Abstract
Phospholipases A2 (PLA2) play important roles in metabolic processes, and the Group VI PLA2 family is comprised of intracellular enzymes that do not require Ca2+ for catalysis. Mice deficient in Group VIA PLA2 (iPLA2β) develop more severe glucose intolerance than wild-type (WT) mice in response to dietary stress. Group VIB PLA2 (iPLA2γ) is a related enzyme distributed in membranous organelles, including mitochondria, and iPLA2γ knockout (KO) mice exhibit altered mitochondrial morphology and function. We have compared metabolic responses of iPLA2γ-KO and WT mice fed a Western diet (WD) with a high fat content. We find that KO mice are resistant to WD-induced increases in body weight and adiposity and in blood levels of cholesterol, glucose, and insulin, even though WT and KO mice exhibit similar food consumption and dietary fat digestion and absorption. KO mice are also relatively resistant to WD-induced insulin resistance, glucose intolerance, and altered patterns of fat vs. carbohydrate fuel utilization. KO skeletal muscle exhibits impaired mitochondrial β-oxidation of fatty acids, as reflected by accumulation of larger amounts of long-chain acylcarnitine (LCAC) species in KO muscle and liver compared with WT in response to WD feeding. This is associated with increased urinary excretion of LCAC and much reduced deposition of triacylglycerols in liver by WD-fed KO compared with WT mice. The iPLA2γ-deficient genotype thus results in a phenotype characterized by impaired mitochondrial oxidation of fatty acids and relative resistance to the metabolic abnormalities induced by WD.
Keywords: insulin resistance, glucose intolerance, diabetes, lipids, mass spectrometry
phospholipases a2 (PLA2) were once considered to be primarily digestive enzymes and noxious venom components, but the discovery of new members of this superfamily since 1991 has revealed that they play myriad roles in regulating biological processes (15, 27, 31, 37, 38, 41, 48, 70, 77, 79). PLA2s catalyze hydrolysis of phospholipids to yield a free fatty acid, e.g., arachidonic acid (AA), and a 2-lysophospholipid, e.g., 2-lysophosphatidylcholine (LPC), and can generate signals by both product generation and substrate loss or modification. For example, AA has intrinsic mediator functions (36) and can also be converted to bioactive eicosanoids (26), and lysophospholipids can also serve as signaling molecules (57, 80, 81) or as biosynthetic intermediates (66). In addition, substrate hydrolysis can compromise the integrity of membranous organelles, e.g., mitochondria, and lead to release of their contents and initiation of intracellular signaling cascades (7).
At least 15 mammalian PLA2 groups are recognized, which fall into five main categories (15, 70). Secretory PLA2s (sPLA2, Groups I–III, V, IX–XIV) were the first recognized and have low (14–18 kDa) molecular masses, require millimolar [Ca2+] for catalysis, and play roles in nutrient digestion, inflammation, and pathophysiological states, e.g., cancer and atherosclerosis (41, 70). Various members of the platelet-activating factor (PAF) acetylhydrolase (Groups VII and VIII) PLA2 category have anti-inflammatory and/or proatherogenic properties or may participate in brain development and spermatogenesis (15, 70). The recently recognized lysosomal PLA2 (Group XV) is important in cellular phospholipid clearance, and mice deficient in its expression develop macrophage phospholipidosis (31).
The first recognized cytosolic PLA2 is now designated cPLA2α (Group IVA), and five other Group IV family members have been identified (27). The cPLA2α requires micromolar [Ca2+] to associate with its membrane substrates and is also regulated by phosphorylation. It prefers substrates with sn-2 arachidonoyl substituents and provides AA for conversion to eicosanoids in many signaling and inflammatory processes. Mice deficient in its expression exhibit female reproductive abnormalities and modified responses in several disease models (27).
The Group VI PLA2s (iPLA2s) are also intracellular enzymes and do not require Ca2+ for catalysis (37, 48, 77, 79). They are now recognized to belong to a “patatin-like phospholipase domain-containing lipase” (PNPLA) family with nine members that share a GXSXG lipase consensus motif (37, 38). PNPLA2 is also designated adipose triglyceride lipase (ATGL) and catalyzes the first step in triacylglycerol (TAG) hydrolysis. Mice deficient in its expression exhibit massive TAG accumulation in multiple tissues and cardiomyopathy, although they have improved insulin sensitivity and glucose tolerance (38). PNPLA3 is also designated adiponutrin and is expressed in adipocytes, where its levels increase during differentiation and decrease with fasting, and PNPLA5 exhibits similar properties. PNPLA4 hydrolyzes keratinocyte retinyl esters, and PNPLA6 and PNLAP7 are lysophospholipases expressed in brain and in muscle and fat, respectively. Human mutations in the former are associated with neurodegenerative disease (38).
PNPLA9 and 8 correspond to Group VIA and VIB PLA2, respectively (38), and are also designated iPLA2β and iPLA2γ (37, 48). The former plays an important role in glucose homeostasis (6, 8). Male iPLA2β-null mice exhibit impaired glucose tolerance, but transgenic male mice that overexpress iPLA2β in insulin-secreting β-cells of pancreatic islets exhibit improved glucose tolerance (6). Female iPLA2β-null mice exhibit normal glucose tolerance when fed standard chow (SC) but develop more severe glucose intolerance than wild-type female mice when fed a Western diet (WD) (8). In each of those cases, glucose tolerance correlates with insulin secretion by pancreatic islets isolated from the animals (6, 8), and a similar relationship is observed in insulinoma cells, in which iPLA2β expression is manipulated genetically (5, 46). This appears to reflect the effect of iPLA2β reaction products to attenuate β-cell Kv2.1 currents and thereby to amplify glucose-induced insulin secretion (6, 36). In addition, iPLA2β deficiency affects insulin sensitivity in a sex-dependent manner (6, 8).
iPLA2γ-null mice have also been generated (49, 50) and exhibit phenotypic abnormalities that include altered mitochondrial morphology, function, and lipid composition associated with reduced exercise tolerance, cardiac dysfunction, and hippocampal degeneration, among other features, although their glucose tolerance is not obviously perturbed when fed SC (50). Here, we have examined the metabolic responses of these animals to a WD and find that, in contrast to iPLA2β-null mice (8), iPLA2γ-null mice exhibit remarkable resistance to obesity and metabolic abnormalities that consumption of the WD induces in wild-type littermates, suggesting that the enzyme plays an important and previously unrecognized role in nutritional effects on metabolic regulation.
EXPERIMENTAL PROCEDURES
Materials.
[14C]palmitic acid (50 mCi/mmol) was obtained from American Radiolabeled Chemicals, St. Louis, MO; [3H]triolein from PerkinElmer (Waltham, MA); enhanced chemiluminescence (ECL) reagents from GE Healthcare (Piscataway, NJ); sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) supplies from Bio-Rad (Richmond, CA); ATP, common reagents, and salts from Sigma Chemical (St. Louis, MO); culture media, penicillin, streptomycin, Hanks' balanced salt solution (HBSS), l-glutamine, agarose, molecular mass standards, and RT-PCR reagents from Invitrogen (Carlsbad, CA); fetal bovine serum from Hyclone (Logan, UT); Pentex bovine serum albumin (BSA, fatty acid free, fraction V) from MP Biomedicals (Solon, OH); and forskolin from Calbiochem (La Jolla, CA). Krebs-Ringer bicarbonate buffer (KRB) contained (in mM) 25 HEPES (pH 7.4), 115 NaCl, 24 NaHCO3, 5 KCl, 1 MgCl2, and 2.5 CaCl2. Synthetic phospholipids used as internal standards in mass spectrometric analyses were purchased from Avanti Polar Lipids (Alabaster, AL), Nu-Chek Prep (Elysian, MN), and Cambridge Isotope Laboratories (Cambridge, MA). Solvents for sample preparation and for mass spectrometric analyses were purchased from Fisher Scientific (Fair Lawn, NJ).
Generating and genotyping iPLA2γ-null mice.
Mice with iPLA2γ-null alleles were obtained from the laboratory of Richard Gross, and their generation is described elsewhere (50). This had involved preparing an iPLA2γ knockout construct, introducing it into 129/SvJ mouse embryonic stem (ES) cells, their selection with G418, characterization by Southern blotting, injection into C57BL/6 mouse blastocysts, production of chimeras and then heterozygotes, and mating of heterozygotes to yield wild-type, heterozygous, and iPLA2γ-null mice, which were genotyped by Southern blotting of tail clipping genomic DNA (50). Genotyping was also performed by PCR with tail clipping DNA as template, using two pairs of primers. The primer “NEO forward” (AAAGGCCTACCCGCTTCCATTGCTCA) was used with a common primer (CCATCTAATCACCTACCACTGC) to amplify a 608-nt product from the knockout allele. The common primer was used with the primer “iPLA2 FOR” (CTGTTCACAGAGGTGTGGTTGC) to amplify a 435-nt product of the wild-type allele. The genetic background of the resultant mice is mixed 129/SvJ-C57BL/6 (50). All experiments were performed using sex- and age-matched littermate controls.
General animal studies.
Animal protocols were in accordance with the National Institutes of Health guidelines for humane treatment of animals and were reviewed and approved by the Animal Care Committee of Washington University.
WD feeding studies.
Mice were housed in a pathogen-free barrier facility with unrestricted access to water and standard mouse chow (Purina Mills Rodent Chow 5053) containing 6% fat, which is designated Standard Chow (SC) hereafter. For dietary intervention studies, mice were fed SC until age 8 wk and thereafter were randomized into groups that were fed either SC or a WD with a high fat content, as described (8, 21). The composition of the WD (Harlan Teklad catalog no. TD88137) is energy from fat 42%, casein 195 g/kg, sucrose 341 g/kg, corn starch 150 g/kg, cellulose 50 g/kg, anhydrous milk fat 210 g/kg, cholesterol 1.5 g/kg, and caloric content 18.95 kJ/g. Body weights of iPLA2γ-knockout (KO) and wild-type (WT) mice were determined gravimetrically at weekly to monthly intervals, as described (6), and blood samples were also obtained after a 4-h fast for determination of cholesterol, insulin, and glucose concentrations.
Blood concentrations of cholesterol, insulin, leptin, glucose, TAG, free fatty acid, β-hydroxybutyrate, and glycerol.
As described (6, 8), blood samples were obtained from the lateral saphenous vein in heparinized capillary tubes, and glucose concentrations were measured in whole blood with a blood glucose monitor (Becton-Dickinson, Franklin Lakes, NJ) or an Ascensia ELITE XL blood glucose meter. As described (9, 18), plasma was prepared from heparinized blood by centrifugation, and cholesterol, TAG, free fatty acids, β-hydroxybutyrate, and glycerol were measured by enzymatic assays with kits from Sigma (St. Louis, MO), insulin by ELISA with a kit from Crystal Chem (Downer's Grove, IL), and leptin and resistin by ELISA with kits from Millipore (Billerica, MA).
Body composition.
Mice were anesthetized with pentobarbital sodium (40 mg/kg ip) and weighed. Body composition determinations were obtained by dual-energy X-ray absorptiometry (DEXA Lunar, Madison, WI) scanning, as described (9, 67), to determine percentage of body fat.
Food consumption.
Individually housed age- and sex-matched mice were given preweighed amounts of food, and the amount of food consumed was determined over a 24-h period for 5 days, as described (35), and expressed as grams consumed per day.
Stool weight and extractable lipid content and composition.
Mice of a given genotype were housed three to a cage with unrestricted access to food and water. Stool was collected at 24-h intervals, and both initial (wet) weight and weight after drying (1 h, 70°C, LabConco CentriVap Concentrator) were determined as described (35). Dried fecal samples (60 mg) were homogenized in water, and lipids were extracted with 4 ml of chloroform-methanol (1:1, vol/vol) and 1.8 ml of water as described (36, 70). Extractable lipid content was determined as described (72) in a process involving vortex mixing, centrifugation, and collection of the chloroform (lower) phase. Additional chloroform (2 ml) was added and extraction repeated. Combined chloroform extracts were concentrated to dryness under nitrogen, and the residue was reconstituted in chloroform and again extracted. Resultant chloroform extracts were placed in preweighed test tubes and concentrated to dryness. The residue was further desolvated (1 h, 70°C, CentriVap Concentrator), and tubes were again weighed (72). Dried lipid residues were reconstituted in chloroform (100 μl), and aliquots (5 μl) were analyzed by TLC with standard lipids in parallel lanes as described (30, 31) Lipid species were visualized by charring (11, 45) and analyzed by densitometry (AlphaInnotech FluorChem 8900 apparatus with AlphaEaseFC software).
Postprandial fat absorption.
Triolein absorption was measured as described (35). Briefly, mice were fasted overnight, and [3H]triolein (1 μCi, PerkinElmer) in olive oil was administered by gavage. Blood samples were obtained 1, 2, 4, and 6 h thereafter by tail bleeding. Radioactivity appearing in plasma was determined by liquid scintillation counting.
Glucose and insulin tolerance tests.
As described (6, 8, 62), intraperitoneal glucose tolerance tests were performed in mice fasted overnight from which a baseline blood sample was obtained followed by intraperitoneal injection of d-glucose (2 mg/g) and subsequent (30, 60, and 120 min) collection of blood for glucose measurement. Insulin tolerance tests were performed in mice with free access to water and chow until intraperitoneal administration of human regular insulin (0.75 U/kg; Lilly, Indianapolis, IN). Blood was collected at 0, 30, 60, and 120 min for glucose measurements (6, 8, 62).
Insulin secretion in vivo.
Mice were fasted overnight, and baseline blood samples were obtained from saphenous vein, followed by intraperitoneal injection of d-glucose (3 mg/g), and a blood sample was obtained 15 min thereafter for measurement of plasma insulin levels.
Insulin secretion by isolated pancreatic islets.
Islets were isolated from pancreata of mice by collagenase digestion after mincing, followed by Ficoll step density gradient separation, and manual selection under stereomicroscopic visualization to exclude contaminating tissues, as described (6, 8, 54). Mouse islets were counted and used to measure ex vivo secretion of insulin (30 islets per incubation), as described (6, 8). Islets were rinsed with KRB medium containing 3 mM glucose and 0.1% BSA and placed in silanized tubes (12 × 75 mm) in that buffer, through which 95% air-5% CO2 was bubbled before incubations. Tubes were capped and incubated (37°C, 30 min, shaking water bath). Buffer was then replaced with KRB medium containing 3, 8, or 20 mM glucose and 0.1% BSA without or with forskolin (2.5 μM), and samples were incubated for 30 min. Secreted insulin was measured by radioimmunoassay.
Indirect calorimetry and determination of the respiratory exchange ratio (RER) and measurements of activity by infrared sensor monitoring.
SC-fed or WD-fed mice (age 6 mo) were studied in the fed state in a single-chamber indirect calorimetry system for 24 h (Columbus Instruments, Columbus, OH), as described (9), to determine V̇o2, V̇co2, heat generation, and RER. Activity was measured with an infrared sensor in an InfraMot apparatus (TSE Systems, Midland, MI).
Substrate oxidation by gastrocnemius muscle.
SC-fed or WD-fed mice (age 6 mo) were euthanized with pentobarbital sodium, and the gastrocnemius muscle was excised. Oxidation of [1-14C]palmitate and of [1-14C]glucose by muscle homogenate was then performed as described (16). Briefly, gastrocnemius specimens (70 mg) were minced with scissors in homogenization buffer (200 μl: 250 mM sucrose, 1 mM EDTA, 10 mM Tris·HCl, and 2 mM ATP, pH 7.4), diluted 20-fold, and transferred to a 3-ml Teflon-on-glass homogenization vessel. Homogenization was performed twice (on ice, 12 passes, 30 s, 1,500 rpm), and the resultant homogenates were used for oxidation experiments after removal of an aliquot for protein determination. To measure [14C]O2 production from [1-14C]palmitate, palmitate (100 μM) was bound to 0.5% BSA (final concentration), and acetyl-CoA and glucose were added. Each substrate was dissolved in reaction buffer (in mM: 100 sucrose, 10 Tris·HCl, 10 KPO4, 100 KCl, 1 MgCl2·6H2O, 1 l-carnitine, 0.1 malate, 2 ATP, 0.05 coenzyme A, 1 dithiothreitol, pH 7.4). Aliquots (80 μl, in triplicate) of diluted muscle homogenates were plated in a 100-well trapping device. Reaction buffer (325 μl) was placed in the bottom of each well. In addition, 1 N NaOH (400 μl) was placed in a 500-μl uncapped microcentrifuge tube, which was also placed in each well to trap CO2. The trapping device was then sealed with parafilm, a siliconized rubber gasket, and the trapping device lid and allowed to incubate (with shaking, 37°C, 1 h). Reactions were terminated by adding 70% perchloric acid (100 μl) via injection ports in the trapping device lid. The trapping device was again placed in the shaking incubator, and released [14C]O2 was trapped (1 h, room temperature), quantitated by liquid scintillation spectrometry, and expressed as CO2 produced per milligram of protein (16).
Electron microscopy of gastrocnemius muscle sections.
Electron microscopic examination of muscle tissue was performed as described (47). Briefly, euthanized mice were perfused via cardiac puncture with modified Karnovsky's fixative buffer containing 3% glutaraldehyde and 1% paraformaldehyde in 0.1 M sodium cacodylate (pH 7.4). The gastrocnemius was excised and cut into strips (1 × 1 mm) along the fiber length and immersed in fixative buffer (24 h). Muscle strips were washed, placed in postfixative solution (1% osmium tetroxide, 1 h), dehydrated with alcohols, and embedded in PolyBed 812 (Polysciences, Warrington, PA). Sections (90 nm) were prepared and viewed with a JEOL model 1200EX electron microscope.
Immunostaining and fluorescence microscopy of gastrocnemius muscle sections.
Immunocytochemical analyses with mouse antibody MS502 (MitoSciences, Eugene, OR) directed against mitochondrial ATP synthase (complex V) α-subunit (57) were performed essentially as described (44). Briefly, euthanized mice were perfused via cardiac puncture with PBS (20 ml) and then with 4% paraformaldehyde (20 ml). Gastrocnemius was then excised and immersed in 30% sucrose (4°C, overnight). Fixed tissue was frozen in embedding medium and cut into 14-μm sections (LEICA CM1850), which were mounted on l-polylysine-coated glass slides, allowed to dry overnight, and washed with PBS. Microwave-assisted (power level 2, 5 min) antigen retrieval was performed in 1 mM EDTA buffer, and the slides were allowed to cool to room temperature, washed (PBS, 5 min, thrice), fixed (4% paraformaldehyde, 1 h, room temperature), permeabilized (0.1% Triton X-100 and 0.1% sodium citrate in PBS), again washed (PBS, 5 min, twice), and blocked (5% goat serum and 3% BSA in TBS-T). Slides were incubated with primary antibody directed against ATP synthase (complex V) α-subunit (20 μl; MS502, Mitoscience) at 1:25 dilution (overnight, 4°C), washed (PBS, 20 min, 4 times), and incubated (1 h) with secondary antibody (20 μl, 1:100 dilution, goat anti-mouse IgG-TR, SC-2781; Santa Cruz Biotechnology, Santa Cruz, CA), and again washed (PBS, 10 min, thrice). A drop of Prolong Gold antifade reagent with DAPI (P36935, Invitrogen) was applied, and tissue sections were covered with glass coverslips. Sealed (Nail oil) slides were examined with a Nikon TE300 microscope.
Tissue cardiolipin content.
Euthanized mice were perfused via cardiac puncture with PBS (20 ml), and liver and gastrocnemius muscle were excised and frozen by immersion in liquid nitrogen. Tissues were processed essentially as described (43) with a tissue pulverization apparatus (Spectrum Laboratories, Rancho Dominguez, CA). Tissue powder (20–100 mg) was homogenized in 1 ml of ice-cold mitochondrial isolation buffer (20 mM HEPES-KOH buffer, pH 7.4, containing 100 mM KCl, 5 mM MgCl2, 1 mM EGTA, 250 mM sucrose) containing protease inhibitors (Sigma) in a Teflon-on-glass homogenization vessel. Homogenate was centrifuged (1,000 g, 5 min), and the supernatant was collected and its protein content determined (Coomassie kit; Pierce, Rockford, IL). Supernatant was again centrifuged (10,000 g, 10 min), and the mitochondria-containing pellet was suspended in 20 mM LiCl (1.8 ml), to which cardiolipin internal standard was added. Mitochondrial lipids were extracted by a modified Bligh-Dyer method (12) by vortex mixing in methanol-chloroform (1:1, vol/vol, 4 ml) followed by centrifugation (2,500 rpm, 10 min). The organic (lower) phase was concentrated to dryness under nitrogen, and the residue was reconstituted in methanol-chloroform (1:1, vol/vol, 200 μl). Appropriate dilutions of the resultant solution were analyzed by ESI-MS on a Finnigan TSQ 7000 instrument as described below. Cardiolipin species were quantified relative to internal standard by interpolation from a standard curve, normalized to sample protein content, and expressed as nanomoles of cardiolipin per milligram of protein.
Tissue acylcarnitine content.
Acylcarnitines were measured by direct injection tandem mass spectrometry as described (3), with minor modification. Briefly, gastrocnemius muscle was harvested, frozen, and pulverized as above. Tissue powder (20–100 mg) was homogenized in H2O (1 ml, ice-cold, double distilled) in a Teflon-on-glass homogenization vessel, and the homogenate was sonicated (15 s, twice, on ice) and centrifuged (14,000 rpm, 10 min). An aliquot of supernatant was removed to measure protein content, and the rest was diluted appropriately. To an aliquot (400 μl) of diluted supernatant was added an internal-standard solution (2 μl, 0.2 nmol/μl [2H3]acetylcarnitine). Protein was precipitated with acetonitrile (1,600 μl) and the mixture centrifuged (2,000 g, 5 min). The supernatant was collected, and two aliquots (400 μl) were transferred to two centrifuge tubes (1.5 ml), and the samples were concentrated to dryness (50°C, 20 min, under nitrogen). The residues were incubated with either 3 M methanol-HCl (200 μl, 50°C, 15 min, Supelco) or 3 M n-butanol-HCl (200 μl, 65°C, 15 min; Regis Chemical, Morton Grove, Il) to form the corresponding acylcarnitine methyl or t-butyl ester derivatives. Samples were concentrated to dryness (50°C, 20 min, under nitrogen) and the residues reconstituted in methanol-water (200 μl, 85:15, vol/vol), diluted (1:3, vol/vol) with 85% methanol, and analyzed by ESI-MS-MS on a Finnigan TSQ 7000 mass spectrometer,- as described below. MS-MS scans for precursors of m/z 99 or 85 were performed for methyl or butyl esters, respectively. Each molecular species was quantified relative to internal standards, normalized to sample protein content, and expressed as nanomoles of acylcarnitine per milligram of protein.
Urine acylcarnitine content.
Mice were placed in metabolic cages (Tecniplast, Buguggiate-VA, Italy), and, after 24-h acclimation, four consecutive 24-h urine specimens were collected. Samples were prepared as described (22). Briefly, urine was centrifuged (10,000 g, 10 min) to remove particulates, and to an aliquot (50 μl) was added [2H3]acetylcarnitine internal-standard solution (0.2 nmol/μl, 4 μl). After dilution (0.1% phosphoric acid, 500 μl), samples were passed over strong cation exchange cartridges (Waters, Milford, MA) and concentrated to dryness (under nitrogen, 60°C). Residue was incubated with n-butanol-HCl (200 μl, 65°C, 15 min), and samples were concentrated to dryness (40°C, under nitrogen), reconstituted (85% methanol, 300 μl), diluted 1:2 with 85% methanol, and analyzed by ESI-MS-MS scanning for precursors of m/z 85 on a Finnigan TSQ7000 mass spectrometer. Quantitation of molecular species relative to internal standards was performed by interpolation from a standard curve, and quantities were normalized to mouse body weight and expressed as nanomoles per gram.
Tissue TAG content.
Gastrocnemius muscles and liver specimens were obtained from mice as described above, flash frozen in liquid nitrogen, processed in a precooled Bessman tissue pulverizer, and homogenized in a Dounce apparatus in extraction buffer (in mM: 20 HEPES-KOH, 100 KCl, 5 MgCl2, 1 EGTA, 250 sucrose, pH 7.4, phosphatase inhibitor at 1:200). An aliquot was removed to measure protein (Coomassie kit, ThermoScientific). Samples were extracted as above and the organic residue was resuspended in chloroform-methanol (1:1). Quantitation of TAG was performed as described (53), using Infinity Triglyceride Reagent (ThermoScientific) in an assay based on enzymatic triglyceride hydrolysis and colorimetric measurement of liberated glycerol by coupled enzymatic reactions (25, 55).
Electrospray ionization mass spectrometric analyses of lipids.
As described, cardiolipin species (30, 34) were analyzed as [M − H]− ions by negative ion ESI-MS(-MS), acylcarnitines as methyl or n-butyl ester derivatives (3, 22) by positive ion ESI-MS-MS scanning for precursors of m/z 99 or 85, respectively, and TAG (33) as Li+ adducts by positive ion ESI-MS(-MS) on a Finnigan (San Jose, CA) TSQ-7000 triple-stage quadrupole MS with an ESI source controlled by Finnigan ICIS software operated on a DEC Alpha work station.
For cardiolipins, lipid extracts in methanol-chloroform solution (1:1, vol/vol) were infused (1.2 μl/min) into the ESI source with skimmer set at ground potential, electrospray needle at 4.5 kV, and heated capillary temperature 250°C. The first quadrupole (Q1) was scanned in negative mode from m/z 1200 to 1600 (rate 3 s/scan). Mass spectra were accumulated (5 min) in profile mode for quantitation. To identify molecular species, ESI-MS-MS spectra were obtained by selecting precursor [M − H]− ions in Q1, accelerating them (46–50 eV) into the rf-only second quadrupole (Q2) containing Ar (2.3 mTorr) to induce collisionally activated dissociation (CAD), and analyzing product ions in the third quadrupole (Q3). Both Q1 and Q3 were tuned to unit mass resolution and scanned at a rate of 3 s/scan. Tandem mass spectra were accumulated (20 min) in profile mode.
For acylcarnitines, methyl or butyl ester derivatives in 85% methanol were infused (1.2 μl/min) into the ESI source, using instrumental parameters specified above. The mass spectrometer was operated in positive precursor ion scan mode. Q1 scanned from m/z 150 to 600 (3 s/scan), and precursor ions were accelerated (30 eV for butyl esters or 40 eV for methyl esters) into Q2 to induce CAD. For acylcarnitine butyl ester derivatives, Q3 was set to detect the product ion m/z 85, and for methyl ester derivatives Q3 was set to detect the product ion m/z 99. For quantitation, the peak intensity of each acylcarnitine species was normalized to that of internal-standard [2H3]acetylcarnitine (m/z 221 for methyl esters or m/z 263 for butyl esters). That ratio was then used to quantify the species by interpolation from standard curves generated with varied amounts (0–0.08 nmol) of standard acetyl-, myristoyl-, palmitoyl- and oleoyl-carnitine species and a fixed amount (0.4 nmol) of internal standard. The resultant quantities were normalized to sample protein content.
For TAG, tissue lipid extracts were dissolved in chloroform-methanol (1:1) to which LiCl was added (final [Li+] 2 mM). Samples were infused (1.2 μl/min) into the ESI source using instrumental parameters as above except that the heated capillary temperature was 260°C. Q1 was scanned in positive ion mode from m/z 600 to 900 (3 s/scan). Mass spectra were accumulated (5 min) in profile mode. For ESI-MS-MS, TAG [M+Li]+ ions were selected in Q1 and accelerated (40–50 eV) into the argon-containing (2.3 mTorr) Q2 collision cell to induce CAD. Product ions were analyzed in Q3. Both Q1 and Q3 were tuned to unit mass resolution and scanned at a 3 s/scan rate. Tandem mass spectra were accumulated (10 min) in profile mode.
Tissue staining for neutral lipids with oil red O and microscopic analysis.
As described (17), euthanized mice were perfused with PBS and then 4% paraformaldehyde, and liver was harvested, immersed in 30% sucrose (4°C, overnight), frozen with embedding medium, and cut into 16-μm sections (LEICA CM1850), which were placed on slides that were then immersed in 60% isopropanol (10 min), followed by Oil red O solution (10 min). Slides were then washed several times with doubly distilled H2O, and tissue sections were covered with glass coverslips and examined with a Nikon TE300 microscope (3).
Statistical methods.
Results are presented as means ± SE. Data were evaluated by unpaired, two-tailed Student's t-test or by analysis of variance with appropriate post hoc tests (6). Significance levels are described in figure legends.
RESULTS
To determine whether genetic deficiency of iPLA2γ affects dietary influences on metabolic processes, we compared effects of feeding an SC or a WD with a high fat content to iPLA2γ knockout (KO) mice and their wild-type (WT) littermates. All mice were fed SC from the time of weaning until age 8 wk and were then separated into groups of male or female WT or KO mice that were fed SC or WD thereafter.
Differential effects of WD on weight gain and body fat content of WT and iPLA2γ-KO mice.
Figure 1 illustrates that on this dietary regimen weight gain for a given sex was similar for WT and KO mice up to the age of 8 wk (Fig. 1). Thereafter, WD-fed WT mice gained more weight than SC-fed WT mice, and both groups gained more weight than did KO mice fed either diet. By the end of the 6 mo differential feeding interval, the mean body weight of WD-fed males (38.61 ± 0.95 g) was 22% greater than that of SC-fed males (31.7 ± 0.95 g), and both exceeded the body weights of KO males fed SC (20.94 ± 0.61 g) or WD (21.82 ± 0.42 g) (Fig. 1A).
Fig. 1.
Body weights of Group VIB phospholipase A2 (iPLA2γ) knockout (KO) and wild-type (WT) mice fed Standard Chow (SC) or a Western diet with a high fat content (WD). Male (A) or female (B) iPLA2γ-KO (squares) and WT (circles) mice were fed SC from the time of weaning until 8 wk of age and thereafter were fed either SC (open symbols) or a WD (closed symbols), as described in experimental procedures and elsewhere (6, 21). Mice were weighed between 0900 and 1100 on a top-loading balance. Mean values ± SE are indicated (n = 72). *Significantly (P < 0.05) greater weight in genotype and diet comparisons; +significantly greater weight only in genotype comparisons.
Although female body weights were lower than those of males in each genotype/diet group, comparisons among groups were similar to those with males, and weights of WD-fed (31.75 ± 1.20 g) and SC-fed (25.92 ± 0.76 g) WT females were significantly different from each other and from the weights of WD-fed (18.44 ± 0.27 g) and SC-fed (17.54 ± 0.33 g) KO females. The body weights of SC-fed and WD-fed KO mice did not differ from each other for males (20.94 ± 0.62 vs. 21.87 ± 0.43 g) or females (17.54 ± 0.33 vs. 18.44 ± 0.0.27 g) by the end of the differential feeding interval (Fig. 1).
The 22% greater body weight of WD-fed compared with SC-fed male WT mice was associated with a 21% greater body fat content (37.89 ± 1.25% for WD vs. 16.77 ± 1.0% for SC; Fig. 2A), as determined by DEXA measurements. The body fat percentage of male SC-fed KO mice (16.2 ± 1.22%) was not significantly different from either WD-fed KO (19.92 ± 1.68%) or SC-fed WT mice (Fig. 2A). Females exhibited similar trends of smaller magnitude (Fig. 2B).
Fig. 2.
Body fat percentage of iPLA2γ-KO and WT mice fed SC or a WD. Male (A) or female (B) iPLA2γ-KO and WT mice were fed SC (light bars) or WD (dark bars) as in Fig. 1 and were anesthetized with pentobarbital sodium at age 6 mo. Body composition was then determined by DEXA as described in experimental procedures and elsewhere (9). Mean values for %body fat ± SE are indicated (n = 18). *Significantly (P < 0.05) higher value in genotype and diet comparisons.
Compared with WT, iPLA2γ-KO mice are thus relatively resistant to diet-induced adiposity, as reflected by much smaller rises in body weight (Fig. 1) and body fat percentage (Fig. 2) when fed a WD. There were also smaller diet-induced rises in adipokines and body brown fat content in KO compared with WT mice. WD-fed WT male and female mice had higher serum leptin levels than did SC-fed WT mice (Fig. S1; supplementary materials are found in the online version of this article). There was no significant WD-induced rise in serum leptin levels for KO males (Fig. S1), and that for KO females was significantly lower than for WT. Similar trends for dietary and genotype effects were observed for serum resistin levels and body brown fat content with some sex heterogeneity (not shown).
Similarity of food consumption and fat digestion and absorption of WT and iPLA2γ-KO mice.
The possibilities were considered that the relative resistance of iPLA2γ-KO mice to diet-induced adiposity might be attributable to failure to consume the WD or to absorb its fat content. Food consumption by SC-fed or WD-fed KO mice was found not to differ significantly from that of WT littermates (Table 1). Unabsorbed dietary fat is reflected by stool-extractable lipid content, and neither this parameter nor stool weight was significantly different for WT or KO mice fed SC or WD in the case of males or females (Table 2), with the single exception of a slightly lower stool lipid content (reflecting more complete fat absorption) for SC-fed KO male mice compared with WT. In addition, TLC analyses of stool lipid components revealed no significant differences between WD-fed WT and KO mice in stool content of free fatty acids, cholesterol-diacylglycerol, cholesterol esters, or total lipids (Table 3). Although the SC-fed KO mice had a slightly lower stool content of some of these lipids, this again reflects absorption that is more (rather than less) complete for KO than for WT mice.
Table 1.
Food consumption by WT and iPLA2γ-KO mice fed standard chow or a Western diet
| Genotype | WT | KO | WT | KO | WT | KO | WT | KO |
|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | F | F | F | F |
| Diet | SC | SC | WD | WD | SC | SC | WD | WD |
| Food consumption | 4.77 | 4.99 | 3.16 | 3.12 | 4.47 | 4.22 | 2.75 | 2.77 |
| SE | 0.16 | 0.22 | 0.11 | 0.09 | 0.16 | 0.11 | 0.05 | 0.06 |
| n | 35 | 35 | 26 | 20 | 30 | 30 | 30 | 25 |
| P WT vs. KO | 0.42 | 0.76 | 0.25 | 0.83 | ||||
Male (M) or female (F) Group VIB phospholipase A2 (iPLA2γ)-knockout (KO) and wild-type (WT) mice were fed standard chow (SC) or a Western diet (WD) as in Figure 1. Individually housed mice age 12 wk were given preweighed amounts of food, and the amounts consumed per 24 h were determined daily for 5 days and expressed as grams consumed per day, as described in experimental procedures and elsewhere (35).
Table 2.
Stool weight and extractable lipid content for WT and iPLA2γ-KO mice fed SC or WD
| Genotype | WT | KO | WT | KO | WT | KO | WT | KO |
|---|---|---|---|---|---|---|---|---|
| Sex | M | M | M | M | F | F | F | F |
| Diet | SC | SC | WD | WD | SC | SC | WD | WD |
| Stool lipid extract | 3.08 | 2.31 | 3.68 | 3.26 | 2.19 | 2.19 | 1.81 | 1.43 |
| SE | 0.24 | 0.15 | 0.45 | 0.21 | 0.20 | 0.26 | 0.12 | 0.17 |
| P WT vs. KO | 0.02 | 0.42 | 0.98 | 0.09 | ||||
| n | 8 | 8 | 12 | 12 | 8 | 8 | 12 | 12 |
| Stool weight | 3.38 | 3.97 | 1.28 | 1.32 | 3.12 | 3.48 | 0.75 | 1.06 |
| SE | 0.43 | 0.26 | 0.14 | 0.10 | 0.40 | 0.09 | 0.26 | 0.10 |
| P WT vs. KO | 0.30 | 0.83 | 0.43 | 0.33 | ||||
Male or female iPLA2γ-KO and WT mice were fed SC or WD as in Table 1. Individually housed mice age 12 wk had unrestricted access to food and water, and all stool was collected for 24 h intervals. Stool wet (expressed in grams and tabulated) and dry weights and extractable lipid content (expressed in mg lipid per 60 mg stool dry weight) were determined as in experimental procedures and elsewhere (35, 72).
Table 3.
TLC analyses of stool lipid species for WT and iPLA2γ-KO mice fed SC or WD
| Genotype | WT | KO | WT | KO |
|---|---|---|---|---|
| Diet | SC | SC | WD | WD |
| Free fatty acids | 324 | 304 | 420 | 435 |
| SE | 11 | 19 | 23 | 41 |
| P WT vs. KO | 0.39 | 0.75 | ||
| Cholesterol/DAG | 588 | 485 | 541 | 474 |
| SE | 28 | 27 | 71 | 77 |
| P WT vs. KO | 0.02 | 0.53 | ||
| Cholesterol esters | 315 | 152 | 216 | 213 |
| SE | 20 | 23 | 25 | 20 |
| P WT vs. KO | <0.01 | 0.94 | ||
| Total stool lipids | 1,227 | 941 | 1,177 | 1,123 |
| SE | 40 | 54 | 85 | 82 |
| P WT vs. KO | <0.01 | 0.65 | ||
| n | 8 | 8 | 16 | 16 |
Stool lipid extracts obtained as in Table 2 were analyzed by TLC, and amounts of separated lipid classes were determined by densitometry after charring as described in experimental procedures and elsewhere (11) and expressed in IDV units.
That KO mice digested and absorbed dietary fat content as efficiently as did WT mice was also reflected by the time course of appearance of [3H]oleate in plasma after administration of [3H]triolein into the gastrointestinal tract by gavage (Fig. 3). Data in Tables 1–3 and Fig. 3 thus indicate that differences between iPLA2γ-KO mice and their WT littermates in metabolic responses to a WD do not reflect impaired consumption of the diet or impaired digestion and absorption of its fat content.
Fig. 3.
Triolein absorption by iPLA2γ-KO and WT mice. After an overnight fast, [3H]triolein (1 μCi) in olive oil was administered to WT (○) or iPLA2γ-KO (●) mice by gavage, and blood samples were taken at 1, 2, 4, and 6 h thereafter, as described in experimental proceduresand elsewhere (35). Plasma was prepared, and its 3H content was determined by liquid scintillation spectrometry and expressed as dpm/10 μl plasma. Mean values ± SE (n = 9) are displayed. There were no significant differences between genotypes.
Differential effects of a WD on blood levels of cholesterol, insulin, glucose, and other analytes for wild-type and iPLA2γ-knockout mice.
Expected sequelae of consuming a WD include hypercholesterolemia and insulin resistance, and fasting serum cholesterol levels in WD-fed WT male mice rose with time after initiation of differential feeding (Fig. 4). After 22 wk on the diet, serum cholesterol levels were significantly higher for WD-fed WT (368 ± 45 mg/dl) than for KO (194 ± 12 mg/dl) male mice (Fig. 4A). Cholesterol levels of both WD-fed groups were significantly higher than those of the corresponding SC-fed groups of the same genotype (170 ± 21 mg/dl for WT and 140 ± 9 mg/dl for KO), although the levels for SC-fed WT and KO male mice did not differ significantly from each other. Similar trends of smaller magnitude were observed with females (Fig. 4B).
Fig. 4.
Diet effects on blood cholesterol levels of iPLA2γ-KO and WT mice. Cholesterol concentration (mg/dl) of plasma from 4-h-fasted WT (circles) or iPLA2γ-KO (squares) male (A) and female (B) mice fed SC (open symbols) or WD (closed symbols) as in Fig. 1 was determined enzymatically as described in experimental procedures and elsewhere (9). Mean values ± SE (n = 23) are displayed. *Significantly (P < 0.05) greater cholesterol level in genotype and diet comparisons; +significantly greater value only in diet comparisons.
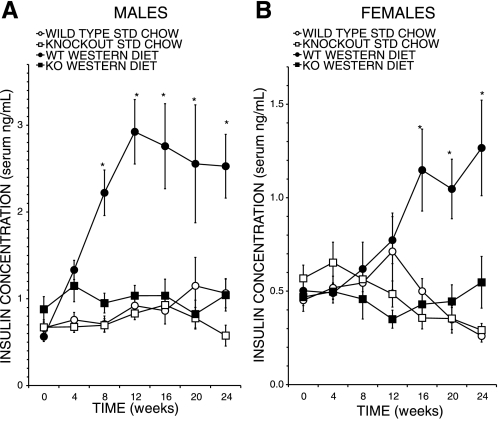
Similarly, rises in fasting serum insulin levels reflecting diet-induced insulin resistance were observed in WD-fed male (Fig. 5A) and female (Fig. 5B) WT mice, and the levels were significantly higher than those for SC-fed WT mice and for SC-fed or WD-fed KO mice of the corresponding sex. Interestingly, no significant WD feeding-induced rise in fasting serum insulin levels was observed in male or female KO mice (Fig. 5), suggesting that the development of diet-induced insulin resistance is attenuated with the iPLA2γ-KO genotype.
Fig. 5.
Diet effects on plasma insulin concentrations of iPLA2γ-KO and WT mice. Insulin concentration (ng/ml) of plasma from 4-h-fasted WT (circles) or iPLA2γ-KO (squares) male (A) and female (B) mice fed SC (open symbols) or WD (closed symbols) as in Fig. 1 was determined by ELISA as described in experimental proceduresand elsewhere (6, 9). Mean values ± SE (n = 21) are displayed. *Significantly (P < 0.05) greater insulin level in genotype and diet comparisons.
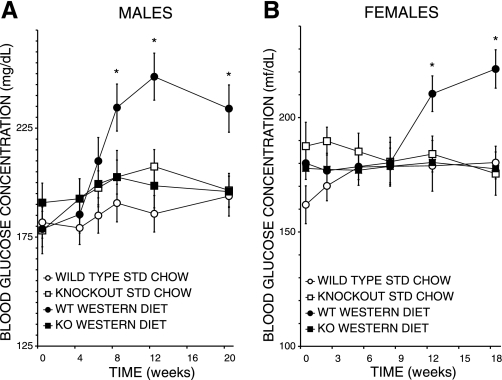
Rising fasting blood glucose levels reflecting diet-induced deterioration in glucose tolerance were also observed in WD-fed male (Fig. 6A) and female (Fig. 6B) WT mice, and these levels were also significantly higher than those for SC-fed WT mice and for SC-fed or WD-fed KO mice of the corresponding sex. As with fasting insulin levels, no significant WD feeding-induced rise in fasting blood glucose levels was observed in male or female KO mice (Fig. 6), suggesting that diet-induced deterioration in glucose tolerance is attenuated with the iPLA2γ-KO genotype.
Fig. 6.
Diet effects on blood glucose concentrations of iPLA2γ-KO and WT mice. Glucose concentration (mg/dl) of blood from 4-h-fasted WT (circles) or iPLA2γ-KO (squares) male (A) and female (B) mice fed SC (open symbols) or WD (closed symbols) as in Fig. 1 was determined enzymatically as described in experimental proceduresand elsewhere (6, 9). Mean values ± SE (n = 33) are displayed. *Significantly (P < 0.05) greater glucose level in genotype and diet comparisons.
No significant effects of this dietary intervention were observed on TAG, free fatty acid, or β-hydroxybutyrate levels in WT or KO mice (Table S1). Glycerol levels were slightly but significantly higher in SC-fed male WT compared with SC-fed male KO mice and in WD-fed female WT compared with WD-fed KO mice, but glycerol levels were statistically indistinguishable in WD-fed WT and KO male mice and in SC-fed WT and KO female mice (Table S1).
Dietary effects on glucose tolerance.
Glucose tolerance testing (GTT) confirmed the diet-induced deterioration in WD-fed WT compared with SC-fed male mice (Fig. 7A), but no significant diet-induced deterioration in glucose tolerance was observed for male KO mice (Fig. 7B). Similar trends of smaller magnitude were observed with female mice (Fig. 8). Compared with SC-fed mice, WD-fed male (Fig. 8A) and female (Fig. 8B) mice experienced a significant increase in GTT area under the curve (AUC), but significant diet-induced increases in GTT AUC were not observed for male (Fig. 8A) or female (Fig. 8B) KO mice, again indicating that diet-induced deterioration in glucose tolerance is attenuated with the iPLA2γ-KO genotype.
Fig. 7.
Glucose tolerance tests of iPLA2γ-KO and WT mice fed SC or a WD. Male WT (A) or iPLA2γ-KO (B) WT (circles) mice were fed SC (●) or WD (■), as in Fig. 1. At age 6 mo, a baseline (time 0) blood sample was obtained; a glucose (2 mg/kg ip) solution was administered, and blood samples were collected 30, 60, and 120 min thereafter, as described in experimental proceduresand elsewhere (6, 9). Glucose concentrations were determined as in Fig. 6. Mean values ± SE (n = 23) are displayed. *Significantly (P < 0.05) greater glucose level in diet and genotype comparisons.
Fig. 8.
Diet effects on glucose tolerance tests area under the curve (AUC) for iPLA2γ-KO and WT mice. Experiments were performed as in Fig. 7, and the area under the glucose concentration vs. time curve was calculated as described (8). Delta (gray bars) represents the difference between AUC values for mice of a given sex and genotype fed SC (light bars) or WD (dark bars) as in Fig. 1. Mean values ± SE are indicated (n = 23). *Significantly (P < 0.05) greater value in genotype and diet comparisons; x, significantly greater value only in genotype comparisons.
Dietary effects on insulin secretion and sensitivity.
To examine the relative contributions of altered insulin secretion and insulin sensitivity to diet-induced glucose intolerance in the mouse WD feeding model, insulin secretion in vivo was assessed by the rise in serum insulin concentration after intraperitoneal glucose administration (Fig. 9A), and insulin secretion in vitro from isolated pancreatic islets was also determined (Fig. 9B). Insulin sensitivity was evaluated by insulin tolerance testing (ITT; Fig. 10).
Fig. 9.
Insulin secretion in vivo and from pancreatic islets isolated from iPLA2γ-KO and WT mice fed SC or WD. A (in vivo secretion): experiments were performed as in Fig. 7, except that the glucose dose was 3 mg/kg ip, and blood samples for insulin determinations were collected at baseline (time 0) and 15 min after glucose administration as described in experimental procedures and elsewhere (8, 62). Mean values ± SE are indicated (n = 45). *Significantly (P < 0.05) greater value in genotype and diet comparisons; x, significantly greater value only in genotype comparisons. B: islets isolated from pancreata of WT (1st and 3rd bars in each set) or iPLA2γ-KO (2nd and 4th bars in each set) male mice were incubated (30 min, 37°C) in buffer containing 3, 8, or 20 mM d-glucose without or with 2.5 μM forskolin, and an aliquot of medium was then removed for measurement of insulin, as described in experimental procedures and elsewhere (6, 8, 62). Mean values ± SE are indicated (n = 39). *Significantly (P < 0.05) greater value in diet comparisons.
Fig. 10.
Insulin tolerance tests of iPLA2γ-KO and WTmice fed SC or WD. Human regular insulin (0.75 U/kg) was administered by ip injection to WT (■) or iPLA2γ-KO (●) male mice age 6 mo fed SC (A) or WD (B) as in Fig. 1, and blood was collected at baseline (time 0) and at 30, 60, and 120 min after injection to measure glucose concentration, as described in experimental procedures and elsewhere (6, 8, 62). Mean values ± SE are indicated (n = 39). *Significantly (P < 0.05) greater value in genotype and diet comparisons.
SC-fed WT mice exhibited an increase in serum insulin levels 15 min after glucose administration of about twofold for females and nearly threefold for males (Fig. 9A). These responses were significantly reduced in WD-fed WT mice of the corresponding gender (Fig. 9A) by the time that diet-induced deterioration in glucose tolerance had occurred (Figs. 7 and 8). Serum insulin levels in KO mice did not change appreciably in this model and were not significantly affected by diet. Insulin secretion by isolated islets tended to be lower for KO than for WT mice and lower for WD-fed than for SC-fed mice (Fig. 9B). These differences were not statistically significant, with the single exception of the greater secretion of SC-fed compared with WD-fed KO islets stimulated with 20 mM glucose and 2.5 μM forskolin (Fig. 9B).
Genotype-dependent differences in insulin secretion thus do not appear to explain the differential WD diet-induced deterioration of glucose tolerance for WT compared with KO mice, and this suggests that diet-induced changes in insulin sensitivity differ between the genotypes. In ITT, insulin administration resulted in statistically indistinguishable time courses of decline and recovery of blood glucose levels for SC-fed WT and KO mice (Fig. 10A). In contrast, WD-fed WT mice exhibited a significantly smaller decline in blood glucose concentrations than KO mice after insulin administration, and WD-fed WT but not KO mice exhibited rebound hyperglycemia at the ITT 120 min time point (Fig. 10B). WT mice thus experience a greater WD feeding-induced decrease in insulin sensitivity than do KO mice.
Dietary effects on diurnal variation in utilization of carbohydrate and fat as fuels.
Indirect calorimetric measurements performed with an Oxymax apparatus demonstrated that SC-fed WT mice exhibit the expected diurnal increase in RER in their active (dark cycle) phase compared with their resting (light cycle) phase (Fig. 11), which reflects increased metabolism of carbohydrate compared with fat in the active phase. This diurnal variation was greatly reduced for WD-fed WT mice (Fig. 11), reflecting greater metabolism of fat and lower RER during the active phase for WD-fed than for SC-fed mice. SC-fed KO mice exhibited diurnal variations in RER that were indistinguishable from that of SC-fed WT mice, but WD-fed KO mice exhibited a much smaller diminution in dark cycle RER compared with SC-fed mice than did WT mice (Fig. 11). This indicates that the WD feeding-induced shift in active-phase fuel metabolism from carbohydrate to fat is attenuated with the iPLA2γ-KO genotype.
Fig. 11.
Diurnal variation of respiratory exchange ratio (RER) for iPLA2γ-KO and WT mice fed SC or WD. WT and iPLA2γ-KO mice were fed SC (STD CHOW) or WD (WEST DIET) as in Fig. 1, and at age 6 mo were studied in the fed state in a single-chamber indirect calorimetry system for 24 h with 12:12-h light-dark cycles, as described in experimental procedures and elsewhere (9). RER values were calculated hourly by instrumental software from V̇o2 and V̇co2 rates and are expressed as mean values for the entire 12-h light or dark cycle. SE are indicated (n = 80). *Significantly (P < 0.05) greater value in dark cycle vs. light cycle.
WD-fed WT mice exhibited a significant increase in heat production compared with SC-fed WT mice, and there was little diurnal variation in this parameter (Fig. S2). KO mice generated less heat than WT mice, but WD-fed KO mice increased heat production, V̇o2, and V̇co2 during their active phase compared with SC-fed KO mice (Fig. S2). There were trends for dark cycle activity to decline in WD-fed compared with SC-fed WT mice and for dark cycle activity to be higher in WD-fed KO compared with WT mice, but these trends did not achieve statistical significance (Fig. S2).
Oxidation of palmitate and glucose by skeletal muscle mitochondria and their morphology and distribution within myocytes.
Skeletal muscle is a principal site of insulin action and substrate oxidation and is rich in mitochondria. Examination of mitochondrial β-oxidation of [14C]palmitate to [14C]O2 by excised gastrocnemius muscle specimens in vitro revealed lower oxidation rates with KO than with WT muscle for SC-fed and WD-fed male and female mice (Fig. 12). β-Oxidation rates were higher for male than for female muscle specimens, and muscle from both WD-fed WT and KO male mice exhibited higher β-oxidation rates than did specimens from SC-fed male mice of the corresponding genotype (Fig. 12). In contrast, rates of glucose oxidation were similar for skeletal muscle specimens from both SC-fed and WD-fed WT and KO mice (Table S2).
Fig. 12.
β-Oxidation of palmitic acid by gastrocnemius muscle of iPLA2γ-KO and WT mice fed SC or WD. WT (light bars) and iPLA2γ-KO (dark bars) mice were fed SC or WD as in Fig. 1, and at age 6 mo gastrocnemius muscle specimens were obtained and 14CO2 production from [14C]palmitate by muscle homogenates was determined as described in experimental procedures and elsewhere (16) and expressed as nmol CO2/mg muscle protein. Mean values ± SE are indicated (n = 12). *Significantly (P < 0.05) greater value in genotype comparisons; x, significantly greater weight in diet comparisons.
The overall reduction in mitochondrial β-oxidation of palmitate by KO compared with WT muscle specimens was associated with differences in mitochondrial morphology and distribution within muscle cells. KO mitochondria were generally larger (Fig. S3) than WT mitochondria, as reported by others (49), and KO mitochondria appeared to be distributed more homogeneously throughout muscle fibers than did WT (Fig. S4).
Cardiolipin composition of mitochondria and tissue content.
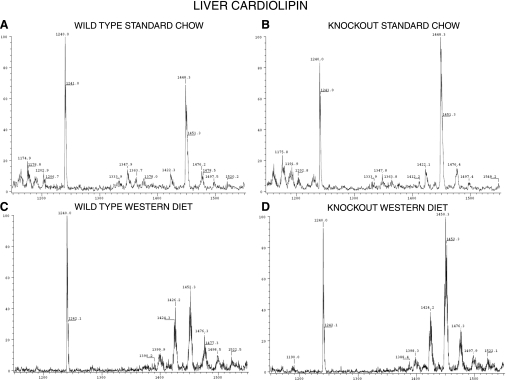
Cardiolipins (CL) are phospholipids that occur virtually exclusively within mitochondria in mammalian tissues and are important in mitochondrial function. Several CL molecular species with different [M − H]− ion m/z values were observed upon ESI-MS analyses of lipid extracts from gastrocnemius skeletal muscle (Fig. 13) and liver (Fig. 14) of WT and KO mice (Table S3). Liver from SC-fed WT (Fig. 14A) and KO (Fig. 14B) mice exhibited a predominant CL molecular species with an [M − H]− ion of m/z 1448 (Fig. 14, A and C) that corresponded to (18:2)4-CL, as demonstrated by tandem MS (Fig. S5A). CL from gastrocnemius muscle (hereafter designated only “muscle”) of SC-fed WT (Fig. 13A) and KO (Fig. 13C) mice comprised a more heterogeneous mixture of several species of comparable abundance.
Fig. 13.
ESI-MS display of cardiolipin (CL) molecular species in gastrocnemius muscle from iPLA2γ-KO and WT mice fed SC or WD. Gastrocnemius muscle specimens were obtained as in Fig. 12 from WT (A and B) and iPLA2γ-KO (C and D) male mice age 6 mo fed Standard Chow (A and C) or a WD (B and D) as in Fig. 1, and mitochondrial lipid extracts were analyzed by negative ion ESI-MS to visualize [M − H]− ions of native CL molecular species and of (14:0)4-CL internal standard (m/z 1240) as described in experimental procedures and elsewhere (30, 34).
Fig. 14.
ESI-MS display of CL molecular species in liver from iPLA2γ-KO and WT mice fed SC or WD. Liver specimens were obtained from WT (A and B) and iPLA2γ-KO (C and D) male mice age 6 mo fed SC (A and C) or WD (B and D) as in Fig. 1, and mitochondrial lipid extracts were analyzed by negative ion ESI-MS as in Fig. 13.
ESI-MS signal for endogenous CL species relative to internal standard (14:0)4-CL tended to be greater for tissues from WD-fed compared with SC-fed mice (Figs. 13 and 14 and Table S3), and tissue total CL contents were significantly greater for WD-fed compared with SC-fed KO mice by ∼2-fold for muscle and 1.5-fold for liver (Fig. 15). There were no significant differences between WD-fed and SC-fed WT mouse tissue CL content, but the heterogeneity of CL molecular species tended to increase in tissues from WD-fed compared with SC-fed mice (Figs. 13 and 14 and Table S3). In liver from WD-fed mice, the [M − H]− ion m/z values for the most abundant CL species shifted from 1448 to 1450/1452, and satellite clusters centered around m/z 1424 and 1476 became more prominent than in CL spectra from SC-fed mouse livers (Fig. 14). Ions of m/z 1450/1452 represent CL species that contain 18:1 fatty acid substituent(s) in addition to the usual 18:2 (Fig. S5). Ion clusters centered on m/z 1424 and 1476 represent mixtures of species with 16:1 and 20:3 fatty acid substituents, respectively, as indicated by tandem MS (Fig. S5). The abundance of these species also increased in muscle of WD-fed compared with SC-fed KO mice (Fig. 13).
Fig. 15.
CL content of gastrocnemius muscle and liver of iPLA2γ-KO and WT mice fed SC or WD. Gastrocnemius muscle (A) or liver (B) specimens were obtained from WT and iPLA2γ-KO male mice age 6 mo fed SC (light bars) or WD (dark bars) as in Fig. 1, and mitochondrial lipid extracts were analyzed by negative ion ESI-MS as in Figs. 13 and 14. Native CL species were quantified relative to (14:0)4-CL internal standard as described in experimental procedures and elsewhere (30, 34). Mean values ± SE (n = 8) are indicated for the sum of endogenous CL molecular species expressed as nmol/mg tissue protein. *Significantly (P < 0.05) higher value in diet comparisons; x, significantly higher value in genotype comparisons.
Dietary and genotype effects on tissue profiles and urinary excretion of LCAC species.
Mitochondrial β-oxidation of long-chain fatty acyl-CoA esters yields shorter-chain species that equilibrate with pools of corresponding carnitine esters, and tissue acylcarnitine profiles reflect mitochondrial oxidative activity. ESI-MS signal for endogenous LCAC species relative to the internal standard tended to be greater for muscle (Fig. 16 and Table S4) and liver (Fig. S6) from WD-fed than from SC-fed mice, and amounts of tissue total LCAC were significantly greater for WD-fed than for SC-fed KO mice by 3.8-fold in muscle and 2.2-fold in liver (Fig. 17, A and C). Trends for tissue LCAC content to be greater in WD-fed compared with SC-fed WT mice did not achieve statistical significance, and LCAC contents of muscle and liver of WD-fed KO mice were significantly greater than those of WD-fed WT mice (Fig. 17, A and C).
Fig. 16.
ESI-MS display of acylcarnitine molecular species in gastrocnemius muscle from iPLA2γ-KO and WT mice fed SC or WD. Gastrocnemius muscle specimens were obtained as in Fig. 12 from WT (A and B) and iPLA2γ-KO (C and D) male mice age 6 mo fed SC (A and C) or WD (B and D) as in Fig. 1, and acylcarnitines in homogenates were analyzed as methyl esters relative to [2H3]acetylcarnitine internal standard by positive ion ESI-MS-MS scanning as described in experimental procedures and elsewhere (3, 22).
Fig. 17.
Acylcarnitine content and mean carbon chain length in gastrocnemius muscle and liver of iPLA2γ-KO and WT mice fed SC or WD. Gastrocnemius muscle (A and B) and liver (C and D) specimens were obtained as in Figs. 12 and 14, respectively, from WT (light bars) and iPLA2γ-KO (dark bars) male mice age 6 mo fed SC or WD as in Fig. 1, and acylcarnitine species in homogenates were quantitated by ESI-MS as in Fig. 16. A and C: values represent the sum of moles of acyl chain carbon for endogenous long-chain acylcarnitine (LCAC) species/mg tissue protein normalized to value for WTmice fed SC. B and D: values represent mean carbon chain length as computed from the ratio moles of acyl chain carbon relative to moles of acylcarnitine. Mean values ± SE (n = 10) are indicated. *Significantly (P < 0.05) higher value in genotype comparisons; x, significantly higher value in diet comparisons.
The mean carbon chain length of LCAC in both liver and muscle of WD-fed KO mice was also significantly greater than that of SC-fed KO mice, and this was true for liver but not muscle of WT mice (Fig. 17, B and D). The increasing length of the LCAC carbon chain is likely to reflect less complete mitochondrial β-oxidation of fatty acid substrates in WD-fed mice. The increased tissue content of LCAC in WD-fed compared with SC-fed KO mice was accompanied by significantly greater urinary excretion of LCAC in WD-fed compared with SC-fed KO mice (Fig. 18). WT mice exhibited a similar trend of smaller magnitude that did not achieve statistical significance. Increased urinary excretion of LCAC may represent one means by which KO mice dispose of the excess caloric and fat load of the WD and the possibility that tissue TAG deposition might represent another was considered.
Fig. 18.
Acylcarnitine content of urine from iPLA2γ-KO and WT mice fed SC or WD. Urine was collected for 24 h on each of 4 consecutive days from male (A) or female (B) iPLA2γ-KO (dark bars) and WT (light bars) mice age 6 mo fed SC or WD as described in Fig. 1, and urine acylcarnitine content/g body wt was measured as in Fig. 17. Mean values ± SE are indicated (n = 25). *Significantly (P < 0.05) higher value in genotype comparisons; x, significantly higher value in diet comparisons.
Dietary and genotype effects on tissue accumulation and molecular composition of TAG.
The TAG content of liver from WT WD-fed mice was 8- to 10-fold higher than that of WT SC-fed mice and 4- to 6-fold higher than that of WD-fed KO mice (Fig. 19). Gastrocnemius muscle TAG content was comparable to that of liver for SC-fed WT and KO mice but did not increase appreciably upon WD feeding. Despite the differing tissue contents of TAG, ESI-MS profiles of WT and KO TAG molecular species in a given tissue, i.e., liver (Fig. 20) or muscle (Fig. S7), were similar, and there was a tendency for tissues of WD-fed mice to contain more saturated TAG species than those for SC-fed mice, as indicated by the relative abundances of members of the ion pairs m/z 863/865 and of m/z 889/891 (Fig. 20, Fig. S7, and Table S5). Tandem MS analyses revealed that this reflected the presence of more saturated fatty acid substituents, e.g., 16:0, and fewer polyunsaturated substituents, e.g., 18:2, in TAG from tissues from WD-fed mice (Fig. S8).
Fig. 19.
Triacylglycerol (TAG) content of liver and gastrocnemius muscle of iPLA2γ-KO and WT mice fed SC or WD. Gastrocnemius muscle and liver specimens were obtained as in Figs. 12 and 14, respectively, from WT and iPLA2γ-KO male (A) and female (B) mice age 6 mo fed SC (light bars) or WD (dark bars) as in Fig. 1, and TAG content of lipid extracts was measured enzymatically as described in experimental procedures and elsewhere (25, 53, 55) and expressed as μg TAG/mg tissue protein. Mean values ± SE are indicated (n = 15). *Significantly (P < 0.05) higher value in genotype comparisons; x, significantly higher value in diet comparisons.
Fig. 20.
ESI-MS display of TAG molecular species in liver from iPLA2γ-KO and WT mice fed SC or WD. Liver specimens were obtained as in Figs. 14 from WT (A and B) and iPLA2γ-KO (C and D) male mice age 6 mo fed SC (A and C) or WD (B and D) as in Fig. 1, and lipid extracts were prepared as in Fig. 19 and analyzed by positive ion ESI-MS in infusion solution containing LiCl to visualize TAG [M+Li]+ ions as described in experimental procedures and elsewhere (33).
Oil red O staining of liver sections to visualize neutral lipid content (Fig. S9) confirmed conclusions from quantitative TAG analyses (Fig. 19) and indicated that there is a low level of staining in liver from SC-fed mice and much greater staining in sections from WD-fed WT mice, reflecting greater neutral lipid content. Staining is also greater in sections from WD-fed compared with SC-fed KO mice but is much less intense than that in sections from WD-fed WT mice (Fig. S9). Deposition of TAG in nonadipose tissues is thus another metabolic effect of WD-feeding in WT mice that is attenuated in the iPLA2γ-KO genotype, similar to increased body weight and adiposity, insulin resistance, glucose intolerance, and disruption of the normal diurnal variation in RER that reflects preferential active-phase oxidation of fatty acid over carbohydrate substrates by WD-fed WT mice.
DISCUSSION
Male WT mice that consume a WD achieve a body weight 23% greater than that of littermates fed SC (39), increased adipose tissue mass (18, 21), and hepatic steatosis (18). This is associated with insulin resistance reflected by hyperinsulinemia, elevated fasting blood glucose levels, impaired glucose tolerance, reduced insulin responsiveness, and rebound hyperglycemia after insulin administration (39). WD-fed mice also lose the normal ability to switch from utilizing fat as fuel during periods of fasting and inactivity to utilizing carbohydrate during periods of activity and food consumption, as reflected by a loss of normal diurnal variation in RER (18, 39). Our observations with WD-fed WT mice agree with these reports, but iPLA2γ-null mice clearly differ in their responses to WD consumption.
Events similar to those for WD-fed WT mice occur in human obesity-related insulin resistance and metabolic syndrome (59, 60), precursors of overt type 2 diabetes precipitated by pancreatic islet β-cell failure to maintain compensatory hypersecretion of insulin (61). Whole body knockout of iPLA2γ yields a complex phenotype affecting multiple organ systems and physiological abnormalities that include growth retardation, neurodegeneration, and cognitive dysfunction. This makes it difficult to determine the precise mechanisms that defend against diet-induced insulin resistance, but published evidence suggests that iPLA2γ might play important physiological roles in tissues involved in obesity and glucose homeostasis (47, 49, 50, 74).
Among tissues affected by WD consumption are adipose tissue, liver, and skeletal muscle. Increased adipose tissue in WD-fed WT mice accounts for most of the diet-induced increase in body weight and is greatly reduced in iPLA2γ-null mice, as are blood levels of the adipokine leptin, and this might reflect an impaired ability of iPLA2γ-null mice to expand their adipocyte pool. When 3T3-L1 preadipocytes are induced to differentiate into adipocytes, iPLA2γ mRNA levels rise, and differentiation is prevented by iPLA2γ siRNA (74). This suggests that iPLA2γ facilitates adipocyte differentiation, and iPLA2γ-deficiency might impair it. The reduced whole body adiposity and tissue TAG content of iPLA2γ-null mice, despite normal food intake, suggests energetic inefficiency that results in resistance to weight gain. Such leanness may protect against the adverse effects of obesity, including tissue lipid accumulation, inflammation, and endoplasmic reticulum stress, and this may enhance insulin sensitivity.
Human type 2 diabetes, the metabolic syndrome, and animal models of insulin resistance are marked by hepatic steatosis and TAG deposition (14, 23, 42, 69), and this also occurs in our WD-fed WT mice. Liver TAG content increased 8- to 10-fold in WD-fed compared with SC-fed WT mice, and this was greatly attenuated in iPLA2γ-null mice. For both genotypes, there was a shift in TAG molecular species distribution toward a higher content of saturated fatty acid substituents, which also occurs with myocardial TAG in mice with streptozotocin-diabetes (28).
Increased hepatic lipogenesis is a classical insulin response, and its occurrence in the setting of insulin resistance has been considered paradoxical (63). Potential explanations include selective insulin resistance (13). Insulin-resistant humans with insulin receptor (IR) mutations and mice with liver-specific IR deletion exhibit hyperglycemia and hyperinsulinemia but not hepatic steatosis (10, 73), suggesting that insulin-resistance does not involve all signaling pathways and that those governing lipid synthesis are preserved. Hyperinsulinemia from β-cell compensation for muscle insulin resistance might thus drive hepatic lipid deposition, which is consistent with our findings that WD-fed iPLA2γ-null mice, unlike WT mice, do not develop hyperinsulinemia and experience much less diet-induced hepatic steatosis.
A converse effect occurs with transgenic mice that overexpress iPLA2γ in myocardium, in which diet-induced cardiac TAG accumulation is augmented and precipitates cardiac dysfunction (47). That is attributed to iPLA2γ-mediated accumulation of endocannabinoid precursors (76) and signaling molecules, e.g., (1-lyso, 2-arachidonoyl)-GPC (47, 82), that drive expression of the lipid biosynthetic enzyme diacylglycerol acyltransferase-1 (DGAT1), whose mRNA increases severalfold in myocardium of iPLA2γ transgenic mice subjected to dietary stress (47). Genetic ablation of iPLA2γ might thus result in reduced tissue levels of these signaling lipids and of DGAT1, and deficiency of endocannabinoid receptors (40) or of DGAT1 (20) also protects mice from diet-induced obesity (20, 40). These findings suggest that iPLA2γ plays a physiological role in regulating tissue lipid deposition that might relate to effects on mitochondrial bioenergetics and fat oxidation.
Protection of iPLA2γ-KO mice against diet-induced obesity and glucose intolerance is associated with a sparing of skeletal muscle from WD feeding-induced deterioration in insulin responsiveness observed in WT mice, as reflected by greater responsiveness in insulin tolerance tests for WD-fed KO compared with WT mice. KO skeletal muscle resistance to WD-induced deterioration in insulin sensitivity is associated with impaired mitochondrial fatty acid β-oxidation reflected by reduced [14C]palmitate oxidation ex vivo and by muscle accumulation of LCAC in vivo. Altered fatty acid processing by KO mitochondria is associated with morphological abnormalities and an altered distribution of mitochondria in muscle cells.
Cardiolipin (CL) is a mitochondrial inner membrane phospholipid with two negative charges at physiological pH that is enriched in linoleate (18:2) and has a large aliphatic chain to polar head group volume. These features allow CL to interact with electron transport chain proteins, e.g., cytochrome c oxidase (31, 64, 71, 84). Streptozotocin-diabetes induction in mice results in a decline in myocardial CL content, an increase in CL longer-chain fatty acid substituents, and cardiac dysfunction (29, 74). CL alterations and cardiac dysfunction also occur in iPLA2γ-null mice and transgenic mice that overexpress iPLA2γ in myocardium, and this may reflect a role for iPLA2γ in CL biosynthesis/remodeling that affects mitochondrial bioenergetics and cardiac physiology (47, 50). A neurophysiological role of iPLA2γ is also suggested by the increased hippocampal CL content and altered CL fatty acid composition of iPLA2γ-null mice, which is associated with mitochondrial degeneration and cognitive dysfunction (49).
We find that muscle and liver CL content increases in WD-fed compared with SC-fed iPLA2γ-null mice, and this rise is greater in KO than in WT mice. WD feeding is also associated with an increase in muscle and liver CL heterogeneity, and this is more pronounced in WT than in KO livers. Such diet-induced changes in CL content and composition may contribute to the differential effects of WD feeding on fuel selection in mitochondria of WT vs. iPLA2γ-null mice and in the impaired ability of the latter to process fatty acids oxidatively.
Such impaired processing is reflected by increased WD-induced accumulation of LCAC in iPLA2γ-null mouse skeletal muscle compared with WT. Moreover, the average muscle LCAC chain length increases significantly in WD-fed compared with SC-fed iPLA2γ-null mice. This indicates that muscle mitochondria of iPLA2γ-null mice are less effective than those of WT mice in dietary fatty acid β-oxidative processing. LCAC also accumulate in myocardium of mice with streptozotocin-induced diabetes (74).
Muscle insulin resistance has been thought to involve impaired carnitine palmitoyltransferase-1 (CPT I)-mediated mitochondrial fatty acid uptake and oxidation (58, 68) and preferential channeling of fatty acids into synthesis of the lipid mediators diacylglycerol and ceramide, and of TAG storage molecules (58, 68) that activate stress kinases, which interfere with insulin signaling (32, 83). Genetic manipulations that increase tissue levels of such lipids have been found not to induce insulin resistance (3, 56), however, and emerging evidence indicates that obesity-associated glucose intolerance arises from increased rather than decreased flux of fatty acids through mitochondrial β-oxidation (3, 39, 59–61).
Nutritional, pharmacological, and genetic maneuvers that suppress mitochondrial β-oxidation can protect against lipid-induced insulin resistance, and dietary fat is less damaging to skeletal muscle metabolic function when β-oxidation is constrained (39). Overexpressing malonyl-CoA decarboxylase results in increased levels of the CPT I inhibitor malonyl-CoA, which suppresses mitochondrial fatty acid import and oxidation, but this reverses muscle insulin resistance (3). In contrast, PPARα transgenic overexpression increases expression of genes involved in lipid oxidation and produces glucose intolerance and insulin resistance, and this phenotype is reversed by pharmacologic CPT I blockade (24). Moreover, PPARα-null mice have diminished muscle fatty acid β-oxidation and resist lipid-induced diabetes (78).
This has led to the hypothesis that WD consumption results in excessive mitochondrial β-oxidation and lipid-induced mitochondrial stress and consequent insulin resistance and glucose intolerance (39). With inborn mitochondrial diseases, acylcarnitine production is considered a detoxification mechanism that permits mitochondrial efflux of excess acyl groups (4). Muscle accumulation of these intermediates could represent a means to relieve redox stress imposed by mitochondrial overload (4), and their subsequent urinary excretion could represent a means to eliminate excess fat and caloric load.
Consistent with the hypothesis that inhibiting muscle fat oxidation protects against diet-induced metabolic dysregulation is a recent report that mice with genetic disruption of oxidative phosphorylation are resistant to diet-induced obesity and diabetes (65). Deleting the mitochondrial flavoprotein apoptosis-inducing factor in mouse skeletal muscle impairs electron transport chain function and is associated with improved glucose uptake and insulin signaling when animals are fed a high-fat diet (65). This and similar reports (3, 24, 39, 60, 65, 78) suggest that insulin action in skeletal muscle is coupled to mitochondrial energetics and substrate selection and that impairing fat oxidation causes muscle to use glucose as primary fuel. Improved glucose tolerance and resistance to diet-induced obesity conferred by mitochondrial oxidative defects might reflect inefficient anaerobic metabolism and consequent increase in fuel utilization to meet metabolic demands (65). Such inefficient fuel utilization could account for the failure of mice with mitochondrial oxidative defects to increase their body fat mass in the face of increased caloric intake (65).
By analogy with these models in which impaired mitochondrial fatty acid β-oxidation protects against diet-induced obesity and glucose intolerance (3, 24, 39, 60, 65, 78), a similar mechanism might operate in iPLA2γ-null mice, whose muscle mitochondria have impaired ability to oxidize dietary fatty acids. This reduces production of stress signals from mitochondrial β-oxidative overload compared with WT mice. The identity of mitochondrion-derived signal(s) that link excessive β-oxidation to insulin resistance is not yet known, but specific β-oxidation intermediates (e.g., 3-hydroxyoctanoic acid) and shorter-chain hydroxycarboxylic acids are ligands of a subfamily of G protein-coupled receptors that regulate adipocyte lipolysis (1, 2). Such compounds might be circulating mediators that coordinate metabolic activities of different tissues (1, 2) and represent candidate mitochondrion-derived signals of excessive β-oxidation. Since the average chain length of fatty acid β-oxidation intermediates that accumulate in muscle of WD-fed iPLA2γ-null mice is substantially greater than that of WT mice, it is possible that a specific set of shorter-chain signaling molecules generated by WT mitochondria are not produced by iPLA2γ-null mitochondria because of their less complete fatty acid β-oxidation.
GRANTS
This work was supported by United States Public Health Service Grants R37 DK-34388, P41 RR-00954, P60 DK-20579, P30 DK-56341, and 1 R01 DK-076729 (C. F. Semenkovich).
DISCLOSURES
No conflicts of interest are reported by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Alan Bohrer and Min Tan for excellent technical assistance, Dr. Trey Coleman for helpful technical advice, Drs. Sasanka Ramanadham and Kevin Yarasheski for a critical reading of the manuscript, and Drs. Richard Gross and David Mancuso for providing breeder mice that we used to establish our colony of iPLA2γ-null mice and wild-type littermates.
REFERENCES
- 1.Ahmed K, Tunaru S, Langhans CD, Hanson J, Michalski CW, Kölker S, Jones PM, Okun JG, Offermanns S. Deorphanization of GPR109B as a receptor for the beta-oxidation intermediate 3-OH-octanoic acid and its role in the regulation of lipolysis. J Biol Chem 284: 21928– 21933, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol 30: 557– 62, 2009 [DOI] [PubMed] [Google Scholar]
- 3.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10: 268– 274, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 70: 200– 214, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Bao S, Bohrer A, Ramanadham S, Jin W, Zhang S, Turk J. Effects of stable suppression of Group VIA Phospholipase A2 expression on phospholipid content and composition, insulin secretion, and proliferation of INS-1 insulinoma cells. J Biol Chem 281: 187– 198, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao S, Jacobson D, Wohltmann M, Bohrer A, Jin W, Philipson LH, Turk J. Glucose homeostasis, insulin secretion, and islet phospholipids in mice that overexpress iPLA2β in pancreatic β-cells and in iPLA2β-null mice. Am J Physiol Endocrinol Metab 294: E217– E229, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao S, Li Y, Lei X, Wohltmann M, Bohrer A, Jin W, Ramanadham S, Tabas I, Turk J. Attenuated free cholesterol loading-induced apoptosis and preserved phospholipid composition of peritoneal macrophages from mice that do not express Group VIA phospholipase A2. J Biol Chem 282: 27100– 27114, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao S, Song H, Wohltmann M, Bohrer A, Turk J. Insulin secretory responses and phospholipid composition of pancreatic islets from mice that do not express Group VIA Phospholipase A2 and effects of metabolic stress on glucose homeostasis. J Biol Chem 281: 20958– 20973, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernal-Mizrachi C, Weng S, Li B, Nolte LA, Feng C, Coleman T, Holloszy JO, Semenkovich CF. Respiratory uncoupling lowers blood pressure through a leptin-dependent mechanism in genetically obese mice. Arterioscler Throm Vasc Biol 22: 961– 968, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Biddinger SB, Hernandez-Ono A, Rask-Madsen C, Haas JT, Alemán JO, Suzuki R, Scapa EF, Agarwal C, Carey MC, Stephanopoulos G, Cohen DE, King GL, Ginsberg HN, Kahn CR. Hepatic insulin resistance is sufficient to produce dyslipidemia and susceptibility to atherosclerosis. Cell Metab 7: 125– 134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bitman JK, Wood DL, Ruth JM. Two-stage, one-dimensional thin layer chromatographic method for separation of lipid classes. J Liquid Chrom Rel Tech 4: 1007– 1021, 1981 [Google Scholar]
- 12.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911– 917, 1959 [DOI] [PubMed] [Google Scholar]
- 13.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab 7: 95– 96, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114: 147– 152, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. J Lipid Res 50: S237– S242, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carper MJ, Zhang S, Turk J, Ramanadham S. Skeletal muscle Group VIA Phospholipase A2(iPLA2β): expression and role in fatty acid oxidation. Biochemistry 47: 12241– 12249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chakravarthy MV, Pan Z, Zhu Y, Tordjman K, Schneider JG, Coleman T, Turk J, Semenkovich CF. “New” hepatic fat activates PPARα to maintain glucose, lipid, and cholesterol homeostasis. Cell Metab 1: 309– 322, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chakravarthy MV, Zhu Y, Lopez M, Yin L, Wozniak DF, Coleman T, Hu Z, Wolfgang M, Vidal-Puig A, Lane MD, Semenkovich CF. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest 117: 2539– 2552, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chakravarthy MV, Zhu Y, Yin L, Coleman T, Pappan KL, Marshall CA, McDaniel ML, Semenkovich CF. Inactivation of hypothalamic FAS protects mice from diet-induced obesity and inflammation. J Lipid Res 50: 630– 640, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen HC, Smith SJ, Ladha Z, Jensen DR, Ferreira LD, Pulawa LK, McGuire JG, Pitas RE, Eckel RH, Farese RV., Jr Increased insulin and leptin sensitivity in mice lacking acyl CoA:diacylglycerol acyltransferase 1. J Clin Invest 10: 1049– 1055, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheverud JM, Ehrich TH, Hrbek T, Kenney JP, Pletscher LS, Semenkovich CF. Quantitative trait loci for obesity- and diabetes-related traits and their dietary responses to high-fat feeding in LGXSM recombinant inbred mouse strains. Diabetes 53: 3328– 3336, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Duranti G, Boenzi S, Rizzo C, Ravà L, Di Ciommo V, Carrozzo R, Meschini MC, Johnson DW, Dionisi-Vici C. Urine acylcarnitine analysis by ESI-MS/MS: a new tool for the diagnosis of peroxisomal biogenesis disorders. Clin Chim Acta 398: 86– 89, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Erion DM, Ignatova ID, Yonemitsu S, Nagai Y, Chatterje P, Weismann D, Hsiao JJ, Zhang D, Iwasaki T, Stark R, Flannery C, Kahn M, Carmean CM, Xing Yu X, Murray SF, Bhanot S, Monia BP, Cline GW, Samuel VT, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein. Cell Metabolism 10: 499– 506, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finck BN, Bernal-Mizrachi C, Han DH, Coleman T, Sambandam N, LaRiviere L, Holloszy JO, Semenkovich CF, Kelly DP. A potential link between muscle peroxisome proliferator-activated receptor-alpha signaling and obesity-related diabetes. Cell Metab 1: 133– 144, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem 28: 2077– 2080, 1982 [PubMed] [Google Scholar]
- 26.Funk CD. Prostaglandiuns and leukotrienes: advances in eicosanoid biology. Science 294: 1871– 1875, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV Phospholipase A2 family. Prog Lipid Res 45: 487– 510, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Han X, Abendschein DR, Kelley JG, Gross RW. Diabetes-induced changes in specific lipid molecular species in rat myocardium. Biochem J 352: 79– 89, 2000 [PMC free article] [PubMed] [Google Scholar]
- 29.Han X, Yang J, Cheng H, Yang K, Abendschein DR, Gross RW. Shotgun lipidomics identifies cardiolipin depletion in diabetic myocardium linking altered substrate utilization with mitochondrial dysfunction. Biochemistry 44: 16684– 16694, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Han X, Yang J, Yang K, Zhao Z, Abendschein DR, Gross RW. Alterations in myocardial cardiolipin content and composition occur at the very earliest stages of diabetes: a shotgun lipidomics study. Biochemistry 46: 6417– 6428, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hiraoka M, Abe A, Lu Y, Yang K, Han X, Gross RW, Shayman JA. Lysososmal Phospholipase A2 and phospholipidosis. Mol Cell Biol 26: 61139– 61148, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167– 179, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Hsu FF, Turk J. Structural characterization of triacylglycerols as lithiated adducts by electrospray ionization mass spectrometry using low energy collisionally activated dissociation on a triple stage quadrupole instrument. J Am Soc Mass Spectrom 10: 587– 600, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Hsu FF, Turk J, Rhoades ER, Russell DG, Shi Y, Groisman EA. Structural characterization of cardiolipin by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectrom 16: 491– 504, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Huggins KW, Boileau AC, Hui DY. Protection against diet-induced obesity and obesity- related insulin resistance in Group 1B PLA2-deficient mice. Am J Physiol Endocrinol Metab 283: E994– E1001, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Jacobson DA, Webber C, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta cell delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem 282: 7442– 7449, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem 279: 48968– 48975, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res 50: S63– S68, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45– 56, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Kunos G, Osei-Hyiaman D, Liu J, Godlewski G, Bátkai S. Endocannabinoids and the control of energy homeostasis. J Biol Chem 283: 33021– 33025, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted Phospholipases A2. Annu Rev Biochem 77: 495– 520, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Leavens KF, Easton RM, Shulman GI, Previs SF, Birnbaum MJ. Akt2 is required for hepatic lipid accumulation in models of insulin resistance. Cell Metab 10: 405– 418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2β)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem 283: 34819– 34832, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu JL, Gall JG. U bodies are cytoplasmic structures that contain uridine-rich small nuclear ribonucleoproteins and associate with P bodies. Proc Natl Acad Sci USA 104: 11655– 11659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lowe ME, Kaplan MH, Jackson-Grusby L, D'Agostino D, Grusby MJ. Decreased neonatal dietary fat absorption and T-cell cytotoxicity in pancreatic lipase-related protein 2-deficient mice. J Biol Chem 273: 31215– 31221, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma Z, Ramanadham S, Wohltmann M, Bohrer A, Hsu FF, Turk J. Studies of insulin secretory responses and of arachidonic acid incorporation into phospholipids of stably transfected insulinoma cells that overexpress Group VIA Phospholipase A2 (iPLA2β) indicate a signaling rather than a housekeeping role for iPLA2β. J Biol Chem 276: 13198– 13208, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Mancuso DJ, Han X, Jenkins CM, Lehman JJ, Sambandam N, Sims HF, Yang J, Yan W, Yang K, Green K, Abendschein DR, Saffitz JE, Gross RW. Dramatic accumulation of triglycerides and precipitation of cardiac hemodynamic dysfunction during brief caloric restriction in transgenic myocardium expressing human calcium-independent Phospholipase A2γ. J Biol Chem 282: 9216– 9227, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Mancuso DJ, Jenkins CM, Gross RW. The genomic organization, complete mRNA sequence, cloning, and expression of a novel human intracellular membrane-associated calcium-independent Phospholipase A2. J Biol Chem 275: 9937– 9945, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Mancuso DJ, Kotzbauer PT, Wozniak DF, Sims HF, Jenkins CM, Guan S, Han X, Yang K, Sun G, Malik I, Conyers S, Green KG, Schmidt RE, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in hippocampal cardiolipin content and molecular species distribution, mitochondrial degeneration, autophagy and cognitive dysfunction. J Biol Chem 284: 35632– 35644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancuso DJ, Sims HF, Han X, Jenkins CM, Guan SP, Yang K, Moon SM, Pietka T, Abumrad NA, Schlesinger PH, Gross RW. Genetic ablation of calcium-independent phospholipase A2γ leads to alterations in mitochondrial lipid metabolism and function resulting in a deficient mitochondrial bioenergetic phenotype. J Biol Chem 282, 34611– 34622, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandieau V, Martin I, Ruysschaert JM. Interaction between cardiolipin and the mitochondrial presequence of cytochrome c oxidase subunit IV favours lipid mixing without destabilizing the bilayer structure. FEBS Lett 368: 15– 18, 1995 [DOI] [PubMed] [Google Scholar]
- 52.Margineantu DH, Emerson CB, Diaz D, Hockenbery DM. Hsp90 inhibition decreases mitochondrial protein turnover. PLoS One 2: e1066, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marshall BA, Tordjman K, Host HH, Ensor NJ, Kwon G, Marshall CA, Coleman T, McDaniel ML, Semenkovich CF. Relative hypoglycemia and hyperinsulinemia in mice with heterozygous lipoprotein lipase (LPL) deficiency. Islet LPL regulates insulin secretion. J Biol Chem 274: 27426– 27432, 1999 [DOI] [PubMed] [Google Scholar]
- 54.McDaniel ML, Colca JR, Kotagal N, Lacy PE. A subcellular fractionation approach for studying insulin release mechanisms and calcium metabolism in islets of Langerhans. Methods Enzymol 98: 182– 200, 1983 [DOI] [PubMed] [Google Scholar]
- 55.McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem 29: 538– 542, 1983 [PubMed] [Google Scholar]
- 56.Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, Hevener AL, Farese RV., Jr Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab 6: 69– 78, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Moolenaar WH, van Meeteren LA, Giepmanns BNG. The ins and outs of lysophosphatidic acid signaling. BioEssays 26: 870– 871, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55, Suppl 2: S9– S15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem 75: 367– 401, 2006 [DOI] [PubMed] [Google Scholar]
- 60.Muoio DM, Newgard CB. Fatty acid oxidation and insulin action: when less is more. Diabetes 57: 1455– 1456, 2008 [DOI] [PubMed] [Google Scholar]
- 61.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193– 205, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Pappan KL, Pan Z, Kwon G, Marshall CA, Coleman T, Goldberg IJ, McDaniel ML, Semenkovich CF. Pancreatic beta-cell lipoprotein lipase independently regulates islet glucose metabolism and normal insulin secretion. J Biol Chem 280: 9023– 9029, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Petersen KF, Dufour S, Savage DB, Bilz S, Solomon G, Yonemitsu S, Cline GW, Befroy D, Zemany L, Kahn BB, Papademetris X, Rothman DL, Shulman GI. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 104: 12587– 12594, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfeiffer K, Gohil V, Stuart RA, Hunte C, Brandt U, Greenberg ML, Schagger H. Cardiolipin stabilizes respiratory chain supercomplexes. J Biol Chem 278: 52873– 52880, 2003 [DOI] [PubMed] [Google Scholar]
- 65.Pospisilik JA, Knauf C, Joza N, Benit P, Orthofer M, Cani PD, Ebersberger I, Nakashima T, Sarao R, Neely G, Esterbauer H, Kozlov A, Kahn CR, Kroemer G, Rustin P, Burcelin R, Penninger JM. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell 131: 476– 491, 2007 [DOI] [PubMed] [Google Scholar]
- 66.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem 69: 419– 445, 2000 [DOI] [PubMed] [Google Scholar]
- 67.Ramanadham S, Yarasheski KE, Silva MJ, Wohltmann M, Veis-Novack D, Lei XY, Turk J. Age-related changes in bone morphology are accelerated in group VIA calcium-independent Phospholipase A2β (iPLA2β)-null mice. Am J Pathol 172: 868– 881, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruderman NB, Saha AK, Vavvas D, Witters LA. Malonyl-CoA, fuel sensing, and insulin resistance. Am J Physiol Endocrinol Metab 276: E1– E18, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 87: 507– 520, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schaloske RH, Dennis EA. The Phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta 1761: 1246– 1259, 2006 [DOI] [PubMed] [Google Scholar]
- 71.Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem 268: 74– 79, 1993 [PubMed] [Google Scholar]
- 72.Schwarz M, Lund EG, Setchell KD, Kayden HJ, Zerwekh JE, Bjorkhem I, Herz J, Russell DW. Disruption of cholesterol 7alpha-hydroxylase gene in mice. II. Bile acid deficiency is overcome by induction of oxysterol 7alpha-hydroxylase. J Biol Chem 271: 18024– 18031, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Semple RK, Sleigh A, Murgatroyd PR, Adams CA, Bluck L, Jackson S, Vottero A, Kanabar D, Charlton-Menys V, Durrington P, Soos MA, Carpenter TA, Lomas DJ, Cochran EK, Gorden P, O'Rahilly S, Savage DB. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest 119: 315– 322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry 44: 5234– 5245, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Su X, Mancuso DJ, Bickel PE, Jenkins CM, Gross RW. Small interfering RNA knockdown of calcium-independent phospholipases A2 beta or gamma inhibits the hormone-induced differentiation of 3T3-L1 preadipocytes. J Biol Chem 279: 21740– 21748, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Sugiura T, Yoshinaga N, Kondo S, Waku K, Ishima Y. Generation of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand, in picrotoxinin-administered rat brain. Biochem Biophys Res Commun 271: 654– 648, 2009 [DOI] [PubMed] [Google Scholar]
- 77.Tang J, Kriz RW, Wolfman N, Shaffer M, Seehra J, Jones SS. A novel cytosolic calcium-independent Phospholipase A2 contains eight ankyrin motifs. J Biol Chem 272: 8567– 8575, 1997 [DOI] [PubMed] [Google Scholar]
- 78.Tordjman K, Bernal-Mizrachi C, Zemany L, Weng S, Feng C, Zhang F, Leone TC, Coleman T, Kelly DP, Semenkovich CF. PPARalpha deficiency reduces insulin resistance and atherosclerosis in apoE-null mice. J Clin Invest 107: 1025– 1034, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turk J, Ramanadham S. Expression and function of Group VIA Phospholipase A2 (iPLA2β) in β-cells. Can J Physiol Pharmacol 82: 824– 832, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Williams SD, Ford DA. Activation of myocardial cAMP-dependent protein kinase by lysoplasmenylcholine. FEBS Lett 420: 33– 38, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Xie Z, Gong MC, Su W, Turk J, Guo Z. Group VIA Phospholipase A2 (iPLA2β) participates in angiotensin II-induced transcriptional upregulation of RGS2 in vascular smooth muscle cells. J Biol Chem 282: 25278– 25289, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yan W, Jenkins CM, Han X, Mancuso DJ, Sims HF, Yang K, Gross RW. The highly selective production of 2-arachidonoyl lysophosphatidylcholine catalyzed by purified calcium-independent phospholipase A2gamma: identification of a novel enzymatic mediator for the generation of a key branch point intermediate in eicosanoid signaling. J Biol Chem 280: 26669– 26679, 2005 [DOI] [PubMed] [Google Scholar]
- 83.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem 277: 50230– 50236, 2002 [DOI] [PubMed] [Google Scholar]
- 84.Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for super-complex formation in the inner mitochondrial membrane. J Biol Chem 277: 43553– 43556, 2002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.