Abstract
Introduction
The new view of cognitive neuropsychology that considers not just case studies of rare severe disorders but also common disorders, as well as normal variation and quantitative traits, is more amenable to recent advances in molecular genetics, such as genome-wide association studies, and advances in quantitative genetics, such as multivariate genetic analysis. A surprising finding emerging from multivariate quantitative genetic studies across diverse learning abilities is that most genetic influences are shared: they are ‘generalist’, rather than ‘specialist’.
Methods
We exploited widespread access to inexpensive and fast Internet connections in the United Kingdom to assess over 5000 pairs of 12-year-old twins from the Twins Early Development Study (TEDS) on four distinct batteries: reading, mathematics, general cognitive ability (g) and, for the first time, language.
Results
Genetic correlations remain high among all of the measured abilities, with language as highly correlated genetically with g as reading and mathematics.
Conclusions
Despite developmental upheaval, generalist genes remain important into early adolescence, suggesting optimal strategies for molecular genetic studies seeking to identify the genes of small effect that influence learning abilities and disabilities.
Keywords: Learning Ability, Intelligence, Reading, Mathematics, Language, Development, Adolescence, Genetics, Twins
Learning abilities and disabilities: Generalist genes in early adolescence
In the past, cognitive neuropsychology has tended to focus on case studies or relatively small samples (Caramazza & Coltheart, 2006), which limited the field's ability to take advantage of developments in genetics. Where genetic factors were considered, they were generally characterized as chromosomal or single-gene abnormalities, or innate species-wide processes. A new, broader view of cognitive neuropsychology is that it represents the cognitive level of analysis that lies between the brain and behavior and aims to provide a full description and explanation, not just of normative species-wide processes and dramatic disruptions of these normal processes, but also of normal variation within species. Although chromosomal abnormalities and single-gene disorders in humans and genetic engineering studies in non-human species can be used to investigate genetics at the normative level of analysis, recent developments in genetics are most useful for investigating normal variation in human behavior seen as quantitative traits and as common disorders that represent the extremes of these normal distributions (Plomin & Davis, in press). For example, in molecular genetics, an advance that has revolutionized research is genome-wide association studies in which hundreds of thousands of common variations in DNA sequence are used to screen the genome for the DNA causes of genetic influence (Wellcome Trust Case Control Consortium, 2007). One consistent finding emerging from these studies is that complex traits and disorders are likely to be influenced by very many genetic variants, each of small effect size (McCarthy et al., 2008).
Quantitative genetic methods, such as inbred strain and selection studies in non-human animals and twin and adoption studies in the human species, emerged from the synthesis between Mendelian and biometric genetics more than a century ago (Fisher, 1918). After the rediscovery of Mendel's laws of inheritance in the early 1900s, Mendelians looked for single-gene effects seen in Mendelian segregation ratios, whereas biometricians argued that Mendel's laws could not apply to complex traits in plants or animals because these traits are distributed quantitatively, not qualitatively, and showed no simple pattern of inheritance. The resolution to this often bitter decade-long debate came with the realization that Mendel's laws of inheritance of single genes also apply to complex traits if the traits are influenced by several genes, each of which are inherited according to Mendel's laws. With just a few genes, phenotypes begin to approach a normal distribution in the population. This notion that multiple-gene effects lead to quantitative traits is the cornerstone of quantitative genetic theory and methods (Fisher, 1918; Wright, 1921; Falconer & MacKay, 1996).
Quantitative genetic methods have primarily been used to discover the ubiquitous influence of genetics on normal variation at all levels of analysis from the brain to cognition to behavior (Plomin, DeFries, McClearn, & McGuffin, 2008). Importantly, quantitative genetics provides the ‘bottom line’ of genetic influence on a trait regardless of how many genetic variants affect the trait or how small and complex their effects might be. Much remains to be learned even about this rudimentary question of how much genetics affects many cognitive and behavioral domains. However, the greatest impact of quantitative genetics will come from research that goes beyond this basic question to investigate how genes have their effect. A major example is multivariate genetic analysis, which investigates not only the variance of traits considered one at a time but also the covariance among traits. In this way, it indicates the extent to which the same or different genes affect several traits, using a statistic known as a genetic correlation (Neale, Boker, Xie, & Maes, 2006), which can be thought of as the probability that a gene associated with one trait is also associated with another trait. The genetic correlation can constrain explanations of cognitive neuropsychology. For example, it is reasonable to suppose that genetic effects will be specific to the substantially different cognitive processes involved in reading and mathematics, which would produce a low genetic correlation between the cognitive processes. A low genetic correlation indicating genetic specificity would lead to attempts to identify the genetically driven differences in brain processes that underlie these cognitive differences.
However, a very different result is emerging from multivariate genetic research on learning abilities and disabilities: Most genetic effects appear to be general in that the same genes affect different learning abilities and disabilities. A review of multivariate genetic research on learning abilities found that genetic correlations varied from 0.47 to 0.98 between reading and mathematics (three studies), from 0.67 to 1.0 between reading and language (five studies), and from 0.59 to 0.98 between language and mathematics (two studies) (Plomin & Kovas, 2005). The average genetic correlation was about 0.70, suggesting that a gene associated with reading, for example, would have a 70% chance of also being associated with mathematics or with language.
Moreover, the general effects of genes appear to extend beyond specific learning abilities such as reading and mathematics to other cognitive abilities such as verbal abilities (e.g., vocabulary and word fluency) and non-verbal abilities (e.g., spatial and memory). The average genetic correlation between specific learning abilities and general cognitive ability (g), which encompasses these verbal and non-verbal cognitive abilities, is about 0.60 (Plomin & Kovas, 2005). These findings have led to a Generalist Genes hypothesis (Plomin & Kovas, 2005), which has far-reaching implications for cognitive neuropsychology (Kovas & Plomin, 2006).
Although the Generalist Genes hypothesis has consistent support from multivariate genetic analyses, it was only recently tested by direct test measures in a sample large enough to conclusively establish the magnitude of the genetic correlations between learning abilities. In order to be able to test large numbers of individuals efficiently and inexpensively, we developed an online test battery that includes measures of reading, mathematics and g. One major advantage of this method of administration is that adaptive branching within each test allows the use of hundreds of items to test the full range of ability, while requiring individual children to complete only a relatively small number of items to ascertain their level of performance. We used this test battery to assess a UK-representative population sample of 2541 pairs of 10-year-old twins from the Twins Early Development Study (TEDS), by far the largest twin sample with cognitive test data (Haworth et al., 2007). Multivariate genetic analysis showed substantial genetic correlations between learning abilities: 0.57 between reading and mathematics, 0.61 between reading and g, and 0.75 between mathematics and g, providing strong support for the Generalist Genes hypothesis (Davis et al., 2008).
The purpose of the present study was to test the Generalist Genes hypothesis at age 12 in an even larger sample of twins. The difference between our previous analysis at age 10 and our present analysis at age 12 is greater than suggested by the interval of two years because 12 years marks the transition to adolescence, which involves brain changes (Ernst & Mueller, 2008; Spear, 2000) in addition to obvious hormonal changes. Underlying these physiological transformations are altered patterns of gene expression. Although changes in gene expression profiles do not necessarily entail changes in which DNA variants are associated with learning abilities, the upheaval may herald a shift in the relative importance of each of the variants, with some becoming more important while others, previously influential, become relatively ineffectual. These shifts have the potential to fundamentally affect the genetic architecture of learning abilities and disabilities at this age.
Moreover, three other improvements increased the scope of the present study to test the Generalist Genes hypothesis. First, at age 10, we assessed reading with a single test, whereas the present study at age 12 included a battery of four reading measures. Second, our previous study at age 10 did not include measures of language, nor has any other genetic research after infancy and early childhood. For this reason, we developed a language battery suitable for 12-year-olds that assesses both receptive spoken language and metalinguistic ability through three tests: syntactic, semantic, and pragmatic language; the language battery presents material orally to avoid confounding with reading ability, which is assessed through tests of reading comprehension, fluency and accuracy (Haworth et al., 2007). A final improvement is the use of a latent factor approach in our model-fitting analyses. In our previous study, we created composite measures of mathematics and g and conducted an analysis of just three measures – reading, mathematics and g. In contrast, in the present study we used a latent factor approach that included information from 14 tests, not just composite measures: four tests of reading, three tests of mathematics, three tests of language, and four tests of g. This latent factor approach made it possible to conduct more powerful multivariate genetic analyses at the level of the latent factors representing reading, mathematics, language and g because the latent factors are independent of test-specific and uncorrelated error variance associated with each method of measurement.
Our hypothesis was that the Generalist Genes hypothesis would be supported despite the major transformations that occur during the transition from childhood to adolescence. Because we used a latent factor approach that excludes uncorrelated measurement error, we expected genetic correlations to be even greater in our present study at age 12 than in our previous study at age 10. Because language has not previously been included in tests of the Generalist Genes hypothesis after the language-learning era of early childhood, we had no hypothesis about the extent to which language conforms to the Generalist Genes hypothesis.
METHODS
Participants
TEDS recruited families of twins born in England and Wales in 1994, 1995 and 1996 (Oliver & Plomin, 2007). Since then, the sample has remained representative of the UK population (ascertained by comparison with census data from the Office of National Statistics; (Kovas, Haworth, Dale, & Plomin, 2007). Although twins have the option of participating or not during each phase of data collection, the pairs that do participate remain representative of the larger sample. Informed consent is obtained by post or online consent forms, and a test administrator is then assigned who telephones the family and generally assists and encourages. Ethical approval for TEDS has been provided by the Institute of Psychiatry ethics committee, reference number 05/Q0706/228.
We excluded from the analyses children with severe current medical problems and children who had suffered severe problems at birth or whose mothers had suffered severe problems during pregnancy. We also excluded twins whose zygosity was unknown or uncertain or whose first language was other than English. Finally, we included only twins whose parents reported their ethnicity as ‘white’, which is 93% of this UK sample. The present analyses are based on 5434 twin pairs (1945 monozygotic pairs, 1760 same-sex dizygotic and 1729 opposite-sex dizygotic).
Measures
At age 12, the twins participated in web-based testing. Widespread access to inexpensive and fast internet connections in the UK has made online testing an attractive possibility for collecting data on the substantial samples necessary for genetic research, especially for multivariate genetic research. The advantages and potential pitfalls of data collection over the internet have been reviewed in detail elsewhere (Birnbaum, 2004). For older children, most of whom are competent computer users, it is an interactive and enjoyable medium. As described above, adaptive branching allows the use of hundreds of items to test the full range of ability, while requiring individual children to complete only a relatively small number of items. In tests where it is appropriate, streaming voiceovers can minimize the necessary reading. In addition, the tests can be completed over a period of several weeks, allowing children to pace the activities themselves, although they are not allowed to return to items previously administered. Finally, it is possible to intersperse the activities with games. All of these factors help to maintain children's engagement with the tests. More details about the measures and their psychometric properties are available in Haworth et al. (2007).
General cognitive ability (g)
The twins were tested on two verbal tests, WISC-III-PI Multiple Choice Information (General Knowledge) and Vocabulary Multiple Choice subtests (Wechsler, 1992), and two non-verbal reasoning tests, the WISC-III-UK Picture Completion (Wechsler, 1992) and Raven's Standard and Advanced Progressive Matrices (Raven, Court, & Raven, 1996; Raven, Court, & Raven, 1998), all administered online.
Reading
Four measures of reading ability were used: two measures of reading comprehension and a measure of reading fluency presented on the Web, and a fourth measure administered over the telephone.
Reading comprehension
The twins completed an adaptation of the reading comprehension subtest of the Peabody Individual Achievement Test (Markwardt, 1997), which we will refer to as PIATrc. The PIATrc assesses literal comprehension of sentences. The sentences were presented individually on the computer screen. Children were required to read each sentence and were then shown four pictures. They had to select the picture that best matched the sentence they had read, using the mouse. All children started with the same items, but an adaptive algorithm modified item order and test discontinuation depending on the performance of the participant. The internet-based adaptation of the PIATrc contained the same practice items, test items and instructions as the original published test.
As well as the PIATrc, we assessed reading comprehension using the GOAL Formative Assessment in Literacy for Key Stage 3 (GOAL plc., 2002). The GOAL is a test of reading achievement that is linked to the literacy goals for children at Key Stage 3 of the National Curriculum. Questions are grouped into three categories: Assessing Knowledge and Understanding (e.g. identifying information, use of punctuation and syntax), Comprehension (e.g. grasping meaning, predicting consequences), and Evaluation and Analysis (e.g. comparing and discriminating between ideas). Within each category, questions about words, sentences, and short paragraphs are asked. Because we were primarily interested in comprehension skills, we used questions from the two relevant categories, Comprehension, and Evaluation and Analysis, with 20 items from each category. Correct answers were summed to give a total comprehension score.
Reading fluency
Reading fluency was assessed using an adaptation of the Woodcock-Johnson III Reading Fluency Test (Woodcock, McGrew, & Mather, 2001) and the Test of Word Reading Efficiency (TOWRE, Form B; (Torgesen, Wagner, & Rashotte, 1999). The Woodcock-Johnson is a measure of reading speed and rate that requires the ability to read and comprehend simple sentences quickly e.g. “A flower grows in the sky? - Yes/No”. The online adaptation consists of 98 yes/no statements; children need to indicate yes or no for each statement as quickly as possible. There is a time limit of 3 minutes for this test. Correct answers were summed to give a total fluency score.
The TOWRE, a standardized measure of fluency and accuracy in word reading skills, includes two subtests, each printed on a single sheet: A list of 85 words, called Sight-word Efficiency (SWE), which assesses the ability to read aloud real words; and a list of 54 non-words, called Phonemic Decoding Efficiency (PDE), which assesses the ability to read aloud pronounceable printed nonwords. The child is given 45 seconds to read as many words as possible. Twins were individually assessed by telephone using test stimuli that had been mailed to families in a sealed package with separate instructions that the package should not be opened until the time of testing. The same tester, who was blind to zygosity, assessed both twins in a pair within the same test session.
Mathematics
In order to assess mathematics, we developed an internet-based battery that included questions from three components of mathematics. The items were based on the National Foundation for Educational Research 5-14 Mathematics Series, which is linked closely to curriculum requirements in the UK and the English Numeracy Strategy (NferNelson Publishing Co. Ltd., 1999). The presentation of items was streamed, so that items from different categories were mixed, but the data recording and branching were done within each category. The items were drawn from the following three categories: Understanding Number, Non-Numerical Processes and Computation and Knowledge. Understanding Number requires an understanding of the numerical and algebraic process to be applied when solving problems (e.g., understanding that multiplication and division are inverse operations). For example, “Look at the number 6085. Change the order of the figures around to make the biggest number possible”. Another example is, “Type the missing number in the box: 27 + 27 + 27 + 27 + 27 + 27 = 27 × ___”. Non-Numerical Processes do not rely solely on memory but rather require understanding of non-numerical mathematical processes and concepts, such as rotational or reflective symmetry and other spatial operations. The questions do not have any significant numerical content that pupils need to consider. Three examples follow: “Which is the longest drinking straw? Click on it.” “One of these shapes has corners that are the same. Click on this shape”. “Which card appears the same when turned upside down? Click on it”. Computation and Knowledge assesses the ability to perform straightforward computations using well-rehearsed pencil-and-paper techniques and the ability to recall mathematical facts and terminology. These questions either are mechanistic or rely on memorization of mathematical facts and terminology. The operation is stated or is relatively unambiguous. Three examples follow: “Type in the answer: 76 - 39”. “All 4-sided shapes are called? Click on the answer (Squares, Rectangles, Parallelograms, Kites, Quadrilaterals)”. “Type in the answer: 149 + 785 = ?”. The mathematics battery is described in detail elsewhere (Kovas, Petrill, & Plomin, 2007).
Language
In order to assess receptive spoken language, standardized tests were selected that would discriminate children with language disability as well as being sensitive to individual differences across the full range of ability. Furthermore, an aspect of language that becomes increasingly important in adolescence – and which shows interesting variability at this age – is metalinguistic ability, which is knowledge about language itself (Nippold, 1998). For this reason, the three measures selected for testing included one with low metalinguistic demands designed to assess syntax (Listening Grammar) and two with higher demands that assess semantics (Figurative Language) and pragmatics (Making Inferences).
Syntax
Syntax was assessed using the Listening Grammar subtest of the Test of Adolescent and Adult Language (TOAL-3) (Hammill, Brown, Larsen, & Wiederholt, 1994). This test requires the child to select two sentences that have nearly the same meaning, out of three options. The sentences are presented orally only.
Semantics
Semantics were assessed using Level 2 of the Figurative Language subtest of Test of Language Competence (Wiig, Secord, & Sabers, 1989), which assesses the interpretation of idioms and metaphors; correct understanding of such non-literal language requires rich semantic representations. The child hears a sentence orally and chooses one of four answers, presented in both written and oral form.
Pragmatics
Level 2 of the Making Inferences subtest of the Test of Language Competence (Wiig et al., 1989) assessed an aspect of pragmatic language, requiring participants to make permissible inferences on the basis of existing (but incomplete) causal relationships presented in short paragraphs. The child hears the paragraphs orally and chooses two of four responses, presented in both written and oral form.
Statistical analyses
According to the quantitative genetic model (Plomin et al., 2008), twins reared together resemble each other due to the additive effects of shared genes (A) or shared (common) environmental factors (C). For identical or monozygotic (MZ) twins, the correlation between their genes is 1.00, whereas for non-identical or dizygotic (DZ) twins, the correlation is .50 because DZ twins on average share half of their segregating alleles. The correlation between twins for shared environment is, by definition, 1.00 for both MZ and DZ twins growing up in the same family, while non-shared environmental influences (E) are uncorrelated and contribute to differences between twins. For the twin analyses, standardized residuals correcting for age and sex were used because the age of twins is perfectly correlated across pairs, which means that, unless corrected, variation within each age group at the time of testing would contribute to the correlation between twins and be misrepresented as shared environmental influence. The same applies to the sex of the twins, since MZ twins are always of the same sex. The assumptions of the classical twin model, and their validity, have been discussed in detail elsewhere (Boomsma, Busjahn, & Peltonen, 2002; Visscher, Hill, & Wray, 2008).
As well as examining twin correlations in R (http://www.r-project.org), we used standard ACE model-fitting analysis in Mx (Neale et al., 2006) where ACE stands for additive genetic influences (A), shared or common environmental influences (C), and non-shared environmental (E) influences, as above. Model-fitting analysis specifies a correlational structure (a model) using matrix algebra. This model is a hypothesis about the structure of the dataset, and is derived from what we know about how MZ and DZ twins are related to each other (see above). By fitting the model to the data using an iteration process, we can assess its ‘goodness of fit’ and estimate the contributions of A, C and E.
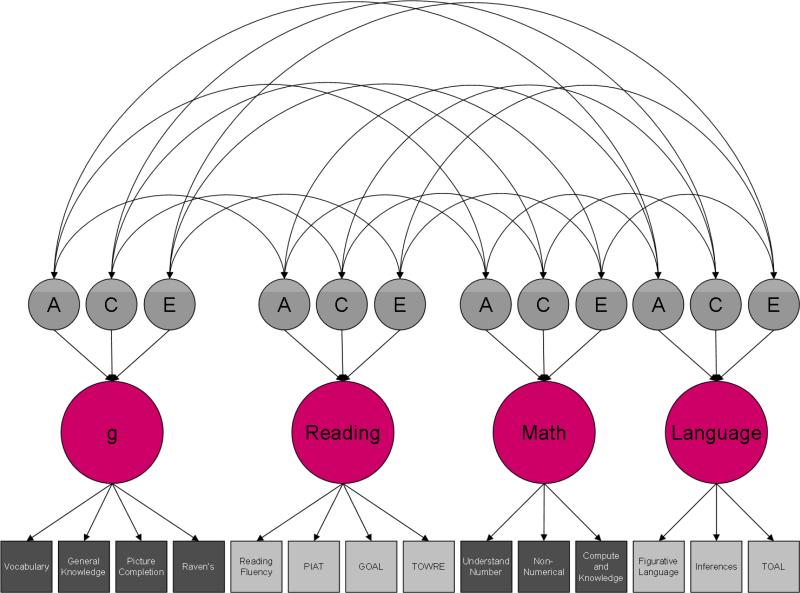
To explore shared genetic and environmental etiology, we fitted a common pathway model to raw data (Figure 1; Neale et al., 2006). This model derives latent factors for each domain using maximum-likelihood factor analysis. It fixes the variance of these latent factors at 1 and partitions them into A, C and E components. It also partitions the covariance between the latent factors in the same way. Similarly, residual variance at each age is partitioned into A, C and E components. Earlier studies indicated very little difference in ACE estimates between males and females (Kovas, Haworth, Dale, & Plomin, 2007), implying no significant differences in etiology, so we combined DZ same-sex and DZ opposite-sex twin pairs for the individual differences analysis. Even though the genetic and environmental etiology of the sexes is similar, this does not preclude differences in mean performance for males and females.
Figure 1. Common pathway model.
A = Additive genetic effects; C = Shared (common) environmental effects; E = non-shared environmental effects. Squares represent measured traits; circles represent latent factors. The lower tier of arrows represents factor loadings; the second tier represents genetic and environmental path coefficients; the curved arrows at the top represent correlations between genetic and environmental latent factors.
RESULTS
Phenotypic analyses
Table 1 presents the measure means and standard deviations, subdivided by sex and zygosity. It also presents the results of an analysis of variance testing the effects of sex and zygosity on the measures. Although our large sample gives us around 90% power to detect a difference in means as small as 0.1 standard deviations, in each measure age and sex accounted for less than 3% of the variance, and more often less than 1% (indexed by the coefficient of determination, R2). Factor loadings of the individual measures onto the latent factors (the bottom of Figure 2) are consistently high ranging from 0.44 to 0.87 with a mean of 0.67. They are also generally similar, indicating that each constituent measure contributes similarly to the factor.
Table 1.
Measure means and standard deviations by sex and zygosity
| Battery | Measure | Male |
Female |
MZ |
DZ |
ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | Sex | Zyg. | Sex*Zyg. | R2 | N pairs | ||
| g | Vocabulary | 0.03 | 0.96 | -0.01 | 1.01 | -0.05 | 1.02 | 0.04 | 0.97 | 0.18 | 0.02 | 0.46 | 0.00 | 4001 |
| General knowledge | 0.19 | 0.97 | -0.15 | 1.01 | -0.10 | 1.01 | 0.06 | 1.00 | 0.00* | 0.00* | 0.05 | 0.03 | 4285 | |
| Picture completion | 0.03 | 1.03 | -0.02 | 0.97 | -0.05 | 1.01 | 0.04 | 0.99 | 0.04 | 0.00 | 0.15 | 0.00 | 3846 | |
| Raven's | -0.01 | 1.03 | 0.00 | 0.99 | -0.03 | 1.00 | 0.01 | 1.02 | 0.81 | 0.28 | 0.97 | 0.00 | 4149 | |
| Reading | Reading fluency | -0.06 | 0.99 | 0.07 | 1.01 | -0.05 | 1.00 | 0.05 | 1.00 | 0.04 | 0.00* | 0.35 | 0.01 | 4757 |
| PIAT | 0.06 | 1.00 | -0.04 | 1.00 | -0.07 | 1.01 | 0.05 | 0.99 | 0.00 | 0.00* | 0.22 | 0.01 | 4875 | |
| GOAL | 0.02 | 1.03 | 0.01 | 0.96 | -0.03 | 1.02 | 0.04 | 0.98 | 0.10 | 0.00* | 0.04 | 0.00 | 4711 | |
| TOWRE | -0.01 | 1.02 | 0.01 | 0.98 | -0.04 | 1.00 | 0.03 | 0.99 | 0.79 | 0.02 | 0.28 | 0.00 | 4061 | |
| Mathematics | Understand number | 0.09 | 1.00 | -0.08 | 1.00 | -0.04 | 0.99 | 0.02 | 1.01 | 0.00* | 0.22 | 0.80 | 0.01 | 4627 |
| Non-numerical | 0.04 | 1.01 | -0.03 | 0.99 | -0.03 | 1.00 | 0.02 | 1.01 | 0.07 | 0.19 | 0.66 | 0.00 | 4655 | |
| Compute & knowledge | 0.05 | 1.03 | -0.03 | 0.98 | -0.03 | 1.00 | 0.03 | 1.00 | 0.02 | 0.06 | 0.26 | 0.00 | 4624 | |
| Language | Figurative language | 0.05 | 0.98 | 0.00 | 0.99 | -0.01 | 0.98 | 0.04 | 0.99 | 0.23 | 0.19 | 0.72 | 0.00 | 4136 |
| Inferences | -0.02 | 1.02 | 0.04 | 0.99 | 0.00 | 1.01 | 0.02 | 1.00 | 0.54 | 0.28 | 0.44 | 0.00 | 3953 | |
| TOAL | -0.06 | 0.99 | 0.03 | 1.00 | -0.06 | 0.99 | 0.02 | 1.00 | 0.22 | 0.02 | 0.50 | 0.00 | 3849 | |
M = mean; SD = Standard deviation; MZ = Monozygotic twins; DZ = Dizygotic twins; Sex = p-value for sex effect; Zyg. = p-value for zygosity effect; Sex*Zyg. = p-value for sex by zygosity interaction; R2 = proportion of total variance accounted for by sex and zygosity; Analysis of variance performed using one member of each twin pair
significant at α = 0.05 after a Bonferroni correction for multiple testing (p < 0.00357).
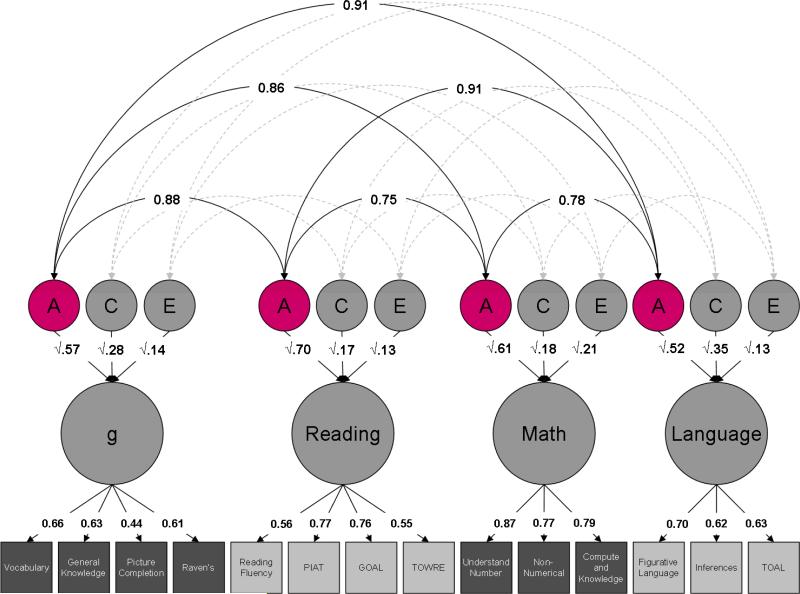
Figure 2. Cross-trait genetic effects.
Estimates of cross-trait additive genetic effects (A) are highlighted.
Univariate genetic analyses
Intraclass correlations (twin similarity coefficients) are presented in Table 2 for the MZ and DZ twins at each age. Correlations between MZ twins were consistently higher than those between DZ twins, suggesting a genetic contribution to each measure. The significance of genetic influence is indicated by the lack of overlap between the confidence intervals for MZ and DZ twins. As a first estimate of the effect size (heritability), doubling the difference between the MZ and DZ correlations yields heritability estimates ranging from 22% to 74% with a mean of 39%. Shared environmental influences are estimated as the extent to which MZ resemblance exceeds heritability: they range from 1% to 26%, with a mean of 14%. The remainder of the variance is attributed to non-shared environmental influences (plus error of measurement): ranging from 28% to 60%, with a mean of 47%.
Table 2.
Twin (intraclass) correlations
| Battery | Measures | MZ | N pairs | DZ | N pairs |
|---|---|---|---|---|---|
| g | Vocabulary | 0.43 (0.38-0.47) | 1421 | 0.25 (0.21-0.29) | 2389 |
| General knowledge | 0.56 (0.52-0.59) | 1500 | 0.41 (0.38-0.44) | 2524 | |
| Picture completion | 0.46 (0.41-0.50) | 1367 | 0.31 (0.27-0.34) | 2275 | |
| Raven's | 0.48 (0.44-0.52) | 1456 | 0.29 (0.26-0.33) | 2474 | |
| Reading | Reading fluency | 0.72 (0.70-0.75) | 1667 | 0.42 (0.38-0.45) | 2844 |
| PIAT | 0.55 (0.52-0.58) | 1694 | 0.35 (0.31-0.38) | 2950 | |
| GOAL | 0.49 (0.45-0.52) | 1635 | 0.32 (0.29-0.35) | 2812 | |
| TOWRE | 0.75 (0.73-0.77) | 1488 | 0.38 (0.35-0.41) | 2540 | |
| Mathematics | Understand number | 0.58 (0.55-0.61) | 1573 | 0.40 (0.36-0.43) | 2709 |
| Non-numerical | 0.55 (0.51-0.58) | 1596 | 0.35 (0.32-0.38) | 2734 | |
| Compute & knowledge | 0.54 (0.51-0.58) | 1581 | 0.30 (0.27-0.34) | 2712 | |
| Language | Figurative language | 0.53 (0.49-0.57) | 1462 | 0.35 (0.32-0.39) | 2455 |
| Inferences | 0.40 (0.36-0.44) | 1398 | 0.29 (0.25-0.33) | 2351 | |
| TOAL | 0.44 (0.40-0.48) | 1377 | 0.29 (0.25-0.33) | 2312 |
95% confidence intervals in parentheses.
Common pathway model
Figures 2, 3 and 4 depict results for the common pathway model used to investigate the shared etiology. As explained above, this model partitions variance into latent factors representing g, reading, mathematics and language, and residual variance specific to each measure. The variance is then further partitioned into additive genetic (A), shared (common) environmental (C) and non-shared environmental (E) influences. Confidence intervals for the estimates in Figures 2, 3 and 4 are presented in Table 3.
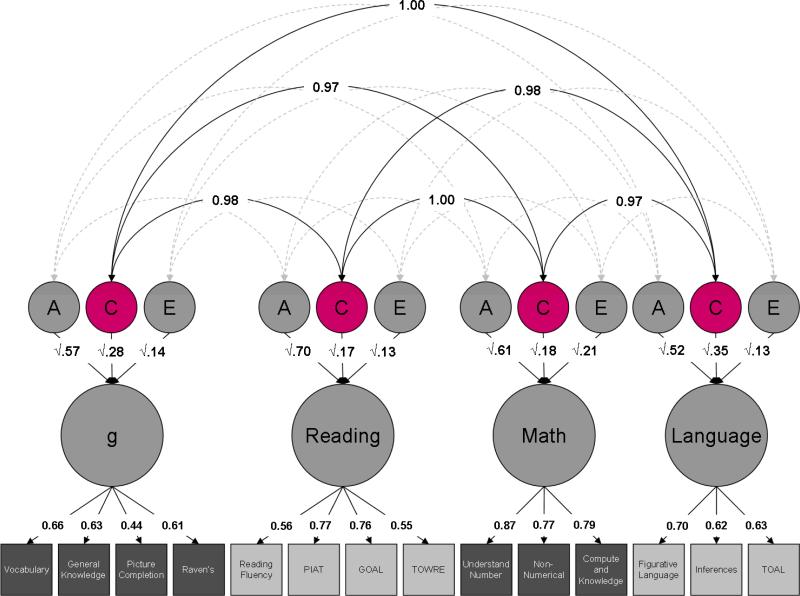
Figure 3. Cross-trait shared environmental effects.
Estimates of cross-trait shared environmental effects (C) are highlighted.
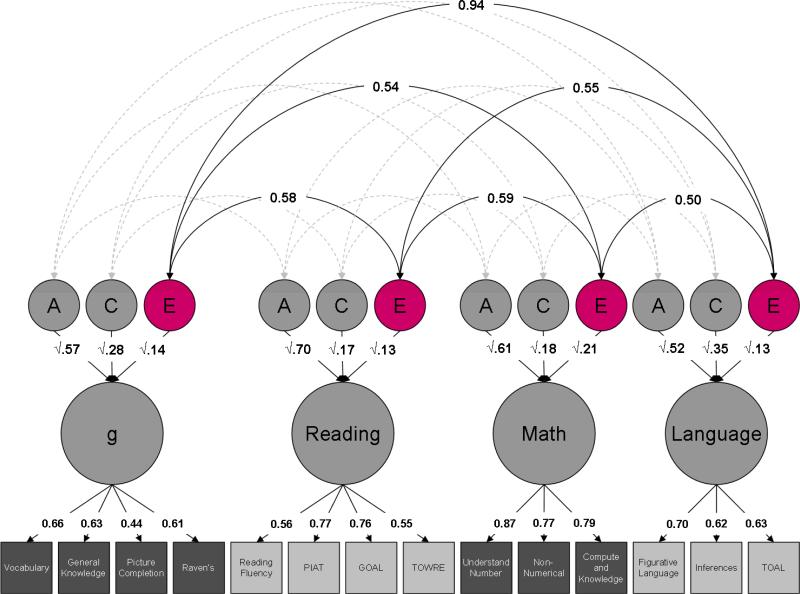
Figure 4. Cross-trait non-shared environmental effects.
Estimates of cross-trait non-shared environmental effects (E) are highlighted.
Table 3.
Common pathway model estimates
| Measures | Common | |||
|---|---|---|---|---|
| A | C | E | ||
| g | 0.57 (0.49-0.57) | 0.28 (0.21-0.28) | 0.14 (0.12-0.17) | |
| Reading | 0.70 (0.63-0.74) | 0.17 (0.11-0.19) | 0.13 (0.11-0.14) | |
| Mathematics | 0.61 (0.53-0.67) | 0.18 (0.12-0.20) | 0.21 (0.19-0.24) | |
| Language | 0.52 (0.41-0.55) | 0.35 (0.25-0.40) | 0.13 (0.09-0.17) | |
| Specific |
Factor Loading | ||||
|---|---|---|---|---|---|
| A | C | E | |||
| g | Vocabulary | 0.02 (0.01-0.02) | 0.01 (0.00-0.01) | 0.51 (0.48-0.53) | 0.66 (0.64-0.67) |
| General knowledge | 0.01 (0.01-0.02) | 0.00 (0.00-0.00) | 0.40 (0.37-0.43) | 0.63 (0.62-0.64) | |
| Picture completion | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.52 (0.48-0.55) | 0.44 (0.42-0.46) | |
| Raven's | 0.01 (0.01-0.02) | 0.00 (0.00-0.00) | 0.44 (0.41-0.45) | 0.61 (0.60-0.63) | |
| Reading | Reading fluency | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.25 (0.23-0.27) | 0.56 (0.54-0.56) |
| PIAT | 0.01 (0.01-0.01) | 0.01 (0.00-0.01) | 0.37 (0.34-0.39) | 0.77 (0.75-0.77) | |
| GOAL | 0.01 (0.01-0.01) | 0.01 (0.01-0.01) | 0.40 (0.38-0.42) | 0.76 (0.75-0.77) | |
| TOWRE | 0.00 (0.00-0.00) | 0.00 (0.00-0.00) | 0.21 (0.19-0.22) | 0.55 (0.53-0.56) | |
| Mathematics | Understand number | 0.03 (0.01-0.03) | 0.02 (0.02-0.03) | 0.22 (0.21-0.24) | 0.87 (0.86-0.87) |
| Non-numerical | 0.01 (0.01-0.02) | 0.02 (0.01-0.02) | 0.32 (0.29-0.33) | 0.77 (0.75-0.78) | |
| Compute & knowledge | 0.01 (0.01-0.02) | 0.02 (0.02-0.02) | 0.30 (0.28-0.32) | 0.79 (0.78-0.80) | |
| Language | Figurative language | 0.03 (0.03-0.03) | 0.00 (0.00-0.00) | 0.40 (0.37-0.41) | 0.70 (0.69-0.72) |
| Inferences | 0.02 (0.01-0.03) | 0.00 (0.00-0.00) | 0.54 (0.50-0.57) | 0.62 (0.60-0.64) | |
| TOAL | 0.02 (0.02-0.03) | 0.00 (0.00-0.00) | 0.51 (0.47-0.52) | 0.63 (0.61-0.64) | |
95% confidence intervals in parentheses; sample-size adjusted Bayesian Information Criterion = -180921.
Heritability of the latent factors (a2) is consistently high, accounting for more than half of the variance: 57% for g, 70% for reading, 61% for mathematics and 52% for language. The remainder of the variance in the latent factors is accounted for by environmental influences, split between the shared (common) environment (c2) – 28%, 17%, 18% and 35% – and the non-shared (unique) environment (e2): 14%, 13%, 21% and 13%.
Specific variance components (i.e., the variance not accounted for by the latent factors) are shown alongside the latent factor variance components in Table 3. The specific heritability is consistently low, ranging from nearly 0 to 0.03, with a mean of 0.013, indicating that all the genetic variance on these measures is subsumed in the latent factors. Specific shared environment is also low (0 to 0.02, with a mean of 0.0064). In contrast, specific non-shared environment ranges from 0.21 to 0.54, with a mean of 0.39. As well as measure-specific non-shared environmental effects, this component partly represents measure-specific measurement error that is not subsumed into the latent factor for each domain.
Genetic correlations between latent factors are uniformly high, ranging from 0.75 to 0.91, with a mean of 0.85 (Figure 2). The shared environmental correlations are even higher, ranging from 0.97 to 1.00, with a mean of 0.98 (Figure 3). Finally, non-shared environmental correlations are moderate, ranging from 0.50 to 0.94, with a mean of 0.62. Interestingly, the non-shared environmental correlation between g and language is substantially higher than the other non-shared environmental correlations: 0.94, compared to a mean of 0.55 (Figure 4).
The genetic and environmental correlations are summarized in Table 4, alongside the bivariate heritability and environmentality of the latent factors. Whereas the genetic correlation indexes the genetic overlap between the latent factors independent of the heritabilities of the latent factors, the bivariate heritability indexes the proportion of the phenotypic correlation between the latent factors that is mediated by genetic effects. Genetic effects consistently account for over half of the phenotypic correlation, ranging from 53% to 65%, with a mean of 61%. Shared environment accounts for just over a quarter of the phenotypic correlation, ranging from 23% to 34%, with a mean of 28%. Although the shared environmental correlation is even higher than the genetic correlation, the mediation of the phenotypic correlation is lower because the shared environment accounts for a smaller proportion of the variance of each of the latent factors. Finally, the non-shared environment accounts for the remainder of the phenotypic correlation, contributing from 8% to 14%, with a mean of 11%.
Table 4.
Cross-trait correlations and bivariate estimates from common pathway model
| Measures | Correlations | |||
|---|---|---|---|---|
| rA | rC | rE | rP | |
| g and reading | 0.88 (0.84-0.88) | 0.98 (0.88-0.99) | 0.58 (0.48-0.68) | 0.85 (0.83-0.86) |
| g and mathematics | 0.86 (0.81-0.90) | 0.97 (0.87-1.00) | 0.54 (0.45-0.60) | 0.82 (0.80-0.83) |
| g and language | 0.91 (0.87-0.94) | 1.00 (0.95-1.00) | 0.94 (0.80-1.00) | 0.94 (0.93-0.95) |
| Reading and mathematics | 0.75 (0.71-0.75) | 1.00 (0.90-1.00) | 0.59 (0.52-0.67) | 0.76 (0.75-0.78) |
| Reading and language | 0.91 (0.86-0.96) | 0.98 (0.88-1.00) | 0.55 (0.43-0.55) | 0.86 (0.84-0.87) |
| Mathematics and language | 0.78 (0.73-0.78) | 0.97 (0.87-1.00) | 0.50 (0.41-0.55) | 0.77 (0.75-0.78) |
| Mediation of rP | |||
|---|---|---|---|
| axayrA/rP | cxcyrC/rP | exeyrE/rP | |
| g and reading | 0.65 (0.58-0.72) | 0.25 (0.19-0.31) | 0.09 (0.07-0.09) |
| g and mathematics | 0.62 (0.54-0.67) | 0.27 (0.20-0.32) | 0.11 (0.09-0.14) |
| g and language | 0.53 (0.45-0.60) | 0.34 (0.26-0.39) | 0.14 (0.11-0.16) |
| Reading andmathematics | 0.64 (0.56-0.70) | 0.23 (0.16-0.29) | 0.13 (0.11-0.15) |
| Reading and language | 0.64 (0.57-0.66) | 0.28 (0.21-0.34) | 0.08 (0.06-0.83) |
| Mathematics and language | 0.57 (0.50-0.65) | 0.32 (0.25-0.37) | 0.11 (0.09-0.13) |
rA = genetic correlation; rC = shared environmental correlation; rE = non-shared environmental correlation; rP = phenotypic correlation; axayrA/rP = proportion of phenotypic correlation accounted for by genetic factors; cxcyrC/rP = proportion accounted for by shared environmental factors; exeyrE/rP = proportion accounted for by non-shared environmental factors; 95% confidence intervals in parentheses.
DISCUSSION
Generalist Genes
The high heritability of our latent factors confirms that genetic effects continue to be important in the etiology of cognitive abilities and disabilities into early adolescence. However, going beyond this, the high genetic correlations between the latent factors representing g, reading, mathematics and language confirm that the genetic influences on these domains continue to be largely shared, in spite of accompanying hormonal and brain changes, with genes accounting for most of the phenotypic correlation between these domains. This implies that when genes influencing reading are found, for example, they are very likely to also be associated with mathematics, general cognitive ability and language. These correlations are consistent with various possible causal pathways (Kovas & Plomin, 2006); so far we do not have the evidence to distinguish between them, but future research combining genetics with neuropsychiatry will bring us closer to uncovering the mechanisms through which these genes have their generalist influence. Finding strong genetic correlations suggests that in our search for genes influencing learning abilities, we should not be looking for genetic variants influencing a specific trait independent of other traits, because there will be relatively few such genes. Instead, we should be seeking the generalist genes and exploring how they interact developmentally with the environment through changes in gene expression and methylation to influence diverse brain and cognitive outcomes. Despite this, a mean genetic correlation of 0.85 is not the same as a correlation of 1.00; although the vast majority of genes are generalist, there are likely to be a few that remain specific to one domain or another. Finding these genes will be an even greater challenge than identifying the generalist genes, because they account for a smaller proportion of the genetic variance.
Although the current paper considers the entire distribution of variation including the low end of the distribution in g, reading, mathematics and language, our previous research has shown that genetic factors influencing variation in the normal range also influence the extremes (Kovas, Haworth, Dale & Plomin, 2007), so in addition to genes being generalist between domains, they also make little distinction between ability and disability; in terms of cognition, the abnormal is normal.
Shared Environment
Although the shared environment accounted for a relatively small proportion of the variance in each of our domains, and mediated only around a quarter of the phenotypic correlation, the very high shared environmental correlations (higher even than the genetic correlations) imply that the shared environmental influences on each of the traits are almost entirely the same. Although there has been some success, identifying the specific shared environments that influence cognition is difficult, in part because what appears to be an environmental effect is often partly genetically mediated, through the process of gene-environment correlation (Plomin & Davis, 2006; Jaffee & Price, 2007). This correlation can be passive, where an individual inherits both genes and the childhood family environment from a parent; active, where an individual seeks out particular environments influenced by their genetic propensities; or evocative, where genetically influenced behavior leads an individual to evoke certain reactions from others in the environment. For this reason, if we are to identify the specific environments that contribute to the development of cognition, it is best to couch the study within a genetically sensitive design. It is likely that when we identify the shared environments – those that make children growing up in the same family more similar – they will be substantially the same for g, reading, mathematics and language.
Non-shared Environment
The non-shared environment has relatively little influence on our latent factors because uncorrelated environments and measurement error are not included in the factor. This variance appears as measurement-specific variance instead, in contrast to the genetic and shared environmental influences where almost all of the variance is subsumed into the latent factor. Similarly, although the non-shared environment is still substantially correlated across traits, it is less correlated than genetic and shared environmental factors, implying that it is largely non-shared environments that bring about unevenness in cognitive ability profiles. However, there is one exception: the non-shared environmental correlation between g and language is much higher than the correlations among the other abilities. Because this is the first study to explore the etiology of language in relation to other cognitive abilities at this age, this is an exciting finding; it will be interesting to see whether this pattern continues as the TEDS twins progress through adolescence. If so, it could have important implications for our understanding of the development of human cognition and how it relates to the development of language, both throughout childhood and throughout the course of evolution.
Conclusion
By testing g, reading, mathematics and language in a large sample of 12-year-old twins, we have demonstrated that the Generalist Genes hypothesis continues to hold true into early adolescence. This has implications affecting the hunt for the many genes of small effect that are expected to influence human cognitive abilities, and also our conception of the pathway from genes to brain to behavior. By understanding that the same genes act similarly on quite different domains, we can begin to explore the mechanisms by which this genetic variation influences the biochemical pathways and neurological networks involved in the day-to-day life of the brain. In addition, we have made an unexpected finding – the non-shared environment, which so often differentiates performance in different domains, appears to act similarly on general cognitive ability and language performance in early adolescence: a clear reminder that both genes and dynamic environments are important influences in the development of human cognition.
ACKNOWLEDGEMENTS
We thank the parents of the twins in TEDS and the twins themselves for making this study possible. TEDS is supported by a program grant from the UK Medical Research Council (G500079), and this research is also supported by grants from the US National Institute of Child Health and Human Development (HD44454, HD46167, HD49861).
REFERENCES
- Birnbaum MH. Human research and data collection via the internet. Annual Review of Psychology. 2004;55:803–832. doi: 10.1146/annurev.psych.55.090902.141601. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Coltheart M. Cognitive Neuropsychology twenty years on. Cognitive Neuropsychology. 2006;23(1):3–12. doi: 10.1080/02643290500443250. [DOI] [PubMed] [Google Scholar]
- Davis OSP, Kovas Y, Harlaar N, Busfield P, McMillan A, Frances J, Petrill SA, Dale PS, Plomin R. Generalist genes and the Internet generation: etiology of learning abilities by web testing at age 10. Genes, Brain and Behavior. 2008;7:455–462. doi: 10.1111/j.1601-183X.2007.00370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Mueller SC. The adolescent brain: Insights from functional neuroimaging research. Developmental Neurobiology. 2008;68(6):729–743. doi: 10.1002/dneu.20615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer DS, MacKay TFC. Introduction to quantitative genetics. Fourth Edition Longman; Harlow, UK: 1996. [Google Scholar]
- Fisher RA. The correlation between relatives on the supposition of Mendelian inheritance. Transactions of the Royal Society of Edinburgh. 1918;52:399–433. [Google Scholar]
- GOAL plc. GOAL Formative Assessment: Key Stage 3. Hodder & Stoughton; London: 2002. [Google Scholar]
- Hammill DD, Brown VL, Larsen SC, Wiederholt JL. Test of Adolescent and Adult Language (TOAL-3) Pro-Ed.; Austin, TX: 1994. [Google Scholar]
- Haworth CMA, Harlaar N, Kovas Y, Davis OSP, Oliver B, Hayiou-Thomas ME, Frances J, Busfield P, McMillan A, Dale PS, Plomin R. Online Internet Testing of Large Samples Needed in Genetic Research. Twin Research and Human Genetics. 2007;10(4):554–563. doi: 10.1375/twin.10.4.554. [DOI] [PubMed] [Google Scholar]
- Jaffee SR, Price TS. Gene-environment correlations: a review of the evidence and implications for prevention of mental illness. Molecular Psychiatry. 2007;12(5):432–442. doi: 10.1038/sj.mp.4001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Haworth CM, Dale PS, Plomin R. The genetic and environmental origins of learning abilities and disabilities in the early school years. Monographs of the Society for Research in Child Development. 2007;72:1–144. doi: 10.1111/j.1540-5834.2007.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Petrill SA, Plomin R. The origins of diverse domains of mathematics: Generalist Genes but Specialist Environments. Journal of Educational Psychology. 2007;99(1):128–139. doi: 10.1037/0022-0663.99.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Plomin R. Generalist genes: implications for the cognitive sciences. Trends in Cognitive Sciences. 2006;10:198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Markwardt FC., Jr. Peabody Individual Achievement Test-Revised (Normative Update) Manual. American Guidance Service; Circle Pines: 1997. [Google Scholar]
- McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JPA, Hirschhorn JN. Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nature Reviews Genetics. 2008;9:356–369. doi: 10.1038/nrg2344. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. 7th ed. Department of Psychiatry; Richmond, VA: 2006. VCU Box 900126. 23298. [Google Scholar]
- NferNelson Publishing Co. Ltd. Mathematics 5-14 series. Windsor, UK: 1999. [Google Scholar]
- Nippold MA. Later language development: The school-age and adolescent years. Pro-Ed.; Austin, TX: 1998. [Google Scholar]
- Oliver BR, Plomin R. Twins Early Development Study (TEDS): A multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Research and Human Genetics. 2007;10:96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral Genetics. Fifth Edition Worth; New York: 2008. [Google Scholar]
- Plomin R, Davis OSP. The future of genetics in psychology and psychiatry: Microarrays, genome-wide association, and non-coding RNA. Journal of Child Psychology & Psychiatry. doi: 10.1111/j.1469-7610.2008.01978.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Davis OSP. Gene-environment interactions and correlations in the development of cognitive abilities and disabilities. In: J., O'Daly O, Murray RM, McGuffin P, Wright P, editors. Beyond Nature and Nurture in Psychiatry: Genes, Environment and their Interplay. Informa Healthcare Medical Books; Oxford: 2006. [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychol.Bull. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Raven JC, Court JH, Raven J. Manual for Raven's Progressive Matrices and Vocabulary Scales. Oxford University Press; Oxford: 1996. [Google Scholar]
- Raven JC, Court JH, Raven J. Oxford Psychologists Press Ltd.; Oxford: 1998. Manual for Raven's Advanced Progressive Matrices. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency. Pro-Ed.; Austin, TX: 1999. [Google Scholar]
- Visscher PM, Hill WG, Wray NR. Heritability in the genomics era -- concepts and misconceptions. Nature Reviews Genetics. 2008;9:255–266. doi: 10.1038/nrg2322. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children - Third Edition UK (WISC-IIIUK) Manual. The Psychological Corporation; London: 1992. [Google Scholar]
- Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447:661–678. doi: 10.1038/nature05911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig EH, Secord W, Sabers D. Test of Language Competence. Expanded Edition The Psychological Corporation; San Antonio, TX: 1989. [Google Scholar]
- Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Riverside Publishing; Itasca , IL: 2001. [Google Scholar]
- Wright S. Systems of mating. Genetics. 1921;6:111–178. doi: 10.1093/genetics/6.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]