Abstract
The tumor suppressor liver kinase B1 (LKB1), also called STK11, is a protein kinase mutated in Peutz-Jeghers syndrome. LKB1 phosphorylates AMP-activated protein kinase (AMPK) and several related protein kinases. Whereas deletion of both catalytic isoforms of AMPK from the pancreatic β-cell and hypothalamic neurons using the rat insulin promoter (RIP2).Cre transgene (βAMPKdKO) diminishes insulin secretion in vivo, deletion of LKB1 in the β-cell with an inducible Pdx-1.CreER transgene enhances insulin secretion in mice. To determine whether the differences between these models reflect genuinely distinct roles for the two kinases in the β-cell or simply differences in the timing and site(s) of deletion, we have therefore created mice deleted for LKB1 with the RIP2.Cre transgene. In marked contrast to βAMPKdKO mice, βLKB1KO mice showed diminished food intake and weight gain, enhanced insulin secretion, unchanged insulin sensitivity, and improved glucose tolerance. In line with the phenotype of Pdx1-CreER mice, total β-cell mass and the size of individual islets and β-cells were increased and islet architecture was markedly altered in βLKB1KO islets. Signaling by mammalian target of rapamycin (mTOR) to eIF4-binding protein-1 and ribosomal S6 kinase was also enhanced. In contrast to Pdx1-CreER-mediated deletion, the expression of Glut2, glucose-induced changes in membrane potential and intracellular Ca2+ were sharply reduced in βLKB1KO mouse islets and the stimulation of insulin secretion was modestly inhibited. We conclude that LKB1 and AMPK play distinct roles in the control of insulin secretion and that the timing of LKB1 deletion, and/or its loss from extrapancreatic sites, influences the final impact on β-cell function.
Keywords: AMP-activated protein kinase, β-cell, insulin secretion, food intake, liver kinase B1, pancreas
liver kinase b1 (LKB1, also called STK11) is a potent tumor suppressor whose inactivation in Peutz-Jeghers syndrome (PJS) (21) is characterised by melanotic macules, hamartomatous polyps in the gastrointestinal tract, and increased risk of all cancers (7). LKB1 is a partial mammalian homolog of the Saccharomyces cerevisae kinases Elm1, Pak1, and Tos3, which phosphorylate yeast snf1 (47), the yeast homolog of mammalian AMP-activated protein kinase (AMPK) (47). Activation of AMPK, a target of several glucose-lowering agents used in diabetes treatment, including metformin and the thiazolidenediones (31), stimulates insulin action in peripheral tissues, acting to phosphorylate and stimulate the TSC1:TSC2 complex, which subsequently inactivates mammalian target of rapamycin/regulatory associated protein of mTOR (mTOR/Raptor) (3). AMPK is also implicated in the control of β-cell survival (27, 39, 40) and insulin secretion (11, 12, 42). In the mediobasal hypothalamus, changes in AMPK activity in proopiomelanocortin (POMC)-, agouti-related peptide (AgRP)-, and neuropeptide Y (NPY)-expressing neurons are also implicated in the control of feeding and body weight (9, 33). Recent data (4) have suggested that calmodulin-dependent protein kinase kinase-β (CaMKKβ), another upstream kinase for AMPK (20), is involved in these cells.
Compelling evidence for a conserved role for an LKB1-AMPK signaling cassette comes from studies in Caenorhabditis. elegans, where inactivation of the corresponding homologs leads to reversal of the dauer phenotype (36), characterized by metabolic inhibition and growth arrest. LKB1 and AMPK are also implicated in the control of cell polarity. Thus, deletion of the LKB1 homologues in Drosophila melanogaster (dLKB1) and C. elegans (par4) disrupts epithelial cell polarity (26, 32), and in Drosophila this change is rescued by transgenic overexpression of AMPKα (48). Although forced overexpression of LKB1 induces cell polarization in intestinal epithelial cancer cell lines (5), the requirement for LKB1 in maintaining the polarity of mammalian cells is less clear (44). However, there is also growing evidence that the effects of LKB1 in mammalian cells may be, at least in part, independent of AMPK, since LKB1 phosphorylates 11 further kinases of the AMPK subfamily in vitro (30, 41).
We (46) have recently demonstrated that deletion of both catalytic isoforms of AMPK from the pancreatic β-cell by use of the rat insulin promoter (RIP2).Cre transgene (βAMPKdKO mouse) leads to impaired glucose tolerance and defective insulin secretion in vivo but enhanced glucose-stimulated insulin secretion from isolated islets. However, and in marked contrast, Fu et al. (16) and Granot et al. (18) have demonstrated that deletion of LKB1 from the pancreatic β-cell (and probably intestinal incretin-producing cells) from adult mice by means of an inducible Pdx1-CreER transgene leads to increased insulin production and enhanced β-cell size. To determine whether these markedly divergent phenotypes may reflect true biological differences in the roles of LKB1 and AMPK in the pancreatic β-cell rather than being the result of differences in the timing and sites of deletion, using the RIP2.Cre transgene, we have therefore generated mice in which LKB1 is deleted in the β-cell.
Inactivation of LKB1 in β-cells with this strategy led to marked increases in β-cell size and insulin production in vivo, consistent with the findings using Pdx1-CreER-mediated deletion (16, 18). However, and in contrast to the latter model, β-cell glucose signaling and insulin secretion were inhibited in vitro. Importantly, the above alterations in βLKB1 mice contrast sharply with the effects of deleting both AMPKα subunits using the same RIP2.Cre transgene (46), wherein unaltered β-cell mass and a decrease in mean β-cell size are observed. In addition, we observed a decrease in body weight and improved glycemia but unaltered insulin sensitivity, suggestive of a role for LKB1 in RIP2.Cre neurons to control satiety distinct from that of AMPK. Overall, the present findings indicate that LKB1 and AMPK control distinct signaling pathways in the β-cell to regulate insulin production. The results also support the view that inhibition of LKB1, or its downstream targets, may be a useful approach to increase β-cell mass in some forms of insulin-secretory insufficiency, including type 2 diabetes, and that these changes are likely to be mediated by member(s) of the AMPK superfamily distinct from AMPKα.
METHODS
Generation of Mutant Mice Selectively Lacking LKB1 in Pancreatic β-Cells and RIP2.Cre Neurons
Mice homozygous for flox’d alleles of the lkb1/stk11 gene (Mouse Models of Human Cancer Consortium, http://mouse.ncifcrf.gov/) were first crossed with heterozygous RIP2.Cre-expressing transgenic mice (expressing Cre recombinase under the rat insulin 2 promoter; Jackson Laboratory). The resulting double heterozygous LKB1fl/+,Cre+ were intercrossed with LKB1fl/+ mice to generate βLKB1KO (LKB1fl/fl,Cre+), βLKB1het (LKB1fl/+,Cre+), and βLKB1Wt (LKB+/+,Cre+). βLKB1KO, βLKB1het, and βLKB1Wt mice were born at the expected Mendelian ratios and kept on a mixed FVB/129S6 and C57BL/6 background.
Mouse Maintenance and Diet
Mice were housed with 2–5 animals per cage in a pathogen-free facility on a 12:12-h light-dark cycle. Mice were fed ad libitum with a standard mouse chow diet or a high-fat diet [60% (wt/wt) fat content; Research Diet, New Brunswick, NJ]. Where indicated, 4-wk-old mice were transferred onto high-fat diet for a period of 6 wk. All in vivo procedures stated were performed in the Imperial College Central Biomedical Service (CBS) and approved by the UK Home Office according to the Animals Scientific Procedures, Act of 1986.
Body Weight and Food Intake
Fed or 15-h overnight-fasted mice were weighed at the age of 6–8 wk. Food intake was measured daily at fed status either for 3 consecutive days or at 30 min and 1 h after refeeding the mice fasted for 15 h using a metabolic cage.
In Vivo Physiological Studies
Intraperitoneal glucose tolerance test.
Mice fasted for 15 h (water allowed) were intraperitoneally injected with 1 g glucose/kg mouse wt. Blood from the tail vein was obtained at 0, 15, 30, 60, 90, and 120 min after injection. Blood glucose levels were measured with an automatic glucometer (Accuchek; Roche, Burgess Hill, UK).
Plasma insulin measurement.
Mice fasted for 15 h were intraperitoneally injected with 3 g glucose/kg mouse wt. Blood from mice's tail veins was collected into a heparin-coated tube (Sarstedt, Beaumont Leys, UK) at 0, 15, and 30 min after injection. Plasma was separated by centrifuging the blood at 2,000 g for 5 min. Plasma insulin levels were measured using an ultrasensitive mouse insulin ELISA kit (Mercodia, Uppsala, Sweden). Normal fed plasma insulin levels were measured from blood collected from 6- to 8-wk-old mice's tail veins between 10:00 and 11:00 AM.
Insulin tolerance tests.
Bovine insulin (Sigma, Dorset, UK; 0.75 U/kg) was intraperitoneally injected into fed mice. Blood glucose levels were measured at 0, 15, 30, and 60 min after injection as above.
Islet Isolation and In Vitro Insulin Secretion Measurement
Islet isolation by in situ collagenase digestion, and in vitro insulin secretion measurement, were performed as previously described (28).
RNA Extraction and RT-PCR
Total cellular RNA from mouse islets or other tissues was obtained using TRIzol reagent (Invitrogen, Paisley, UK), and RNA was further purified against DNA contamination with a DNA-free kit (Applied biosystems, Warrington, UK). Total RNA (1.5–2 μg) was then reverse transcribed into cDNA with a high-capacity reverse transcription kit (Applied Biosystems) according to the manufacturer's instructions. To detect deletion of LKB1 exons 3 to 6, two pairs of primers within exon 1 (LKB fwd: AGGTGAAGGAGGTGCTGG) and 8 (LKB rev: TCTGGGCTTGGTGGGATA) were designed (Fig. 1A). The PCR reaction was preheated at 95°C for 2 min, and amplification was performed for 30 cycles under the following conditions: 94°C for 30 s, 57°C for 30 s, and 72°C for 1 min. At the end of the last cycle, a prolonged extension step was carried out at 72°C for 10 min.
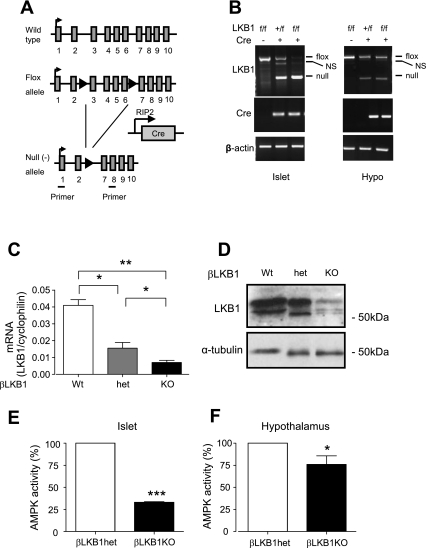
Fig. 1.
Generation of βLKB1KO mice. A: schematic representation of deletion of flox’d lkb1 exons (exons 3–6) driven by RIP2.Cre expression. Location of primers used for PCR as indicated. Black arrows, flox sites; gray bar, LKB1 exons. B: RT-PCR analysis of effects on LKB1 transcript levels of deleting exons 3–6 in pancreatic islets and hypothalamus (Hypo). Product sizes were 864 and 300 bp for flox’d and null alleles, respectively. NS, nonspecific. C: qRT-PCR (C) and Western blot analysis (D) of LKB1 mRNA and protein levels in pancreatic islets of βLKB1KO mice and their heterozygous (het) and wild-type (Wt) controls. Total AMPK activity in islets (E) and hypothalamus (F) of βLKB1KO and βLKB1het mice. Data are expressed as means ± SE; n = 4–5 mice per genotype. *P < 0.05, **P < 0.01, ***P < 0.001.
qRT-PCR
The expression levels of lkb1/stk11, kir6.2, sur1, glut2, ki67, insulin (ins1 and ins2), glucagon, gck, NPY, POMC, and AgRP genes were quantitated by real-time PCR using the SYBR Green method (11).
Immunoprecipitation, AMPK Activity Measurement, and Western (Immuno-) Blot Analysis
To determine total AMPK activity in islet extracts, 10 μg of total protein from 150–200 islets isolated from fed mice was incubated in RPMI supplemented with 11 mM glucose for 3–5 days prior to AMPK assay with synthetic SAMS peptide (HMRSAMSGLHLVKRR) as substrate (28). To determine AMPKα1 and -α2 activities in the hypothalamus, dissected tissue was placed in liquid nitrogen immediately after extraction and lysed in 800 μl of ice-cold lysis buffer [in mM: 50 Tris·HCl (pH 7.4, 4°C), 250 sucrose, 50 NaF, 1 Na pyrophosphate, 1 EDTA, 1 EGTA, 1 DTT, 0.1 benzamidine, and 0.1 PMSF, 5 μg/ml soybean trypsin inhibitor, and 1% (vol/vol) Triton X-100] [with 10% (wt/vol) sucrose in lysis buffer for hypothalamus]. Total extracts (100 μg of protein) were used for immunoprecipitating with anti-AMPKα1/α2 antibodies (Upstate, Dundee) conjugated to protein G-Sepharose and were subjected to AMPK activity measurement as above. For Western blot analysis, 50 μg of protein from 300–400 islets was used.
Immunohistochemistry and Analysis of Islet Architecture
Isolated pancreata were fixed in 10% (vol/vol) formalin and embedded in paraffin wax within 24 h of removal. Head-to-tail sections (5 μm lengthwise) were cut and incubated at 37°C overnight on superfrost slides. Slides were submerged sequentially in 100% (vol/vol) xylene followed by decreasing concentrations of industrial methylated spirits for removal of paraffin wax. Antigen epitopes were then retrieved (de-cross-linked) in Tris-EDTA-0.05% (vol/vol) Tween buffer (pH 9.0). Slides were subsequently blocked in 5% (vol/vol) goat serum in Tris-bbuffered saline with 0.05% (vol/vol) Tween (TBS-T) for 20 min at room temperature and then incubated in a mixture of primary antibodies at the concentrations indicated at 4°C overnight, After being washed in TBS-T for three times of 5 min each time, slices blotted with primary antibodies were visualized with Alexa fluor 568- or 488-conjugated IgG (1:500; Invitrogen, Paisley, UK) under fluorescent microscopy using a Zeiss Axiovert-200 confocal microscope with an Improvision/Nokigawa spinning disc and running Volocity 5.0 (Improvision, Coventry, UK) software (10).
To quantify the number of “rosette-like” structures (i.e., 8–10 cells arranged concentrically around an identifiable central “hub”; see results) in islets, we used E-cadherin and DAPI staining of pancreatic sections. Structures were included where the void at the center was negative for DAPI. Ten islets from three pairs of mice per genotype were assessed.
Antibodies
Antibodies used in Western (immuno-)blot analysis and immunohistochemistry were the following: rabbit anti-LKB1 (Millipore, Watford, UK), rabbit anti-E-cadherin, anti-phospho-S6 ribosomal protein (Ser235/236), anti-VEGFR2, rabbit anti-phospho-4E-BP1 (New England Biolabs, Hitchin, UK), mouse anti-actin (C2) (Santa Cruz Biotechnology, Heidelburg, Germany), rabbit anti-mouse GLUT2 (kind gift from Dr. Bernard Thorens, Lausanne), guinea pig anti-insulin, rabbit anti-glucagon (Dako, Ely, UK), rabbit monoclonal anti-Ki67 (Epitomics, Burlingame, USA), mouse anti-α-tubulin, anti-β-tubulin (Sigma Aldrich, Dorset, UK), and rat anti-ZO1 (kind gift from Dr. Paolo Meda, Geneva).
Optical Projection Tomography (OPT) and Determination of Relative β-Cell Mass, Single β-Cell Size, and Proliferation
Whole pancreatic optical projection tomography, to 19 μm resolution, was performed as described (2). Briefly, whole pancreata fixed in 4% (wt/vol) paraformaldehyde for 2–3 h at 4°C were dehydrated and subjected to five cycles of freezing and thawing (−80°C to room temperature). Pancreata were then blocked overnight in Tris-buffered saline, pH7.5, containing 0.05% (vol/vol) Tween 20, 0.01% (wt/vol) sodium azide and 10% (vol/vol) goat serum, (Dako) before incubation with guinea pig anti-swine insulin antibody (Dako, 1:1,000) dissolved in the above buffer further supplemented with 5% (vol/vol) dimethylsulfoxide overnight at 4°C. To visualize insulin-positive staining, Alexa 594 goat anti-guinea pig antibody (Invitrogen, 1:1,000) was applied. The sample was then embedded in 1% (wt/vol) low melting temperature agarose, dehydrated in methanol, and cleared in benzyl alcohol-benzyl benzoate (1:2) for optical tomographical scanning.
All specimens were scanned using two fluorescent channels (excitation: 580 ± 15 nm and emission: 650 ± 15 nm for insulin-positive staining; excitation: 480 ± 9 nm and emission: 650 ± 25 nm for autofluorescence). The raw data for these two channels were reconstructed in a pair of 3-D voxel data sets (voxel to μm = 1:19.5) using Matlab software, and cell volumes (μm3) from each channel were measured using Volocity 5.0 (Improvision). Data from Volocity 5.0 were further analyzed in Microsoft Excel to provide size frequency histograms and total volume graphs. Relative β-cell mass was calculated by dividing the volume of insulin-positive area (total β-cell mass) by that of the autofluorescence area (total pancreatic mass).
To calculate single β-cell size, the area of 100 single β-cells from five islets of each genotype from E-cadherin- and insulin-costained pancreatic sections were measured using Image J (http://rsbweb.nih.gov/ij/). To measure relative number of proliferating β-cells, the number of Ki67-positive β-cells per islet was divided by total β-cell number; 15–20 islets per genotype were analyzed.
Electrophysiological Measurements and Ca2+ Imaging
The plasma membrane potential of β-cells was recorded in perforated-patch whole cell configuration using an EPC9 patch clamp amplifier controlled by Pulse acquisition software (HEKA Elektronik, Lambrecht/Pfalz, Germany). The pipette tip was dipped into pipette solution (see below),and then back-filled with the same solution containing 0.24 mg/ml amphotericin B. Recordings were initiated after 30-min exposure to substrate-free solutions at 37°C, and the duration of exposure to each concentration of effector(s) was ≥2 min. Cells that were not responsive to tolbutamide were excluded from analysis.
Series resistance and cell capacitance were compensated for automatically by the acquisition software. Experiments were carried out by periodically switching from current-clamp to voltage-clamp mode, thus obtaining pseudo-simultaneous recordings of cell membrane potential (Vm) and KATP conductance (GKATP). This controlled for the leaks of the patch and verified that the depolarization (hyperpolarization) of the membrane was linked to KATP channel closure (opening). The current-clamp protocol involved continuous recording without electrical stimulation. In the voltage clamp, the membrane potential was held at −70 mV, and whole cell currents were evoked by ±10-mV 0.5-Hz pulses. Data were filtered at 0.2 kHz and digitized at 0.5 kHz.
The pipette solution contained (in mmol/l): 76 K2SO4, 10 NaCl, 10 KCl, 1 MgCl2, and 5 HEPES (pH7.35 with KOH). The bath solution contained (in mmol/l): 137 NaCl, 5.6 KCl, 10 HEPES (pH 7.4 with NaOH), 2.6 CaCl2, and 1.1 MgCl2. All experiments were conducted at 33–37°C, and the bath solution was perifused continuously.
For Ca2+ imaging, dispersed islets were incubated for 30 min in Krebs-Ringer Buffer [KRB; in mmol/l: 125 NaCl, 3.5 KCl, 1.5 CaCl2, 0.5 NaH2PO4, 0.5 MgSO4, 3 glucose, 10 HEPES, and 2 NaHCO3, pH 7.4, and equilibrated with O2-CO2 (95:5) and supplemented with 0.1% (wt/vol) BSA] containing 3 mmol/l glucose and 200 nM FURA-RED AM (Invitrogen UK). Cells were stimulated using the conditions indicated and excited at 480/440 nm using an Olympus IX-81 microscope coupled to an F-view camera and captured using Cell^R software (Olympus UK) on a ×40 oil objective. Data were expressed at the ratio of the fluorescence emission at 440/480 nm.
Statistical Analysis
Data are expressed as means ± SE. Significance was tested by Student's two-samples unpaired or paired Student's t-tests using Excel, or ANOVA test using Graphpad 4.0. P < 0.05 was considered significant.
Results
βLKB1KO Mice Are Lean and Hypophagic and Display Improved Glucose Tolerance
To generate mice lacking LKB1 selectively in the pancreatic β-cell from midgestation (E9–11.5) and in a subset of hypothalamic neurons (“RIP2.Cre neurons”) (17), we crossed animals bearing flox’d Lkb1/Stk11 alleles with RIP2.Cre mice (Fig. 1A). Demonstrating efficient deletion from β-cells, levels of endogenous LKB1 mRNA were markedly reduced in islets from homo- (fl/fl) vs. heterozygous (+/fl) mouse islets in the presence of the Cre transgene, with the consequent increase in the level mRNA encoded by the null allele (Fig. 1B). Similarly, the null transcript was also present in hypothalamic extracts of hetero- and homozygous mice (Fig. 1B). Levels of LKB1 mRNA (Fig. 1C) and immunoreactivity (Fig. 1D) in islets from mice of each genotype were compared next. Each parameter was decreased by 70–80% in islets from βLKB1KO (fl/fl,Cre+) vs. wild-type (+/+,Cre+) mice, consistent with deletion from the islet β-cell compartment. Levels of LKB1 mRNA and immunoreactivity in βLKB1het mouse islets were intermediate between those in βLKB1Wt and βLKB1KO mouse islets (Fig. 1, C and D).
Since LKB1 is likely to be the major upstream kinase for LKB1 in islets (I. Leclerc and G. A. Rutter, unpublished results), AMPK activities were also measured as a further readout of LKB1 activity. Total cellular AMPK activity in isolated islets was reduced by ∼75% in βLKB1KO vs. βLKB1het (Fig. 1E) (comparison with levels of AMPK activity in wild-type islets was not performed). AMPKα1/α2 activities were reduced by ∼25% in hypothalamic extracts from βLKB1KO vs. βLKB1het mice (Fig. 1F), consistent with deletion from a subset of RIP2.Cre neurons within this tissue and/or regulation of AMPK in this tissue by distinct upstream kinases (e.g., CaMKKβ) (20).
Examined at 6–8 wk, male (Fig. 2) and female (not shown) βLKB1KO mice displayed decreased fed body weight (Fig. 2A) and daily food intake (Fig. 2B) compared with βLKB1Wt or βLKB1het mice. In addition, after 15 h of starvation, βLKB1KO mice tended to eat less in the first 30 min after refeeding (Fig. 2C). Reduced hypothalamic expression of the orexigenic peptide NPY mRNA and the anorexigenic peptide POMC were also observed (Suppl. Fig. 1A; supplementary materials are found in the online version of this paper at the Jounal website), although in each case the differences were only apparent between wild-type and heterozygous mice, suggesting they are unlikely to drive the marked differences in feeding between knockout and heterozygous animals (Fig. 1). Interestingly, we have observed in preliminary experiments increases in the immunoreactivity of POMC in hypothalamic extracts of βLKB1KO mice (data not shown), suggesting that LKB1-dependent posttranscriptional mechanisms may modify the levels of this factor. Further studies will be required to explore this possibility in detail.
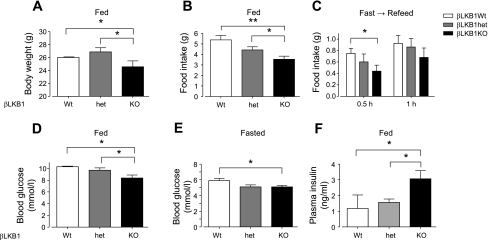
Fig. 2.
Food intake and glucose homeostasis in βLKB1KO mice. A–C: body weight (A), and food intake of βLKB1KO mice fed (B) or refed after fasting for 15 h (C). D–F: blood glucose (D and E) and plasma insulin (F) of βLKB1KO mice fed or fasted for 15 h. Male mice from 6–8 wk old were used. Data are expressed as means ± SE; *P < 0.05, **P < 0.01; n = 7–10 mice per genotype.
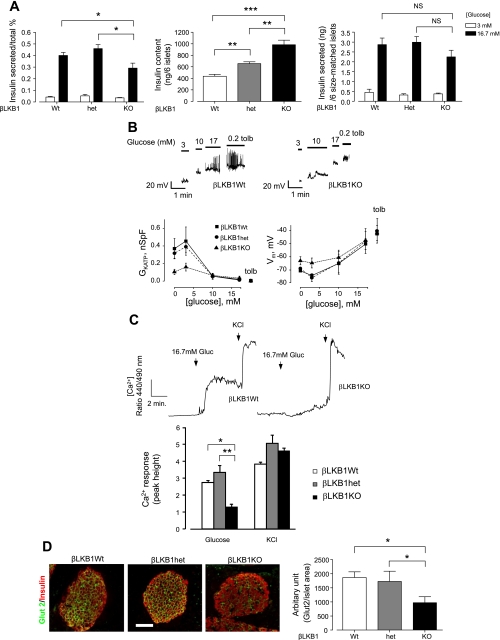
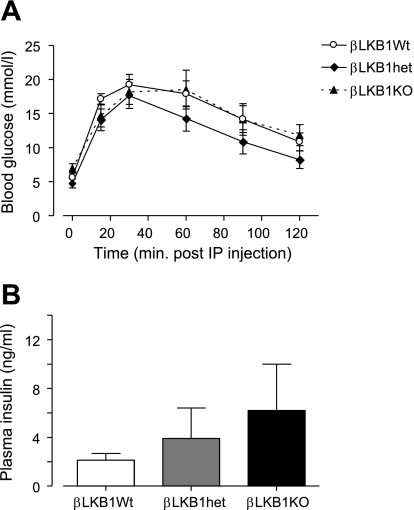
Fasting and fed glycemia were significantly decreased in βLKB1KO mice (Fig. 2, D and E), a change accompanied by substantial increases in plasma insulin levels in the fed state (Fig. 2F). Correspondingly, βLKB1KO mice displayed dramatically improved glucose tolerance (Fig. 3A) and insulin release (Fig. 3B) in vivo compared with βLKB1Wt or βLKB1het mice but unchanged insulin sensitivity (Fig. 3C).
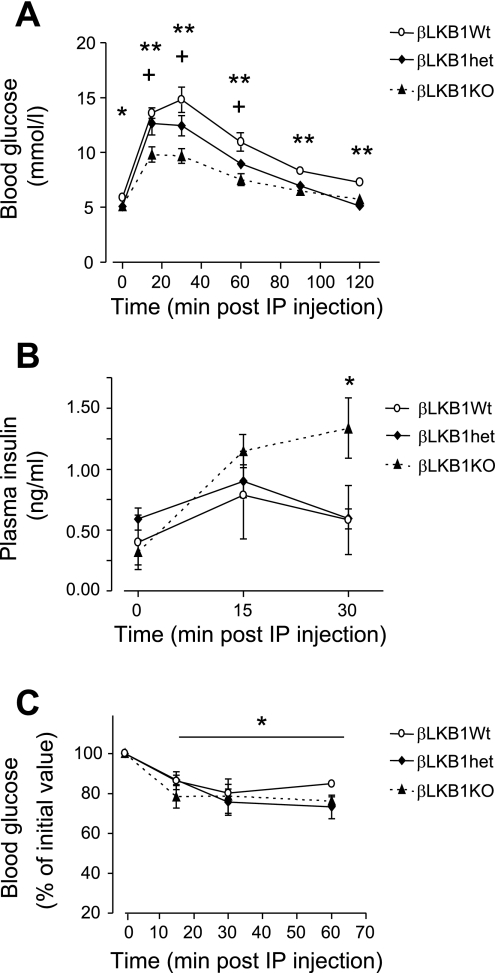
Fig. 3.
Glucose and insulin tolerance of βLKB1KO mice. Glucose tolerance (A), and plasma insulin response (B) after intraperitoneal (ip) glucose injection, and whole body insulin sensitivity (C) monitored after ip insulin injection of βLKB1KO or control mice. Male 6- to 8-wk-old mice old were used. Data are expressed as means ± SE. For A and B, *P < 0.05, **P < 0.01 for βLKB1KO vs. βLKB1Wt, +P < 0.05 for βLKB1KO vs. βLKB1het; in C, *P < 0.05 with respect to time 0 for all genotypes; n = 7–10 mice per genotype.
LKB1 Controls β-Cell Size, Morphology, and Islet Architecture
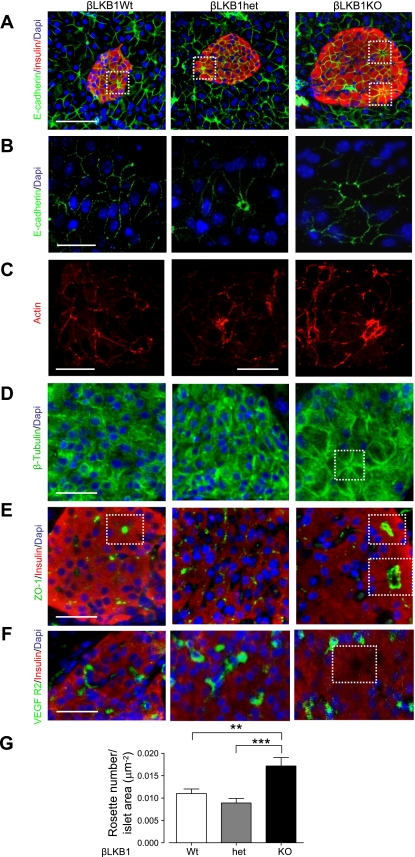
To examine in detail the possible mechanisms behind the hyperinsulinism in βLKB1KO mice, we used OPT (2). This technique, which we have previously validated by comparison with conventional measurements of β-cell mass based on histochemistry (46), allowed us to determine the mean and total volume, size, distribution, and number of islets within the entire pancreas (Fig. 4, A–D, Suppl. Fig. 2, and Supplementary movies: betaLKB1wt, betaLKB1Het, betaLKB1KO). Total β-cell mass was markedly increased with respect to βLKB1wt mice, and the mean volume of individual islets was significantly elevated by ∼30% in βLKB1KO vs. βLKB1het and ∼20% in βLKB1KO vs. βLKB1wt pancreata. The most marked increase was apparent in the number of the largest islets present (>107 μm3; ∼10,000 cells) and certain small islets. Although there were no evident changes in the ratio of β- to α-cells within islets (Fig. 4E), we observed a significant 40% increase in the surface area of individual β-cells, as identified by staining with E-cadherin antibodies (see Methods; Fig. 4F). Demonstrating an increase in β-cell proliferation, Ki67 staining was markedly increased in βLKB1KO islets (Fig. 4G).
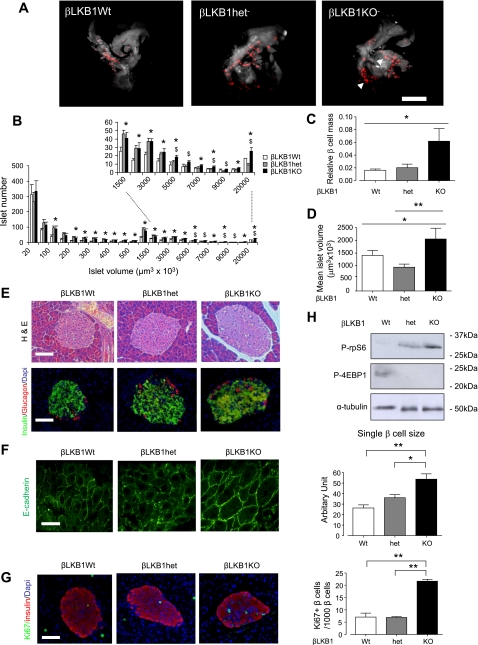
Fig. 4.
Altered islet morphology in βLKB1KO mice. A: representative optical projection tomographic (OPT) images of whole pancreas. OPT was performed as described under methods. Red staining indicates insulin-positive structures (islets), while the outline of the whole pancreas was apparent as autofluorescence and is presented as white/gray shading. Images shown correspond to 3-D projections; (see also Suppl. Fig. 2 and Suppl. Movies betaLKB1wt, betaLKB1het, and betaLKB1KO.avi.) Note presence of a particularly large islet in the βLKB1KO pancreas (right image, arrow). B: distribution of islet volumes with marked (dotted lines) section magnified. C: relative β-cell mass; D: mean islet volume. Data are from 5–6 mice per genotype. Scale bar, 500 μm. E: hematoxylin-eosin (H&E) and immunofluorescent staining of pancreatic sections using guinea pig anti-insulin (1:200; green) and rabbit anti-glucagon (1:100; red) antibodies. Nuclei are shown with DAPI (blue) staining. Scale bar, 75 μm. F: representative E-cadherin staining of pancreatic sections and quanification of single islet β-cell sizes. Average area of 100 single β-cells from 5 islets of each genotype, costained in pancreatic sections for E-cadherin and insulin, were analyzed. Scale bar, 12 μm. G: staining for proliferation marker Ki67 and quantification, based on 15–20 islets per pancreas; n = 3 mice per genotype. H: Western blot analysis of mTOR signaling pathway markers phospho-ribosomal protein (rp)S6 and phospho-4E-BP1 of pancreatic islet extracts from βLKB1KO and control mice. Islets extracted from fed mice were incubated in RPMI supplemented with 11 mM glucose for 16 h and lysed for analysis. Data are expressed as means ± SE. *P < 0.05, **P < 0.01. In B, *P < 0.05, for βLKB1KO vs. βLKB1Wt, $P < 0.05 for βLKB1KO vs. βLKB1het.
Since mTOR signaling (3) is implicated in controlling cell growth and protein synthesis, phosphorylation of ribosomal protein subunit S6 (rpS6) and eIF4-binding protein-1 (4E-BP1) within islets was measured. As expected, increased phospho-rpS6 and decreased phospho-4E-BP1 were detected in βLKB1KO mice islets compared with βLKB1Wt or βLKB1het mouse islets (Fig. 4H).
To determine whether LKB1 may be critical for the establishment of basolateral-apical polarity of β-cells, we examined the distribution of the adherens junction marker E-cadherin, as well as actin, tubulin, and the tight junction protein zona occludins 1 (ZO1). Unexpectedly, polarization was not compromised in LKB1-deficient β-cells (Fig. 5). Instead, mutant islets displayed a significant increase in the number of rosette-like structures or “islet acini” (15) identified by E-cadherin and ZO1 staining in which 8–10 cells were arranged around a central void (6) (Fig. 5, A, B, E, and G). Strikingly, nuclei were positioned in the cells comprising these rosettes away from the apical pole, while actin and tubulin staining were concentrated at the junctional zones (Fig. 5, C and D). Whereas the apical “voids” were devoid of staining for VEGFR, this was enriched at the basolateral surface, indicating the presence of capillaries at the latter site (Fig. 5F).
Fig. 5.
β-Cell polarity is enhanced in βLKB1KO mice with increased rosette-like (“islet acini”) structures and accumulation of cell junction proteins. A–E: immunofluorescence staining of pancreatic sections for the adherens junction marker (A and B) E-cadherin (1:100, green), (C) actin filament (1:50, red; note imaged areas were confirmed as lying over an islet by inspection of corresponding bright field images), (D) β-tubulin (1:100, green), (E) tight junction marker zona occludins 1 (ZO1, whole serum; green) or capillary marker (F) VEGFR2 (1:100, green). White square, voids at center of rosettes. G: quantification of no. of rosette-like structures per islet in βLKB1KO mice based on E-cadherin and DAPI staining. Such structure in islet is counted as 1 by E-cadherin staining where the voids at the center of the rosette is absent of DAPI staining. Ten islets from 3 pairs of mice per genotype were assessed. Data are expressed as means ± SE. **P < 0.01, ***P < 0.001. Scale bars, 50 μm (A), 12 μm (B), 10 μm (C), and 25 μm (D–F).
LKB1 Deletion Diminishes β-Cell Responses to Glucose Ex Vivo
To determine whether the dramatic improvements in glucose tolerance in vivo might also in part reflect enhanced β-cell glucose sensitivity, we isolated islets and single cells from βLKB1KO and βLKB1Wt or βLKB1het mice. Glucose-stimulated insulin secretion (GSIS) under static incubation was diminished in βLKB1KO mouse islets by ∼30% compared with βLKB1Wt mouse islets (Fig. 6A), although the absolute insulin output, as assessed from six size-matched islets, was not significantly different between the genotypes (Fig. 6A). By contrast, the insulin content of similarly sized islets from βLKB1KO mice was substantially increased (2.5-fold vs. βLKB1Wt and 1.5-fold vs. βLKB1Het; Fig. 6A). Glucose-stimulated changes in the conductance of ATP-sensitive K+ channels (GKATP), membrane depolarization (Vm), and increases in intracellular free Ca2+ concentration were all markedly attenuated in β-cells from βLKB1KO islets (Fig. 6, B and C). In addition, membrane electrical activity was elevated at basal and 3 mM glucose levels in βLKB1KO islet β cells, suggesting blunted glucose sensing in these cells (Fig. 6B).
Fig. 6.
βLKB1KO β-cells display abnormal electrical, Ca2+, and secretory responses and decreased GLUT2 immunoreactivity at the plasma membrane. A: glucose-stimulated insulin secretion and total insulin content of 6 size-matched islets statically incubated with indicated glucose for 0.5 h from βLKB1KO, heterozygous, and WT mice; n = 3 mice per genotype. B: representative traces of whole cell KATP channel conductance (GKATP) and plasma membrane potential (Vm) from perforated patch clamp measurements. Three to six β-cells from 3 pairs of mice of each genotype were recorded. C: representative traces and quantification of free [Ca2+] with fura 2-AM in dissociated β-cells in βLKB1KO mice. Cells (38–60) from 3 pairs of mice of each genotype were analyzed. D: immunofluorescence staining and quantification of Glut2 expression at the plasma membrane of pancreatic islet β-cells. Fifteen islets from 2 pairs of mice per genotype were examined. Scale bar, 75 μm. Data are expressed as means ± SE. *P < 0.05, **P < 0.01, ***P < 0.001.
Providing a possible contribution to the impaired GSIS from βLKB1KO islets, we observed a substantial decrease in the level of GLUT2 (slc2A2) immunoreactivity at the plasma membrane of LKB1-deleted cells (Fig. 6D) and a correspondingly large (>90%) reduction in GLUT2 mRNA in isolated islets (Suppl. Fig. 1B). Likewise, the levels of mRNAs encoding the ATP-sensitive KATP channel subunit kir6.2 (Suppl. Fig. 1B) were substantially (>80%) decreased in βLKB1KO vs. βLKB1wt islets. By contrast, we observed no significant decreases in the expression of other genes involved in maintaining the differentiated function of β cells, including Pdx1, nor in SUR1 (ABCC8) or glucokinase (Gck; Suppl. Fig. 1), nor in neuroD1 or MafA (LKB1fl/fl.RIP2.Cre+ vs. LKB1fl/+.RIP2.Cre+, not shown) suggesting that a generalized decrease in β-cell differentiation did not occur.
Effect of Metabolic Stress on βLKB1KO vs. Wild-Type Mice
Since LKB1 and AMPK are implicated in the cellular stress responses, we determined how LKB1 deficiency in β-cells and hypothalamus may impact on the diabetogenic effects of a high-fat diet (1). After 6 wk on a high-fat diet, differences in glucose tolerance (Fig. 7A) or insulin secretion (Fig. 7B) were no longer apparent between genotypes, consistent with a greater susceptibility of βLKB1KO mice to the effects of this metabolic stress.
Fig. 7.
In vivo metabolic advantages of LKB1 deletion from insulin-expressing cells are lost on a high-fat diet. Glucose tolerance (A) and plasma insulin changes (B) after ip glucose injection of βLKB1KO mice on a high-fat diet for 6 wk (see methods).
DISCUSSION
LKB1 Regulates β-Cell Size and Islet Architecture
The principal aim of the present study was to determine whether, when compared using a near-identical strategy for deletion from β-cells with that used previously for AMPKα subunits (46), the loss of LKB1 may lead to similar changes in insulin secretion and glucose homeostasis in vivo and in vitro. This has seemed important given the dramatically different phenotypes of mice deleted for LKB1 in adults using a Pdx-1-CreER transgene (16, 18) and those deleted for AMPK using the RIP2.Cre transgene (46), with the latter strategy resulting in earlier (midgestational) deletion and some loss from hypothalamic nuclei. Although the genetic background of the LKB1-deleted mice generated here (FVB/129S6/C57BL6) and in earlier studies (16, 18) differs slightly from that of those deleted for AMPK (C57BL6) (46), it seems unlikely to us that this difference could explain the markedly different phenotypes of mice deleted for LKB1 vs. AMPK in the β-cell.
Our findings confirm the view that LKB1 is a critical regulator of β-cell development and function. First, we demonstrated that the size of individual β-cells was considerably increased, as was that of individual islets, as assessed by OPT. Interestingly, and as shown in Fig. 4D, the mean islet volume, as determined by OPT, was increased ∼40% in βLKB1 KO vs. wild-type islets. This compares with an increase in average (single) β-cell area of ∼60% (Fig. 4F), equivalent to an increase in volume of more than two fold. The difference between these values seems likely to be due to the contribution of an unchanged volume of other cell types within the islets, and/or a lowered number of β-cells per islet.
As also recently reported (2), the distribution of islets in wild-type mouse pancreata was highly nonuniform, with the largest islets representing as much as 50% of the total β-cell mass. Using the same approach in βLKB1KO islets, we demonstrated that the abundance of the largest islets was significantly increased. This shift seems likely to be the result, at least in large part, of an increase in the average volume of individual β-cells (∼60%), as well as increases in β-cell proliferation. TSC1/2 deletion in β-cells, using the same RIP2.Cre mice, leads to increased β-cell mass (35), a phenotype similar to what we describe here in LKB1KO mice. Increased phosphorylation of rpS6 and decreased phosphorylation of 4E-BP1 in βLKB1KO mice islets suggested that the increased β-cell size and islet volume are likely to be due to elevated mTOR signaling.
The present results also confirm the view (16, 18) that LKB1 is involved in the establishment of cell polarity in mammalian epithelial cells, consistent with findings from Shorning et al. (44) in intestinal paneth and goblet cells, as well as the findings in β-cells (16, 18). Thus, we found that rosette-like arrangements of β-cells (6), containing strikingly polarized β-cells, were twice as frequently observed in βLKB1KO islets. We considered the possibility that this may be due simply to an increase in the size of individual cells. Arguing against this possibility, Hamada et al. (19) demonstrated an increase in β-cell volume of ∼60% after transgenic overexpression of mTORC1 in β-cells, similar to the increase observed after lkb1 knockout. However, no obvious increase in the number of rosettes was apparent in the case of mTORC1-deleted β-cells in contrast to the findings described here, nor after induction of β-cell hypertrophy by dexamethasone (38). Similarly, such structures did not appear to be more abundant in the islets of transgenic mice expressing the cell cycle regulator CDK4/R24C under the rat insulin promoter, in which a massive increase in β-cell mass (to almost 20% of pancreatic volume) was observed, in this case due to enhanced proliferation (34).
We have considered the possibility that the effects of LKB1 deletion reflect the requirement for this enzyme to activate AMPK. However, deletion of the AMPKα1 and AMPKα2 subunits in β-cells by use of the RIP2.Cre transgene leads to defective insulin release in vivo, with none of the changes in β-cell mass or cellular architecture observed here in βLKB1KO islets (46). Instead, and as previously proposed (16, 18) the increased number of islet acini most likely involves alternative kinases such as MARKS1–3. In this context, Par-1b (MARK2) was recently shown to promote cell-cell adhesion and association of E-cadherin with the actin cytoskeleton (13). A loss of this interaction may be involved in the relocalization of the latter to the apical pole as observed in βLKB1KO β-cells. Par1b/MARK2 has also recently been shown to regulate glucose metabolism by adipose tissue in vivo (25), possibly by interacting with syntaxin-4 (14) to control vesicular trafficking of GLUT4 to the plasma membrane. As syntaxin-4 is implicated in the second phase of GSIS (45), it is possible that abnormal phosphorylation of Par1b/MARK2 in the absence of LKB1 may also influence the trafficking of β-cell secretory granules to the cell surface. Most importantly, β-cells from mice deleted globally for Par1b/MARK2−/− display a similar redistribution of nuclei to that described here (18).
The results of the two very recent studies mentioned above (16, 18), contrast with our own findings in several important respects. Notably, LKB1-deficient β-cells were found in the present studies to display abnormalities in the expression of the glucose transporter GLUT2, and the KATP channel subunit Kir6.2 (but not of several other genes important in maintaining the glucose responsiveness of β-cells; see results), with corresponding alterations in basal and glucose-induced changes in membrane potential and intracellular free Ca2+. Although neither of the earlier studies examined the levels of expression of β-cell-specific genes in detail or involved studies of glucose sensing at the single β-cell level, the subcellular distribution of GLUT2 immunoreactivity within individual β-cells was reportedly altered by LKB1 deficiency (18). We suspect that the relatively well-preserved GSIS from isolated islets may in both cases therefore represent alterations in β-cell-β-cell (or β-cell-α-cell contact) or between β-cells and the capillary network. Moreover, the well-preserved stimulation of insulin secretion by glucose observed here in LKB1-deleted islets (Fig. 6A), despite changes in KATP channel conductance, resting membrane potential, and a marked (∼50%) decrease in the rise in intracellular free Ca2+ in response to the sugar (Fig. 6, B–D), may suggest a compensatory increase in “KATP-independent” (22) mechanisms of activation, serving to enhance the sensitivity of the secretory machinery to Ca2+ changes. A more detailed assessment of this possibility will require further analysis, including a comparison of the transcriptome of islets from βLKBKO and wild-type islets. It should also be stressed, however, that the substantial increase in β-cell size and mass (∼4-fold; Fig. 2, C and D) seems likely to predominate over the relatively more minor changes in β-cell glucose sensing in LKB1-deleted β-cells and to underlie the substantial increase in vivo in insulin release under glucose challenge.
Does LKB1 Control Neuronal Function to Regulate Food Intake and Body Weight?
A striking finding in the present study was that deletion of LKB1 using the RIP2.Cre transgene led to a decrease in body weight and feeding. We have considered the possibility that these changes may simply reflect the increased levels of insulin, with the latter serving as a satiety factor (37). However, we suspect that the metabolic and cellular changes resulting from LKB1 deficiency in the brain and in the pancreas are, at least in large part, independent. Thus, mice inactivated for LKB1 by Pdx1.CreER-mediated excision, which leads to deletion in all pancreatic lineages but not in the hypothalamus, show similar increases in circulating insulin levels and marked improvements in glucose tolerance (23) without a change in body weight.
Importantly, we show that changes in food intake and body mass in βLKB1KO mice were accompanied by significant decreases in the hypothalamic expression of the orexigenic peptide NPY and of the anorexigenic peptide POMC at the mRNA level, suggesting that RIP2.Cre neurons may control the activity of neighboring cells in the melanocortin circuitry (43). On the other hand, in preliminary experiments (not shown) we have observed by Western blotting a small but detectable increase in POMC immunoreactivity in hypothalamic extracts from βLKB1KO mice vs. wild-type controls, suggesting possible posttranslational modifications through which LKB1 may control the levels of this peptide in feeding centers. Further studies will be necessary to explore this possibility and the mechanisms through which deletion of LKB1 in RIP2.Cre neurons, not usually thought to express either NPY or POMC (24), may influence the levels of these peptides in the ventromedial hypothalamus. Interestingly, deletion of insulin receptor substrate-2 (IRS-2), using an identical strategy to that used here, has previously been shown to cause hyperphagia, diminished β-cell mass, and glucose intolerance in mice (29). Conversely, potentation of insulin signaling by deletion in RIP2.Cre cells of the inositol phospholipid 5′-phosphatase PTEN (8) led to diminished growth and body weight. LKB1 deletion in the same nuclei thus leads to a strikingly similar phenotype to the latter model. This similarity suggests that LKB1 may also oppose insulin, or possibly leptin, signaling in RIP2.Cre neurons to enhance appetite and body weight. Surprisingly, however, deletion of TSC1, also expected to enhance mTOR signaling in the same neuronal population, led to hyperphagia and obesity (35), suggesting that LKB1 may engage other signaling pathways to influence the activity of these and associated neurons in the melanocortin network.
Interestingly, although βLKB1-null mice displayed clear decreases in hypothalamic and β-cell AMPK activity (Fig. 1), mice deleted for both AMPK catalytic subunits in the same cells do not display a feeding or body weight phenotype (46), indicating that the hypothalamic effects of LKB1 may be mediated by alternative downstream kinases (30) (Table 1) or other effectors. In any case, we stress that further studies will be required in the future, perhaps involving the selective deletion of LKB1 in the hypothalamus by the injection of Cre recombinase into this brain region in LKB1 flox’d mice, to formally confirm or refute the possibility of distinct roles for central and pancreatic islet LKB1 in the control of glucose homeostasis and food intake.
Table 1.
Relative abundance of LKB1 target kinases in pancreatic islets
| Protein Kinase | mRNA Level (Relative Intensity) |
|---|---|
| LKB1/STK11 | 404 |
| CaMKK2β | 152 |
| AMPKα1 (PRKAA1) | 416 |
| AMPK α2 (PRKAA2) | 104 |
| Snf-related kinase (SNRK) | 396 |
| Nuak1/Snf-1 line kinase-1/ARK5 | 314 |
| Nuak2/SNARK | 172 |
| Salt-inducible kinase 1 (SIK1/MSK/SNF1LK1) | 35 |
| Salt-inducible kinase (SIK2/QIK/ SNF1LK2) | 54 |
| MARK1 | 214 |
| MARK2 (PAR1B) | 422 |
| MARK3 (PAR1A/TAK1/MAP3K7) | 568 |
| MARK4 | A |
| BRSK1 (SAD-A) | A |
| BRSK2 (SAD-B) | A |
Pancreatic islets isolated from 12-wk-old wild-type male C57Bl/6 mice by collagenase digestion and histopaque gradient purification as described (27) were incubated in RPMI supplemented with 11 mM glucose for 16 h. Total cellular RNA (400-600 ng) extracted from islets by use of RNAeasy kit (Qiagen UK) was used for microarray analysis. Four independent hybridizations were performed on Mouse 430 2.0 chip (Affymetrix, Santa Clara, CA). Data were exported to GeneSpringX to perform quality control and subsequent downstream analysis. Robust multichip average (RMA) summary and quantile normalization was employed to produce expression values for the probe sets. Data are expressed as means of observations on islets from 4 separate mice, which agreed within 10%. A, absent.
Conclusion
Using an identical strategy for tissue-selective deletion in mice, we have shown that LKB1 plays a distinct role(s) from that of AMPK in controlling β-cell mass and insulin secretion in vivo and in vitro. Further dissection of the signaling pathway(s) through which LKB1 acts at each site may provide exciting new modalities for the treatment of metabolic disease including type 2 diabetes.
GRANTS
This work was supported by grants to G. A. Rutter from the Wellcome Trust (Programme Grant 081958/2/07/Z), The European Union (FP6 “Save Beta”), the Medical Research Council (G0401641) and National Institutes of Health (RO1 DK-071962-01), and a JDRFI PostDoctoral Fellowship to A. I. Tarasov.
DISCLOSURES
No conflicts of interest were reported by the authors.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Blerina Kola (Queen Mary, University of London) for useful discussion and Lorraine Lawrence for the preparation of pancreatic slices.
REFERENCES
- 1.Ahren B, Simonsson E, Scheurink AJ, Mulder H, Myrsen U, Sundler F. Dissociated insulinotropic sensitivity to glucose and carbachol in high-fat diet-induced insulin resistance in C57BL/6J mice. Metabolism 46: 97–106, 1997 [DOI] [PubMed] [Google Scholar]
- 2.Alanentalo T, Asayesh A, Morrison H, Loren CE, Holmberg D, Sharpe J, Ahlgren U. Tomographic molecular imaging and 3D quantification within adult mouse organs. Nat Methods 4: 31–33, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Sakamoto K, Bayascas JR. LKB1-dependent signaling pathways. Annu Rev Biochem 75: 137–163, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Anderson KA, Ribar TJ, Lin F, Noeldner PK, Green MF, Muehlbauer MJ, Witters LA, Kemp BE, Means AR. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 7: 377–388, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Baas AF, Boudeau J, Sapkota GP, Smit L, Medema R, Morrice NA, Alessi DR, Clevers HC. Activation of the tumour suppressor kinase LKB1 by the STE20-like pseudokinase STRAD. EMBO J 22: 3062–3072, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonner-Weir S. Morphological evidence for pancreatic polarity of beta cells within islets of Langerhans. Diabetes 37: 616–621, 1988 [DOI] [PubMed] [Google Scholar]
- 7.Boudeau J, Sapkota G, Alessi DR. LKB1, a protein kinase regulating cell proliferation and polarity. FEBS Lett 546: 159–165, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Choi D, Nguyen KT, Wang L, Schroer SA, Suzuki A, Mak TW, Woo M. Partial deletion of Pten in the hypothalamus leads to growth defects that cannot be rescued by exogenous growth hormone. Endocrinology 149: 4382–4386, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Claret M, Smith MA, Batterham RL, Selman C, Choudhury AI, Fryer LG, Clements M, Al-Qassab H, Heffron H, Xu AW, Speakman JR, Barsh GS, Viollet B, Vaulont S, Ashford ML, Carling D, Withers DJ. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 117: 2325–2336, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva XG, Loder MK, McDonald A, Tarasov AI, Carzaniga R, Kronenberger K, Barg S, Rutter GA. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes 58: 894–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Silva XG, Leclerc I, Salt IP, Doiron B, Hardie DG, Kahn A, Rutter GA. Role of AMP-activated protein kinase in the regulation by glucose of islet beta-cell gene expression. Proc Natl Acad Sci USA 97: 4023–4028, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.da Silva XG, Leclerc I, Varadi A, Tsuboi T, Moule SK, Rutter GA. Role for AMP-activated protein kinase in glucose-stimulated insulin secretion and preproinsulin gene expression. Biochem J 371: 761–774, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elbert M, Cohen D, Musch A. PAR1b promotes cell-cell adhesion and inhibits dishevelled-mediated transformation of Madin-Darby canine kidney cells. Mol Biol Cell 17: 3345–3355, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elbert M, Rossi G, Brennwald P. The yeast par-1 homologs kin1 and kin2 show genetic and physical interactions with components of the exocytic machinery. Mol Biol Cell 16: 532–549, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esni F, Taljedal IB, Perl AK, Cremer H, Christofori G, Semb H. Neural cell adhesion molecule (N-CAM) is required for cell type segregation and normal ultrastructure in pancreatic islets. J Cell Biol 144: 325–337, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu A, Ng AC, Depatie C, Wijesekara N, He Y, Wang GS, Bardeesy N, Scott FW, Touyz RM, Wheeler MB, Screaton RA. Loss of Lkb1 in adult beta cells increases beta cell mass and enhances glucose tolerance in mice. Cell Metab 10: 285–295, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Gannon M, Shiota C, Postic C, Wright CVE, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis 26: 139–142, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Granot Z, Swisa A, Magenheim J, Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK, Stoeckert CJ, Jr, Meyuhas O, Seino S, Permutt MA, Piwnica-Worms H, Bardeesy N, Dor Y. LKB1 regulates pancreatic beta cell size, polarity, and function. Cell Metab 10: 296–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, Nagata M, Yokono K. Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes 58: 1321–1332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, Jarvinen H, Kristo P, Pelin K, Ridanpaa M, Salovaara R, Toro T, Bodmer W, Olschwang S, Olsen AS, Stratton MR, de la Chapelle A, Aaltonen LA. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature 391: 184–187, 1998 [DOI] [PubMed] [Google Scholar]
- 22.Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes 49: 1751–1760, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Hezel AF, Gurumurthy S, Granot Z, Swisa A, Chu GC, Bailey G, Dor Y, Bardeesy N, DePinho RA. Pancreatic LKB1 deletion leads to acinar polarity defects and cystic neoplasms. Mol Cell Biol 28: 2414–2425, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hisadome K, Smith MA, Choudhury AI, Claret M, Withers DJ, Ashford ML. 5-HT inhibition of rat insulin 2 promoter Cre recombinase transgene and proopiomelanocortin neuron excitability in the mouse arcuate nucleus. Neuroscience 159: 83–93, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurov JB, Huang M, White LS, Lennerz J, Choi CS, Cho YR, Kim HJ, Prior JL, Piwnica-Worms D, Cantley LC, Kim JK, Shulman GI, Piwnica-Worms H. Loss of the Par-1b/MARK2 polarity kinase leads to increased metabolic rate, decreased adiposity, and insulin hypersensitivity in vivo. Proc Natl Acad Sci USA 104: 5680–5685, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen M, Ten Klooster JP, Offerhaus GJ, Clevers H. LKB1 and AMPK family signaling: the intimate link between cell polarity and energy metabolism. Physiol Rev 89: 777–798, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Kefas BA, Heimberg H, Vaulont S, Meisse D, Hue L, Pipeleers D, Van de Casteele M. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46: 250–254, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Leclerc I, Woltersdorf WW, da Silva XG, Rowe RL, Cross SE, Korbutt GS, Rajotte RV, Smith R, Rutter GA. Metformin, but not leptin, regulates AMP-activated protein kinase in pancreatic islets: impact on glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 286: E1023–E1031, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Lin X, Taguchi A, Park S, Kushner JA, Li F, Li Y, White MF. Dysregulation of insulin receptor substrate 2 in beta cells and brain causes obesity and diabetes. J Clin Invest 114: 908–916, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lizcano JM, Goransson O, Toth R, Deak M, Morrice NA, Boudeau J, Hawley SA, Udd L, Makela TP, Hardie DG, Alessi DR. LKB1 is a master kinase that activates 13 kinases of the AMPK subfamily, including MARK/PAR-1. EMBO J 23: 833–843, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116: 1776–1783, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin SG, St Johnston D. A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature 421: 379–384, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Miyawaki K, Inoue H, Keshavarz P, Mizuta K, Sato A, Sakamoto Y, Moritani M, Kunika K, Tanahashi T, Itakura M. Transgenic expression of a mutated cyclin-dependent kinase 4 (CDK4/R24C) in pancreatic beta-cells prevents progression of diabetes in db/db mice. Diabetes Res Clin Pract 82: 33–41, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Mori H, Inoki K, Munzberg H, Opland D, Faouzi M, Villanueva EC, Ikenoue T, Kwiatkowski D, MacDougald OA, Myers MG, Jr, Guan KL. Critical role for hypothalamic mTOR activity in energy balance. Cell Metab 9: 362–374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narbonne P, Roy R. Caenorhabditis elegans dauers need LKB1/AMPK to ration lipid reserves and ensure long-term survival. Nature 457: 210–214, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest 116: 1761–1766, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased β-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab 296: E681–E689, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Riboulet-Chavey A, Diraison F, Siew LK, Wong FS, Rutter GA. Inhibition of AMP-activated protein kinase protects pancreatic (beta)-cells from cytokine-mediated apoptosis and CD8+ T cell-induced cytotoxicity. Diabetes 57: 415–423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards SK, Parton LE, Leclerc I, Rutter GA, Smith RM. Over-expression of AMP-activated protein kinase impairs pancreatic beta-cell function in vivo. J Endocrinol 187: 225–235, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Rutter GA, Leclerc I. The AMP-regulated kinase family: Enigmatic targets for diabetes therapy. Mol Cell Endocrinol 297: 41–49, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Salt IP, Johnson G, Ashcroft SJ, Hardie DG. AMP-activated protein kinase is activated by low glucose in cell lines derived from pancreatic beta cells, and may regulate insulin release. Biochem J 335: 533–539, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science 307: 375–379, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Shorning BY, Zabkiewicz J, McCarthy A, Pearson HB, Winton DJ, Sansom OJ, Ashworth A, Clarke AR. Lkb1 deficiency alters goblet and paneth cell differentiation in the small intestine. PLoS ONE 4: e4264, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spurlin BA, Thurmond DC. Syntaxin 4 facilitates biphasic glucose-stimulated insulin secretion from pancreatic beta-cells. Mol Endocrinol 20: 183–193, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Sun G, Tarasov AI, McGinty J, McDonald A, DaSilva Xavier G, Gorman T, Marley A, French PM, Parker H, Gribble F, Reimann F, Prendiville O, Carzaniga R, Viollet B, Leclerc I, Rutter GA. Ablation of AMP-activated protein kinase alpha1 and alpha2 from pancreatic beta-cells and RIP.Cre neurons suppresses insulin release in vivo. Diabetologia In press2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sutherland CM, Hawley SA, McCartney RR, Leech A, Stark MJ, Schmidt MC, Hardie DG. Elm1p is one of three upstream kinases for the Saccharomyces cerevisiae SNF1 complex. Curr Biol 13: 1299–1305, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Li J, Young LH, Caplan MJ. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA 103: 17272–17277, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.