Abstract
Hepatic ketogenesis provides a vital systemic fuel during fasting because ketone bodies are oxidized by most peripheral tissues and, unlike glucose, can be synthesized from fatty acids via mitochondrial β-oxidation. Since dysfunctional mitochondrial fat oxidation may be a cofactor in insulin-resistant tissue, the objective of this study was to determine whether diet-induced insulin resistance in mice results in impaired in vivo hepatic fat oxidation secondary to defects in ketogenesis. Ketone turnover (μmol/min) in the conscious and unrestrained mouse was responsive to induction and diminution of hepatic fat oxidation, as indicated by an eightfold rise during the fed (0.50+/−0.1)-to-fasted (3.8+/−0.2) transition and a dramatic blunting of fasting ketone turnover in PPARα−/− mice (1.0+/−0.1). C57BL/6 mice made obese and insulin resistant by high-fat feeding for 8 wk had normal expression of genes that regulate hepatic fat oxidation, whereas 16 wk on the diet induced expression of these genes and stimulated the function of hepatic mitochondrial fat oxidation, as indicated by a 40% induction of fasting ketogenesis and a twofold rise in short-chain acylcarnitines. Together, these findings indicate a progressive adaptation of hepatic ketogenesis during high-fat feeding, resulting in increased hepatic fat oxidation after 16 wk of a high-fat diet. We conclude that mitochondrial fat oxidation is stimulated rather than impaired during the initiation of hepatic insulin resistance in mice.
Keywords: hepatic insulin resistance, liquid chromatography-tandem mass spectrometry, nuclear magnetic resonance
the liver maintains multiple metabolic pathways critical for the adaptive response to variable macronutrient consumption. During feeding, liver accumulates excess carbohydrate as glycogen or converts it to lipid for storage in peripheral adipose tissue. In the postabsorptive state, glycogen breakdown (glycogenolysis) buffers plasma glucose levels, and adipose-derived nonesterified fatty acids (NEFA) are oxidized by the liver to support endergonic fasting pathways such as gluconeogenesis and ureagenesis. During extended fasting or limited carbohydrate availability, the liver adapts to dwindling glycogen stores by increasing β-oxidation and converting the resulting acetyl-CoA to ketone bodies, primarily acetoacetate and its chemically reduced analog β-hydroxybutyrate. Since hepatic glucose production can drop by as much as 40% during extended fasting (52), the ∼10-fold rise in plasma ketone concentration that occurs under these conditions (19) provides a critical alternative substrate for oxidation by central nervous and other tissues normally dependent on glucose metabolism. Ketogenesis occurs almost exclusively in liver mitochondria (23) and during extended fasting can account for roughly two-thirds of total hepatic fat oxidation in rodents, with terminal oxidation of acetyl-CoA in the citric acid cycle accounting for the rest (31, 44, 48). Hepatic ketogenesis is influenced by a number of mechanisms, including hormonal signaling (31), direct substrate metabolism (31), and autonomic control (7).
Abnormal mitochondrial fat oxidation is a key component of numerous diseases, including inborn errors of metabolism (51), infection (12, 37), and alcohol toxicity (27), and is closely associated with insulin resistance and related pathophysiologies (29, 30, 36, 49). Insulin-resistant skeletal muscle contains mitochondria that inefficiently metabolize fat (22, 25, 42, 50), a defect that may precede the onset of insulin resistance (42) and likely contributes to impaired insulin signaling. A similar deficiency in mitochondrial fat oxidation may be present in the insulin-resistant liver (43, 53). Evidence that hepatic fat oxidation, and specifically ketogenesis, is affected by the metabolic manifestations of insulin resistance is demonstrated by elevated ketone bodies in humans with nonalcoholic fatty liver disease (47) and impaired fasting ketosis in the Zucker Diabetic fatty (ZDF) rat, which develops fatty liver in conjunction with impaired fasting hepatic fat oxidation and a fourfold reduction in ketogenesis (48, 54). However, systematic investigation of whether hepatic fat metabolism is altered during in insulin resistance has been limited because few in vivo approaches can be applied against mouse models.
Here, we report the effect of a high-fat diet-induced insulin resistance on hepatic fat oxidation in mice, using stable isotope tracers and liquid chromatography-tandem mass spectrometry (LC-MS/MS) to monitor ketogenesis and acylcarnitine profiles. Ketone turnover increased robustly during longitudinal fasting studies in mice and was markedly blunted in fed and fasted PPARα−/− mice, a well-described model of impaired hepatic fat oxidation (24). In contrast, mice fed a high-fat diet to induce insulin resistance had normal ketone body turnover after 8 wk, and elevated ketone body turnover after 16 wk of high-fat feeding, in conjunction with increased hepatic expression of genes principal to fat oxidation and ketone body formation. The data indicate a progressive increase in hepatic fat oxidation in diet-induced obese mice and demonstrate that impaired fat oxidation is not necessarily required for the development of hepatic insulin resistance.
MATERIALS AND METHODS
Animals
All of the animal protocols were approved by the University of Texas Southwestern Institutional Animal Care and Use Committee.
Comparison of 13C NMR and LC-MS/MS.
Long-Evans rats (∼250 g; n = 5) were fasted for 24 h, and ketone body turnover was determined by LC-MS/MS (described below) or by a 13C NMR method previously used by our laboratory (48) and compared to confirm that the two methods provide identical results.
Effect of fasting on ketone body turnover in mice.
Serial measurements of ketone body turnover during feeding and 16 and 24 h of fasting were performed in 12-wk-old female C57BL/6 mice (n = 5) maintained on normal laboratory chow. Mice were infused in the late afternoon while given full access to chow, the next morning (16 h after chow was removed), and then again in the afternoon of the 2nd day (24 h after chow was removed).
Effect of impaired fat oxidation on ketone body turnover in mice.
Ketone body turnover during the fed-to-16-h-fasted transition (n = 3–5) was studied in male PPARα+/+ (129S1/SvlmJ, Jaxlab 002448) and PPARα−/− (Ppara-tm1Gonz/J, Jaxlab 003580) mice to determine whether ketone body turnover responded to known defects in fat oxidation.
Effect of diet-induced obesity on ketone body turnover in mice.
Four- to six-week-old male C57BL/6 mice (n = 7–10) were maintained on either a control 10% fat calorie diet (TD06416; Harlan-Teklad) or a 60% fat calorie diet (TD06414; Harlan-Teklad) for 8 or 16 wk to induce obesity and insulin resistance (40). Unless noted otherwise, fasted measurements were made after an overnight, 16-h fast, and fed measurements were made in the morning from mice with normal access to food.
Tracer Infusion
Rats and mice were implanted with an indwelling jugular vein catheter and allowed to recover for 5 days before stable isotope tracer infusion while they were awake and unrestrained (11). Ketone body turnover by NMR was determined using stable isotope tracer dilution of [3,4-13C]acetoacetate and [1,2-13C4]β-hydroxybutyrate. The latter stable isotope tracer was replaced with [1,2,3,4-13C]β-hydroxybutyrate for LC-MS/MS analysis of ketone body turnover. Preparation and infusion of these stable isotope tracers were carried out as described previously (48). Briefly, ∼2 h prior to stable isotope infusion, 28 mg (∼212 μmol) of [3,4-13C]ethylacetoacetate was dissolved in 4 ml of deionized water and 80 μl of 4 M NaOH. This solution was incubated at 40°C for 75 min to hydrolyze the ethyl acetoacetate ester. The solution was neutralized and placed on ice, and 21 mg (∼164 μmol) of either [1,2-13C]- or [1,2,3,4-13C]β-hydroxybutyrate was added and the volume adjusted to 8 ml with saline. Tracers were infused as bolus (2.25 ml/h for rats and 0.30 ml/h for mice) for the initial 10 min and as continuous infusion (0.5 ml/h for rats and 0.12 ml/h for mice) for the remaining 80 min. Animals were allowed unrestrained movement within the cage during the entire infusion period. For NMR analysis, blood samples from rats were collected from the descending aorta until exsanguination. For LC-MS/MS analysis, which required 25 μl of blood, samples were collected after a tail clip. Samples were immediately frozen at −80°C until analysis.
Hepatic Insulin Sensitivity Index
Hepatic insulin sensitivity index was calculated as described previously (15, 28) using the following equation: 1/(endogenous glucose production × fasting insulin concentration). Endogenous glucose production was determined by steady-state dilution of infused [3,4-13C]glucose, as described previously (11), and expressed in μmol/min. Plasma insulin was measured by enzyme-linked immunoassay using the Mouse Insulin ELISA kit (ALPCO Diagnostics, Salem, NH) and expressed in nanograms per milliliter.
LC-MS/MS Analysis of Ketone Body Tracer Dilution
Thawed samples were immediately spiked with one volume of 1 M sodium borodeuteride and two volumes of acetonitrile to reduce plasma acetoacetate to β-hydroxybutyrate. Sodium borodeuteride converts labile acetoacetate to stable β-hydroxybutyrate but preserves the acetoacetate information by tagging the resulting β-hydroxybutyrate with a single deuterium (2H) (16, 17). The samples were then centrifuged at 14,000 rpm for 10 min, and the supernatant was loaded on a cation exchange column, washed off the column with 4 ml of water, and lyophilized overnight. The lyophilized sample was dissolved in 50 μl of mobile phase, of which 1–5 μl was injected to optimize β-hydroxybutyrate isotopomer abundances.
Analysis was done on an API 3200 triple quadrupole LC-MS/MS mass spectrometer (Applied Biosystems/Sciex Instruments) in positive electrospray ionization mode. The mass spectrometer was equipped with a Shimadzu LC-20AD liquid chromatograph and a SIL-20AC auto sampler. A reverse-phase C18 column (T3, 150 × 2.1 mm, 3 μm; Waters Atlantis) was used with liquid chromatograph mobile phase consisting of water-methanol (2:98, vol/vol) with 0.025% acetic acid (eluent A) and water-methanol (40:60, vol/vol) with 0.025% acetic acid (eluent B). The gradient program was 0% eluent B in eluent A, which was increased to 15% eluent B over 10 min, followed by an increase to 90% eluent B rapidly over 0.5 min. This gradient was held for 2 min before being returned to 0% eluent B in 0.5 min and for 7 min to equilibrate the column. The flow rate was held constant at 0.17 ml/min throughout the run.
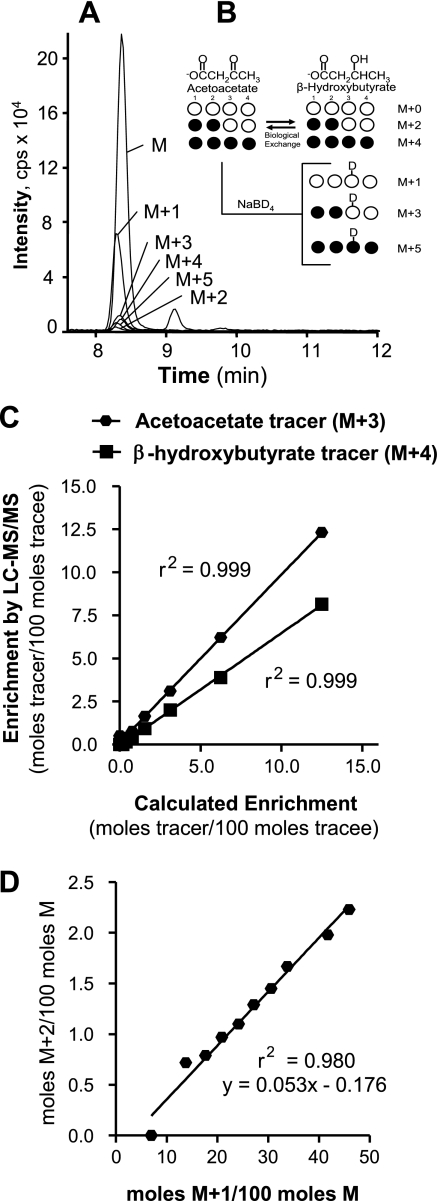
For qualitative mass measurements, full scan and product ion scan analyses were performed by direct infusion of β-hydroxybutyrate solution to the mass spectrometer. Acquisition parameters, including ionization source voltage, source temperatures, and gas flow, were adjusted to maximize the signals of ions of interest. Quantitative measurements were performed in multiple reaction monitoring (MRM) mode, where the protonated molecular ion [M + H]+ was monitored via the first quadrupole filter (Q1) and its product fragment [M + H]+ minus 18 via the third quadrupole filter (Q3). The source was operated at 300°C, and the mass spectrometer was operated with the following parameters: declustering potential 26 V; entrance potential 2.5 V; collision cell entrance potential 7.5 V; collision cell exit potential 8 V; collision energy 8.1 V; ion spray voltage 4,000 V; channel electron multiplier 2,000 V. Nitrogen was used for the nebulizing gas, and the ion source gas 1, ion source gas 2, curtain gas, and collision gas were 30, 40, 20, and 5, respectively. The following MRM transitions were monitored to quantify β-hydroxybutyrate isotopomers (Fig. 1, A and B): detection of β-hydroxybutyrate originating from β-hydroxybutyrate 105/87 (M + 0); reduced acetoacetate 106/88 (M + 1); [3,4-13C]β-hydroxybutyrate 107/89 (M + 2); reduced [3,4-13C]acetoacetate 108/90 (M + 3); [1,2,3,4-13C]β-hydroxybutyrate 109/91 (M + 4); reduced [1,2,3,4-13C]acetoacetate 110/92 (M + 5).
Fig. 1.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) detection of plasma acetoacetate and β-hydroxybutyrate isotopomers. A: LC-MS/MS spectrum of β-hydroxybutyrate isotopomers isolated from mouse blood. B: relationship between infused ketone tracers and mass isotopomers detected in processed blood. M + 2 acetoacetate and M + 4 β-hydroxybutyrate were infused but are interconverted to M + 4 acetoacetate and M + 2 β-hydroxybutyrate by biological exchange through β-hydroxybutyrate dehydrogenase. Upon processing of the blood with sodium borodeuteride, plasma acetoacetate is chemically reduced to β-hydroxybutyrate but with a single mass unit increase in its molecular weight due to the addition of a deuterium. Thus, detection of M + 0, M + 2, and M + 4 mass isotopomers of β-hydroxybutyrate quantifies blood enrichment of β-hydroxybutyrate, whereas detection of M + 1, M + 3, and M + 5 mass isotopomers of β-hydroxybutyrate quantifies blood enrichment of acetoacetate. C: enrichment curves for acetoacetate (M + 3) and β-hydroxybutyrate (M + 4) tracers constructed with increasing tracer-to-tracee ratios for correction of measured enrichments by LC-MS/MS. D: a spillover curve was constructed with varying proportions of acetoacetate and β-hydroxybutyrate to calculate the contribution of M + 1 acetoacetate to the M + 2 of β-hydroxybutyrate after processing with sodium borodeuteride. The M + 1/M on the x-axis is a function of acetoacetate/β-hydroxybutyrate, whereas the M + 2/M on the y-axis is the resulting spillover correction for M + 2. This correction was applied as a function of M + 1/M in blood samples.
The peak areas of β-hydroxybutyrate isotopomers were quantified by Analyst Software version 1.4.2. Tracer-to-tracee ratios were calculated after correcting for isotope natural abundance. Tracer enrichments measured by LC-MS/MS were also corrected using enrichment curves constructed with increasing tracer-to-tracee ratios (Fig. 1C). A spillover curve was also constructed to correct for the contribution of the deuterated acetoacetate (M + 1) to the M + 2 and M + 3 β-hydroxybutyrate isotopomers by determining the M + 2/M and M + 1/M ratios in solutions containing varying proportions of acetoacetate reduced with sodium borodeuteride and unlabeled β-hydroxybutyrate, and these ratios were plotted to obtain a linear regression equation (Fig. 1D). The corrected enrichments of the β-hydroxybutyrate mass isotopomers and infusion rates were fit to a two-pool model to determine β-hydroxybutyrate and acetoacetate turnover and exchange rates (3, 9, 32). The sum of β-hydroxybutyrate and acetoacetate turnover rates is reported as ketone body turnover.
NMR Analysis of Ketone Body Tracer Dilution
Sample processing and analysis were carried out as described previously (48). Briefly, ∼5 ml of thawed plasma was deproteinized with 70% perchloric acid and the supernatant passed through cation (H+) resin and neutralized with LiOH. The plasma extract was then lyophilized to ∼500 μl, and 100 μl of D2O was added for 13C NMR analysis of acetoacetate and β-hydroxybutyrate multiplets on a 14T spectrometer equipped with a 5-mm broad-band probe. Peak areas were analyzed using 1D NMR software ACD/Labs 9.0 (Advanced Chemistry Development, Toronto, ON, Canada). The enrichments and infusion rates were fit to a two-pool model, as described previously (3, 9, 32), but modified slightly for NMR (48) to determine ketone body turnover.
LC-MS/MS Analysis of Liver Acylcarnitines
Following tracer infusion and blood collection, free carnitine and acylcarnitines were extracted from liver, derivatized, and quantified as described previously (1, 34) with some minor modification. Detection was performed after LC separation using API 3200 triple quadrupole LC-MS/MS mass spectrometer (Applied Biosystems/Sciex Instruments) in positive ionization MRM mode. Free carnitine was monitored using the 176 to 117 MRM transition. Acylcarnitines were monitored using a precursor of 99 Da. Acylcarnitines were quantified by comparison of the individual ion peak area with that of an internal 13C standard (Cambridge Isotope Laboratories).
Gene Expression Analysis
Primers were designed using Primer Express software (Applied Biosystems, San Jose, CA) on the basis of GenBank sequence data. Quantitative real-time PCR reactions (10 μl) contained 25 ng of cDNA, 150 nM of each primer, and 5 μl of SYBR Green PCR Master Mix (Applied Biosystems). Reactions were performed in triplicate on an Applied Biosystems Prism 7900HT Sequence Detection System, and relative mRNA levels were calculated by the comparative threshold cycle method by using cyclophilin as the internal control.
Reagents and Materials
[3,4-13C]ethyl acetoacetate (98%), [1,2-13C]sodium β-hydroxybutyrate (98%), and [1,2,3,4-13C]β-hydroxybutyrate were purchased from Isotec (St. Louis, MO). Ketone body, NEFA, and triglyceride concentrations in plasma or tissue samples were determined using commercially available analytical kits (Wako). Other common chemicals were obtained from Sigma (St. Louis, MO).
Statistical Analysis
Results are expressed as means ± SE. Statistical differences were analyzed using ANOVA for multiple groups or an unpaired t-test between two groups. Means were considered significantly different at P ≤ 0.05. Pearson's correlation and regression analysis was performed to determine whether a linear or nonlinear relationship existed between two variables of interest.
RESULTS
LC-MS/MS Determination of β-Hydroxybutyrate Enrichment
All six β-hydroxybutyrate isotopomers were easily detected and quantified from mouse blood (Fig. 1, A and B). Quantification of β-hydroxybutyrate was linear over a wide range of injected mass (10 pg to 50 ng); r2 = 0.9993. Injection of ∼0.75 ng of β-hydroxybutyrate was enough to maintain a signal-to-noise ratio of >25 in all the samples. Enrichment curves constructed with [1,2,3,4-13C]β-hydroxybutyrate (M + 4) and reduced [3,4-13C]acetoacetate (M + 3) were linear over a wide range (0–12.5 mole%, r2 = 0.999; Fig. 1C) and were used to correct the measured enrichments. Reduction of acetoacetate with sodium borodeuteride introduces a significant spillover of M + 2 enrichment that was corrected using a linear (r2 = 0.980) equation generated from standards of variable acetoacetate/β-hydroxybutyrate ratios (Fig. 1D).
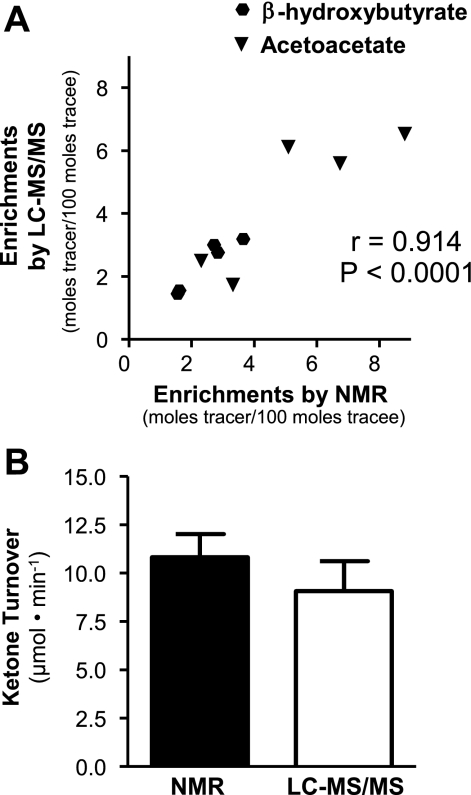
Determination of Ketone Body Tracer Enrichment by LC-MS/MS and NMR Provides Equivalent Values
Carbon-13 NMR analysis of positional enrichment provides a very accurate readout of ketone body tracer dilution without interference from background enrichments or requirement for spillover corrections (48). However, 13C NMR is several orders of magnitude less sensitive and requires long scan times for analysis compared with MS, and therefore, it is less practical for mouse experiments. To confirm that ketone body tracer dilution measured by LC-MS/MS and NMR agree, we infused rats with [1,2,3,4-13C]β-hydroxybutyrate and [1,2-13C]acetoacetate and measured plasma enrichments by NMR and LC-MS/MS in samples from identical rats. Both methods provided similar quantification of ketone body enrichment with a correlation coefficient between the two methods of 0.91 and a slope equaling 0.90 (Fig. 2A). To further confirm that LC-MS/MS and NMR measurements provide equivalent measurement of ketone body turnover, we infused two separate groups of fasted rats with either NMR tracers ([3,4-13C]β-hydroxybutyrate, [1,2-13C]acetoacetate) or LC-MS/MS tracers ([1,2,3,4-13C]β-hydroxybutyrate, [1,2-13C]acetoacetate). Ketone body turnover determined by both NMR and LC-MS/MS gave identical values in these two groups of rats (Fig. 2B). These data demonstrate that the LC-MS/MS approach provides identical measurements of ketone body dilution and turnover compared with the NMR approach we reported earlier (48), but with much improved sensitivity.
Fig. 2.
Ketone tracer enrichment measured by LC-MS/MS is corroborated by NMR measurements. A: comparison of a previously validated NMR method for determining ketone enrichment with the LC-MS/MS method demonstrates that the 2 methods provide identical measurements of β-hydroxybutyrate and acetoacetate tracer enrichments in rat plasma. B: ketone turnover in 24-h-fasted Long-Evans rats was not different when determined by NMR or LC-MS/MS.
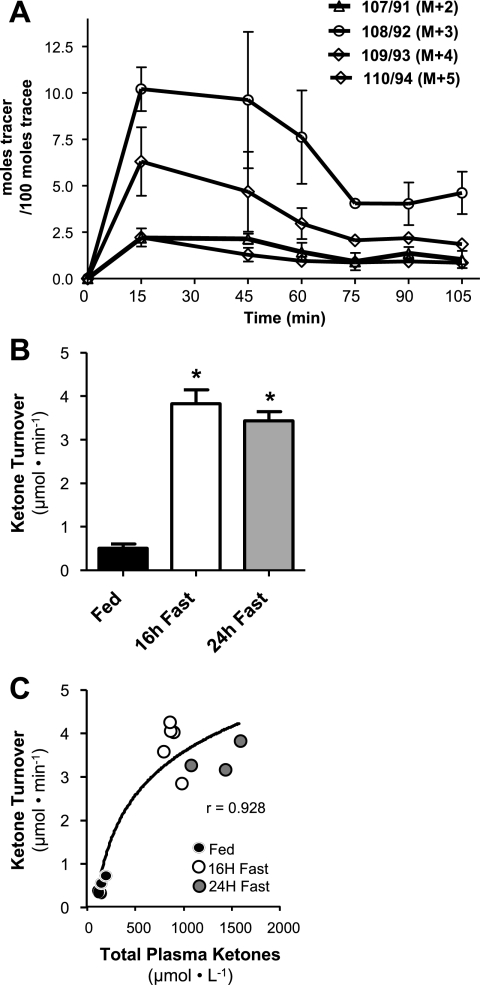
Steady-State Infusion of Ketone Body Tracers in Mice
Before application to mice, we confirmed steady-state enrichment of ketone body tracers during a 120-min infusion (Fig. 3A). The initial tracer bolus resulted in supra-steady-state enrichment of the infused tracers (M + 3 and M + 4), but enrichment of the conversion products (M + 2 and M + 5) was nearly identical to steady-state enrichment. All tracers reached steady state within 75 min of the infusion being started. Subsequent experiments used similar bolus infusion rates for 90 min.
Fig. 3.
Steady-state infusion of ketone tracers demonstrates induction of ketogenesis during fasting in mice. A: enrichment curves of β-hydroxybutyrate isotopomers in mouse blood during a 105-min infusion of ketone tracers demonstrates that steady state is reached by 75 min of infusion. B: 3 separate infusion experiments were repeated in the same C57BL/6 mice during the fed-to-24-h-fasted transition. Ketone turnover was stimulated substantially during the transition from feeding (n = 6) to 16 h of fasting (n = 5) but did not increase further by 24 h of fasting (n = 3) (attrition over the experiment was due to loss of catheter patency). C: total ketone concentration continued to rise throughout the fed-to-fasted transition despite a leveling of ketone turnover. The data are represented by means ± SE. *P ≤ 0.05.
Ketone Body Turnover in Mice is Stimulated During the Fed-to-Fasted Transition
To determine whether apparent ketone body turnover in mice is reflective of normal hepatic physiology associated with induction of ketogenesis, we performed experiments in C57BL/6 mice during the fed-to-fasted transition. Mice fitted with indwelling jugular vein catheters were infused with ketone body tracers without food being withheld and then 16 and 24 h later in the same mice after food was removed. Ketone body turnover was very low when mice had access to food but was induced fivefold after a 16-h fast (Fig. 3B), in agreement with the known effects of fasting on hepatic ketogenesis in mice and concomitant with an increase in plasma ketone body concentration from 173.9 ± 11.74 to 857.5 ± 30.49 μM. An additional 8 h of fasting did not induce any further increase in ketone body turnover, although ketone body concentration did increase by an additional 50% to 1,365.3 ± 83.29 μM (Fig. 3C). These data indicate that apparent ketone body turnover in mice accurately reflects the induction of hepatic fat oxidation in response to fasting but that extending fasting from 16 to 24 h does not further induce ketogenic rates. Subsequent studies used 16-h fasting protocols.
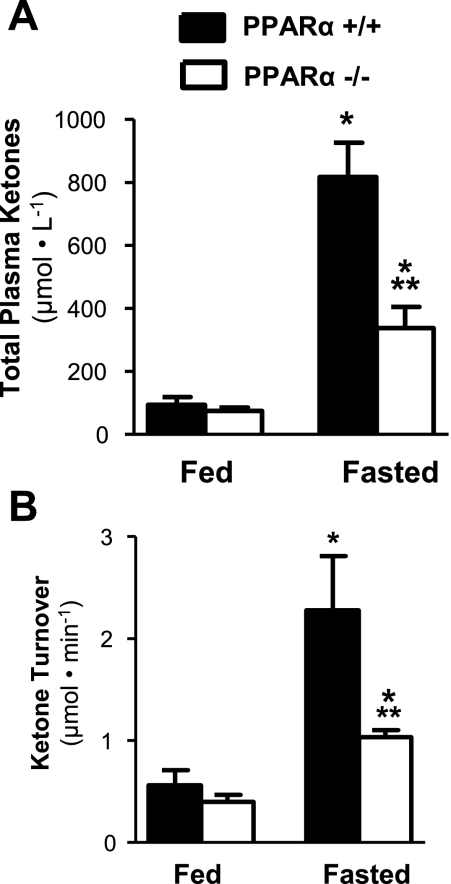
Ketone Body Turnover is Impaired in PPARα−/− Mice
To confirm that ketone body turnover is sensitive to a pathophysiological impairment of hepatic fat oxidation and ketogenesis, we performed experiments in PPARα−/− and PPARα+/+ mice before and after a 16-h fast. Not surprisingly, fasting ketosis was impaired in PPARα−/− mice, as indicated by impaired fasting plasma ketone body concentration (Fig. 4A). As expected, PPARα−/− mice also had a 40% reduction in ketone body turnover in the fed state and a twofold reduction in the fasted state compared with control mice (Fig. 4B). These results are consistent with the known impairment of hepatic fat oxidation in these mice (24) and, taken with the former results, indicate that steady-state ketone body dilution reflects alterations in hepatic ketogenesis within the physiological window encompassed by feeding, fasting, and PPARα-targeted impairment of hepatic fat oxidation.
Fig. 4.
In vivo ketone turnover in mice reflects alterations of hepatic fat oxidation associated with PPARα loss of function. A: fasting for 16 h induced an 8-fold rise in total plasma ketone bodies in wild-type mice, which were severely blunted in PPARα−/− mice. B: ketone turnover measured by ketone tracer dilution was likewise impaired in PPARα−/− mice after a 16-h fast, consistent with the known effects of PPARα loss of function and indicative that the method accurately reports alterations of hepatic ketogenesis. The data are represented by means ± SE. *P ≤ 0.05 between fed and fasted groups; **P ≤ 0.05 between PPARα+/+ and PPARα−/−.
Effect of High-Fat Diet on Ketogenesis in Mice Depends on Duration of Exposure
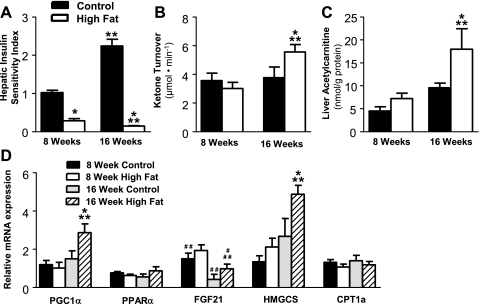
Since defects in hepatic fat oxidation and ketogenesis have been implicated in insulin-resistant rat models (48) and in humans (47), we investigated whether ketogenesis is altered in a diet-induced mouse model of obesity and insulin resistance. The metabolic characteristics of these mice are presented in Table 1. After 8 wk of a 60% high-fat diet, mice were obese (30.86 ± 0.962 vs. 40.5 ± 1.18 g, P < 0.0001), hyperinsulinemic (0.40 ± 0.039 vs. 1.93 ± 0.329 ng/ml, P < 0.001), and glucose intolerant (Supplemental Fig. S2; Supplemental Material for this article can be found on the AJP-Endocrinology and Metabolism web site) and had a severely suppressed hepatic sensitivity index (Fig. 5A) compared with mice on the control diet. These data are consistent with previous reports on the diet-induced obese C57BL/6 mouse (40). Despite hepatic insulin resistance and substantial hepatic steatosis (Table 1), fasting plasma ketone body concentration (1,220 ± 103.7 vs. 1,178 ± 119.2 μM) and turnover (Fig. 5B) were normal after 8 wk of a high-fat diet. Additionally, the liver acylcarnitine profile was unremarkable (Fig. 5C and Supplemental Fig. S1). These data are consistent with unaltered hepatic fat oxidation during 8 wk of diet-induced insulin resistance in these mice.
Table 1.
Metabolic characteristics of mice maintained on control (10% fat calories) or high-fat (60% fat calories) diets for either 8 or 16 wk
| 8 Wk |
16 Wk |
|||
|---|---|---|---|---|
| Control | High Fat | Control | High Fat | |
| Body weight, g | 30.9 ± 0.96 | 40.5 ± 1.2* | 37.4 ± 0.61† | 47.4 ± 0.98*† |
| Fasting glucose, mg/dl | 67.7 ± 2.2 | 101.8 ± 6.9* | 72.5 ± 3.0 | 129.4 ± 13* |
| Fed total ketones, μmol/l | 144.2 ± 12 | 106.1 ± 6.5* | 69.1 ± 3.6† | 100.4 ± 4.9* |
| Fasting total ketones, μmol/l | 1,220 ± 104 | 1,178 ± 119 | 854 ± 123 | 1,189 ± 161 |
| Fasting NEFA, mEq/l | 0.61 ± 0.036 | 0.67 ± 0.062 | 0.81 ± 0.071 | 1.05 ± 0.065*† |
| Fasting liver triglycerides, mg/g | 80.9 ± 16 | 110.2 ± 19 | 94.1 ± 22 | 201.1 ± 43*† |
| Fasting insulin, ng/ml | 0.40 ± 0.039 | 1.93 ± 0.33* | 1.02 ± 0.078† | 3.24 ± 0.71* |
Data are means ± SE. NEFA, nonesterified fatty acids.
P < 0.05 between control and high-fat groups;
P < 0.05 between 8- and 16-wk groups.
Fig. 5.
High-fat feeding induces insulin resistance and increased hepatic fat oxidation in mice. A: mice fed a high-fat diet for either 8 or 16 wk had a dramatically impaired hepatic insulin sensitivity index. B: ketone turnover was unchanged after 8 wk of a 60% high-fat diet but was significantly elevated after 16 wk of the high-fat diet. C: liver acetylcarnitine was assayed by LC-MS/MS in mice fed a synthetic control diet (10% fat calories) or a high-fat diet (60% fat calories) for either 8 or 16 wk. D: high-fat feeding for 8 wk resulted in normal expression of genes that regulate hepatic fat oxidation relative to cyclophilin, whereas 16 wk on the diet induced expression of these genes. Data are presented as means ± SE. *P ≤ 0.05 between control and high-fat diet. **P ≤ 0.05 between 8 and 16 wk of diet intervention; #P ≤ 0.1 between control and high-fat diet; ##P ≤ 0.1 between 8 and 16 wk of diet intervention. HMGCS, hydroxylmethylglutaryl-CoA synthase; CPT Ia, carnitine palmitoyltransferase Ia.
In view of the fact that we previously found that hepatic fat oxidation is impaired in severely insulin-resistant rats (48), we investigated whether a longer duration of high-fat feeding results in impaired hepatic ketogenesis. Mice maintained on a high-fat diet for 16 wk gained an additional 25% (47.4 ± 0.98 g) of body weight and had elevated insulin levels (3.24 ± 0.71 ng/ml), higher plasma NEFA and liver triglyceride levels (Table 1), deteriorated glucose tolerance (Supplemental Fig. S2), and a further suppression of hepatic insulin sensitivity index compared with mice on the diet for 8 wk (Fig. 5A). This deterioration of insulin sensitivity was accompanied by a 40% increase in ketone body turnover (Fig. 5B), demonstrating that hepatic fat oxidation through the ketogenic pathway is induced during this period of diet-induced insulin resistance. To further characterize hepatic β-oxidation in mice fed a high-fat diet for 16 wk, we assayed the C2–C16 acylcarnitine profile in liver extracts of these mice. There were no differences in free carnitines or medium- and long-chain carnitines (C5–C16) (Supplemental Fig. S1). However, short-chain acylcarnitines, particularly acetylcarnitine (Fig. 5C), were significantly elevated in the livers of mice fed a high-fat diet for 16 wk, consistent with increased fatty acid oxidation and in agreement with findings in other mouse models with increased fat oxidation (1, 25, 36, 38). Together, these data indicate that the function of hepatic fat oxidation is normal after 8 wk of high-fat feeding but induced after 16 wk of high-fat feeding in mice.
To probe the molecular origin of these functional findings, we measured the expression of genes that regulate fat oxidation in the liver. High-fat feeding for 16 but not 8 wk significantly induced PPARγ coactivator-1α (P = 0.03) and a trend for increased PPARα (P = 0.13) expression in liver (Fig. 5D), suggesting that hepatic fat oxidation is molecularly upregulated by 16 wk of a high-fat diet in these mice. To specifically address ketogenesis, we measured hepatic expression of fibroblast growth factor 21 (FGF21), a newly described factor critical for regulation of ketogenesis (2, 44) and hydroxylmethylglutaryl (HMG)-CoA synthase, the rate-limiting step in ketone formation. Whereas 8 wk of a high-fat diet had no effect on the expression of either of these genes, 16 wk of a high-fat diet resulted in a twofold induction of both FGF21 expression (P = 0.08) and HMG-CoA synthase (P = 0.03) (Fig. 5D). Taken together with the flux measurements, these data demonstrate a progression of both expression and function of hepatic fat oxidation during high-fat diet-induced insulin resistance.
DISCUSSION
Ectopic lipid accumulation is a primary risk factor for the development of insulin resistance and related metabolic maladies (30). The mechanistic link between intracellular lipid accumulation and insulin resistance appears to involve substrate level mitochondrial pathways of fat oxidation and the malformation of lipid-derived intermediates that inhibit the insulin-signaling cascade (22, 25, 36, 42, 49, 50). Inasmuch as most investigations along these lines have focused on skeletal muscle, it remains unclear whether the same factors prevail in liver. Since the largest proportion of mitochondrial fat oxidation in the fasted rodent liver is directed toward the synthesis and efflux of ketone bodies (31), the aim of this study was to determine whether hepatic mitochondrial function, as indicated by ketogenic flux and acylcarnitine profiles, is altered in insulin-resistant, high-fat-fed mice. We first confirmed that ketone body tracer dilution in conscious and unrestrained mice accurately reflects the anticipated changes in hepatic fat oxidation with feeding, fasting, and PPARα loss of function. The most important finding was that hepatic insulin resistance induced by 8 wk of a high-fat diet in mice had no remarkable effect on the expression or function of hepatic fat oxidation, although the livers of these mice were steatotic and clearly insulin resistant. Furthermore, 16 wk of high-fat feeding worsened hepatic steatosis, insulin resistance, lipidemia, and glycemia but also resulted in the induction of hepatic fat oxidation gene expression, elevated ketogenic flux, and increased short-chain acylcarnitine content in the liver. These findings establish that impaired mitochondrial fat oxidation is not an early feature of hepatic insulin resistance in diet-induced obese mice and that, in fact, there appears to be an induction of hepatic fat oxidation in advance of any decline in mitochondrial function.
The finding of increased ketone body production in high-fat diet-induced insulin resistance was contrary to our initial expectation, which was based on impaired mitochondrial function in insulin-resistant skeletal muscle (22, 36, 42, 49) and causative links between impaired mitochondrial fat oxidation and diminished hepatic insulin signaling. Induction of hepatic mitochondrial dysfunction by loss of long-chain acyl-CoA dehydrogenase was sufficient to cause hepatic insulin resistance (57), whereas increasing hepatic mitochondrial fat oxidation by malonyl-CoA decarboxylase gain of function (1) or acetyl-CoA carboxylase loss of function (14) ameliorated PKC-dependent hepatic insulin resistance. In addition, hepatic insulin resistance is improved by treatment with pharmacological interventions, such as PPARα agonists (56) or the newly described hepatokine FGF21 (6), that induce flux through hepatic ketogenesis (44, 48). With regard to hepatic energy metabolism during insulin resistance, humans with type 2 diabetes mellitus were found to have impaired rates of hepatic ATP synthesis (53), whereas insulin-resistant humans with fatty liver disease have been reported to have both decreased (41) and increased (47) hepatic mitochondrial metabolism, with the latter indicated by elevated plasma ketone concentration. In contrast, the frankly diabetic ZDF rat has severely blunted fasting ketone turnover, leading to impaired hepatic fat oxidation during fasting (48). Taken together with our present findings, these data suggest that impaired hepatic mitochondrial fluxes may occur with severe insulin resistance/diabetes but not in the milder setting of diet-induced insulin resistance studied here. It is possible that the induction of frank hyperglycemia itself could impair hepatic ketogenesis during severe insulin resistance (45).
We postulate that induction of ketone body production during high-fat feeding in mice reflects a metabolic compensation in response to increased lipid delivery to the liver. Acute lipid overload in rodent models, either by lipid infusion (26) or short-term (3-day) high-fat diet (46), induces hepatic insulin resistance through PKC-dependent phosphorylation of insulin receptor substrates. Increased short-chain acylcarnitines in the skeletal muscle of high-fat-fed rodents suggests that skeletal muscle β-oxidation is increased secondary to mitochondrial overload of fat (38), and similarly, mitochondrial fat oxidation is increased the hearts of high-fat-fed mice (55). We found that circulating NEFA levels increased between 8 and 16 wk of exposure to the high-fat diet, corresponding to the timing in which ketone production increased. The induction of mitochondrial fat oxidation in response to lipid overload provides a plausible mechanism for the induction of oxidative stress and mitochondrial damage during later stages of insulin resistance. Indeed, the initial metabolic compensation for increased lipid delivery is followed by pathological gene expression in skeletal muscle (50). Likewise, hepatic gene expression of fat oxidation initially increases with high-fat feeding but then decreases after prolonged exposure to a high-fat diet in rodents (13). Our findings of normal ketogenesis after 8 wk, followed by elevated ketogenesis after 16 wk of high-fat feeding, demonstrate that changes in hepatic mitochondrial metabolism evolve during progressing insulin resistance and establish that impaired hepatic fat oxidation is not a required feature of hepatic insulin resistance. A caveat of the present study is that we do not report mitochondrial fat oxidation in the TCA cycle, but given the requirement of the TCA cycle for gluconeogenesis (10), it is unlikely that this pathway is impaired during insulin resistance.
The measurement of in vivo metabolic flux in mice is challenging because of blood volume and sampling limitations. The LC-MS/MS method used here provided a measurement of ketone body enrichment and turnover identical to the 13C NMR approach we used previously (48) but required 300 times less blood (20 μl vs. ∼6 ml), allowing ketone turnover to be measured in awake and urestrained mice. Many previous studies used gas chromatography (GC)-MS to measure ketone body enrichment and turnover (3, 8, 9, 16, 17, 32, 35) with similar sensitivity, but the approach has not been applied to mice. Although there is no inherent reason that GC-MS could not be used, an advantage of LC-MS/MS is that sample derivatization is not required, resulting in lower molecular weight fragments and thus less natural abundance background enrichment and attendant correction factors than standard silane derivatives used for GC-MS (3, 9, 16, 17, 32). We note that other GC-MS derivatives provide similarly low background correction by splitting off in the source before detection (20). The method used here also takes advantage of a technique to sequester labile acetoacetate as stabile β-hydroxybutyrate by treating plasma samples with sodium borodeuteride prior to analysis (16, 17), allowing samples to be stored and analyzed without risk of sample degradation while retaining acetoacetate enrichment information as deuterium-tagged β-hydroxybutyrate.
Regardless of the analytical technique used to measure ketone body tracer enrichment, the relationship between apparent ketone body turnover and hepatic ketogenesis may be complicated by exchange reactions in extrahepatic tissue (termed pseudoketogenesis) that can artificially dilute ketone tracers (17, 18). Despite this potential complication, ketone body turnover and net hepatic ketone arterial-venous difference were equivalent in both normal and diabetic dogs (3, 21, 32). Since a similar validation is not possible in mice, we measured ketone body turnover during the fed-to-fasted transition and in gene-altered mice with impaired hepatic fat oxidation to determine whether ketone body turnover reflects hepatic fat oxidation in mice as expected. Fasted mice had an eightfold increase in ketone body turnover compared with fed mice, approximately equal to the increase in ketone body concentration with fasting. We also found that ketone body turnover was robustly blunted in PPARα−/− mice, consistent with impaired hepatic fat oxidation in these mice (24). Although we cannot ascertain the absolute contribution of pseudoketogenesis to ketone tracer dilution, it appears that ketone body turnover is largely reflective of hepatic ketogenesis under the conditions studied here.
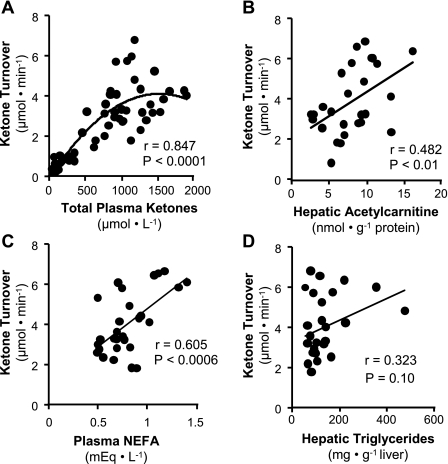
To probe the factors that influence ketogenesis during diet-induced obesity, we analyzed correlations between ketone turnover and other metabolic parameters related to liver fat oxidation. When ketone body turnover (57 mice: fed, fasting, PPARα−/−, 8- and 16-wk control, and high-fat diet mice) was compared with plasma ketone concentration, we found a curvilinear relationship (Fig. 6A) with a strong correlation at ketone concentrations <1,000 μM that tended to level off at higher ketone concentrations. This finding recapitulated the data in individual mice during the fed-to-starved transition shown in Fig. 3C, where ketone body turnover leveled off after 16 h of fasting, but ketone body concentration rose by another 50% between 16 and 24 h of fasting. A similar curvilinear relationship between plasma ketone concentration and production was identified in the dog during fasting ketosis and type I diabetes (21). The origin of this effect is unclear but has been explained as either a regulatory pacing of ketone body production when plasma ketone concentrations rise above maximal peripheral ketone utilization (5, 39) or inhibition of ketone utilization at elevated plasma ketone concentrations (21). Additionally, hepatic acetylcarnitine correlated positively with ketone body turnover in mice from control and high-fat-fed groups (Fig. 6B). This finding is significant because acetylcarnitine reflects cellular acetyl-CoA, the end product of β-oxidation and precursor of ketone body synthesis. These correlations provide some additional support that ketone turnover in mice is reflective of hepatic fat oxidation.
Fig. 6.
Correlations of ketone turnover with liver and plasma metabolite concentrations. A: ketone turnover in all mice reported in this study (57 mice) correlates with plasma ketone concentration. The best correlation was obtained by a polynomial trend line, indicating that the highest ketone concentrations may be influenced by suppressed ketone utilization rather than increased hepatic ketogenesis. B: liver acetylcarnitine levels determined by LC-MS/MS correlated positively with ketone turnover in mice fed control or high-fat diets for 8 and 16 wk. C: ketone turnover correlated positively with nonesterified fatty acids (NEFA) in plasma of mice fed a control or high-fat diet for 8 and 16 wk. D: ketone turnover and hepatic triglyceride content trended toward a positive correlation but did not reach significance from mice fed a control or high-fat diet for 8 and 16 wk. Correlations were determined by Pearson's correlation and regression analysis. P < 0.05 was considered significant.
We also determined whether lipid metabolites correlate with ketone turnover in control and high-fat-fed mice. Plasma NEFA concentration strongly correlated with ketone turnover (Fig. 6C), a finding that reflects both the dependence of ketogenesis on hepatic fatty acid delivery and the reality that factors governing hepatic fat oxidation also influence adipose fatty acid release. In contrast, hepatic triglyceride content tended to but did not strongly correlate with ketone turnover (Fig. 6D). This finding suggests that altered ketogenesis during diet-induced obesity is not strongly dependent on the development of hepatic steatosis and that the onset of fatty liver is dependent on other factors besides altered mitochondrial function.
In conclusion, apparent ketone body turnover in mice reflects alterations in hepatic fat metabolism within the metabolic window of feeding, fasting, and PPARα loss of function. High-fat feeding for 8 wk induces insulin resistance without altered hepatic fat oxidation, whereas 16 wk of high-fat feeding induces both hepatic gene expression and in vivo function of fat oxidation. These findings demonstrate that hepatic fat oxidation adapts progressively to high-fat feeding and establishes that diet-induced insulin resistance is not accompanied initially by impaired mitochondrial fat oxidation.
GRANTS
Support for this work was provided by National Institute of Diabetes and Digestive and Kidney Diseases Grant RO1-DK-078184 (S. C. Burgess) and American Diabetes Association Grant 7-09-BS-24 (S. C. Burgess). N. E. Sunny is supported by Postdoctoral Training Grant RL9-DK-081180. Core support was provided by Grants UL1-DE019584 (Task Force for Obesity Research), RR-02584 (National Center for Research Resources), and DK-076269 (University of Texas Southwestern Mouse Metabolic Phenotyping Center).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Dr. Bob Stevens of Duke University for providing guidance in implementing the acylcarnitine profile assay. Dr. Henri Brunengraber of Case Western University provided invaluable discussions and guidance in assaying ketone body enrichment by mass spectrometry. Sreeraman Katikaneni and Josh Liu provided excellent technical assistance.
REFERENCES
- 1.An J, Muoio DM, Shiota M, Fujimoto Y, Cline GW, Shulman GI, Koves TR, Stevens R, Millington D, Newgard CB. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med 10: 268–274, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bailey JW, Haymond MW, Miles JM. Validation of two-pool model for in vivo ketone body kinetics. Am J Physiol Endocrinol Metab 258: E850–E855, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Balasse EO, Neef MA. Inhibition of ketogenesis by ketone bodies in fasting humans. Metabolism 24: 999–1007, 1975. [DOI] [PubMed] [Google Scholar]
- 6.Berglund ED, Li CY, Bina HA, Lynes SE, Michael MD, Shanafelt AB, Kharitonenkov A, Wasserman DH. Fibroblast growth factor 21 controls glycemia via regulation of hepatic glucose flux and insulin sensitivity. Endocrinology 150: 4084–4093, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuers U, Beckh K, Jungermann K. Control of ketogenesis in the perfused rat liver by the sympathetic innervation. Eur J Biochem 158: 19–24, 1986 [DOI] [PubMed] [Google Scholar]
- 8.Beylot M, Beaufrere B, Normand S, Riou JP, Cohen R, Mornex R. Determination of human ketone body kinetics using stable-isotope labelled tracers. Diabetologia 29: 90–96, 1986 [DOI] [PubMed] [Google Scholar]
- 9.Bougneres PF, Ferre P. Study of ketone body kinetics in children by a combined perfusion of 13C and 2H3 tracers. Am J Physiol Endocrinol Metab 253: E496–E502, 1987 [DOI] [PubMed] [Google Scholar]
- 10.Burgess SC, He T, Yan Z, Lindner J, Sherry AD, Malloy CR, Browning JD, Magnuson MA. Cytosolic phosphoenolpyruvate carboxykinase does not solely control the rate of hepatic gluconeogenesis in the intact mouse liver. Cell Metab 5: 313–320, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess SC, Jeffrey FM, Storey C, Milde A, Hausler N, Merritt ME, Mulder H, Holm C, Sherry AD, Malloy CR. Effect of murine strain on metabolic pathways of glucose production after brief or prolonged fasting. Am J Physiol Endocrinol Metab 289: E53–E61, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chambrier C, Laville M, Rhzioual Berrada K, Odeon M, Boulétreau P, Beylot M. Insulin sensitivity of glucose and fat metabolism in severe sepsis. Clin Sci (Lond) 99: 321–328, 2000 [PubMed] [Google Scholar]
- 13.Chan MY, Zhao Y, Heng CK. Sequential responses to high-fat and high-calorie feeding in an obese mouse model. Obesity (Silver Spring) 16: 972–978, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Choi CS, Savage DB, Abu-Elheiga L, Liu ZX, Kim S, Kulkarni A, Distefano A, Hwang YJ, Reznick RM, Codella R, Zhang D, Cline GW, Wakil SJ, Shulman GI. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci USA 104: 16480–16485, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37: 667–687, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Des Rosiers C, Montgomery JA, Desrochers S, Garneau M, David F, Mamer OA, Brunengraber H. Interference of 3-hydroxyisobutyrate with measurements of ketone body concentration and isotopic enrichment by gas chromatography-mass spectrometry. Anal Biochem 173: 96–105, 1988 [DOI] [PubMed] [Google Scholar]
- 17.Des Rosiers C, Montgomery JA, Garneau M, David F, Mamer OA, Daloze P, Toffolo G, Cobelli C, Landau BR, Brunengraber H. Pseudoketogenesis in hepatectomized dogs. Am J Physiol Endocrinol Metab 258: E519–E528, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Fink G, Desrochers S, Des Rosiers C, Garneau M, David F, Daloze T, Landau BR, Brunengraber H. Pseudoketogenesis in the perfused rat heart. J Biol Chem 263: 18036–18042, 1988 [PubMed] [Google Scholar]
- 19.Foster DW. Studies in the Ketosis of Fasting. J Clin Invest 46: 1283–1296, 1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hachey DL, Patterson BW, Reeds PJ, Elsas LJ. Isotopic determination of organic keto acid pentafluorobenzyl esters in biological fluids by negative chemical ionization gas chromatography/mass spectrometry. Anal Chem 63: 919–923, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Keller U, Cherrington AD, Liljenquist JE. Ketone body turnover and net hepatic ketone production in fasted and diabetic dogs. Am J Physiol Endocrinol Metab Gastrointest Physiol 235: E238–E247, 1978 [DOI] [PubMed] [Google Scholar]
- 22.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes 51: 2944–2950, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Kennedy EP, Lehninger AL. Oxidation of fatty acids and tricarboxylic acid cycle intermediates by isolated rat liver mitochondria. J Biol Chem 179: 957–972, 1949 [PubMed] [Google Scholar]
- 24.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest 103: 1489–1498, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Lam TK, Yoshii H, Haber CA, Bogdanovic E, Lam L, Fantus IG, Giacca A. Free fatty acid-induced hepatic insulin resistance: a potential role for protein kinase C-δ. Am J Physiol Endocrinol Metab 283: E682–E691, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34: 9–19, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470, 1999 [DOI] [PubMed] [Google Scholar]
- 29.McGarry J. What if Minkowski had been ageusic? An alternative angle on diabetes. Science 258: 766–770, 1992 [DOI] [PubMed] [Google Scholar]
- 30.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 51: 7–18, 2002 [DOI] [PubMed] [Google Scholar]
- 31.McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem 49: 395–420, 1980 [DOI] [PubMed] [Google Scholar]
- 32.Miles JM, Schwenk WF, McClean KL, Haymond MW. A dual-isotope technique for determination of in vivo ketone body kinetics. Am J Physiol Endocrinol Metab 251: E185–E191, 1986 [DOI] [PubMed] [Google Scholar]
- 34.Millington DS, Kodo N, Norwood DL, Roe CR. Tandem mass spectrometry: a new method for acylcarnitine profiling with potential for neonatal screening for inborn errors of metabolism. J Inherit Metab Dis 13: 321–324, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Moreau NM, Goupry SM, Antignac JP, Monteau FJ, Le Bizec BJ, Champ MM, Martin LJ, Dumon HJ. Simultaneous measurement of plasma concentrations and 13C-enrichment of short-chain fatty acids, lactic acid and ketone bodies by gas chromatography coupled to mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 784: 395–403, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9: 193–205, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Neufeld HA, Pace JG, Kaminski MV, George DT, Jahrling PB, Wannemacher RW, Jr, Beisel WR. A probable endocrine basis for the depression of ketone bodies during infectious or inflammatory state in rats. Endocrinology 107: 596–601, 1980 [DOI] [PubMed] [Google Scholar]
- 38.Noland RC, Koves TR, Seiler SE, Lum H, Lust RM, Ilkayeva O, Stevens RD, Hegardt FG, Muoio DM. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J Biol Chem 284: 22840–22852, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Owen OE, Reichard GA, Jr, Markus H, Boden G, Mozzoli MA, Shuman CR. Rapid intravenous sodium acetoacetate infusion in man. Metabolic and kinetic responses. J Clin Invest 52: 2606–2616, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parekh PI, Petro AE, Tiller JM, Feinglos MN, Surwit RS. Reversal of diet-induced obesity and diabetes in C57BL/6J mice. Metabolism 47: 1089–1096, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Pérez-Carreras M, Del Hoyo P, Martín MA, Rubio JC, Martín A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 38: 999–1007, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med 119, 5 Suppl 1: S10–S16, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potthoff MJ, Inagaki T, Satapati S, Ding X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA, Burgess SC. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci USA 106: 10853–10858, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riou JP, Beylot M, Laville M, De Parscau L, Delinger J, Sautot G, Mornex R. Antiketogenic effect of glucose per se in vivo in man and in vitro in isolated rat liver cells. Metabolism 35: 608–613, 1986 [DOI] [PubMed] [Google Scholar]
- 46.Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem 279: 32345–32353, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology 120: 1183–1192, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Satapati S, He T, Inagaki T, Potthoff M, Merritt ME, Esser V, Mangelsdorf DJ, Kliewer SA, Browning JD, Burgess SC. Partial resistance to peroxisome proliferator-activated receptor-alpha agonists in ZDF rats is associated with defective hepatic mitochondrial metabolism. Diabetes 57: 2012–2021, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Stanley CA. New genetic defects in mitochondrial fatty acid oxidation and carnitine deficiency. Adv Pediatr 34: 59–88, 1987 [PubMed] [Google Scholar]
- 52.Steele R, Winkler B, Rathgeb I, Bjerknes C, Altszuler N. Plasma glucose and free fatty acid metabolism in normal and long-fasted dogs. Am J Physiol 214: 313–319, 1968 [DOI] [PubMed] [Google Scholar]
- 53.Szendroedi J, Chmelik M, Schmid AI, Nowotny P, Brehm A, Krssak M, Moser E, Roden M. Abnormal hepatic energy homeostasis in type 2 diabetes. Hepatology 50: 1079–1086, 2009 [DOI] [PubMed] [Google Scholar]
- 54.Triscari J, Greenwood MR, Sullivan AC. Oxidation and ketogenesis in hepatocytes of lean and obese Zucker rats. Metabolism 31: 223–228, 1982 [DOI] [PubMed] [Google Scholar]
- 55.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, Ilkun O, Theobald HA, Cooksey RC, Kandror KV, Abel ED. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res 82: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye JM, Doyle PJ, Iglesias MA, Watson DG, Cooney GJ, Kraegen EW. Peroxisome proliferator-activated receptor (PPAR)-alpha activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats: comparison with PPAR-gamma activation. Diabetes 50: 411–417, 2001 [DOI] [PubMed] [Google Scholar]
- 57.Zhang D, Liu ZX, Choi CS, Tian L, Kibbey R, Dong J, Cline GW, Wood PA, Shulman GI. Mitochondrial dysfunction due to long-chain Acyl-CoA dehydrogenase deficiency causes hepatic steatosis and hepatic insulin resistance. Proc Natl Acad Sci USA 104: 17075–17080, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.