The midbrain dopamine system has been traditionally associated with reward-related phenomena; however, recent studies suggest a more generalized role for this transmitter system. In this issue of Biological Psychiatry, Krebs, Schott and Düzel use imaging studies in humans to provide evidence for a fascinating modulation of presumably dopaminergic midbrain neurons, depending on personality traits of the individuals performing the tasks. The authors suggest that the personality traits “reward dependence” and “novelty seeking” modulate neuronal activation elicited by reward-predicting versus novel non-reward-associated pictures in the substantia nigra/ventral tegmental area (1). This finding may help to redefine neurotransmitter correlates of partially heritable personality traits and thus help to explain how two apparently independent personality traits (i.e. “novelty seeking” and “reward dependence”) can both be associated with behaviourally relevant phasic activation of midbrain dopamine neurons. In 1987, Cloninger suggested that “novelty seeking”, “reward” dependence” and “harm avoidance” are independent and partially heritable personality factors, which are modulated by dopamine, norepinephrine, and serotonin, respectively (2). Further studies partially confirmed this hypothesis: “harm avoidance” was indeed associated with negative mood states such as depression and anxiety, which are modulated by serotonin function in human and non-human primates (3). However, animal experiments provided little proof for direct associations between norepinephrine neurotransmission and “reward dependence” as a personality trait. Instead, animal experiments suggested that activation of midbrain dopaminergic neurons can be elicited by both novel and reward-predicting stimuli (4,5). If dopaminergic neurotransmission impacts on central processing of both reward and novelty, is massive dysfunction of dopaminergic neurotransmission, e.g. as seen in alcoholism, associated with closely correlated alterations in both “novelty seeking” and “reward dependence”? The answer is no (6), and the question why this was not the case remained unsolved for more than a decade.
The study of Krebs et al. (1) now sheds new light on the neurobiological correlates of “exploratory excitability”, a core component of “novelty seeking”, and “reward dependence”. In accordance with animal studies (4,5), they confirm that both reward-predicting and novel, non-reward predicting stimuli can elicit neuronal activation in the substantia nigra and the closely associated ventral tegmental area. However, whether activation is elicited more strongly by either type of stimuli was itself predicted by the personality traits of “reward dependence” versus “novelty seeking” and the authors suggest that if a larger part of brainstem dopamine neurons is activated by novelty per se, fewer neurons will be stimulated by reward-associated information. Indeed, the higher the midbrain activation to novel stimuli, the lower was the effect of additional reward-related information on midbrain neuronal stimulation (1). This result suggests that at least partially overlapping neuronal networks in the midbrain respond to novel and reward-predicting stimuli and that the relative percentage of neurons responding to novelty versus reward anticipation may determine whether an individual is biased towards seeking novelty or reward.
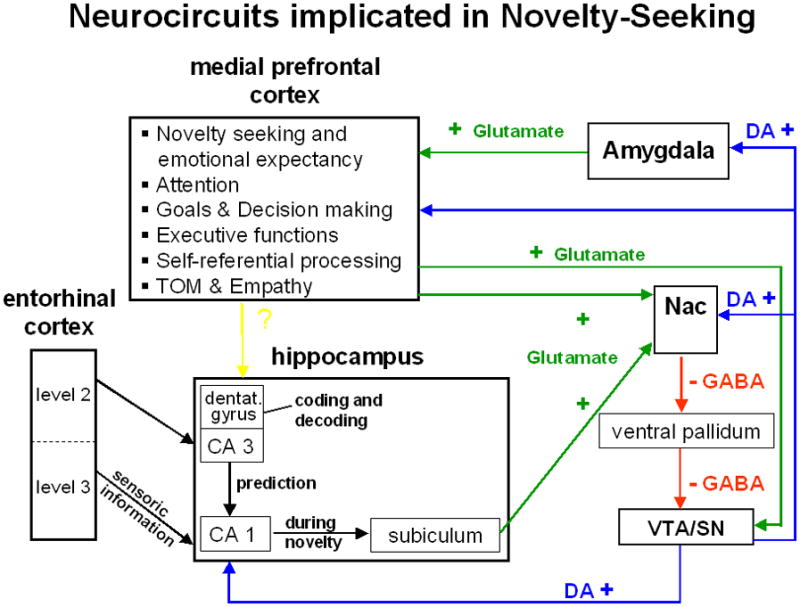
However, it is quite unlikely that computation of stimulus features that distinguish reward-associated from novel cues occurs in a limited number of dopamine neurons (and GABAergic interneurons) in the substantia nigra and ventral tegmental area. The authors point to current data that suggest hippocampal control of striatal activation, a major projection area for dopamine neurons. Specifically, hippocampal information on reward or novelty-related features of sensory input can modify up- and down-states of striatal neurons and thus set the stage for dopamine effects on fronto-striatal-thalamic neurocircuits that control goal-directed behaviour (7). Moreover, it has been shown that hippocampal drive of the striatum, leading to inhibition of the ventral pallidum and disinhibition of dopamine neuron activity, can control the proportion of spontaneously active dopamine neurons. This would enable the ventral hippocampus to control which population of dopamine neurons can elicit behaviourally salient burst firing in response to appropriate reward- or novelty-related stimuli (8) thereby regulating the amplitude of the dopamine signal received by the striatum. Previous experiences with sensory stimuli, particularly related to their reward association or novelty, can thus direct behavioural output.
Furthermore, the medial prefrontal cortex, which directly interacts with ventral striatal neurotransmission, was activated by conditioned cues that signalled emotional stimuli, and the degree of activation by such anticipatory cues predicted “novelty seeking” scores (9). The medial prefrontal cortex responds to conditioned emotional cues via drive from the basolateral amygdala, and this drive is necessary for behavioural expression (10). Recent evidence suggests that amygdala activation elicited by emotionally salient stimuli is itself modulated by dopamine storage capacity in ascending dopaminergic fibers (11). Furthermore, the medial prefrontal cortex can attenuate ventral hippocampal drive of the nucleus accumbens (12). Therefore, the medial prefrontal cortex is positioned to receive information on conditioned emotional cues and to use these cues to control ventral hippocampus-nucleus accumbens drive and thereby shape dopamine neuron firing. Individual differences in e.g. excitability of the medial prefrontal cortex may determine whether a subject responds more strongly to reward-related or novel stimuli. However, while such functional connections are highly plausible, most of them are currently derived from animal experiments and human imaging studies are required to elucidate individual differences in functional connectivity during processing of reward-related versus novel stimuli and their respective impact on trait-like personality features.
Figure 1.
Footnotes
Commentary on “Personality traits differentially modulate reward and novelty processing in the human substantia nigra/ventral tegmental area” by Ruth M. Krebs, Björn H. Schott and Emrah Düzel
Biol Psychiatry 2008
References
- 1.Krebs RM, Schott BH, Düzel E. Personality traits differentially modulate reward and novelty processing in the human substantia nigra/ventral tegmental area. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.08.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry. 1987;44(6):573–588. doi: 10.1001/archpsyc.1987.01800180093014. [DOI] [PubMed] [Google Scholar]
- 3.Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcohol Clin Exp Res. 2001;25(4):487–95. [PubMed] [Google Scholar]
- 4.Rebec GV, Christensen JR, Guerra C, Bardo MT. Regional and temporal differences in real-time dopamine efflux in the nucleus accumbens during free-choice novelty. Brain Res. 1997;776(1–2):61–7. doi: 10.1016/s0006-8993(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 5.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275(5306):1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 6.Heinz A, Dufeu P, Kuhn S, Dettling M, Gräf K, Kürten I, Rommelspacher H, Schmidt LG. Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Arch Gen Psychiatry. 1996;53(12):1123–1128. doi: 10.1001/archpsyc.1996.01830120061011. [DOI] [PubMed] [Google Scholar]
- 7.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46(5):703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30(5):220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Wrase J, Schlagenhauf F, Bauer M, Heinz A, Schlaug G, Northoff G. Novelty seeking modulates medial prefrontal activity during the anticipation of emotional stimuli. Psychiatry Res. 2008;164(1):81–85. doi: 10.1016/j.pscychresns.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 10.Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25(26):6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kienast T, Hariri AR, Schlagenhauf F, Wrase J, Sterzer P, Buchholz HG, Smolka MN, Gründer G, Cumming P, Kumakura Y, Bartenstein P, Dolan RJ, Heinz A. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nat Neurosci. 2008 doi: 10.1038/nn.2222. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47(2):255–66. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]


