Abstract
Maternal obesity (MO) is increasing at an alarming rate. The objective of this study was to evaluate the effect of MO on fibrogenesis in fetal skeletal muscle during maturation in late gestation. Nonpregnant ewes were assigned to a control diet (Con; fed 100% of NRC nutrient recommendations, n = 6) or obesogenic diet (OB; fed 150% of NRC recommendations, n = 6) from 60 days before conception, and fetal semitendenosus (St) muscle was sampled at 135 days of gestation (term 148 days). Total concentration and area of collagen in cross-sections of muscle increased by 27.0 ± 6.0 (P < 0.05) and 105.1 ± 5.9% (P = 0.05) in OB compared with Con fetuses. The expression of precursor TGF-β was 177.3 ± 47.6% higher, and concentration of phospho-p38 74.7 ± 23.6% was higher (P < 0.05) in OB than in CON fetal muscle. Increases of 327.9 ± 168.0 (P < 0.05) and 188.9 ± 82.1% (P < 0.05), respectively, were observed for mRNA expression of Smad7 and fibronectin in OB compared with Con muscles. In addition, enzymes involved in collagen synthesis, including lysyl oxidase, lysyl hydroxylase 2b, and prolyl 4-hydroxylase-α1, were increased by 350.2 ± 90.0 (P < 0.05), 236.5 ± 25.2 (P < 0.05), and 82.0 ± 36.2% (P = 0.05), respectively, in OB muscle. In conclusion, MO-enhanced fibrogenesis in fetal muscle in late gestation was associated with upregulation of the TGF-β/p38 signaling pathway. Enhanced fibrogenesis at such an early stage of development is expected to negatively affect the properties of offspring muscle because muscle fibrosis is a hallmark of aging.
Keywords: collagen, maternal obesity, fetus
obesity is a very serious problem in the United States and many areas around the world. According to a National Health and Nutrition Examination Survey (1999–2002), 29% of nonpregnant women between 20 and 39 yr of age are obese. Maternal obesity (MO) in combination with a high-energy diet is harmful to fetal development, predisposing offspring to hypertension, type 2 diabetes, dyslipidemia, and coronary heart diseases (37, 40, 49). However, mechanisms linking maternal obesity to increased obesity in offspring remain poorly defined.
Skeletal muscle composes 40–50% of body mass and is the body's key insulin-responsive organ (34), constituting the most important tissue for glucose and fatty acid utilization as a result of its basal activity and as well as contractile activity during locomotion. In precocial mammals, which include humans and sheep, fetal life is crucial for skeletal muscle development because there is no net increase in muscle fiber numbers after birth (44). Thus, reduction of prenatal muscle fiber number has irreversible effects on later postnatal life (2). Due to its metabolic importance, impaired fetal skeletal muscle development is considered to play a key role in the influence of MO on progeny obesity (2, 3, 51).
During fetal skeletal muscle development, mesenchymal stem cells differentiate into myogenic cells, adipogenic cells, and fibroblasts. Fibrogenesis forms the endomysium, perimysium, and epimysium of fetal skeletal muscle (13). In addition, fibrogenesis forms the extracellular matrix that provides the environmental niche for differentiation of mesenchymal stem cells (9). Limited studies indicate that maternal nutrition affects fibrogenesis in skeletal muscle. Maternal nutrient restriction in swine increases collagen content in skeletal muscle in offspring (27). Because skeletal muscle fibrosis impairs muscle function and is a hallmark of aging (7), more studies on the association among maternal nutrition, fibrogenesis, and collagen accumulation in fetal muscle are needed.
Transforming growth factor-β (TGFβ) promotes pathological fibrosis via activation of the Smad signaling pathway (12, 21). p38 promotes TGFβ, and its activation is necessary for fibrosis (19, 33). Fibrosis occurs in response to inflammation, and we have reported an increase in inflammatory markers in skeletal muscle of fetuses of obese ewes (51). We hypothesize that inflammation associated with MO promotes fibrogenesis in fetal muscle via TGFβ and p38-mediated signaling pathways. Fetal skeletal muscle in sheep matures at late gestation (after 105 days of gestation) (13), and thus changes in fetal muscle at this stage are likely to have long-term effects on the properties of offspring muscle. The objective of the current study was to assess the effect of MO on TGFβ signaling and fibrogenesis in fetal muscle in late gestation.
MATERIALS AND METHODS
Care and use of animals.
All animal procedures were approved by the University of Wyoming Animal Care and Use Committee. Multiparous Rambouillet/Columbia cross ewes (3–4 yr of age with 2–3 previous pregnancies) were studied. Ewes were all mated with a single ram. Beginning 60 days before conception and continuing to the day of necropsy (1st day of mating = day 0), ewes were individually fed either a highly palatable diet at 100% of National Research Council (NRC) recommendations for energy (Con; n = 6) (38) or 150% of NRC's recommended energy requirements for early gestation (OB; n = 6). Ewes were housed in individual pens within a temperature-controlled room (∼20°C). All ewes were weighed at weekly intervals, and rations were adjusted for weekly changes in metabolic body weight (BW0.75) (15). Body condition was scored at monthly intervals to evaluate changes in fatness. A body condition score of 1 (emaciated) to 9 (obese) was assigned by two trained observers after palpation of the transverse and vertical processes of the lumbar vertebrae (L2 through L5) and the region around the tail head (16). The maternal body weight and body condition scores were reported previously and are shown in Table 1 (51).
Table 1.
Maternal and fetal body weight and fetal St muscle weight
| Category | Con | OB | Significance |
|---|---|---|---|
| Maternal, at the beginning of treatments | |||
| Body condition score | 5.5 ± 0.3 | 5.9 ± 0.3 | NS |
| Body weight, kg | 75.7 ± 7.3 | 65.1 ± 4.7 | NS |
| Maternal, at the end of treatments | |||
| Body condition score | 6.1 ± 0.4 | 8.5 ± 0.4 | P < 0.05 |
| Body weight, kg | 90.4 ± 7.7 | 107.6 ± 7.1 | P < 0.05 |
| Fetal | |||
| Body weight, g | 5,180.3 ± 232.4 | 4,977.1 ± 268.5 | NS |
| St muscle, g | 8.7 ± 0.3 | 7.9 ± 0.5 | NS |
Values are means ± SE; n = 6. St, semitendenosus; Con, control diet; OB, obesogenic diet; NS, not significant.
Immediately before necropsy on day 135 of gestation, ewes were weighed and sedated with intravenous ketamine (10 mg/kg), and general anesthesia was induced and maintained by isofluorane inhalation (1–2%). Ewes and fetuses were exsanguinated while under general anesthesia and fetuses quickly removed. No difference in body weight was observed between twins, so only one fetus of each twin pair was chosen at random for analysis. Fetal muscle from four ewes with twin fetuses and two ewes with singletons was analyzed for each treatment; each group had three male and three female fetuses. No difference was observed in measured fetal, organ, or tissue weights between male and female fetuses. Fetal semitendenosus (St) muscle samples from the left side were analyzed. Surface tissues were trimmed; one piece of muscle was sampled at the anatomic center of the muscle and snap-frozen in liquid nitrogen for biological analyses, and another piece was fixed in fresh paraformaldehyde before being embedded in paraffin. These tissues have been used in a previous study, and the fetal weight and St muscle weight are shown in Table 1 (51).
Antibodies.
Antibodies against tubuin (no. 2128), TGFβ (no. 3711), Smad2/3 (no. 3102), phospho-Smad2/3 at Ser423/425 (no. 9520), p38 (no. 9212), and phospho-p38 at Thr180/182 (no. 9211) were purchased from Cell Signaling (Danvers, MA).
Histochemical analyses.
St muscle samples were fixed in 4% (wt/vol) paraformaldehyde in phosphate buffer (0.12 M, pH 7.4), embedded in paraffin, and sectioned at 10 μm. Sections were rehydrated by a series of incubations in xylene and ethanol solutions and then treated with Masson Trichrome stain (18), which stains muscle fibers red, nuclei black, and collagen blue. Ten fields per sample were randomly selected for the quantification of collagen using Image J 1.30v software (National Institutes of Health). The area of collagen was expressed as the percentage of total view areas.
Collagen concentration analyses.
St muscle samples (0.1 g) were ground and dried in a convection oven at 60°C, and samples were weighed and hydrolyzed in 6 N HCl at 105°C for 16 h. An aliquot was removed for hydroxyproline determination, as described previously (50). Collagen concentration (mg/g dry muscle weight) was calculated assuming collagen weighs 7.25 times the measured weight of hydroxyproline (55).
Real-time quantitative PCR.
Total mRNA was extracted from the fetal St muscle using TRI reagent (Sigma, St. Louis, MO) and reverse transcribed into cDNA by using a kit (Qiagen, Valencia, CA). RT-PCR was performed using an iQ5 RT-PCR detection system (Bio-Rad Laboratories, Hercules, CA). A SYBR Green RT-PCR kit from Bio-Rad Laboratories was used along with the following primers: Ovis aries Smad7, forward 5′-GCAGCAGTTACCCCATCTTC-3′ and reverse 5′-GGCTGTACGCCTTCTCGTAG-3′; Ovis aries fibronectin, forward 5′-GGATGATGCCGCTCAGTATT-3′ and reverse 5′-GGGCCAGACAGTTAAGTGAA-3′; ovis aries collagen type I, forward 5′-GGTGACAGGAAGTCCCAGAA-3′ and reverse 5′-CTGTAGGTGAAGCGGCTGTT-3′; Ovis aries lysyl hydroxylase 2b (LH2b), forward 5′-ATGCCAATCAGGAGGATCTG-3′ and reverse 5′-CAGGTAGCGTTTCCCAATGT-3′; Ovis aries lysyl oxidase, forward 5′-AGCTCAGCATACAGGGGAGA-3′ and reverse 5′-CATCCATGCTGTGGTAATGC-3′; Ovis aries prolyl 4-hydroxylase α-subunit (P4HA), forward 5′-GATAAGGCGCTTTTGCTCAC-3′ and reverse 5′-ATCCACAGCAGCACCTTTCT-3′; tubulin, forward 5′-CGAGAGCTGTGACTGTCTGC-3′ and reverse 5′-GGCATGACGCTAAAGGTGTT-3′. Each reaction yielded amplicons between 80 and 200 bp. PCR conditions were as follows: 20 s at 95°C, 20 s at 56°C, and 20 s at 72°C for 35 cycles. After amplification, a melting curve (0.01 C/s) was used to confirm product purity, and the PCR products were electrophoresed to confirm the targeted sizes. Results are expressed relative to tubulin.
Western blot analysis.
Muscle samples were washed with PBS and lysed in a buffer containing 50 mM HEPES (pH 7.4), 2% SDS, 1% NP-40, 10% glycerol, 2 mM phenylmethylsulfonyl fluoride, 10 mM sodium pyrophosphate, 10 mg/ml aprotinin, 10 mg/ml leupeptin, 2 mM Na3VO4, and 100 mM NaF. Soluble proteins were recovered after a 10-min centrifugation (10,000 g), and their concentrations were determined according to the Bradford method (Bio-Rad Laboratories) (19). For the subcellular fractionation, nuclear and cytoplasmic extracts from muscle samples were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA), following the instructions by the manufacturer. Proteins in cell lysates were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Subsequently, the membranes were treated with blocking buffer (5% nonfat dry milk in Tris-buffered saline-Tween 20 buffer containing 150 mM NaCl, 10 mM Tris, pH 8.0, and 0.1% Tween 20) for 1 h. The blocked membranes were probed with primary antibodies and incubated further with a secondary antibody conjugated with horseradish peroxidase. Membranes were visualized using enhanced chemiluminescence Western blotting reagents (Amersham Bioscience, Piscataway, NJ) and exposure to film (MR; Kodak, Rochester, NY). Density of bands was quantified by using Imager Scanner II and ImageQuant TL softwares (Amersham Bioscience). Band density was normalized according to the tubulin content.
Electrophoretic mobility shift assay.
DNA-binding assays were performed, following the instruction of a kit (LightShift Chemiluminescent EMSA Kit, product no. 20148; Pierce, Rockford, IL). The following oligonucleotide sequences were used as probes in gel shift assays: SBE, forward 5′-GGAGTATGTCTAGACTGACAATGTAC-3′ and reverse 5′-GTACATTGTCAGTCTAGACATACTCC-3′ (53). Briefly, oligonucleotides were biotin labeled using the Biotin 3′-End DNA Labeling Kit (product no. 89818; Pierce), and then biotin-labeled oligonucleotides were heat treated at 90–95°C for 3 min, followed by rapid cooling on ice and addition of a nonspecific competitor, poly(dI·dC), and then the solutions were subjected to electrophoresis in native polyacrylamide gel. The DNA was then electroblotted at 380 mA for 60 min to a positively charged nylon membrane (Biodyne B Membrane) in a MiniProtean II electroblotter (Bio-Rad Laboratories) using 0.5× TBE buffer (450 mM Tris, 450 mM boric acid, and 10 mM EDTA, pH 8.3). The DNA was cross-linked to the membrane by 312 nm of ultraviolet radiation for 10 min. After washing, the membrane was incubated in chemiluminenscent solution, and bands were visualized by exposure to X-ray film.
Statistical analyses.
Statistical analyses were conducted following protocols used in our previous studies with sheep (54). Briefly, one fetus from each pregnant ewe was considered an experimental unit. Data were analyzed as a complete randomized design using a general linear model. Differences in mean values were compared by Tukey's multiple comparison test, and means ± SE are reported. Statistical significance was considered as P < 0.05.
RESULTS
Collagen content in OB and Con fetal muscle.
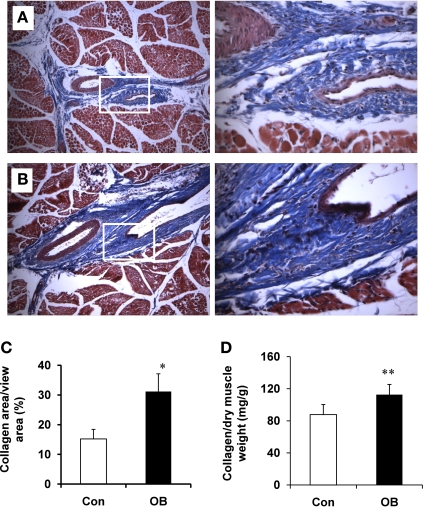
More collagen was detected histochemically in fetal muscle from OB ewes than from Con ewes (Fig. 1, A–C). Total collagen area was 105.1 ± 5.9% (P = 0.05) higher in OB fetal muscle compared with controls (Fig. 1C). Collagen as a function of hydroxyproline concentration increased by 27.0 ± 6.0% (P < 0.05) in OB fetal muscle compared with controls (Fig. 1D).
Fig. 1.
Collagen content for fetal semitendenosus (St) muscle of sheep assigned to control (Con; open bars) and obesogenic diets (OB; filled bars). A: representative images of St muscle from Con sheep (left: ×100 magnification; right: ×400 magnification). B: representative images of St muscle from OB sheep (left: ×100 magnification; right: ×400 magnification). C: %collagen area vs. total view area showing an increase in collagen area. D: collagen content calculated on the basis of the concentration of hydroxylproline showing an increase in collagen concentration. *P < 0.05; **P < 0.01 (means ± SE; n = 6).
TGFβ signaling pathway.
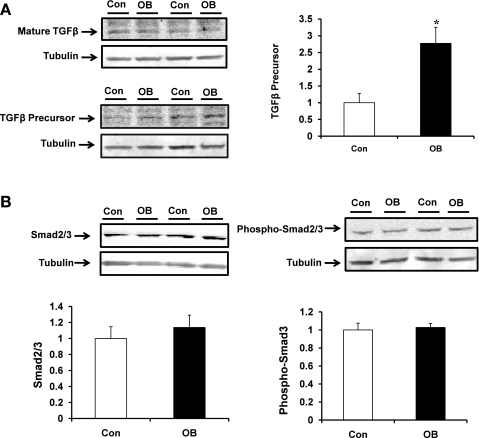
Protein content for precursor TGFβ was 177.3 ± 47.6% higher (P < 0.05) in fetal muscle taken from OB mothers compared with that of Con mothers (Fig. 2A) whereas no differences were observed in Smad2/3 and their phosphorylation between Con and OB groups (Fig. 2B).
Fig. 2.
Transforming growth factor-β (TGFβ)/Smad signaling in fetal St muscle of Con (open bars) and OB (filled bars) sheep. A: TGFβ content in fetal St muscle showing an increase of TGFβ in OB compared with Con fetal muscle. B: Smad2/3 and phospho-Smad2/3 in fetal St muscle showing no difference. *P < 0.05 (means ± SE; n = 6).
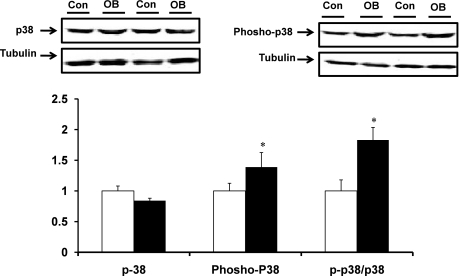
No difference in total p38 protein concentration was observed between OB and Con fetal muscle (Fig. 3). However, p38 phosphorylation was 74.7 ± 23.6% higher (P < 0.05) in fetal muscle in the OB group. The phospho-p38/p38 ratio was also higher (83.2 ± 20.4%, P < 0.05) in OB compared with Con fetal muscle (Fig. 3).
Fig. 3.
TGFβ/p38 signaling in fetal St muscle of Con (open bars) and OB (filled bars) sheep. An increase in p38 phosphorylation was observed in OB compared with Con fetal muscle. *P < 0.05 (means ± SE; n = 6).
mRNA expression of TGFβ target genes.
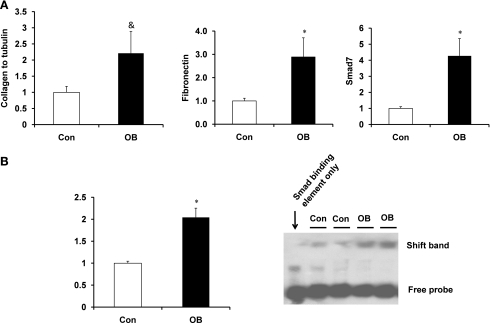
Smad7, fibronectin, and collagen mRNA expression were measured by RT-PCR (Fig. 4A). Fetal muscle mRNA expression of Smad7 and fibronectin in the OB group was increased 327.9 ± 168.0 (P < 0.05) and 188.9 ± 82.1% (P < 0.05), respectively, compared with Con. Furthermore, procollagen type I mRNA expression was 121.3 ± 67.8% higher (P = 0.08) in OB than in Con fetal muscle.
Fig. 4.
The expression of TGFβ signaling targeted genes in fetal St muscle of Con (open bars) and OB (filled bars) sheep. A: the mRNA expression of Smad7, fibronectin, and procollagen type I in fetal St muscle showing the increase of their mRNA expression in OB compared with Con fetal muscle. B: electrophoretic mobility shift assay for Smad response element showing an increased shift of DNA in OB compared with Con fetal muscle. *P < 0.05; &P < 0.1 (means ± SE; n = 6).
To determine whether such enhancement in the expression of TGFβ target genes was due to the increased Smad-mediated gene transcription, the nucleoproteins were extracted and subjected to an electrophoretic mobility shift assay using a DNA fragment corresponding to the Smad response element. Samples from OB fetal muscle had 104.5 ± 20.7% (P < 0.05) more DNA shift than Con samples (Fig. 4B).
Enzymes catalyzing collagen synthesis and cross-linking.
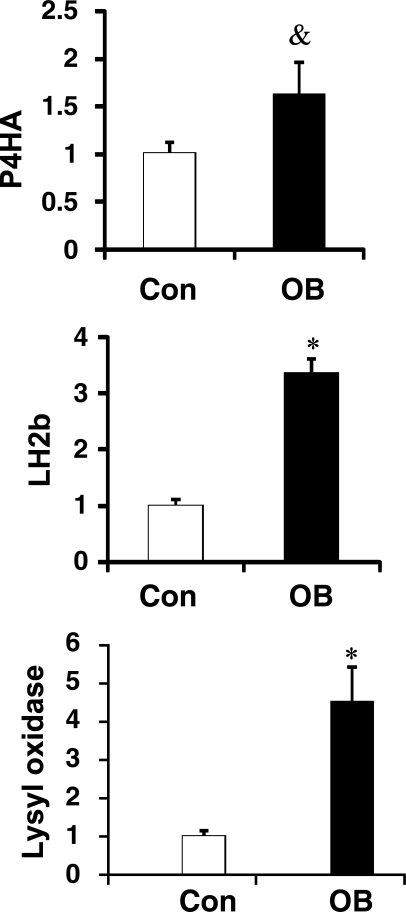
Lysyl oxidase, LH2b, and P4HA were increased by 350.2% ± 90.0 (P < 0.05), 236. 5 ± 25.2 (P < 0.05), and 82.0 ± 36.2% (P = 0.05), respectively, in fetal muscle from OB compared with Con ewes (Fig. 5).
Fig. 5.
mRNA expression of lysyl oxidase, lysyl hydroxylase 2b (LH2b), and prolyl 4-hydroxylase (P4HA) in fetal St muscle of Con (open bars) and OB (filled bars) sheep. Data show the increase of their mRNA expression in OB compared with Con fetal muscle. *P < 0.05; &P < 0.1 (means ± SE; n = 6).
DISCUSSION
Since sheep usually deliver one or two precocial young, the pregnant sheep is one of the most frequently used models for understanding fetal changes that take place in human pregnancy (1, 6, 11, 24, 36, 42). We have reported previously that MO induced inflammation in fetal skeletal muscle, demonstrated by upregulation of nuclear factor κ-light chain enhancer of activated B cells (NF-κB) and c-Jun NH2-terminal kinase (JNK) pathways (45, 51). TGFβ signaling is activated during muscle injury and inflammation (43). During myopathy, inflammation induces the expression of TGFβ, which then leads to connective tissue expansion during muscle regeneration (10, 41). We analyzed TGFβ and the phosphorylation of key downstream signaling mediators in fetal muscle at late gestation. However, no difference in Smad2/3 phosphorylation at Ser423/425 was observed. The absence of any change in Smad2/3 Ser423/425 phosphorylation could be due to feedback inhibition since it is known that the TGFβ-targeted gene Smad7 downregulates TGFβ signaling (22, 48). Indeed, a dramatic increase in Smad7 expression in OB fetal muscle was observed. Expression of Smad7 is induced by inflammation via the JNK cascade (46), and we have previously reported activation of the JNK cascade in OB fetal muscle (45). Smad7 binds to the TGFβ receptor, preventing binding and phosphorylation of Smad2/3 at Ser423/425, thereby downregulating TGFβ/Smad signaling (31). Smad7 also has an anti-inflammatory effect in chronic kidney diseases when it blocks the NF-κB-dependent inflammatory pathway (31). A similar effect might be responsible for the downregulation of inflammatory response in OB fetal muscle in late gestation compared with midgestation (45, 51). Despite this critical role of Smad7 as a negative regulator of TGFβ type I receptor for Smad2/3 activation, recent evidence illustrates the importance of Smad7 as an organizer of additional signaling proteins around the TGFβ type I receptor, which leads to activation of p38 (15), a kinase essential for Smad-dependent gene transcription (14). p38 phosphorylates Smad3 at Ser203 and Ser207 residues, which are required for the full transactivation potential of Smad3 (26). TGFβ receptor-activated p38 MAP kinase mediates TGFβ responses (52), and inhibition of p38 abolishes TGFβ response (35). In this study, enhanced p38 phosphorylation was detected in OB fetal muscle at late gestation, which should be responsible for the increased TGFβ-induced gene expression at late gestation. The combined effect of Smad7 and p38 activation is expected to gradually downregulate TGFβ signaling but prolong the expression of genes mediated by Smad3.
To analyze whether there is a change in TGFβ-induced gene expression, we used an electrophoretic mobility shift assay to analyze the binding of Smads to the conserved Smad binding element (GTCTAGAC) (53). Data showed that there was an increase of proteins binding to the Smad-binding element in OB fetal muscle, showing the enhancement of TGFβ-mediated gene expression.
Fibronectin and type I collagen are profibrotic genes regulated by TGFβ signaling (28), and their increased expression leads to fibrosis (18). In this study, we observed that the expression of both fibronectin and type I collagen was upregulated in OB fetal muscle, which accords with the activation of TGFβ-dependent gene expression. The upregulation of fibronectin expression is also consistent with the inflammatory response in OB fetal muscle (51). Inflammation promotes fibronectin expression through a NF-κB-dependent mechanism (39), and activation of NF-κB in skeletal muscle leads to fibrosis (32).
Collagen synthesis involves extensive intracellular and extracellular posttranslation modifications, including conversion of prolyl residues to hydroxyproline as well as widespread cross-linking. Synthesis of hydroxyprolyl residues is catalyzed by prolyl 4-hydroxylase (P4H). P4H has four subunits, of which the α-subunit is the catalytic subunit. The rate of α-subunit synthesis limits the rate of collagen synthesis (23, 29). Therefore, we analyzed the mRNA expression of P4HA. Expression of P4HA was increased, consistent with the observed increased expression of fibronectin and type I collagen. LH2b and Lysyl oxidase are necessary for the formation of cross-links. LH2b is an LH isoform that hydroxylates selected lysyl residues intracellularly and ultimately promotes the formation of the trivalent cross-link hydroxylysylpyridoline in fibrotic tissues (47). Lysyl oxidase deaminates selected lysyl and hydroxylysyl residues extracellularly and allows the cross-linking process to proceed (16). The expression of both LH2b and lysyl oxidase was higher in OB fetal muscle. Hypoxia induces the expression of LH2b and P4HA, both of which are indispensible for collagen fibril formation (8, 25). MO is likely to induce hypoxia in fetuses (17), which might explain the enhanced expression of genes involved in collagen synthesis. The profibrotic cytokine TGFβ promotes the expression of genes involved in collagen maturation and cross-linking (47). The enhanced expression of enzymes and genes described here would help explain the increased accumulation of collagen in OB compared with Con fetal muscle. Our results are consistent with a previous observation that skeletal muscle in obese adults has a higher collagen content (5).
Skeletal muscle constitutes about 40–50% of body mass and is the main peripheral tissue responsive to insulin-stimulated uptake of glucose and fatty acids (45). It is the only tissue responsible for locomotion. Poor cellular function in skeletal muscle, accompanied by decreased muscle mass, would likely enhance predisposition to related chronic disease conditions such as obesity and diabetes (20). During aging, a progressive loss of muscle mass associated with increased adiposity and fibrosis occurs (7, 30), resulting in the decline in muscle structural integrity and functional capacity (4). In combination with our previous observation that MO promotes intramuscular adipogenesis (45, 51), our observations indicate that MO induces changes in skeletal muscle at the early developmental stage that typically are not observed until later in life, predisposing offspring skeletal muscle to aging characterized by intramuscular adiposity and fibrosis. Such changes might impair skeletal muscle function earlier in life, providing a potential explanation for the increase in obesity and diabetes at a younger age in offspring of obese mothers.
In conclusion, our findings demonstrate that MO induces muscle fibrogenesis and connective tissue accumulation in the skeletal muscle of fetuses of obese mothers. TGFβ and associated p38 signaling in fetal muscle were activated by MO, providing a potential mechanism that would account for the increased collagen accumulation in OB fetal muscle.
GRANTS
This research is supported by the National Center for Research Resources of the National Institutes of Health and the Wyoming IDeA Network of Biomedical Research Excellence (P20RR016474).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reprod Suppl 61: 183–194, 2003 [PubMed] [Google Scholar]
- 2.Bayol SA, Macharia R, Farrington SJ, Simbi BH, Stickland NC. Evidence that a maternal “junk food” diet during pregnancy and lactation can reduce muscle force in offspring. Eur J Nutr 48: 62–65, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 567: 951–961, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggs ML, Nagarajan R, Taylor-Jones JM, Nolen G, Macnicol M, Peterson CA. Alterations in the TGFbeta signaling pathway in myogenic progenitors with age. Aging Cell 3: 353–361, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Berria R, Wang L, Richardson DK, Finlayson J, Belfort R, Pratipanawatr T, De Filippis EA, Kashyap S, Mandarino LJ. Increased collagen content in insulin-resistant skeletal muscle. Am J Physiol Endocrinol Metab 290: E560–E565, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 144: 3575–3585, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Brack AS, Conboy MJ, Roy S, Lee M, Kuo CJ, Keller C, Rando TA. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317: 807–810, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Brinckmann J, Kim S, Wu J, Reinhardt DP, Batmunkh C, Metzen E, Notbohm H, Bank RA, Krieg T, Hunzelmann N. Interleukin 4 and prolonged hypoxia induce a higher gene expression of lysyl hydroxylase 2 and an altered cross-link pattern: important pathogenetic steps in early and late stage of systemic scleroderma? Matrix Biol 24: 459–468, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Campos LS, Decker L, Taylor V, Skarnes W. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem 281: 5300–5309, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology 65: 826–834, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Das UG, Schroeder RE, Hay WW, Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol 276: R809–R817, 1999 [DOI] [PubMed] [Google Scholar]
- 12.Decologne N, Kolb M, Margetts PJ, Menetrier F, Artur Y, Garrido C, Gauldie J, Camus P, Bonniaud P. TGF-beta1 induces progressive pleural scarring and subpleural fibrosis. J Immunol 179: 6043–6051, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Du M, Yan X, Tong JF, Zhao J, Zhu MJ. Maternal obesity, inflammation, and fetal skeletal muscle development. Biol Reprod 82: 4–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dziembowska M, Danilkiewicz M, Wesolowska A, Zupanska A, Chouaib S, Kaminska B. Cross-talk between Smad and p38 MAPK signalling in transforming growth factor beta signal transduction in human glioblastoma cells. Biochem Biophys Res Commun 354: 1101–1106, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Edlund S, Bu S, Schuster N, Aspenstrom P, Heuchel R, Heldin NE, ten Dijke P, Heldin CH, Landstrom M. Transforming growth factor-beta1 (TGF-beta)-induced apoptosis of prostate cancer cells involves Smad7-dependent activation of p38 by TGF-beta-activated kinase 1 and mitogen-activated protein kinase kinase 3. Mol Biol Cell 14: 529–544, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem 53: 717–748, 1984 [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Twinn DS, Ozanne SE. Mechanisms by which poor early growth programs type-2 diabetes, obesity and the metabolic syndrome. Physiol Behav 88: 234–243, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Foidart M, Foidart JM, Engel WK. Collagen localization in normal and fibrotic human skeletal muscle. Arch Neurol 38: 152–157, 1981 [DOI] [PubMed] [Google Scholar]
- 19.Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y, Nishizawa M, Fujisawa J, Inoue K. p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 38: 879–889, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol 5: 87–90, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Gosselin LE, Williams JE, Deering M, Brazeau D, Koury S, Martinez DA. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve 30: 645–653, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Hamzavi J, Ehnert S, Godoy P, Ciuclan L, Weng H, Mertens PR, Heuchel R, Dooley S. Disruption of the Smad7 gene enhances CCI4-dependent liver damage and fibrogenesis in mice. J Cell Mol Med 12: 2130–2144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han XY, Wang W, Myllylä R, Virtanen P, Karpakka J, Takala TE. mRNA levels for α-subunit of prolyl 4-hydroxylase and fibrillar collagens in immobilized rat skeletal muscle. J Appl Physiol 87: 90–96, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Hay WW, Jr, Sparks JW, Wilkening RB, Battaglia FC, Meschia G. Fetal glucose uptake and utilization as functions of maternal glucose concentration. Am J Physiol Endocrinol Metab 246: E237–E242, 1984 [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer KH, Gess B, Lohaus C, Meyer HE, Katschinski D, Kurtz A. Oxygen tension regulates the expression of a group of procollagen hydroxylases. Eur J Biochem 270: 4515–4522, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kamaraju AK, Roberts AB. Role of Rho/ROCK and p38 MAP kinase pathways in transforming growth factor-beta-mediated Smad-dependent growth inhibition of human breast carcinoma cells in vivo. J Biol Chem 280: 1024–1036, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Karunaratne JF, Ashton CJ, Stickland NC. Fetal programming of fat and collagen in porcine skeletal muscles. J Anat 207: 763–768, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy L, Shi-Wen X, Carter DE, Abraham DJ, Leask A. Fibroblast adhesion results in the induction of a matrix remodeling gene expression program. Matrix Biol 27: 274–281, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kivirikko KI, Myllyla R, Pihlajaniemi T. Protein hydroxylation: prolyl 4-hydroxylase, an enzyme with four cosubstrates and a multifunctional subunit. FASEB J 3: 1609–1617, 1989 [PubMed] [Google Scholar]
- 30.Lahoute C, Sotiropoulos A, Favier M, Guillet-Deniau I, Charvet C, Ferry A, Butler-Browne G, Metzger D, Tuil D, Daegelen D. Premature aging in skeletal muscle lacking serum response factor. PLoS ONE 3: e3910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lan HY. Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci 13: 4984–4992, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Li H, Malhotra S, Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J Mol Med 86: 1113–1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Campanale NV, Liang RJ, Deane JA, Bertram JF, Ricardo SD. Inhibition of p38 mitogen-activated protein kinase and transforming growth factor-beta1/Smad signaling pathways modulates the development of fibrosis in adriamycin-induced nephropathy. Am J Pathol 169: 1527–1540, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science 307: 384–387, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Meyer-Ter-Vehn T, Gebhardt S, Sebald W, Buttmann M, Grehn F, Schlunck G, Knaus P. p38 inhibitors prevent TGF-beta-induced myofibroblast transdifferentiation in human tenon fibroblasts. Invest Ophthalmol Vis Sci 47: 1500–1509, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Muhlhausler BS, Duffield JA, McMillen IC. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-gamma, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 148: 878–885, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Nathanielsz PW. Animal models that elucidate basic principles of the developmental origins of adult diseases. ILAR J 47: 73–82, 2006 [DOI] [PubMed] [Google Scholar]
- 38.National Research Council Nutrient Requirements of Sheep Washington, DC: National Academy, 1985 [Google Scholar]
- 39.Reddy VS, Harskamp RE, van Ginkel MW, Calhoon J, Baisden CE, Kim IS, Valente AJ, Chandrasekar B. Interleukin-18 stimulates fibronectin expression in primary human cardiac fibroblasts via PI3K-Akt-dependent NF-kappaB activation. J Cell Physiol 215: 697–707, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Reusens B, Ozanne SE, Remacle C. Fetal determinants of type 2 diabetes. Curr Drug Targets 8: 935–941, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Salvadori C, Peters IR, Day MJ, Engvall E, Shelton GD. Muscle regeneration, inflammation, and connective tissue expansion in canine inflammatory myopathy. Muscle Nerve 31: 192–198, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Sebert SP, Hyatt MA, Chan LL, Patel N, Bell RC, Keisler D, Stephenson T, Budge H, Symonds ME, Gardner DS. Maternal nutrient restriction between early and midgestation and its impact upon appetite regulation after juvenile obesity. Endocrinology 150: 634–641, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen W, Li Y, Zhu J, Schwendener R, Huard J. Interaction between macrophages, TGF-beta1, and the COX-2 pathway during the inflammatory phase of skeletal muscle healing after injury. J Cell Physiol 214: 405–412, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Stickland NC. A quantitative study of muscle development in the bovine foetus (Bos indicus). Anat Histol Embryol 7: 193–205, 1978 [DOI] [PubMed] [Google Scholar]
- 45.Tong JF, Yan X, Zhu MJ, Ford SP, Nathanielsz PW, Du M. Maternal obesity downregulates myogenesis and β-catenin signaling in fetal skeletal muscle. Am J Physiol Endocrinol Metab 296: E917–E924, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uchida K, Suzuki H, Ohashi T, Nitta K, Yumura W, Nihei H. Involvement of MAP kinase cascades in Smad7 transcriptional regulation. Biochem Biophys Res Commun 289: 376–381, 2001 [DOI] [PubMed] [Google Scholar]
- 47.van der Slot AJ, van Dura EA, de Wit EC, De Groot J, Huizinga TW, Bank RA, Zuurmond AM. Elevated formation of pyridinoline cross-links by profibrotic cytokines is associated with enhanced lysyl hydroxylase 2b levels. Biochim Biophys Acta 1741: 95–102, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Verrecchia F, Mauviel A, Farge D. Transforming growth factor-beta signaling through the Smad proteins: role in systemic sclerosis. Autoimmun Rev 5: 563–569, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med 21: 149–157, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys 93: 440–447, 1961 [DOI] [PubMed] [Google Scholar]
- 51.Yan X, Zhu MJ, Xu W, Tong JF, Ford SP, Nathanielsz PW, Du M. Up-regulation of Toll-like receptor 4/nuclear factor-kappaB signaling is associated with enhanced adipogenesis and insulin resistance in fetal skeletal muscle of obese sheep at late gestation. Endocrinology 151: 380–387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu L, Hebert MC, Zhang YE. TGF-beta receptor-activated p38 MAP kinase mediates Smad-independent TGF-beta responses. EMBO J 21: 3749–3759, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zawel L, Dai JL, Buckhaults P, Zhou S, Kinzler KW, Vogelstein B, Kern SE. Human Smad3 and Smad4 are sequence-specific transcription activators. Mol Cell 1: 611–617, 1998 [DOI] [PubMed] [Google Scholar]
- 54.Zhu MJ, Han B, Tong J, Ma C, Kimzey JM, Underwood KR, Xiao Y, Hess BW, Ford SP, Nathanielsz PW, Du M. AMP-activated protein kinase signalling pathways are down regulated and skeletal muscle development impaired in fetuses of obese, over-nourished sheep. J Physiol 586: 2651–2664, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zimmerman SD, Thomas DP, Velleman SG, Li X, Hansen TR, McCormick RJ. Time course of collagen and decorin changes in rat cardiac and skeletal muscle post-MI. Am J Physiol Heart Circ Physiol 281: H1816–H1822, 2001 [DOI] [PubMed] [Google Scholar]