5,33]. Environmental agents, such as the estrogenic substance plastic component BPA, can modulate the Agouti locus DNA methylation state to alter phenotypes in the mouse [11].
As discussed, the majority of the actions of environmental factors are on somatic cells,Table 3. Examples of specific toxicants acting on a given tissue to promote a disease state in many cases involves epigenetic processes. An example is the action of the BPA on the pubertal prostate to promote an epigenetic effect on DNA methylation that is associated with adult-onset prostate disease [12]. Similar effects have also been observed with the fungicide vinclozolin [35] on prostate disease. The majority of environmental factors influencing adult-onset disease will likely involve direct or indirect epigenetic modifications in the tissue or somatic cells involved [1]. These somatic cell effects will be critical in the etiology of disease in the individual exposed, but the phenotype will not become transgenerational without the involvement of the germline. Nevertheless, if multigenerational exposures occur [27,28], the transgenerational process will be extrinsic [22] (Table 3).
Table 3.
Transgenerational versus multigenerational phenotypes and toxicology.
| Phenotype | Exposure | Definition |
|---|---|---|
| Multigenerational | Direct | Coincident direct exposure of multiple generations to an environmental factor or toxicant promoting a toxicology in the multiple generations exposed |
| Transgenerational | None, except the initial (ancestral) generation |
After the initial exposure, the transgenerational phenotype is only transmitted through the germline in the absence of direct exposure |
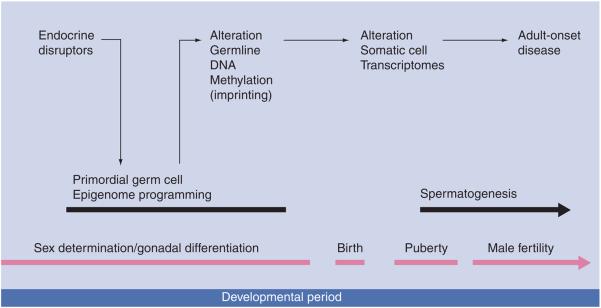
Since the germline is required for the transmission of genetic information between generations, in the event a permanent epigenetic modification of the germline occurred, then an intrinsic epigenetic transgenerational phenomena would be possible. In considering germ-cell biology there are critical periods of development where epigenetic modifications are influenced. During embryonic development in mammalian species, the primordial germ cells migrate down the genital ridge to the developing gonad prior to sex determination as a germline stem cell [36-38]. Upon gonadal sex determination the germ cells develop into a male or female germline. The female germline develops and then enters meiosis in the developing embryonic ovary [36-38]. The male germline continues to proliferate until immediately prior to birth, and then resumes proliferation after birth. In the adult, the female germline undergoes oogenesis during follicle development to generate oocytes. The male germ-line develops from spermatogonial stem cells and requires spermatogenesis for the production of spermatozoa in the testis. Epigenetic programming of the germline occurs during the migration of the primordial germ cells in the embryo. The migrating primordial germ cells undergo an erasure or demethylation of the DNA during genital ridge migration, and colonize the early bipotential gonad prior to gonadal sex determination [36-38]. Then, when gonadal development and sex determination is initiated, the primor-dial germ cells develop female or male-germ-cell lineage and remethylate the DNA in a male or female specific manner. Therefore, the germ-cell DNA is demethylated and remethylated during gonadal sex determination, and appears sensitive to environmental factors [36-38] (Figure 1). Although there are alterations in the male and female germline epigenomes (i.e., DNA methylation) during gametogenesis in the adult gonads [39], the embryonic period of gonadal sex determination is the most critical in establishing the epigenome and cell fate [40]. This developmental period is the most sensitive to environmental insults influencing the epigenome (e.g., imprinting). In contrast to the female germ cell, which could allow the transmission of numerous types of epigenetic processes, the male germ cell during spermatogenesis replaces the majority of histones with protamines, involves DNA condensation to eliminate chromatin structure and silences the genome for expression of the majority of genes and noncoding RNAs [41]. Although the histones retained have recently been suggested to map to critical genome regions [42], the functional impact of altering these histones remains to be determined. Therefore, the primary epigenetic process established that is transmitted through the male germline is DNA methylation. An example of this involves imprinted genes that have a specific pattern of DNA methylation that is transmitted through a parent of origin allele to subsequent progeny [43]. These imprinted sites regulate gene expression in an allele-specific manner. Therefore, DNA methylation has the potential to be a transgenerational epigenetic mechanism [43]. Owing to the reprogramming of the germline that occurs during gonadal sex determination, this is one of the most sensitive periods for environmental exposures to influence germ-cell fate programming and create a transgenerational phenotype. The epigenome modifications during gametogenesis in the adult are critical for germ-cell differentiation and function, but their role in epigenome programming (e.g., imprinting) is less critical. Although epigenetic modifications in the adult germline during gametogenesis can influence the offspring from the affected germ-line, subsequent transgenerational effects have not been documented [39].
Figure 1.
Developmental events of epigenetic transgenerational phenomena.
Transgenerational phenotypes
The majority of known environmentally induced epigenetic effects will involve direct exposures and action on somatic tissues. In contrast, intrinsic transgenerationally induced phenotypes exclude persistent generational exposures and must be transmitted through multiple generations [1,44]. For example, exposure of a gestating female provides direct exposure of the F0 generation female, the F1 generation embryo, and the germline that will generate the F2 generation is also directly exposed [44]. Therefore, investigation of the phenotype in the F3 generation is required to observe a transgenerational effect that is independent of direct exposure. The effects observed in the F0 and F1 generations were due to direct somatic cell exposures, as well as that in the F2 generation germline [1,44]. The ability of a direct exposure to influence multiple generations is defined as a multiple generation phenotype (Table 3), and not a transgenerational phenotype. In contrast, an intrinsic transgenerational process requires the absence of continued direct environmental exposure (Table 3). A class of environmental compounds or toxicants involved in such phenotypes are endocrine disruptors that interfere with normal hormone signaling.
A classic example of a multigenerational toxicology involves the pharmaceutical agent with estrogen agonist activity, diethylstilbesterol (DES) [45]. Exposure of a gestating female to DES was found to promote an abnormal reproductive tract and gonadal dysfunction in the F1 generation males and females, as well as abnormal female reproductive tract function in the F2 generation [46,47]. Interestingly, the phenotype of the F1 and F2 generations have differences. Limited information exists for the F3 generation in humans, but F3 generation rodent models have not exhibited a major phenotype [45-47]. It is possible that DES could promote a transgenerational phenotype, but extended generations need to be investigated [46]. Another example of a multigenerational toxicology is a study with flutamide [48]. This antiandrogenic endocrine disruptor after exposure of a gestating female promoted an F1 generation abnormality in the testis and F2 generation effects in skeletal development, but no F3 generation effects [48]. Again, the F1 and F2 generation phenotypes were distinct. In contrast, the endocrine disruptor vinclozolin did promote transgenerational phenotypes in the F3 generation [48]. These phenotypes include spermatogenic cell defects in the testis [16,49], and a number of other adult-onset diseases [50,51]. Another example of multi generation effect is due to prenatal nutrient restriction. Bertram et al. have shown that changes in heart structure and in the hypothalamo-pituitary-adrenal function can be observed in the F2 generation rodents after prenatal nutrient restriction [52]. Environmental factors and toxicants that promote multiple-generation effects involving direct exposure have been observed for numerous agents [1,3]. These multigenerational exposures and phenotypes are not transgenerational, although they are critical to consider in order to evaluate the toxicology of an environmental agent.
In summary, intrinsic transgenerational phenotypes require transmission between generations through the germline. These transgenerational phenotypes occur in the absence of persistent exposure (Table 3). Somatic cell targets are critical and common in order to promote adult-onset disease and alteration in phenotypes, but are not able to transmit the phenotype transgenerationally without continued direct exposure. Therefore, the critical target cell for an intrinsic transgenerational process is the germline.
Epigenetic transgenerational phenomena
The initial observations of epigenetic transgenerational phenotypes were in plants, and involved DNA methylation and paramutation mechanisms [32,53]. Evidence appeared in mammals when the transient exposure of the environmental compound vinclozolin during gonadal sex determination was found to promote an adult-onset disease in the F1 generation as well as subsequent generations (i.e., F1-F4) [16]. This was found to be due in part to DNA methylation changes in the male germline (i.e., sperm), and promoted transgenerational changes in the transcriptomes of a number of tissues [54-56]. The adult-onset diseases observed included testis abnormalities, prostate disease, kidney disease, immune abnormalities and tumor development [50]. Therefore, the environmental toxicant vinclozolin after exposure of a gestating female during the critical period of gonadal sex determination appears to have modified the male sperm epigenome to allow the transgenerational transmission of adult-onset disease [1,16] (Figure 1). A recent study suggests the transgenerational actions of vinclozolin may not involve its antiandrogen endocrine disruptor actions, but other unknown actions [48]. These studies provide one of the first examples of epigenetic transgenerational inheritance promoting adult-onset disease [16].
Several other environmental factors and models are now being used to further describe these epigenetic transgenerational phenomena [57,58]. The Agouti mouse model has been used to document a transgenerational obesity adult-onset disease phenotype [59]. Modification of the DNA methylation pattern of the Agouti locus with environmental toxicants such as BPA modified this phenotype [11]. Another example involves the ability of BPA to promote testis abnormalities for three generations (F1–F3) [60]. Nutritional deficits in early-life, such as famine in humans, can promote a transgenerational response [61], and influence transgenerational genetic defects [62]. A number of heritable disease states also appear to be of epigenetic origin (e.g., multiple sclerosis) [63]. Therefore, further analysis of the actions of environmental factors and toxicants when exposure could effect the germline need to be performed to elucidate the extent to which epigenetic transgenerational phenomena are involved in adult-onset disease and environmental toxicology.
Conclusion & future perspective
Intrinsic epigenetic transgenerational processes will require the involvement of the germline to allow the transmission of an epigenetic abnormality between multiple generations. The ability of environmental factors or toxicants to promote an alteration in the epigenome will be common for somatic tissues, but less common for the germline due to the limited developmental period in which the germline is sensitive to reprogramming. In the event an altered germline epigenome becomes permanently programmed, an epigenetic transgenerational phenotype would be possible. The phenomena of the fetal basis of adult-onset disease has been established [1,3], and epigenetics will likely play a critical role in this process. In considering the role of environmental agents in disease, both the transient early-life exposures in the individual and/or the potential involvement of the germline need to be considered in the etiology of adult-onset disease. Further investigation into the role of epigenetics in disease etiology is now needed to determine if early-life exposures may be a significant factor in disease. Elucidation of the epigenetics involved in transgenerational processes would provide insights into the diagnosis of exposure and potential therapeutic targets for disease. Although the prevalence of trans generational epigenetic processes needs to be assessed in various disease states, the role of epigenetics will likely be a major factor to consider in toxicology and medicine in the future.
Executive summary.
Environmental factors & disease etilology
■ Environmental factors do not generally promote genetic mutations or change DNA sequence, but have a significant impact in disease etiology.
■ Environmental factors can alter epigenetic processes to promote changes in genome activity (promotion of epimutations) to influence adult-onset disease.
■ During critical periods of development when organ system are rapidly differentiating they are most sensitive to environmental factors altering the epigenome, which later in life promotes adult-onset disease.
Site of action versus phenotypes from environmental factors & exposures
■ The majority of factors and exposures will act on somatic cells and tissues, which will be critical for adult-onset disease in the individual exposed, but not capable of transmitting a transgenerational phenotype.
■ In the event, an exposure or environmental factor permanently alters the germline (i.e., sperm and egg) epigenome, a transgenerational epigenetic phenotype can develop.
Transgenerational phenotype versus multigenerational phenotypes
■ When multiple generations are directly exposed to an environmental factor or toxicant (e.g., F0 generation mother, F1 generation embryo and F2 generation germline) and the phenotype is only observed in those generations directly exposed, then a multigenerational phenotype is observed.
■ In the event, the phenotype is observed in subsequent generations without direct exposure, then a transgenerational phenotype is observed (e.g., transgenerational inheritance).
Acknowledgements
We thank Ms Heather Johnson for assistance in preparation of the manuscript.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1■.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007;8(4):253–262. doi: 10.1038/nrg2045. Review on environment, epigenetics and disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Szyf M. The dynamic epigenome and its implications in toxicology. Toxicol. Sci. 2007;100(1):7–23. doi: 10.1093/toxsci/kfm177. [DOI] [PubMed] [Google Scholar]
- 3■.Hanson MA, Gluckman PD. Developmental origins of health and disease: New insights. Basic Clin. Pharmacol. Toxicol. 2008;102(2):90–93. doi: 10.1111/j.1742-7843.2007.00186.x. Review on developmental origins of adult-onset disease. [DOI] [PubMed] [Google Scholar]
- 4.Morgan DK, Whitelaw E. The role of epigenetics in mediating environmental effects on phenotype. Nestle Nutr. Workshop Ser. Pediatr. Program. 2009;63:109–117. doi: 10.1159/000209976. discussion 117–109; 259–168. [DOI] [PubMed] [Google Scholar]
- 5.Waterland RA. Is epigenetics an important link between early life events and adult disease? Horm. Res. 2009;71(Suppl. 1):13–16. doi: 10.1159/000178030. [DOI] [PubMed] [Google Scholar]
- 6.Cooney CA, Dave AA, Wolff GL. Maternal methyl supplements in mice affect epigenetic variation and DNA methylation of offspring. J. Nutr. 2002;132(8 Suppl.):2393S–2400S. doi: 10.1093/jn/132.8.2393S. [DOI] [PubMed] [Google Scholar]
- 7.Cropley JE, Suter CM, Beckman KB, Martin DI. Germline epigenetic modification of the murine a vy allele by nutritional supplementation. Proc. Natl Acad. Sci. USA. 2006;103(46):17308–17312. doi: 10.1073/pnas.0607090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh KP, Dumond JW., Jr Genetic and epigenetic changes induced by chronic low dose exposure to arsenic of mouse testicular leydig cells. Int. J. Oncol. 2007;30(1):253–260. [PubMed] [Google Scholar]
- 9.Waalkes MP, Liu J, Chen H, et al. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J. Natl Cancer Inst. 2004;96(6):466–474. doi: 10.1093/jnci/djh070. [DOI] [PubMed] [Google Scholar]
- 10.Novikova SI, He F, Bai J, Cutrufello NJ, Lidow MS, Undieh AS. Maternal cocaine administration in mice alters DNA methylation and gene expression in hippocampal neurons of neonatal and prepubertal offspring. PLoS ONE. 2008;3(4):e1919. doi: 10.1371/journal.pone.0001919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11■.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol a-induced DNA hypomethylation in early development. Proc. Natl Acad. Sci. USA. 2007;104(32):13056–13061. doi: 10.1073/pnas.0703739104. Description of how environmental toxicants affects the Agouti mouse model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12■.Ho SM, Tang WY, Belmonte De Frausto J, Prins GS. Developmental exposure to estradiol and bisphenol a increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006;66(11):5624–5632. doi: 10.1158/0008-5472.CAN-06-0516. Description of how environmental toxicants affect epigenetics and prostate disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaoi T, Itoh K, Nakamura K, Ogi H, Fujiwara Y, Fushiki S. Genome-wide ana lysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol a. Biochem. Biophys. Res. Commun. 2008;376(3):563–567. doi: 10.1016/j.bbrc.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 14.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerrero-Bosagna CM, Sabat P, Valdovinos FS, Valladares LE, Clark SJ. Epigenetic and phenotypic changes result from a continuous pre and post natal dietary exposure to phytoestrogens in an experimental population of mice. BMC Physiol. 2008;8:17. doi: 10.1186/1472-6793-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16■■.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308(5727):1466–1469. doi: 10.1126/science.1108190. Description of how environmental toxicants affect epigenetic transgenerational disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17■.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-α1b promoter and estrogen receptor-α expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915. doi: 10.1210/en.2005-1119. Description of how behavior effects brain epigenome transgenerationally. [DOI] [PubMed] [Google Scholar]
- 18.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (nr3c1) and infant cortisol stress responses. Epigenetics. 2008;3(2):97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 19.Heijmans BT, Tobi EW, Stein Ad, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA. 2008;105(44):17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kucharski R, Maleszka J, Foret S, Maleszka R. Nutritional control of reproductive status in honeybees via DNA methylation. Science. 2008;319(5871):1827–1830. doi: 10.1126/science.1153069. [DOI] [PubMed] [Google Scholar]
- 21■.Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr. Opin. Genet. Dev. 2008;18(3):273–279. doi: 10.1016/j.gde.2008.07.001. Review of transgenerational inheritance and role of the germline. [DOI] [PubMed] [Google Scholar]
- 22.Guerrero-Bosagna CM, Skinner M. Epigenetic transgenerational effects of endocrine disruptors on male reproduction. Semin. Reprod. Med. 2009;27(5):403–408. doi: 10.1055/s-0029-1237428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Environ. Health Perspect. 2007;115(9):1264–1270. doi: 10.1289/ehp.9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Constancia M, Pickard B, Kelsey G, Reik W. Imprinting mechanisms. Genome Res. 1998;8(9):881–900. doi: 10.1101/gr.8.9.881. [DOI] [PubMed] [Google Scholar]
- 25.Macphee DG. Epigenetics and epimutagens: Some new perspectives on cancer, germ line effects and endocrine disrupters. Mutat. Res. 1998;400(1–2):369–379. doi: 10.1016/s0027-5107(98)00046-3. [DOI] [PubMed] [Google Scholar]
- 26■.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. Description of the epigenetic programming in the germline. [DOI] [PubMed] [Google Scholar]
- 27.Mcgowan PO, Meaney MJ, Szyf M. Diet and the epigenetic (re)programming of phenotypic differences in behavior. Brain Res. 2008;1237:12–24. doi: 10.1016/j.brainres.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szyf M, Mcgowan P, Meaney MJ. The social environment and the epigenome. Environ. Mol. Mutagen. 2008;49(1):46–60. doi: 10.1002/em.20357. [DOI] [PubMed] [Google Scholar]
- 29■■.Waddington CH. Organisers and genes. Cambridge University Press; Cambridge, UK: 1940. First description of epigenetics. [Google Scholar]
- 30■■.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187(4173):226–232. First identification of DNA methylation. [PubMed] [Google Scholar]
- 31.Turner BM. Histone acetylation as an epigenetic determinant of long-term transcriptional competence. Cell Mol. Life Sci. 1998;54(1):21–31. doi: 10.1007/s000180050122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32■.Cuzin F, Grandjean V, Rassoulzadegan M. Inherited variation at the epigenetic level: Paramutation from the plant to the mouse. Curr. Opin. Genet. Dev. 2008;18(2):193–196. doi: 10.1016/j.gde.2007.12.004. Review of paramutation processes in plants and mammals. [DOI] [PubMed] [Google Scholar]
- 33.Dolinoy DC. The agouti mouse model: an epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008;66(Suppl. 1):S7–S11. doi: 10.1111/j.1753-4887.2008.00056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakyan VK, Chong S, Champ Me, et al. Transgenerational inheritance of epigenetic states at the murine axin(fu) allele occurs after maternal and paternal transmission. Proc. Natl Acad. Sci. USA. 2003;100(5):2538–2543. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowin PA, Foster P, Pedersen J, Hedwards S, Mcpherson SJ, Risbridger GP. Early-onset endocrine disruptor-induced prostatitis in the rat. Environ. Health Perspect. 2008;116(7):923–929. doi: 10.1289/ehp.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36■.Durcova-Hills G, Hajkova P, Sullivan S, Barton S, Surani MA, Mclaren A. Influence of sex chromosome constitution on the genomic imprinting of germ cells. Proc. Natl Acad. Sci. USA. 2006;103(30):11184–11188. doi: 10.1073/pnas.0602621103. Description of epigenetic programming in the germline. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129(2):137–149. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- 38.Trasler JM. Origin and roles of genomic methylation patterns in male germ cells. Semin. Cell Dev. Biol. 1998;9(4):467–474. doi: 10.1006/scdb.1998.0225. [DOI] [PubMed] [Google Scholar]
- 39.Zamudio NM, Chong S, O'Bryan MK. Epigenetic regulation in male germ cells. Reproduction. 2008;136(2):131–146. doi: 10.1530/REP-07-0576. [DOI] [PubMed] [Google Scholar]
- 40■■.Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414(6859):122–128. doi: 10.1038/35102186. Description of the role of epigenetics in germline and impact inheritance. [DOI] [PubMed] [Google Scholar]
- 41.Godmann M, Lambrot R, Kimmins S. The dynamic epigenetic program in male germ cells: Its role in spermatogenesis, testis cancer, and its response to the environment. Microsc. Res. Tech. 2009;72(8):603–619. doi: 10.1002/jemt.20715. [DOI] [PubMed] [Google Scholar]
- 42.Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–478. doi: 10.1038/nature08162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ideraabdullah FY, Vigneau S, Bartolomei MS. Genomic imprinting mechanisms in mammals. Mutat. Res. 2008;647(1–2):77–85. doi: 10.1016/j.mrfmmm.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008;25(1):2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil. Steril. 2008;89(2 Suppl.):E55–E56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 46■.Newbold RR, Padilla-Banks E, Jefferson WN. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology. 2006;147(6 Suppl.):S11–17. doi: 10.1210/en.2005-1164. Description of diethylstilbesterol actions in multiple generations. [DOI] [PubMed] [Google Scholar]
- 47.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, De Gier RP, Roeleveld N. Hypospadias: A transgenerational effect of diethylstilbestrol? Hum. Reprod. 2006;21(3):666–669. doi: 10.1093/humrep/dei398. [DOI] [PubMed] [Google Scholar]
- 48.Anway MD, Rekow SS, Skinner MK. Comparative antiandrogenic actions of vinclozolin and flutamide on transgenerational adult-onset disease and spermatogenesis. Reprod. Toxicol. 2008;26(2):100–106. doi: 10.1016/j.reprotox.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anway MD, Memon MA, Uzumcu M, Skinner MK. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J. Androl. 2006;27(6):868–879. doi: 10.2164/jandrol.106.000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50■.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147(12):5515–5523. doi: 10.1210/en.2006-0640. Description of environmental toxicant effect on epigenetic transgenerational disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult-onset disease. Reproduction. 2008;135(5):713–721. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function. J. Physiol. 2008;586(8):2217–2229. doi: 10.1113/jphysiol.2007.147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mathieu O, Reinders J, Caikovski M, Smathajitt C, Paszkowski J. Transgenerational stability of the arabidopsis epigenome is coordinated by cg methylation. Cell. 2007;130(5):851–862. doi: 10.1016/j.cell.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Anway MD, Rekow SS, Skinner MK. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics. 2008;91(1):30–40. doi: 10.1016/j.ygeno.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3(11):e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Anway MD, Skinner MK. Transgenerational effects of the endocrine disruptor vinclozolin on the prostate transcriptome and adult-onset disease. Prostate. 2008;68(5):517–529. doi: 10.1002/pros.20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing Y, Shi S, Le L, Lee CA, Silver-Morse L, Li WX. Evidence for transgenerational transmission of epigenetic tumor susceptibility in drosophila. PLoS Genet. 2007;3(9):1598–1606. doi: 10.1371/journal.pgen.0030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Katz DJ, Edwards TM, Reinke V, Kelly WG. A C. elegans lsd1 demethylase contributes to germline immortality by reprogramming epigenetic memory. Cell. 2009;137(2):308–320. doi: 10.1016/j.cell.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59■■.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int. J. Obes. (Lond.) 2008;32(9):1373–1379. doi: 10.1038/ijo.2008.100. Description of nutritional effects on transgenerational obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol a. Life Sci. 2009;85(1–2):11–18. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 61.Kaati G, Bygren LO, Pembrey M, Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur. J. Hum. Genet. 2007;15(7):784–790. doi: 10.1038/sj.ejhg.5201832. [DOI] [PubMed] [Google Scholar]
- 62.Pinheiro AR, Salvucci ID, Aguila MB, Mandarim-De-Lacerda CA. Protein restriction during gestation and/or lactation causes adverse transgenerational effects on biometry and glucose metabolism in F1 and F2 progenies of rats. Clin. Sci. (Lond.) 2008;114(5):381–392. doi: 10.1042/CS20070302. [DOI] [PubMed] [Google Scholar]
- 63.Chao MJ, Ramagopalan SV, Herrera BM, et al. Epigenetics in multiple sclerosis susceptibility: difference in transgenerational risk localizes to the major histocompatibility complex. Hum. Mol. Genet. 2009;18(2):261–266. doi: 10.1093/hmg/ddn353. [DOI] [PubMed] [Google Scholar]