Abstract
The utility of the bis-metallating reagent 1,3-dimethyl-2-trimethylstannyl-2-bora-1,3-diazacyclopentane (1) has not been fully realized because of the hydrolytic instability of the products derived from catalyzed vicinal syn-additions to alkynes. The isolation of variety of such adducts derived from alkynes (and also from hitherto unreported additions to 1,3-enynes) as stable boron pinacolates is reported. Examples of the applications of resulting products in tandem cross-coupling reactions and as dienes in Diels-Alder reactions are illustrated.
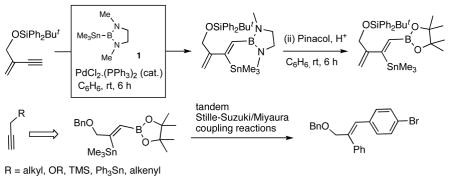
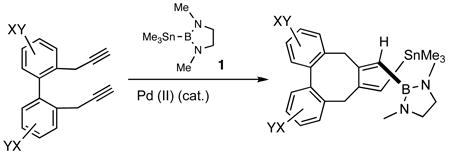
While exploring the applications of bismetallative cyclization of α,ω-diynes using 1,3-dimethyl-2-trimethylstannyl-2-bora-1,3-diazacyclopentane 1 (eq 1) in the synthesis of highly functionalized dibenzocyclooctadienes,1 it became apparent that except for low molecular weight adducts where the products can be isolated by distillation, or in rare cases where they are crystalline, the hydrolytic instability of the primary [BSn]-adducts severely limits the utility of this otherwise useful reagent.2 Further impetus for work in the area came from our recent recognition that despite the extreme sensitivity to moisture and difficulties in its preparation, this reagent could have a much broader substrate scope and improved selectivity in its reactions as compared to the more well known silylstannanes.3 Even though both reagents undergo regio- and stereoselective 1,2-addition to terminal alkynes, giving products in which the stannyl residue is attached to the internal carbon, only the [BSn] reagent reacts with internal alkynes. In this paper we provide examples of a simple protocol for the isolation of the borylstannyl alkenes derived from acetylene, mono-and disubstituted alkynes and enynes. Also illustrated are examples of applications of the products derived from these reactions, including tandem Stille/Suzuki coupling reactions, and the use of a highly functionalized adduct from an enyne as a diene in a Diels-Alder reaction.
 |
(1) |
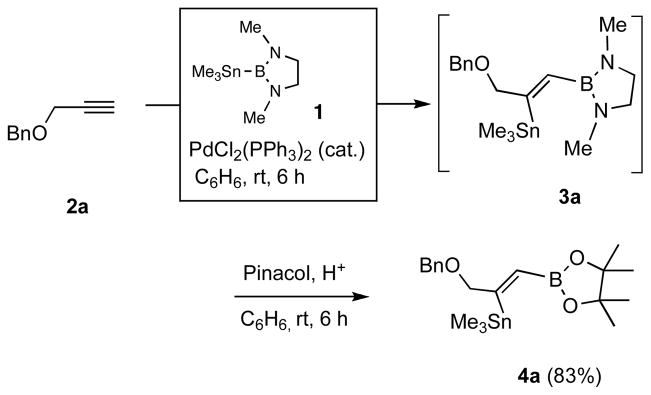
In a typical example, we find that the crude primary product (3a) from 3-benzyloxy-1-propyne (2a) when treated with pinacol in the presence of p-toluenesulfonic acid gives a dioxaborolidine (4a) that can be isolated in high yield (Scheme 1).4 Thus addition of 1 to 2a at room temperature in the presence of 0.05 equivalents of PdCl2.(Ph3P)2 gives the expected adduct (3a) in excellent conversion as judged by in situ NMR spectroscopy. After 6 h at room temperature, a solution of 1.2 equivalents of pinacol dissolved in benzene and 1.2 equivalents of solid p-toulenesulfonic acid are added, and the reaction is stirred for an additional 2 h.5,6 The reaction is quenched by addition of triethylamine (1.6 equiv) and the product 4a is isolated by column chromatography on silica gel after concentration. The (Z)-configuration of the product 4a can be easily established by nOe measurements. Other examples of these transformations are shown in Table 1. In general, excellent regio- and stereoselectivities are observed for these reactions, and yields are surprisingly good for the generation of such a functionalized alkene. The sequence of reactions, especially the acid-catalyzed pinacolate formation, is tolerated by various propargylic substituents such as a benzyl ether (entry 1), a trimethylsilyl group (entry 4), and even a triphenylstannane moiety (entry 5).
Scheme 1.
Borostannylation of an Alkyne
Table 1.
Borostannylation of Alkynes Using 1 Followed by Dioxaborolidene Formationa
| entry | alkyne | adduct (%yield)b |
|---|---|---|
| 1. |
2a |
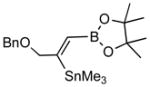 4a (83) |
| 2. |
2b |
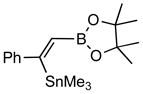 4b (82) |
| 3. |
2c |
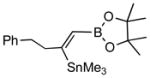 4c (81) |
| 4. |
2d |
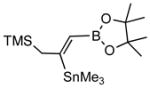 4d (80) |
| 5. |
2e |
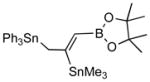 4e (80) |
| 6. |
2f |
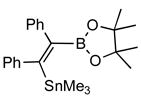 4f (84) |
| 7. |
2g |
 |
| 8. |
2hc |
 |
see Scheme 1 for procedure.
isolated by column chromatography.
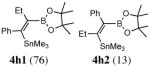
use of PdCl2(CH3CN)2/P(But)3 (60 °C, 12 h) gives a ratio of 4h1: 4h2 = 94:6.
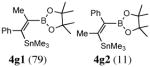
Disubstitued alkynes also give the corresponding (Z)-adducts in very good to excellent yields. Diphenylacetylene gives the (Z)-product 4f in 84% yield (entry 6). 1-Phenylpropyne gave a mixture of products in a ratio of 79:11 with the stannyl moiety occupying the benzylic position as the major isomer. 1-Phenylbutyne also shows comparable regioselectivity (entry 8). An examination of ligand effects5 revealed that use of PdCl2(CH3CN)2/P(But)3 (60 °C, 12 h) gave an improved regioselectivity (94:6), albeit with decreased overall yield (75%).
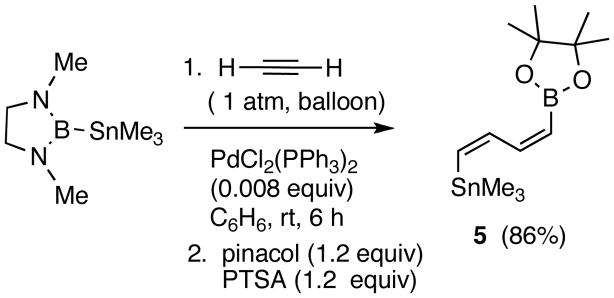
Acetylene itself undergoes facile borostannylation at ambient pressure and temperature to give a 2:1 adduct 5 in 86% yield and with very high regio- and stereoselectivity (Scheme 2).7 The (ZZ)-configuration of the double bonds can be deduced from the coupling constants of the alkene protons (J12 = 12.0 – 13.5 Hz). The corresponding (EE)-diastereomer has been described in the literature,8 and it exhibits J12 values in the range 15.2–18.0 Hz. A similar dimerization reaction has been observed in the Ni-catalyzed reactions of Me2(Ph)Si-B(Pinacolate) with terminal alkynes.9
Scheme 2.
Borostannylation of Acetylene at Ambient Temperature and Pressure
Borostannylation of Enyenes
Pd-catalyzed borostannylation of enynes has not been disclosed in the literature. This class of substrates undergoes the addition reaction with surprisingly high chemo-, regio- and stereoselectivity. No complication from the adjacent alkene has been noted. Under the standard conditions described earlier, a variety of 1,3-enynes give very good yields of highly functionalized bis-metallalated dienes. Only one isomer (Z, with a terminal boronate) is detected by NMR (selectivity >19:1). Typical examples are shown in Table 2. Among the enynes studied, 2-phenyl-buta-1-ene-3-yne (6e) alone gave an unsatisfactory yield.
Table 2.
Borostannylation of Enynesa
| entry | enyne | product (% yield)b |
|---|---|---|
| 1. |
6a |
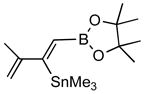 7a (84) |
| 2. |
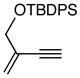 6b |
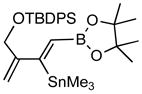 7b (82) |
| 4. |
6c |
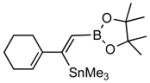 7c (85) |
| 5. |
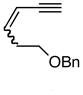 6d |
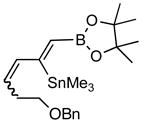 7d (81) |
| 3. |
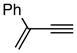 6e |
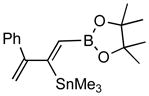 7e (10) |
See Scheme 1 for procedure.
isolated by column chromatography
Applications
The bismetallated alkenes and dienes are valuable intermediates, providing rapid access to stereodefined alkenes and polyalkenes, largely because of the power of cross-coupling reaction such as Stille and Miyaura-Suzuki reactions. The vinyl stannyl group can also be replaced by a bromine or iodine, and the resulting products could serve as electrophilic partners in yet other cross-coupling reactions.4b The value of 1,4-disubstituted borylstannyl-dienes have been amply demonstrated by Coleman who used these compounds to prepare the polyene side-chains of several important natural products.10 The dienes we disclose have different configuratons and are not accessible by previously reported routes.
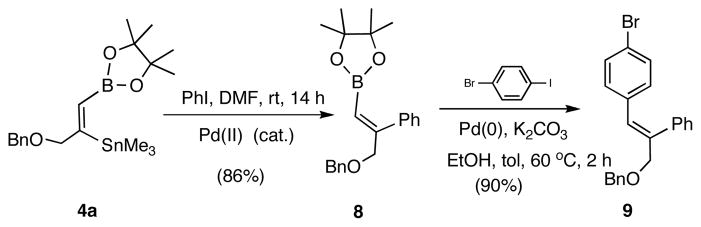
A prototypical application of the 1,2-borylstannyl alkene is illustrated in Scheme 3. The adduct 4a carrying an allylic benzyloxy substituent undergoes Stille reaction with iodobenzene at room temperature giving an 85% yield of 8. The resulting boronate is an excellent substrate for a Suzuki coupling with 4-iodobromobenzene, giving a trisubstituted alkene 9 in over 90% yield. As expected, these reactions proceed with excellent stereoselectivity.
Scheme 3.
Tandem Stille/Suzuki Reactions of a Borylstannyl Alkene
 |
(2) |
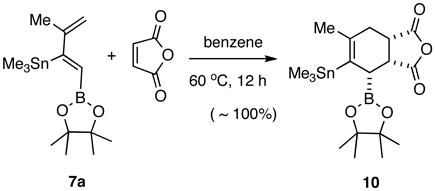
Finally, the utility of the bismetallated dienes for Diels-Alder reaction is illustrated with the example of 7a in eq 2. Formation of a nearly quantitative yield of the endo-adduct 10 suggests that these dienes are quite reactive even with the electron-withdrawing boron substituent.11 Many ways of further elaborating these highly functionalized molecules, including the powerful Vaultier sequence (Diels-Alder followed by allyl boronation),11a can be envisioned.
In summary, we report a simple procedure for the derivatization of hydrolytically unstable primary adducts from borostannylation of alkynes and enynes using a capricious yet very reactive and selective reagent. This procedure preserves the two vinyl metal moieties, still enabling stepwise bidirectional elaboration based on the intrinsically different reactivities of the respective carbon-metal bonds.
Supplementary Material
Acknowledgments
Financial assistance for this research by NSF (CHE-0610349) and NIH (General Medical Sciences, R01 GM075107) is gratefully acknowledged.
Footnotes
Supporting Information. Available full experimental details for the preparation of precursors 6a–6e and 1H and 13C NMR of all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Singidi RR, RajanBabu TV. Org Lett. 2008;10:3351. doi: 10.1021/ol8013792. [DOI] [PubMed] [Google Scholar]
- 2.Palladium-catalyzed additions of 1 to alkynes including reactions that lead to carbocyclic 1,2-bis-alkylidenes were initailly reported by Tanaka et al. See: Onozawa Sy, Hatanaka Y, Sakakura T, Shimada S, Tanaka M. Organometallics. 1996;15:5450.Onozawa Sy, Hatanaka Y, Choi N, Tanaka M. Organometallics. 1997;16:5389.See also: Weber L, Wartig HB, Stammler HG, Stammler A, Neumann B. Organometallics. 2000;19:2891.For the preparaion of the reagent 1, see: Niedenzu K, Rothgery EF. Synth React Inorg Metal-Org Chem. 1972;2:1.For a review, 1,3-Dimethyl-2-trimethylstannyl-2-bora-1,3-diazacyclopentane: Tanaka M. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. John Wiley; New York: 2004.
- 3.Chenard BL, Laganis ED, Davidson F, RajanBabu TV. J Org Chem. 1985;50:3666.Warren S, Chow A, Fraenkel G, RajanBabu TV. J Am Chem Soc. 2003;125:15402. doi: 10.1021/ja035136m.Kumareswaran R, Shin S, Gallou I, RajanBabu TV. J Org Chem. 2004;69:7157. doi: 10.1021/jo049010z.Apte S, Radetich B, Shin S, RajanBabu TV. Org Lett. 2004;6:4053. doi: 10.1021/ol048265w.Trimethylsilyltributylstannane: RajanBabu TV, Shin S. In: Encyclopedia of Reagents for Organic Synthesis. Paquette LA, editor. John Wiley; New York: 2005.
- 4.Bromoboration of an alkyne followed by reaction of the resulting dibromobora-alkene with N, N′-dimethylethylenediamine or pinacol also gives adducts similar to 3 and 4. See: Lallemand JY, Six Y, Ricard L. Eur J Org Chem. 2002:503.Wang C, Tobrman T, Xu Z, Negishi Ei. Org Lett. 2009;11:4092. doi: 10.1021/ol901566e.For another reference to the formation and reactions of borostannylalkenes, see: Lhermitte F, Carboni B. Synlett. 1996:377.
- 5.See Supporting Information for experimental details and full characterization of the products.
- 6.Examples of 2,5-azaborolidine to 2,5-oxaborolidine conversions, see: Biffar W, Nöth H, Schwerthöffer R. Liebigs Ann Chem. 1981:2067.Suginome M, Yamamoto A, Murakami M. Angew Chem Int Ed. 2005;44:2380. doi: 10.1002/anie.200462961.Onozawa Sy, Hatanaka Y, Tanaka M. Tetrahedron Lett. 1998;1998:9043.
- 7.A similar reaction using a Pd-bis-phosphite catalyst has been described in the patent literature. Onozawa, Sy.; Tanaka, M., Jpn. Kokai Tokkyo Koho 2003, 26692. However, experimental and characterization details are not readily available. Trimethylsilyltributylstannane undergoes the expected Pd-catalyzed 1,2-syn-addition to acetylene. See: Murakami M, Matsuda T, Itami K, Ashida S, Terayama M. Synthesis. 2004:1522.
- 8.Coleman RS, Walczak MC. Org Lett. 2005;129:2289. doi: 10.1021/ol050768u. [DOI] [PubMed] [Google Scholar]
- 9.Suginome M, Matsuda T, Ito Y. Organometallics. 1998;17:5233. [Google Scholar]
- 10.(a) Coleman RS, Lu X, Modolo I. J Am Chem Soc. 2007;129:3826. doi: 10.1021/ja070265e. [DOI] [PubMed] [Google Scholar]; (b) Coleman RS, Walczak MC, Campbell EL. J Am Chem Soc. 2005;127:16038. doi: 10.1021/ja056217g. [DOI] [PubMed] [Google Scholar]
- 11.For Diels-Alder reactions of boron substituted 1,3-dienes, see: Vaultier M, Truchet F, Carboni B, Hoffmann RW, Denne I. Tetrahedron Lett. 1987;28:4169.Gao X, Hall DG. Tetrahedron Lett. 2003;44:2231.See also ref 4(a). A recent reviews of the chemistry of boron and silicon substituted dienes, see: Welker ME. Tetrahedron. 2008;64:11529.Toure BB, Hall DG. Chem Rev. 2009;109:4439. doi: 10.1021/cr800296p.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.