Abstract
Diet and exercise are two environmental factors that can alter colon cancer risk. The purpose of this study was to determine if regular moderate intensity treadmill exercise training could attenuate polyp formation in ApcMin/+ mice fed a Western-style diet. Four-week-old male ApcMin/+ mice (n=12/group) were assigned to AIN-76A Control, AIN-76A Exercise, Western Control, or Western Exercise treatment groups. Mice were weaned to these diets and subjected to either regular moderate intensity treadmill exercise (18 m/min, 60 min/d, 6 d/wk) or remained sedentary for 6 weeks. Mice fed the Western-style diet consumed approximately 14% more calories and had 42% more epididymal fat compared to mice fed the AIN-76A diet. Exercise had no effect on fat pad mass with either diet treatment. Exercise reduced total intestinal polyp number 50% and the number of large polyps (≥1 mm diameter) 67% in AIN-76A-fed mice. The Western-style diet increased polyp number 75% when compared to AIN-76A fed mice, but exercise did not decrease polyp number or alter polyp size in mice fed the Western-style diet. Markers of systemic inflammation and immune system function were improved with exercise in mice fed the AIN-76A diet. Mice fed the Western-style diet demonstrated more inflammation and immunosuppression which were not completely ameliorated by exercise. These data suggest that the induction of adiposity, inflammation, and immunosuppression by the Western-style diet may compromise the beneficial effect of moderate intensity exercise on the intestinal polyp burden in ApcMin/+ mice.
Keywords: treadmill running, Western-style diet, colorectal cancer
INTRODUCTION
Colon cancer is the third most common type of cancer among American men and women (1). The lifetime probability of developing colorectal cancer is 1 in 18 for men and 1 in 19 for women (1). The main risk factors for developing colorectal cancer include age, family history, and environmental causes. Two environmental risk factors for colon cancer include a high-fat diet and physical inactivity, which can lead to obesity (1-4). A recent meta-analysis has summarized the epidemiological studies regarding colon cancer risk and obesity. When examining 26 studies including over 70,000 colorectal cancer cases that reported Body Mass Index (BMI), individuals with a BMI ≥ 30 kg/m2 had a 20% greater risk for developing colorectal cancer compared to those with a normal BMI (<25 kg/m2) (5). Since the lack of regular physical activity and poor dietary habits contribute to body weight gain, it remains important to determine how these factors contribute to the initiation and progression of colon cancer.
The preventative effects of physical activity and/or exercise on colorectal cancer risk have been reviewed and the data are convincing that physical activity is protective (3, 6-8). A meta-analysis examining the rates of colon cancer showed that both active men and women have an overall relative risk (RR) of 0.79 and 0.78, respectively, of developing colon cancer (6). The hypothesized mechanisms include improved immune function, decreased gastrointestinal transit time, decreased systemic and intestinal inflammation, and an improved metabolic profile, including decreases in circulating insulin-like growth factor I (IGF-I), cholesterol, and circulating triglycerides (3, 8). Currently, there have not been any human studies employed to use exercise or physical activity as an intervention to prevent colon cancer.
Animal models remain an important tool for examining the direct impacts of physical activity on colorectal cancer prevention (9). The ApcMin/+ mouse has a nonsense mutation at codon 850 in the adenomatous polyposis coli (Apc) gene, which results in a truncated protein, predisposing these mice to both small and large intestinal adenomas (10). Germline mutations in the APC gene in humans are responsible for the initiation and progression of familial adenomatous polyposis (FAP), a genetic form of colon cancer (11). In addition, 60% of all sporadic colon cancers have a mutated APC gene (11). The ApcMin/+ mouse has been extensively used to examine both dietary and physical activity-induced mechanisms related to colon cancer prevention (12, 13).
The effect of exercise on ApcMin/+ mouse intestinal polyp formation has been well investigated (14-18), and the majority of these studies find beneficial effects. Understanding the benefits of regular exercise is complex, because beneficial outcomes can be affected by the type of activity, duration, intensity, and the accumulated amount of weekly activity (19). Studies examining exercise in the ApcMin/+ mouse have varied by the type of physical activity, which have used treadmill running or voluntary wheel running. Treadmill running protocols allow for the application of a defined dose of exercise, and more closely mimics human exercise. The mice run at a constant intensity for a defined period of time. With voluntary wheel running, mice accumulate a considerably greater amount of activity by running intermittently throughout the night at uncontrolled durations and intensities. Nevertheless, both modalities improve oxidative capacity of skeletal muscle (17, 18), but may differentially affect health outcomes due to the different dose of exercise applied. Previous studies have shown a beneficial effect of voluntary wheel running on the number of total intestinal polyps (15, 16) or regional reductions in polyp number in ApcMin/+ mice following treadmill exercise training (14, 18). Our lab has published that 9 weeks of moderate intensity treadmill running can decrease both intestinal polyp number and size in male ApcMin/+ mice, while in this same study, voluntary wheel running was not effective (17). We noted that mice with access to voluntary activity wheels consumed more calories during the study to maintain body weight, and we hypothesized that a negative caloric balance may be a potential mechanism for the exercise-induced reduction in intestinal polyp burden.
Caloric balance is an important factor contributing to tumorigenesis. Negative caloric balance induced by restricting caloric intake can prevent the development of tumors in ApcMin/+ mice (15, 20, 21). Likewise, excess consumption of calories can promote tumorigenesis. Newmark et. al. (22) created the original Western-style stress diet, based on the AIN-76A diet. It contained a higher percentage of calories from dietary fat (20% instead of 5%), increased phosphate, decreased vitamin D, and decreased calcium to mimic the relative percentages consumed by Americans. Administration of this diet to mice and rats induced colonic adenomas and lesions, whereas tumors or other pre-cancerous lesions did not develop in rodents fed a standard defined diet (22-25). Apc1638 mice (mutation at codon 1638 instead of 850) and ApcMin/+ mice fed a Western-style diet developed more adenomas and carcinomas than mice fed the AIN-76A diet (26-29)Ju et al. (16) recently showed that increased physical activity through unrestricted voluntary wheel running in ApcMin/+ mice was effective at reducing the intestinal tumor burden when the mice were fed a Western-style diet. These data suggest that increased dietary fat intake and nutrient deprivation can promote tumorigenesis, but that increased physical activity may be able to prevent polyp development.
One of the mechanisms by which exercise may alter polyp development is through its well-documented effects on improving the function of the immune system and ablating chronic inflammation (30-36). Chronic inflammation arises from improper functioning of the innate and adaptive immune systems, and chronic inflammation enhances tumor development (37). This may be especially important in the ApcMin/+ mouse because they demonstrate chronic inflammation. Our laboratory has shown that circulating interleukin-6 (IL-6), a pro-inflammatory cytokine, is elevated in ApcMin/+ mice, but is reduced with exercise training (17). Haptoglobin is an anti-inflammatory acute phase protein secreted by the liver, its secretion increases during inflammation, and is elevated in the ApcMin/+ mouse. In fact, haptoglobin-null ApcMin/+ mice (38) and lymphocyte-depleted ApcMin/+ mice (39, 40) demonstrate more polyps, and IL-6-null ApcMin/+ mice demonstrate less polyps (41). Splenomegaly occurs in ApcMin/+ mice (42, 43). Spleen size is directly related to the polyp burden, and splenomegaly can be reduced with exercise (17). Together, these data demonstrate the importance of systemic inflammation on polyp formation.
The American Institute of Cancer Research recommends to consume a low-fat diet that is rich in fruits and vegetables, maintain a healthy weight, and to accumulate at least 30 minutes of physical activity daily (44). Furthermore, new health related exercise guidelines released in 2008 call for 30 minutes of moderate intensity exercise most days of the week (19). Since most Americans do not follow these dietary or activity guidelines, it is unknown whether a defined period of moderate exercise would have a preventative effect on intestinal polyp formation when one consumes a high-fat diet. The purpose of this study was to determine if regular moderate intensity treadmill exercise, which can reduce the ApcMin/+ intestinal tumor burden, can also be beneficial when consuming a high-fat diet. Our research design subjected young ApcMin/+ mice to a 6-week dietary and exercise training regimen, and we examined the polyp burden in the nascent stage of dietary-induced obesity. To remove any confounding effects of vitamins or minerals, our study used a Western-style diet that was high in saturated fat and overall caloric density, but retained the same vitamin and mineral content as the control diet. Our hypothesis was that moderate intensity treadmill running would be effective at attenuating the Western diet-induced increase in tumor burden in the ApcMin/+ mouse.
METHODS
Animals and dietary treatments
Male ApcMin/+ mice (Jackson Laboratories) were purchased and bred with female C57BL/6 mice in the University of South Carolina's animal resource facility (17). Offspring were genotyped as heterozygotes by RT-PCR for the Apc gene by taking tail snips at weaning. The room was maintained on a 12:12 light:dark cycle with the light period starting at 0700. Mice were randomly assigned and weaned to the following diets: (1) AIN-76A or (2) Western-style diet (Research Diets, Inc., New Brunswick, NJ). Table 1 lists the components of each diet. Group-housed mice were fed weekly and food was weighed at that time. All animal experimentation was approved by the University of South Carolina's Institutional Animal Care and Use Committee.
Table 1.
Dietary composition of AIN-76A and Western-style diets administered to male ApcMin/+ mice for 6 weeks.
| AIN-76A (g %) | Western (g %) | |
|---|---|---|
| Protein | 20.3 | 20.0 |
| Carbohydrate | 66.0 | 50.0 |
| Fat | 5.0 | 21.0 |
| Kcal/g | 3.9 | 4.7 |
| (g/kg diet) | (g/kg diet) | |
|---|---|---|
| Caesin | 200 | 195 |
| DL-Methionine | 3 | - |
| L-Cysteine | - | 3 |
| Corn starch | 150 | 50 |
| Sucrose | 500 | 341 |
| Maltodextrin | - | 100 |
| Cellulose | 50 | 50 |
| Milk fat | - | 200 |
| Corn oil | 50 | 10 |
| Mineral mix | 35 | 35 |
| Vitamin mix | 10 | 10 |
| Choline bitartrate | 2 | 2 |
| Cholesterol | - | 1.5 |
| Ethoxyquin | - | 0.04 |
| Total | 1000 | 1001.54 |
Treadmill protocol
The treadmill training program was performed as previously described (17, 45), except the length of the training program was for 6 weeks instead of 8 weeks. Briefly, 4-wk-old male mice were randomly divided into Control and Exercise groups making 4 treatment groups total: (1) AIN-76A Control (n=12), (2) AIN-76A Exercise (n=12), (3) Western Control (n=12), and (4) Western Exercise (n=12). The running protocol consisted of a brief 5-min warm-up (10 and 14 m/min), followed by 55 min at 18 m/min and 5% grade. Mice were run at the beginning of their dark cycle (1900 hours) under red lights 6 d/wk for a total of 6 weeks. Controls were kept next to the treadmill, but remained in their cages during training. All mice were sacrificed at 10 weeks of age, at least 36 hours after the last bout of exercise.
Tissue and blood collection
Under fasting conditions, mice were anesthetized with a subcutaneous injection of ketamine/xylazine/acepromazine cocktail (1.2 ml/kg BW). Blood was collected from the retroorbital sinus (~75 μl) and 10 μl of whole blood was immediately analyzed on a veterinary hematology analyzer (VetScan, Abaxis, Union City, CA). The gastrocnemius muscle, epididymal fat pad, and spleen were removed and immediately frozen in liquid nitrogen. The tibia was also removed and the length was measured with vernier calipers to determine animal size. The small intestines were carefully dissected distally to the stomach and proximal to the cecum. The large intestine was removed from the distal end of the cecum to the anus. Mesentery tissue was removed with tweezers and the small intestine was cut into four sections (duodenum, jejunum, proximal ileum, and distal ileum). All intestinal sections were flushed with PBS, opened longitudinally with scissors, and flattened with a cotton swab between 2 pieces of blotting paper. Intestinal sections were fixed in 10% buffered formalin for 24 hours. After fixation, intestinal sections were transferred to 70% ethanol and stored at room temperature. Terminal blood collection was drawn from the inferior vena cava with heparin and stored on ice. Blood samples were centrifuged and plasma was stored at -80°C until further analysis.
Polyp counts
Polyp counts were performed as previously described (17). Formalin-fixed intestinal sections from all animals were briefly stained in 0.1% methylene blue, and they were counted by the same investigator who was blinded to the treatments. Polyps were counted under a dissecting microscope, using tweezers to pick through the intestinal villi and identify polyps. Polyps were categorized as large (≥1 mm in diameter) or small (<1 mm) in the small intestine and large intestine. After counting, intestinal sections were placed in 70% ethanol for further analysis.
Immunohistochemistry
Immunohistochemical detection of β-catenin staining of polyps was performed as previously described (45). Four slides were prepared from each animal, representing 4 different areas of the small intestine: (1) duodenum, (2) jejunum, (3) proximal ileum, and (4) distal ileum. Formalin-fixed, paraffin-embedded intestinal sections were Swiss-rolled and were cut on a microtome in 4 μm sections. Sections were deparaffinized in xylene and rinsed in 100% ethanol. Peroxidase activity was squelched with 6% H2O2 in methanol for 30 minutes. Antigen retrieval was accomplished with 0.1% bovine trypsin for 30 min at 37 °C. Sections were blocked for 30 minutes in rabbit serum. Slides were incubated 1:100 with β-catenin (BD Biosciences, San Jose, CA) for 1 h at 37 °C. Slides were rinsed in PBS and incubated 1:200 with anti-rabbit peroxidase-conjugated antibody for 1 h 37 °C. Color detection was visualized with an ABC detection kit and DAB (Vector Laboratories, Burlingame, CA). Polyps were identified by foci containing intense nuclear β-catenin staining. The number of polyps was counted and polyp diameter was measured at an X400 magnification by a technician blinded to the treatments.
Circulating assays
Plasma leptin was measured via ELISA (Diagnostic Systems Laboratories, Webster, TX) according to manufacturer's directions. A sample size of 5-8 mice per treatment group was analyzed.
Plasma haptoglobin was measured via western blotting. Plasma (1-3 μl) was fractionated on 12% polyacrylamide gels. Gels were transferred to PVDF membranes overnight and blocked in 5% TBS-T milk. Equal protein loading of the gels was qualitatively assessed using Ponceau staining and examining the albumin band. Primary antibody for haptoglobin (MP Biomedicals, Aurora, OH) was incubated at a 1:1000 dilution overnight at 4 °C in 5% TBST milk. Secondary anti-goat IgG conjugated secondary antibody (GE Healthcare, Piscataway, NJ) was incubated with the membranes at a 1:10,000 dilution for 1 hour in 5% TBST milk. Enhanced chemiluminescence (GE Healthcare, Piscataway, NJ) was used to visualize the antibody-antigen interactions and developed by autoradiography (Kodak, Biomax, MR). Digitally scanned blots were analyzed by measuring the integrated optical density (IOD) of each band using digital imaging software (Scion Image, Frederick, MD). A sample size of 11-12 was used per treatment group.
Statistical analyses
Data are reported as means ± SE. All data were analyzed with two-way ANOVAs. If a main effect existed, the means and SE for the main effect are presented in the text. If the assumption of normality failed, a Kruskal-Wallis ANOVA on ranks was performed. Post-hoc analyses were performed with Student-Newman-Keuls methods. Pearson correlations were performed to determine associations. Significance was set at P<0.05.
RESULTS
Polyp burden
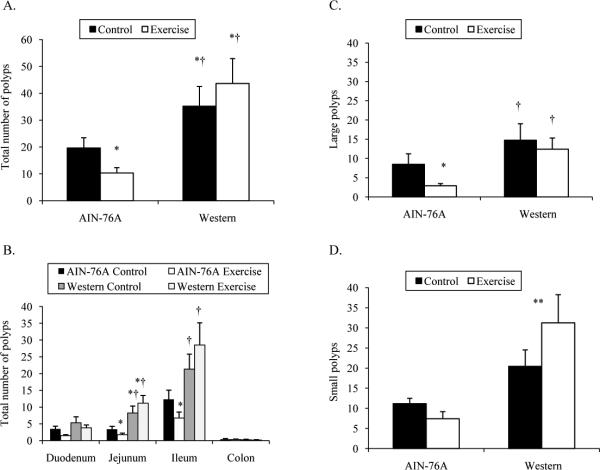
It has been previously shown that a Western-style diet increases the polyp burden in ApcMin/+ mice and induces polyps in wild-type mice (22-28). Since the diet we used did not contain altered amounts of calcium, phosphorous, or Vitamin D, our first main objective was to determine if our Western-style could increase the polyp burden in ApcMin/+ mice. In the current study, control mice fed the Western-style diet had overall polyp counts that were 75% greater compared to control mice fed the AIN-76A diet (Figure 1A). These data suggest that increased fat consumption alone, particularly saturated fat, can increase the intestinal polyp burden in ApcMin/+ mice.
Figure 1.
Total intestinal polyp number, location, and size in 10-week male ApcMin/+ mice fed an AIN-76A or Western-style diet following 6 weeks of treadmill training. A. Total number of polyps. B. Location of polyps. C. Large polyps. Large polyps were defined as ≥1 mm in diameter. B. Small polyps. Small polyps were defined as <1 mm in diameter.
*Denotes significant difference from AIN-76A Control.
†Denotes significant difference from AIN-76A Exercise.
**Denotes main effect of diet.
Our lab has previously shown that moderate-intensity treadmill running decreases the overall intestinal polyp burden in ApcMin/+ mice fed rodent chow. Our second objective was to replicate our previous exercise-induced reduction in polyp burden in ApcMin/+ mice fed a defined diet (AIN-76A) instead of rodent chow. We also wanted to extend this finding using the Western-style diet. In the current study, 6 weeks of moderate intensity treadmill running resulted in 50% fewer polyps in mice fed the AIN-76A diet (Figure 1A), which is similar to our previous results using rodent chow (17). However, when mice were fed the Western-style diet and were exercised, this had no effect on overall polyp number (Figure 1A).
In addition to the total number of polyps, polyp location was also analyzed (Figure 1B). Among control mice, the Western-style diet preferentially increased the number of polyps only in the jejunum by 2.7-fold compared to the AIN-76A diet. ApcMin/+ mice fed the AIN-76A diet and that exercised had 33% fewer polyps in the jejunum and 42% fewer polyps in the ileum compared to sedentary control mice. Control and exercised ApcMin/+ mice fed the Western-style diet had similar polyp counts in each area of the intestinal tract.
Polyp size was also examined in each treatment group (Figures 1C and 1D). Intestinal polyps were counted and classified as being large (≥1 mm in diameter) or small (<1 mm in diameter). The Western-style diet did not induce an increase in large polyps among control mice (Figure 1C). The greater number of overall polyps in mice fed the Western-style diet was mostly due to more small polyps (Figure 1D). There was a main effect of diet (P<0.001) on the number of small polyps. When collapsing the Control and Exercise treatment groups, mice fed Western-style diet (26 ± 4 small polyps) had nearly 3 times as many small polyps than mice fed the AIN-76A diet (9 ± 1 small polyps). The number of large polyps was reduced 67% with treadmill running in mice fed the AIN-76A diet, but the number of small polyps was unaffected. Similar to overall polyp number, treadmill running had no effect on the number of large polyps or small polyps in mice fed the Western-style diet.
In addition to counting polyps with methylene blue staining, we also identified polyps using immunohistochemical staining for β-catenin (Figure 2A). One caveat to this method is that it does not detect all polyps since only 4 μm slices at 4 different locations of the small intestinal tract are examined. Nevertheless, we found very similar results when counting the total number of polyps compared to immunohistochemical detection of polyps. We detected twice as many β-catenin-positive polyps in control mice fed the Western-style diet compared to AIN-76A-fed mice (Figure 2B). Exercised mice fed the AIN-76A diet had 67% less β-catenin-positive polyps compared to sedentary control mice. The number of β-catenin-positive polyps remained the same between control and exercised mice fed the Western-style diet. Taken together, these data show that that exercise is not effective in lowering the overall polyp burden in ApcMin/+ mice when the mice are fed a Western-style diet.
Figure 2.
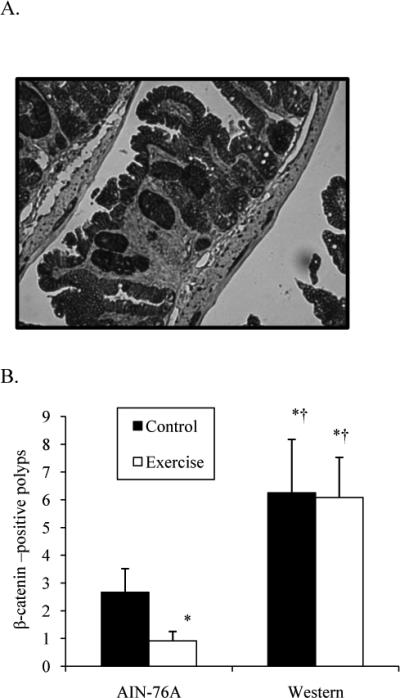
β-catenin-positive polyps in 10-week male ApcMin/+ mice fed an AIN-76A or Western-style diet following 6 weeks of treadmill training. A. Example of β-catenin-positive polyp. B. Number of β-catenin-positive polyps.
*Denotes significant difference from AIN-76A Control.
†Denotes significant difference from AIN-76A Exercise.
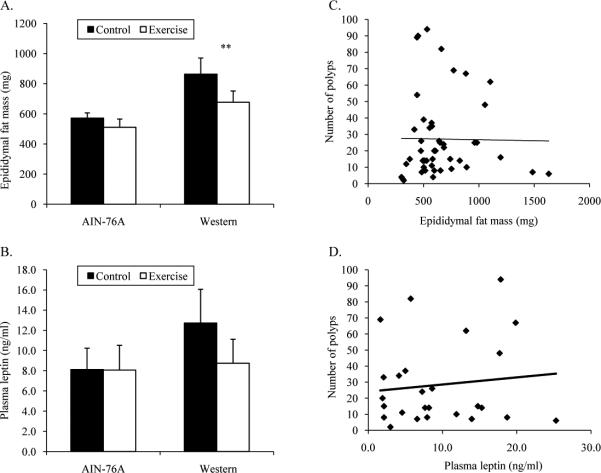
Adiposity measurements
Since increased adiposity is a risk factor for colorectal cancer, we next examined food intake, mouse size, and the quantity of adipose tissue. Food intake was measured weekly for each cage of mice and averaged together for each of the 6 weeks. There were no differences in food intake (P=1.000) between AIN-76A Control (2.6 ± 0.1 g/d), AIN-76A Exercise (2.6 ± 0.0 g/d), Western Control (2.4 ± 0.1 g/d), or Western Exercise (2.4 ± 0.1 g/d) treatment groups. This also means that vitamin and mineral consumption was the same between the treatment groups. However, since the Western-style diet contained approximately 20% more calories than the AIN-76A diet (See Table 1), there was a main effect of diet on daily caloric intake (P=0.003). Mice fed the Western-style diet (11.5 ± 0.3 kcal/d) consumed approximately 14% more calories per day than mice fed the AIN-76A diet (10.1 ± 0.1 kcal/d).
ApcMin/+ mouse body weights, tibia lengths, and gastrocnemius muscle weights are listed in Table 2. There was a trend for a main effect of diet on body mass (P=0.057). When combining Control and Exercise treatment groups, mice being fed the Western-style diet (25.8 ± 0.5 g) tended to be 5% heavier than mice fed the AIN-76A diet (24.6 ± 0.3 g). There were no differences in tibia length (P=0.951) or gastrocnemius muscle mass (P=0.648) between the treatment groups. There was a main effect of diet on epididymal fat pad mass (Figure 3A). When combining both Control and Exercise groups, mice fed the Western-style diet had 42% more fat (770 ± 67 mg) compared to mice fed the AIN-76A diet (541 ± 32 mg; P=0.003). However, exercise training did not lower epididymal fat pad mass in either dietary treatment group. Since we only examined one fat depot, we used circulating leptin as a marker of global adiposity. There was significant correlation between circulating leptin and epididymal fat pad mass (r=0.658; P<0.001). Plasma leptin was not different between the treatment groups (Figure 3B; P=0.473). There were also no correlations between polyp number and epididymal fat pad mass (Figure 3C; r=-0.030; P=0.841) or polyp number and circulating leptin (Figure 3D; r=0.114; P=0.580). These data suggest that the Western-style diet does increase epididymal fat mass, but this is not the lone factor responsible for increased polyp number in ApcMin/+ mice fed the Western-style diet. Furthermore, the mechanism of the reduced polyp burden with exercise training in mice fed the AIN-76A diet appears to be independent of reductions in adiposity.
Table 2.
Body weight, tibia length, and gastrocnemius muscle mass in 10-week male ApcMin/+ mice fed an AIN-76A or Western-style diet following 6 weeks of treadmill training.
| AIN-76A | Western | |||
|---|---|---|---|---|
| Control (n=12) | Exercise (n=12) | Control (n=12) | Exercise (n=12) | |
| Body mass (g) | 24.6 ± 0.4 | 24.5 ± 0.6 | 26.5 ± 0.8 | 25.2 ± 0.7 |
| Tibia length (mm) | 15.8 ± 0.1 | 15.9 ± 0.1 | 15.9 ± 0.1 | 15.8 ± 0.1 |
| Gastrocnemius mass (mg) | 117 ± 2 | 117 ± 3 | 120 ± 4 | 116 ± 5 |
Figure 3.
Relationship between epididymal fat pad mass, circulating leptin, and polyp number in 10-week male ApcMin/+ mice fed an AIN-76A or Western-style diet following 6 weeks of treadmill training. A. Epididymal fat pad mass. B. Circulating leptin. C. Correlation between polyp number and epididymal fat pad mass (r=-0.030; P=0.841). D. Correlation between polyp number and circulating leptin (r=0.114; P=0.580).
**Denotes main effect of diet.
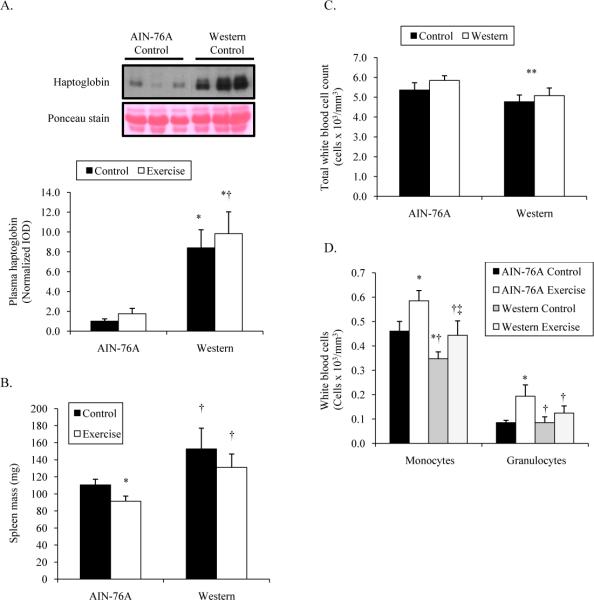
Inflammatory and immune responses
Adipose tissue contains inflammatory cells and can secrete cytokines that influence the systemic inflammatory state of the animal. We determined several inflammatory markers to see if the Western-style diet or exercise could influence them. Blood samples were taken at the end of the exercise training period to examine plasma haptoglobin levels, a known acute-phase protein that influence the polyp burden in ApcMin/+ mice (38), and white blood cell count. The Western-style diet induced plasma haptoglobin levels 8.4 times higher over AIN-76A control mice (Figure 4A). Treadmill running had no effect on circulating haptoglobin levels in mice on either diet. Increased spleen size, or splenomegaly, is another indicator of systemic inflammation. We have previously shown that treadmill running decreases spleen size in ApcMin/+ mice (17). In the current study, spleen mass was 18% lower in exercised mice fed the AIN-76A diet compared to control mice (Figure 4B). However, mice fed the Western-style diet did not see the same improvement with spleen mass. These data suggest that the Western-style diet induces more systemic inflammation, but that exercise training does not alleviate the inflammation when mice are fed this high-fat diet.
Figure 4.
Inflammatory and immune state in 10-week male ApcMin/+ mice fed an AIN-76A or Western-style diet following 6 weeks of treadmill training. A. Plasma haptoglobin. B. Spleen mass. C. Total white blood cell count. D. Monocytes and Granulocytes.
*Denotes significant difference from AIN-76A Control.
†Denotes significant difference from AIN-76A Exercise.
‡Denotes significant difference from Western Control.
**Denotes main effect of diet.
A properly functioning immune system is important for preventing cancer and this functioning of this system is improved with moderate-intensity exercise training (35, 36). There was a main effect of diet on total white blood cell count (Figure 4C; P<0.050). Mice fed the Western-style diet (4.9 ± 0.3 cells × 103/mm3) had a 13% reduction in total white blood cell count compared to AIN-76A-fed mice (5.6 ± 0.2 cells × 103/mm3). The different sub-populations of immune cells were also determined. There were no differences in total lymphocyte number (P=0.908). However, the number of monocytes was 28% higher and granulocytes were 111% greater in exercised mice fed the AIN-76A diet compared to control mice. Control mice fed the Western-style diet had 24% fewer monocytes, but similar granulocytes compared to control mice fed the AIN-76A diet. Similar to the AIN-76A diet, exercise training induced a 26% increase in monocyte number in mice fed the Western-style diet. However, treadmill running did not improve granulocyte number in mice fed the Western-style diet. In both sub-populations of immune cells, exercised mice fed the Western-style diet had fewer immune cells than exercised mice on the AIN-76A diet.
DISCUSSION
The overall goal of this paper was to study the interaction between a high-fat diet and moderate intensity treadmill exercise for the prevention of intestinal adenoma development in the ApcMin/+ mouse. Our main finding was that ApcMin/+ mice undergoing moderate intensity exercise training and fed a Western-style diet were not able to decrease the number of intestinal adenomas. However, when mice were fed the AIN-76A diet, exercise was effective at reducing polyp number and size. Adiposity, chronic inflammation, and immunosuppression were examined to help determine why moderate exercise training failed to reduce the polyp burden in mice fed the Western-style diet. Mice fed the Western-style diet increased epididymal fat pad mass, while moderate exercise training was not sufficient decrease this fat depot. The Western-style diet also induced markers of systemic chronic inflammation, and moderate exercise did not alleviate this inflammatory state. There were also indications of immunosuppression that were induced by the Western-style diet that were not improved by moderate intensity exercise. Together, these data demonstrate that 6 weeks of moderate intensity exercise training cannot suppress the greater tumor burden, adiposity, systemic inflammation, and immunosuppression induced by a Western-style diet.
It has been well-documented that a Western-style diet increases cancerous lesions in both wild-type and ApcMin/+ mice (22-28). We confirmed this by showing that in as little as 6 weeks, a diet high in saturated fat can nearly double the intestinal polyp burden in ApcMin/+ mice. Unfortunately, moderate intensity exercise training was unable to prevent polyp formation when mice were fed the Western-style diet. One reason may be the mechanism of how exercise reduces the polyp burden in mice fed a normal diet. First, we confirmed the results of our 2005 study (17) by showing that male ApcMin/+ mice fed a normal diet and that engage in 6 weeks of moderate intensity treadmill running have a significant reduction in their polyp burden. This decrease in polyp number was mostly due to a reduction in large polyps. These data may suggest that exercise training not only prevents adenoma formation, but also slows adenoma growth. However, the number of large polyps was the same between AIN-76A and Western Control mice. The Western-style diet substantially increased the number of small polyps. Exercise training may not have been effective in decreasing the polyp burden in mice fed the Western-style diet because the Western-style diet did not accelerate polyp growth.
Ju et al. (16) also recently examined the role of exercise when ApcMin/+ mice were fed the Western-style diet. However, their results differed from ours because they found that voluntary wheel running was effective at lowering the intestinal polyp burden in ApcMin/+ mice when mice were fed either a normal or a high-fat diet. There are some differences between the studies to note, such as the mode of exercise training (treadmill vs. voluntary wheel running), gender (male vs. female mice), and length of training (6 wks vs. 9 wks). With treadmill running, our mice ran at 18 m/min for nearly an hour, accumulating 1.1 km in one training session, and having 6 sessions per week. Ju et al. (16) reports that mice fed the Western-style diet voluntarily ran 3.5 km/d for 7 d/wk. When comparing accumulated exercise activity (km/week), mice running on activity wheels ran 3 times more per week and 5 times more over the duration of the study compared to our mice that ran on the treadmill. It is not possible to compare the intensity of these exercise modalities due to the inherent variability with wheel running exercise. These data may suggest that a longer duration of exercise is necessary prevent polyp formation when consuming a high-fat diet.
One advantage to our study was that it was designed to test the effect of both diet and exercise on the number of intestinal polyps. We used the appropriate control diet (AIN-76A) and the length of training was the same between mice fed the AIN-76A diet and the Western-style diet. The Control diet used by Ju et al. (16) contained a different composition of vitamins and minerals compared to the Western-style diet. Also, mice were exercised for different lengths of time on each diet. Therefore, the interaction between a high-fat diet and exercise could not be directly compared. Despite the caveat in research design, sedentary mice fed the each diet had similar tumor counts. This could explain the discrepancy between the two studies, suggesting that exercise is only effective when the intestinal polyp burden is not being accelerated by another factor, such as a dietary component or a carcinogen.
Adipose tissue is a risk factor for cancer (4) and adiposity increases when mice are fed the Western-style diet (16, 46, 47). We determined if the Western-style diet increased adipose tissue mass, if exercise training could decrease adipose tissue mass, and if adipose tissue mass was related to polyp burden. First, the Western-style diet increased epididymal fat pad mass in ApcMin/+ mice, similar to other studies. However, exercise did not decrease epididymal fat pad mass in mice on either diet. This was confirmed by circulating leptin, a marker of global adiposity. We also could not detect correlations between polyp number and epididymal fat pad mass or polyp number and circulating leptin. These data suggest that the overall polyp burden is not dependent on total fat content. In addition, since mice fed the AIN-76A diet did not experience fat loss, but decreased polyp number with exercise training, the mechanism of reduced polyp number via exercise is not mediated through a reduction in fat loss. These data are in agreement with previous work that failed to find an association between fat pad mass and polyp number (16). More work is needed to identify the factors induced by the Western-style diet that promote tumorigenesis.
One possible system that may be modified by a high-fat diet is the immune system. A few investigators have examined the immune response in ApcMin/+ mice. Splenomegaly has been documented in ApcMin/+ mice compared to wild-type mice (42, 43). It has also been reported that there are fewer Peyer's patches in ApcMin/+ mice in small intestine, and more megakaryocytes in the spleen compared to wild-type mice (43). However, circulating white blood cells and the intestinal immune response are similar between ApcMin/+ and wild-type mice (43, 48). Alternatively, this may be a problem for the ApcMin/+ mouse, because the immune system should respond to the intestinal polyp burden. Exercise may help with this response in the ApcMin/+ mouse because immune system function improves with moderate intensity exercise. This is reflected in both an increase in the number of immune cells and an improvement in their function. For example, moderate-intensity treadmill running in wild-type mice improves macrophage anti-tumor cytotoxicity (30-34) and neutrophil respiratory burst activity (49). In the current study, the number monocytes and granulocytes were improved with exercise training in mice fed the AIN-76A diet. Splenomegaly, a marker of systemic inflammation, was also reduced with exercise training. These data suggest that exercise training in the ApcMin/+ mouse improves the immune system and decreases systemic inflammation. These additional immune cells may help prevent malignant cells from developing into polyps or possibly attack formed polyps by limiting their growth.
When mice were fed the Western-style diet, they had lower numbers of both monocytes and granulocytes compared to mice fed the AIN-76A diet. Haptoglobin, another inflammatory marker, was also induced in mice fed the Western-style diet. Exercise training also improved the number of monocytes, but not granulocytes, in mice fed the Western-style diet. If the innate immune system response is important for the exercise-induced reduction in polyp burden, these data could mean two things. First, granulocytes may be more important than monocytes for preventing adenoma formation and growth. Second, a component of the Western-style diet, such as saturated fat, may impair monocyte and granulocyte function. For example, macrophages and neutrophils can be recruited to adipose tissue by saturated fat (50), possibly limiting their ability to prevent tumor formation at the site of the intestine. More work is needed to determine the effect of the Western-style diet on monocyte/macrophage and granulocyte function. Future studies are also needed to determine if the innate immune response is responsible for the exercise-induced reduction in polyp burden in ApcMin/+ mice.
It is important to note some limitations to our study. First, we used relatively young mice in our study. Colorectal cancer typically affects much older adults, and older ApcMin/+ mice may exhibit a different response to both exercise and a high-fat diet. Second, the length of the treadmill running protocol was 6 weeks, rather than 8 weeks long in our previous studies (17, 45). This corresponded to the age of mice being 10 weeks of age at the end of the study, instead of 12 weeks old. The rate of polyp formation is typically much slower in young ApcMin/+ mice, and accelerates after 10 weeks of age (51). The purpose of this change was to build on our previous findings, and determine if exercise can prevent polyp formation prior to the rapid phase of polyp formation. It is possible that our results may have been different if the length of the treadmill training protocol was longer, during this period of rapid polyp formation. Third, the composition of our Western-style diet was slightly different compared to others using the Western-style diet (22-25, 27, 28). The diet that we used was only modified in total fat content. The source of carbohydrate was decreased, and the source of fat, corn oil, was replaced with mostly milk fat. Other diets manipulate calcium, phosphorous, and Vitamin D content in addition to fat (22-25, 27, 28). Our data suggest that manipulating the quantity and composition of dietary fat alone can affect adenoma development, as well as the effect of exercise on adenoma development. Finally, we should note that while epididymal fat pad was increased in mice fed the Western-style diet, overall body mass was not greater. This would suggest that our mice were just starting to become obese. The lifespan of the mouse, anemia, willingness to exercise, and cachexia were all limiting factors that prevented us from significantly lengthening the experiment to study an obese ApcMin/+ mouse.
A healthy diet and regular exercise or physical activity are recommended by the American Institute for Cancer Research to prevent cancer (44). Our data support this concept in that both a healthy diet and exercise are necessary to prevent colorectal cancer. Exercising when still consuming a diet high in fat is not sufficient to prevent adenoma formation in the ApcMin/+ mouse. The exercise stimulus needed to prevent polyp formation may be greater when consuming a high-fat diet. The exercise-induced effect on polyp burden is not mediated through a reduction in adiposity because exercise did not lower epididymal fat pad mass and there was not an association between polyp number and fat pad mass. The interaction of a high-fat diet and exercise could be mediated through positive effects of exercise and the negative effects of the Western-style diet on the immune/inflammatory response.
ACKNOWLEDGEMENTS
The research described in this report was supported by the National Institutes of Health (NIH) Grant P20 RR-017698 from the National Center for Research Resources and a seed grant from the South Carolina Nutritional Research Consortium awarded to Dr. James A. Carson. The authors would like to thank Manish Dave, Tia Davis, Valerie Kennedy, Joseph McClung, and Tyrone Washington for technical assistance.
REFERENCES
- 1.Cancer Facts and Figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91:916–32. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 3.Quadrilatero J, Hoffman-Goetz L. Physical activity and colon cancer. A systematic review of potential mechanisms. J Sports Med Phys Fitness. 2003;43:121–38. [PubMed] [Google Scholar]
- 4.Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr. 2007;86:s836–42. doi: 10.1093/ajcn/86.3.836S. [DOI] [PubMed] [Google Scholar]
- 5.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 6.Samad AK, Taylor RS, Marshall T, Chapman MA. A meta-analysis of the association of physical activity with reduced risk of colorectal cancer. Colorectal Dis. 2005;7:204–13. doi: 10.1111/j.1463-1318.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- 7.Johnson IT, Lund EK. Review article: nutrition, obesity and colorectal cancer. Aliment Pharmacol Ther. 2007;26:161–81. doi: 10.1111/j.1365-2036.2007.03371.x. [DOI] [PubMed] [Google Scholar]
- 8.Slattery ML. Physical activity and colorectal cancer. Sports Med. 2004;34:239–52. doi: 10.2165/00007256-200434040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Hoffman-Goetz L. Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc. 2003;35:1828–33. doi: 10.1249/01.MSS.0000093621.09328.70. [DOI] [PubMed] [Google Scholar]
- 10.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 11.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 12.Corpet DE, Pierre F. Point: From animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- 13.Basterfield L, Reul JM, Mathers JC. Impact of physical activity on intestinal cancer development in mice. J Nutr. 2005;135:3002S–3008S. doi: 10.1093/jn/135.12.3002S. [DOI] [PubMed] [Google Scholar]
- 14.Colbert LH, Mai V, Perkins SN, Berrigan D, Lavigne JA, Wimbrow HH, et al. Exercise and intestinal polyp development in APCMin mice. Med Sci Sports Exerc. 2003;35:1662–9. doi: 10.1249/01.MSS.0000089349.54813.41. [DOI] [PubMed] [Google Scholar]
- 15.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27:2103–7. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 16.Ju J, Nolan B, Cheh M, Bose M, Lin Y, Wagner GC, et al. Voluntary Exercise Inhibits Intestinal Tumorigenesis in ApcMin/+ Mice and Azoxymethane/Dextran Sulfate Sodium-Treated mice. BMC Cancer. 2008;8:316. doi: 10.1186/1471-2407-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–25. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 18.Colbert LH, Davis JM, Essig DA, Ghaffar A, Mayer EP. Exercise and tumor development in a mouse predisposed to multiple intestinal adenomas. Med Sci Sports Exerc. 2000;32:1704–8. doi: 10.1097/00005768-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 19.2008 Physical Activity Guidelines for Americans. U.S. Department of Health and Human Services; Washington, D.C.: 2008. [Google Scholar]
- 20.Kakuni M, Morimura K, Wanibuchi H, Ogawa M, Min W, Hayashi S, et al. Food restriction inhibits the growth of intestinal polyps in multiple intestinal neoplasia mouse. Jpn J Cancer Res. 2002;93:236–41. doi: 10.1111/j.1349-7006.2002.tb02163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mai V, Colbert LH, Berrigan D, Perkins SN, Pfeiffer R, Lavigne JA, et al. Calorie restriction and diet composition modulate spontaneous intestinal tumorigenesis in Apc(Min) mice through different mechanisms. Cancer Res. 2003;63:1752–5. [PubMed] [Google Scholar]
- 22.Newmark HL, Lipkin M, Maheshwari N. Colonic hyperplasia and hyperproliferation induced by a nutritional stress diet with four components of Western-style diet. J Natl Cancer Inst. 1990;82:491–6. doi: 10.1093/jnci/82.6.491. [DOI] [PubMed] [Google Scholar]
- 23.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, et al. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–5. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- 24.Newmark H, Yang K, Kurihara N, Fan K, Augenlicht L, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: A preclinical model for human sporadic colon cancer. Carcinogenesis. 2008 doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, et al. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68:7803–10. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- 26.Fan K, Kurihara N, Abe S, Ho CT, Ghai G, Yang K. Chemopreventive effects of orange peel extract (OPE). I: OPE inhibits intestinal tumor growth in ApcMin/+ mice. J Med Food. 2007;10:11–7. doi: 10.1089/jmf.2006.0214. [DOI] [PubMed] [Google Scholar]
- 27.Yang K, Edelmann W, Fan K, Lau K, Leung D, Newmark H, et al. Dietary modulation of carcinoma development in a mouse model for human familial adenomatous polyposis. Cancer Res. 1998;58:5713–7. [PubMed] [Google Scholar]
- 28.Yang K, Lamprecht SA, Shinozaki H, Fan K, Yang W, Newmark HL, et al. Dietary calcium and cholecalciferol modulate cyclin D1 expression, apoptosis, and tumorigenesis in intestine of adenomatous polyposis coli1638N/+ mice. J Nutr. 2008;138:1658–63. doi: 10.1093/jn/138.9.1658. [DOI] [PubMed] [Google Scholar]
- 29.Dowling S, Cox J, Cenedella RJ. Inhibition of Fatty Acid Synthase by Orlistat Accelerates Gastric Tumor Cell Apoptosis in Culture and Increases Survival Rates in Gastric Tumor Bearing Mice In Vivo. Lipids. 2009 doi: 10.1007/s11745-009-3298-2. [DOI] [PubMed] [Google Scholar]
- 30.Woods JA, Davis JM, Mayer EP, Ghaffar A, Pate RR. Exercise increases inflammatory macrophage antitumor cytotoxicity. J Appl Physiol. 1993;75:879–86. doi: 10.1152/jappl.1993.75.2.879. [DOI] [PubMed] [Google Scholar]
- 31.Woods JA, Davis JM, Mayer EP, Ghaffar A, Pate RR. Effects of exercise on macrophage activation for antitumor cytotoxicity. J Appl Physiol. 1994;76:2177–85. doi: 10.1152/jappl.1994.76.5.2177. [DOI] [PubMed] [Google Scholar]
- 32.Davis JM, Kohut ML, Jackson DA, Colbert LH, Mayer EP, Ghaffar A. Exercise effects on lung tumor metastases and in vitro alveolar macrophage antitumor cytotoxicity. Am J Physiol. 1998;274:R1454–9. doi: 10.1152/ajpregu.1998.274.5.R1454. [DOI] [PubMed] [Google Scholar]
- 33.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. Am J Physiol. 1999;276:R482–9. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- 34.Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol. 2004;97:955–9. doi: 10.1152/japplphysiol.00252.2004. [DOI] [PubMed] [Google Scholar]
- 35.Woods JA, Davis JM. Exercise, monocyte/macrophage function, and cancer. Med Sci Sports Exerc. 1994;26:147–56. doi: 10.1249/00005768-199402000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31:57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 37.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 38.Barbour KW, Davis T, White A, Baumann H, Berger FG. Haptoglobin, inflammation, and tumorigenesis in the MIN mouse. Redox Rep. 2001;6:366–8. doi: 10.1179/135100001101536553. [DOI] [PubMed] [Google Scholar]
- 39.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, et al. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 40.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A. 2007;104:19977–82. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol Regul Integr Comp Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 42.Wood PA, Yang X, Taber A, Oh EY, Ansell C, Ayers SE, et al. Period 2 mutation accelerates ApcMin/+ tumorigenesis. Mol Cancer Res. 2008;6:1786–93. doi: 10.1158/1541-7786.MCR-08-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.You S, Ohmori M, Pena MM, Nassri B, Quiton J, Al-Assad ZA, et al. Developmental abnormalities in multiple proliferative tissues of Apc(Min/+) mice. Int J Exp Pathol. 2006;87:227–36. doi: 10.1111/j.1365-2613.2006.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guidelines for Cancer Prevention . American Institute for Cancer Research. Washington, D.C.: 2007. [Google Scholar]
- 45.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Carson JA. Effect of exercise on biological pathways in ApcMin/+ mouse intestinal polyps. J Appl Physiol. 2008;104:1137–43. doi: 10.1152/japplphysiol.00955.2007. [DOI] [PubMed] [Google Scholar]
- 46.de Wit NJ, Bosch-Vermeulen H, de Groot PJ, Hooiveld GJ, Bromhaar MM, Jansen J, et al. The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics. 2008;1:14. doi: 10.1186/1755-8794-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci U S A. 2007;104:979–84. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kettunen HL, Kettunen AS, Rautonen NE. Intestinal immune responses in wild-type and Apcmin/+ mouse, a model for colon cancer. Cancer Res. 2003;63:5136–42. [PubMed] [Google Scholar]
- 49.Murphy EA, Davis JM, Brown AS, Carmichael MD, Ghaffar A, Mayer EP. Oat beta-glucan effects on neutrophil respiratory burst activity following exercise. Med Sci Sports Exerc. 2007;39:639–44. doi: 10.1249/mss.0b013e3180306309. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139:1–4. doi: 10.3945/jn.108.098269. [DOI] [PubMed] [Google Scholar]
- 51.Tucker JM, Davis C, Kitchens ME, Bunni MA, Priest DG, Spencer HT, et al. Response to 5-fluorouracil chemotherapy is modified by dietary folic acid deficiency in Apc(Min/+) mice. Cancer Lett. 2002;187:153–62. doi: 10.1016/s0304-3835(02)00402-0. [DOI] [PubMed] [Google Scholar]