Abstract
The temporal changes in skeletal muscle mitochondrial content and lipid metabolism that precede type 2 diabetes are largely unknown. Here we examined skeletal muscle mitochondrial fatty acid oxidation (MitoFAOX) and markers of mitochondrial gene expression and protein content in sedentary 20- and 40-wk-old hyperphagic, obese Otsuka Long-Evans Tokushima fatty (OLETF-SED) rats. Changes in OLETF-SED rats were compared with two groups of rats who maintained insulin sensitivity: age-matched OLETF rats given access to voluntary running wheels (OLETF-EX) and sedentary, nonobese Long-Evans Tokushima Otsuka (LETO-SED) rats. As expected, glucose tolerance tests revealed insulin resistance at 20 wk that progressed to type 2 diabetes at 40 wk in the OLETF-SED, whereas both the OLETF-EX and LETO-SED maintained whole body insulin sensitivity. At 40 wk, complete MitoFAOX (to CO2), β-hydroxyacyl-CoA dehydrogenase activity, and citrate synthase activity did not differ between OLETF-SED and LETO-SED but were significantly (P < 0.05) higher in OLETF-EX compared with OLETF-SED rats. Genes controlling skeletal muscle MitoFAOX (PGC-1α, PPARδ, mtTFA, cytochrome c) were not different between OLETF-SED and LETO-SED at any age. Compared with the OLETF-SED, the OLETF-EX rats had significantly (P < 0.05) higher skeletal muscle PGC-1α, cytochrome c, and mtTFA mRNA levels at 20 and 40 wk and PPARδ at 40 wk; however, protein content for each of these markers did not differ between groups at 40 wk. Limited changes in skeletal muscle mitochondria were observed during the transition from insulin resistance to type 2 diabetes in the hyperphagic OLETF rat. However, diabetes prevention through increased physical activity appears to be mediated in part through maintenance of skeletal muscle mitochondrial function.
Keywords: Otsuka Long-Evans Tokushima fatty rats, fatty acid oxidation, insulin resistance, exercise
westernized societies are experiencing a weight gain epidemic and an associated increased risk in type 2 diabetes. It is estimated that more than 300 million people worldwide will have type 2 diabetes by the year 2025 (36), which stands to impose a significant burden on the already strained health care system. The underlying mechanism for the development of obesity-induced insulin resistance and type 2 diabetes remains unknown.
Obesity is associated with impaired skeletal muscle lipid metabolism, which is believed to play a role in the development of insulin resistance and type 2 diabetes (4). In addition, type 2 diabetic humans have reduced skeletal muscle mitochondria (9), reduced peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-1α), and reduced expression of other mitochondrial genes compared with nondiabetic controls (20, 22). Rodent models of high-fat feeding-induced insulin resistance also show decreased skeletal muscle mitochondrial content and function (3, 15, 28). In contrast, other studies have shown fatty acid oxidation and the activity of oxidative enzymes to be elevated in muscle of high-fat-fed or obese rodent models of insulin resistance (8, 13, 34), making the topic controversial (11).
Insulin resistance is thought to be due to the interplay of multiple systemic factors (26), and yet the regulation of insulin sensitivity and mitochondrial lipid metabolism by high or low physical activity is largely understudied. This occurs despite the fact that acute and chronic exercise training can dramatically improve insulin sensitivity (5, 31) and prevents the development of type 2 diabetes (5) while also increasing skeletal muscle mitochondrial size, number (7, 12), and fatty acid oxidative capacity (1, 18). We have previously utilized the hyperphagic (spontaneous null expression of cholecytokinin-1 receptor), obese Otsuka Long-Evans Tokushima fatty (OLETF) rat to examine the impact of physical activity levels on muscle, liver, and adipose mitochondrial metabolism (19, 24, 25). Sedentary OLETF rats display insulin resistance at 10–20 wk, which progresses to overt type 2 diabetes between 30 and 40 wk of age (19); however, when OLETF rats are given access to voluntary running wheels, body weight gain is suppressed (19), insulin sensitivity is enhanced (27), and type 2 diabetes development is prevented (19). Therefore, the OLETF is an excellent model to study obesity-induced outcomes in the context of daily physical activity vs. sedentary conditions.
The majority of available literature in various models of high-fat feeding-induced insulin resistance or type 2 diabetes has examined mitochondrial metabolism at single time points, making it difficult to determine whether mitochondrial dysfunction precedes or is a consequence of type 2 diabetes. Furthermore, sedentary caged animals are commonly used as controls. The study design employed here compares changes in skeletal muscle mitochondrial fatty acid oxidation (MitoFAOX) and mitochondrial gene and protein expression that occur in a transition from insulin resistance to overt type 2 diabetes in the sedentary OLETF rat to measurements made in two groups that maintain whole body insulin sensitivity by either daily exercise or by being nonobese controls. We have measured MitoFAOX, indices of mitochondrial function and oxidative enzymes [citrate synthase and β-hydroxyacyl-CoA dehydrogenase (β-HAD) activity], and expression of genes controlling mitochondrial fatty acid oxidation [PGC-1α, PPARδ, silent mating type information regulation 2 homolog 1 (SIRT1), mitochondrial transcription factor A (mtTFA), and cytochrome c mRNA] at 20 and 40 wk in all three groups. In addition, SIRT1, PGC-1α, PPARδ, cytochrome c, cytochrome c oxidase IV (COX-IV)-subunit I, and AMP-activated protein kinase (AMPK) protein content in skeletal muscle also were examined. Our overall hypothesis was that skeletal muscle MitoFAOX and mitochondrial function and protein expression would be significantly suppressed in sedentary OLETF rats compared with exercising OLETF rats and sedentary Long-Evans Tokushima Otsuka (LETO) controls. We also hypothesized that the transition from insulin resistance to type 2 diabetes in the sedentary OLETF rat would reveal reductions in skeletal muscle mitochondrial function, protein content, and gene expression, effects that would not occur in exercising OLETF rats.
METHODS
Animal protocol.
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri-Columbia. OLETF and LETO male rats at 4 wk of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). OLETF animals were randomly separated into those with (OLETF-EX) or without (OLETF-SED) access to running wheels. All LETO animals remained sedentary (LETO-SED). The OLETF-EX group was immediately housed (at the age of 28 days) in cages equipped with voluntary running wheels outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA) for measuring daily running activity. Voluntary running was selected to approximate the more natural activity state of the animal. Cages were in temperature-controlled animal quarters (21°C) with a 12:12-h light-dark cycle (0600–1800 light, 1800–0600 dark) that was maintained throughout the experimental period. All animals were provided standard rodent chow (Formulab 5008; Purina Mills, St. Louis, MO) in new cages at the beginning of each week when cages were changed, and body weights were obtained between 0800 and 1000. Running activity was obtained daily between 0800 and 1000, and rats in the running groups had daily access to wheels and food and water ad libitum until designated time of euthanization (20 or 40 wk of age). Body mass and food intake were measured weekly throughout the investigation. At 20 and 40 wk of age, rats (OLETF-SED, OLETF-EX, and LETO-SED) were anesthetized with an intraperitoneal injection of pentobarbital sodium (100 mg/kg) following a 5-h fast that began 4 h before the end of the animal's dark cycle. Blood for serum measurements and hemoglobin A1c (Hb A1c) was collected by cardiac puncture at this time. The animal was then euthanized by exsanguination by removal of the heart. This timing was 53 h after locking of the wheels in the OLETF-EX groups and was used to mirror our previous study (25) and to examine the effects of chronic exercise, not the acute effects of the last bout of exercise.
Dual-energy X-ray absorptiometry.
Whole body composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats, as described previously (19).
Glucose transport in the extensor digitorum longus skeletal muscle.
Glucose transport was measured as modified from Ref. 14. Briefly, extensor digitorum longus (EDL) muscles were carefully teased into strips (15–20 mg), placed in Lucite clamps, and immediately placed into separate wells containing preincubation medium [gassed (95% O2-5% CO2) Krebs-Henseleit buffer with 1.2 M pyruvic acid and 1% BSA] for 60 min. In the final 10 min of preincubation, 100 nM of insulin was added to one-half of the samples before the samples were quickly moved to medium containing cold 2-deoxyglucose (5 mM) and mannitol (39 mM) and hot 2-[3H]deoxyglucose (0.5 uCi/ml) and [14C]mannitol (0.032 uCi/ml) for a period of 60 min. Samples previously exposed to insulin during preincubation were placed in corresponding insulin (100 nM) treatment wells. After a 20-min incubation, tissue was removed from the medium, cut out of the Lucite clips, blotted dry, and snap-frozen in liquid nitrogen. Samples were then dissolved in boiling H2O in 2.0-ml screwtop tubes overnight, and the extract was counted on a liquid scintillation counter. Glucose transport into muscle was calculated as reported previously (31).
Mitochondrial isolation and fatty acid oxidation from red gastrocnemius skeletal muscle.
Mitochondrial suspensions from the red gastrocnemius (RG) skeletal muscle were prepared according to modified methods of Koves et al. (16), as described previously (18). Fatty acid oxidation was performed with radiolabeled [1-14C]palmitate (American Radiochemicals) in the freshly isolated RG mitochondria preparation using previously described methods (18, 32). Both 14CO2, representing complete fatty acid oxidation, and 14C-labeled acid-soluble metabolites (ASMs), representing incomplete fatty acid oxidation, were collected in a previously described trapping device and then counted on a liquid scintillation counter.
Fat pad collection and serum assays.
Retroperitoneal and omental adipose tissue fat pads were removed from exsanguinated animals and weighed. Serum glucose (Sigma, St. Louis, MO), triglycerides (TG; Sigma), free fatty acids (FFA; Wako Chemicals, Richmond, VA), and insulin (Linco Research, St. Charles, MO) were measured using commercially available kits according to the manufacturer's instructions. Hb A1c concentrations were determined in EDTA-whole blood by the Diabetes Diagnostics Laboratory at the University of Missouri using a boronate-affinity HPLC method (Primus Diagnostics, Kansas City, MO).
Intraperitoneal glucose tolerance test.
Five days prior to euthanization, animals underwent a fasted intraperitoneal glucose tolerance test (IPGTT). Food was removed from the cages 12 h before each received an intraperitoneal injection of dextrose (50% solution, 2 g/kg body wt). The running wheels were locked 12 h before the IPGTT procedure began. Venipuncture blood samples were collected from the lateral tail vein immediately before dextrose administration and 15, 30, 45, 60, and 120 min after injection. After centrifugation at 4°C at 3,000 g, serum samples were stored at −20°C until glucose and insulin measurement by glucose oxidase kit (Thermo) and ELISA (Linco Research), respectively. Glucose tolerance was estimated as the product of the areas under the curve (AUCs) for glucose and insulin calculated using the trapezoidal method (30), with higher AUCs indicative of reduced glucose tolerance.
PGC-1α, PPARδ, cytochrome c, SIRT1, and mtTFA mRNA expression.
PGC-1α, PPARδ, cytochrome c, SIRT1, and mtTFA mRNA expression was quantified by real-time PCR using the ABI 7000 Sequence Detection System and software. RG samples were pulverized in RLT buffer using the Qiagen TissueLyser system, with subsequent RNA isolation from the RNeasy kit and with the optional DNase I step (Qiagen). Purity was ensured and concentration determined with a spectrophotometer. Reverse transcription was performed by combining RNA with the reverse transcription reaction mixture (nuclease-free water, ImProm-II 5× reaction buffer, MgCl2, dNTP mix, and ImProm-II reverse transcriptase), and cDNA was synthesized. The reaction mixture [nuclease-free water, β-actin, PGC-1α, PPARδ, cytochrome c, SIRT1, and mtTFA primers and probe, both forward and reverse transcriptases, and TaqMan Master Mix (ABI)] was loaded to a 96-well microplate, along with the cDNA sample (50 ng), and placed into the ABI 7000 Sequence Detection System for polymerization. After polymerization, results were quantified using the DdCT method relative to β-actin. Comparison of the differences in raw CT values did not differ (P = 0.4) among groups, indicating that β-actin mRNA was an appropriate normalizer.
Western blotting.
Western blot analyses were performed for the determination of the protein content of SIRT1 (Santa Cruz Biotechnology, Santa Cruz, CA), PGC-1α (Calbiochem, San Diego, CA), PPARδ (Santa Cruz Biotechnology), total cytochrome c (Cell Signaling, Beverly, MA), COX-IV-subunit I (Molecular Probes, Eugene, OR), and AMPKα (Cell Signaling), as described previously (24, 25). To control for equal protein loading and transfer, the membranes were stained with 0.1% amido black (Sigma), as described previously (25). The total protein staining for each lane was quantified, and these values were used to correct for any differences in protein loading or transfer of all band densities.
Superoxide dismutase, catalase, citrate synthase, and β-HAD activity.
Superoxide dismutase (SOD) activity in RG homogenate was determined by commercially available methods (Cayman Chemicals, Ann Arbor, MI). Catalase activity in RG homogenate was determined by commercially available methods (Sigma), as described previously (32). Citrate synthase and β-HAD activities were determined in whole RG homogenate using the methods of Srere (29) and Bass et al. (2), respectively, as described previously (24, 25).
Statistics.
Each outcome measure was examined in six to eight animals. For each outcome measure, a one-way analysis of variance was performed (SPSS 15.0; SPSS, Chicago, IL) to compare animal groups within a respective age. A significant main effect (P < 0.05) was followed up with Student-Newman-Kuels post hoc comparisons. Unpaired t-tests were utilized to examine differences between 20- and 40-wk values within respective animal groups. Values are reported as means ± SE, and P ≤ 0.05 denotes a statistically significant difference.
RESULTS
Animal characteristics.
Similar to what we have reported previously (19, 21), initial running distance at 4 wk of age averaged ∼5 km/day (∼170 min/day, ∼33 m/min), with daily distances peaking between 8 and 11 wk of age (∼11–12 km/day, ∼275 min/day, ∼43 m/min) and declining to ∼6–8 km/day (∼200 min/day, ∼32 m/min) at 20 wk of age, and ∼4 km/day (∼150 min/day, ∼27 m/min) by 40 wk of age in the OLETF-EX rats. It remains unknown why the OLETF animals exhibited decreased voluntary wheel running as they aged, but perhaps the OLETFs appropriately mirror the human condition, in which aging is often associated with reduced physical activity. Consistent with our previous reports (19, 24, 25), body mass, fat pad mass, and body fat percent were significantly greater in the OLETF-SED animals compared with LETO-SED and OLETF-EX at all ages studied (P < 0.001; Table 1). A marker of exercise training, higher heart weight/body weight ratio, was found in all OLETF-EX animals compared with OLETF-SED and LETO-SED (Table 1).
Table 1.
Animal characteristics
| Food Consumption |
||||||
|---|---|---|---|---|---|---|
| Age, wk | Body Weight, g | %Body Fat, g | g/wk | g·wk−1·g body wt−1 | Fat Pad Mass, g | HW/BW, mg/g |
| OLETF-SED | ||||||
| 20 | 614.6 ± 8.3 | 30.6 ± 0.9 | 227.2 ± 7.0 | 0.37 ± 0.01 | 26.3 ± 1.0 | 2.62 ± 0.05 |
| 40 | 693.7 ± 38.8# | 30.9 ± 4.5 | 302.6 ± 20.4# | 0.45 ± 0.05#* | 57.1 ± 8.5# | 2.67 ± 0.15 |
| OLETF-EX | ||||||
| 20 | 425.6 ± 11.7† | 9.4 ± 1.2† | 244.3 ± 9.3* | 0.58 ± 0.02†* | 6.8 ± 1.4† | 3.67 ± 0.11†* |
| 40 | 538.5 ± 16.8#† | 17.9 ± 2.0#† | 228.7 ± 7.4†* | 0.43 ± 0.01#* | 15.1 ± 1.8#† | 3.07 ± 0.06#†* |
| LETO-SED | ||||||
| 20 | 479.3 ± 7.5† | 15.4 ± 1.2† | 159.1 ± 1.5† | 0.33 ± 0.01 | 9.0 ± 0.6† | 2.92 ± 0.07 |
| 40 | 555.8 ± 21.1#† | 21.9 ± 1.5#† | 167.5 ± 5.9† | 0.30 ± 0.01 | 14.3 ± 1.5#† | 2.70 ± 0.04 |
Values are means ± SE (n = 6–8). HW/BW, heart weight/body weight ratio; OLETF-SED, sedentary Otsuka Long-Evans Tokushima fatty rats; OLETF-EX, OLETF rats with access to running wheels; LETO-SED, sedentary Long-Evans Tokushima Otsuka rats.
Significantly different from 20-wk values within respective animal group (P < 0.01).
Significantly different from LETO-SED at respective age (P < 0.01).
Significantly different from OLETF-SED at respective age. Fat pad mass was the combination of omental and retroperitoneal fat pads.
Absolute weekly food consumption was significantly greater (P < 0.05) in OLETF-SED and OLETF-EX rats at 20 wk, with the OLETF-SED animals consuming more than OLETF-EX and LETO-SED by 40 wk (Table 1). Food consumption relative to body weight was significantly greater in the OLETF-EX animals at 20 wk of age and was significantly greater in both OLETF groups compared with LETO animals at 40 wk of age (Table 1).
Serum characteristics.
Serum TG and FFA concentrations were significantly greater in the OLETF-SED animals compared with OLETF-EX and LETO-SED at 20 and 40 wk of age (Table 2). In addition, serum TG levels observed in the OLETF-EX rats were significantly higher than those seen in LETO-SED rats at 20 and 40 wk of age (Table 2). As we have reported previously (19), OLETF-SED animals displayed insulin resistance at 20 wk of age, demonstrated by elevated levels of insulin and glucose compared with OLETF-EX and LETO-SED, and developed overt diabetes mellitus by 40 wk of age with an ∼50% drop in serum insulin and a twofold increase in Hb A1c (Table 2), whereas OLETF-EX animals remarkably maintained whole body glycemic control to the level of the LETO-SED animals at all ages studied (Table 2).
Table 2.
Serum characteristics
| Age, wk | Serum TG, mg/dl | Serum FFA, μmol/l | Serum Glucose, mg/dl | Serum Insulin, ng/ml | Hb A1c, % |
|---|---|---|---|---|---|
| OLETF-SED | |||||
| 20 | 187.1 ± 26.6 | 328.7 ± 50.3 | 582.5 ± 48.1 | 12.9 ± 0.7 | 5.4 ± 0.1 |
| 40 | 256.4 ± 37.8 | 317.3 ± 64.8 | 657.7 ± 47.3 | 5.4 ± 1.3# | 8.5 ± 0.8# |
| OLETF-EX | |||||
| 20 | 72.7 ± 14.2†* | 135.8 ± 32.6† | 410.7 ± 74.5† | 8.3 ± 0.8† | 4.6 ± 0.1† |
| 40 | 82.9 ± 11.0†* | 158.3 ± 20.1† | 305.1 ± 18.7† | 11.5 ± 0.9#† | 4.8 ± 0.1† |
| LETO-SED | |||||
| 20 | 40.4 ± 4.3† | 181.9 ± 23.7† | 328.9 ± 43.7† | 8.7 ± 0.8† | 4.6 ± 0.1† |
| 40 | 41.2 ± 3.5† | 187.9 ± 26.9† | 263.9 ± 24.4† | 10.6 ± 0.8† | 4.6 ± 0.1† |
Values are means ± SE (n = 6–8). TG, triglycerides; FFA, free fatty acids.
Significantly different from 20-wk values within respective animal group (P < 0.01).
Significantly different from LETO-SED at respective age (P < 0.05).
Significantly different from OLETF-SED at respective age (P < 0.05).
IPGTT and glucose transport in skeletal muscle.
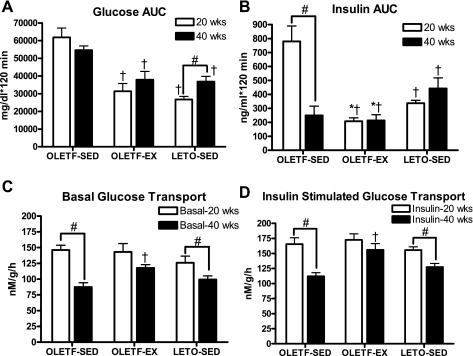
Differences in peripheral insulin sensitivity estimated by serum glucose, insulin, and Hb A1c levels were confirmed with IPGTT analysis and ex vivo assessment of glucose transport in skeletal muscle. At 20 wk of age, OLETF-SED animals displayed significant peripheral insulin resistance, with both glucose and insulin AUCs being twofold higher than in OLETF-EX and LETO-SED rats (Fig. 1, A and B). In addition, insulin AUC was significantly lower in the OLETF-EX compared with the LETO-SED animals (Fig. 1B). By 40 wk of age, glucose AUC remained significantly higher in the OLETF-SED animals compared with the other groups, but the OLETF-SED had an impaired insulin response (Fig. 1, A and B), suggesting loss of pancreatic insulin secretion in the 40-wk-old OLETF-SED rats. Basal and insulin-stimulated skeletal muscle glucose transport did not differ among groups at 20 wk (Fig. 1, C and D). However, the OLETF-SED rats exhibited a significant 40–50% reduction in basal and insulin-stimulated skeletal muscle glucose transport from 20 to 40 wk. Smaller but statistically significant (P < 0.05) reductions were also observed in the sedentary LETO animals from 20 to 40 wk. On the other hand, skeletal muscle glucose transport was maintained from 20 to 40 wk in the OLETF-EX rats (Fig. 1, C and D). This resulted in significantly higher basal and insulin-stimulated glucose transport in the OLETF-EX rats at 40 wk compared with OLETF-SED (Fig. 1, C and D).
Fig. 1.
Changes in systemic and skeletal muscle glucose homeostasis. Glucose (A) and insulin (B) area under curve (AUC) during an intraperitoneal glucose tolerance test. Values are means ± SE (n = 5–6). Basal (C) and insulin-stimulated (D) glucose transport in the extensor digitorum longus. Values are means ± SE (n = 6–8). #Significant difference between 20- and 40-wk values within respective animal group (P < 0.01). †Significantly different from sedentary Otsuka Long-Evans Tokushima fatty rats (OLETF-SED) at respective age (P < 0.05). *Significantly different from sedentary Long-Evans Tokushima Otsuka rats (LETO-SED) at respective age (P < 0.05). OLETF-EX, rats with access to running wheels.
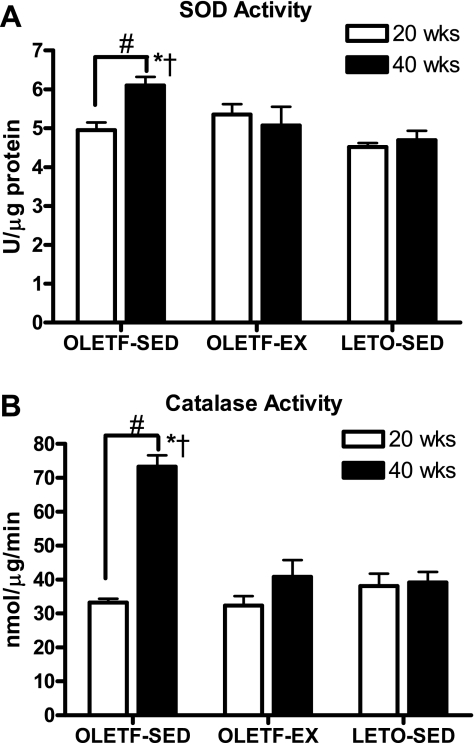
SOD and catalase activities.
SOD activity in the RG did not differ among groups at 20 wk of age but was significantly elevated by 40 wk in the OLETF-SED animals (Fig. 2A). This same pattern was observed in RG catalase activity, with significant increases observed by 40 wk in the OLETF-SED animals (Fig. 2B). Increases in free radical scavengers in the OLETF-SED animals are likely in response to increases in oxidative stress in the skeletal muscle of these animals.
Fig. 2.
Changes in superoxide dismutase (SOD; A) and catalase (B) activity in the red gastrocnemius (RG) muscle. Values are means ± SE (n = 6–8). #Significant difference between 20- and 40-wk values within respective animal group (P < 0.01). †Significantly different from OLETF-EX at respective age (P < 0.05). *Significantly different from LETO-SED at respective age (P < 0.05).
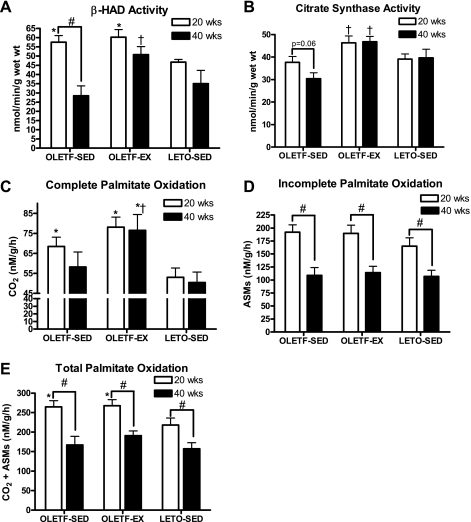
RG citrate synthase and β-HAD enzyme activities and RG mitochondrial fatty acid oxidation.
RG β-HAD activity did not differ between OLETF-SED and OLETF-EX at 20 wk, but activity levels were significantly higher compared with LETO-SED (P < 0.05; Fig. 3A). However, β-HAD activity dropped ∼50% from 20 to 40 wk in OLETF-SED animals, reductions that were not observed in OLETF-EX and LETO-SED rats, and thus led to significantly lower β-HAD levels in the OLETF-SED compared with OLETF-EX at 40 wk (P < 0.01; Fig. 3A). RG citrate synthase activity tended to be higher in OLETF-EX compared with OLETF-SED rats at 20 wk of age (P = 0.06; Fig. 3B) and was significantly higher than in OLETF-SED at 40 wk (P < 0.01). RG citrate synthase activity in LETO-SED animals did not differ from either group at any age examined (Fig. 3B). Both complete (to CO2) and total (CO2 and ASMs) MitoFAOX were significantly higher in both OLETF groups compared with LETO animals at 20 wk (Fig. 3, B and D). By 40 wk, complete MitoFAOX was maintained and significantly higher in the OLETF-EX animals compared with other groups (Fig. 3B); however, incomplete and total MitoFAOX were reduced in all groups and did not differ statistically (Fig. 3, C and D).
Fig. 3.
Changes in skeletal muscle β-hydroxyacyl-CoA dehydrogenase (β-HAD; A) and citrate synthase activity (B) and complete (C), incomplete (D), and total (E) palmitate oxidation from isolated RG mitochondria. Values are means ± SE (n = 6–8). #Significant difference between 20- and 40-wk values within respective animal group (P < 0.01). †Significantly different from OLETF-SED at respective age (P < 0.05). *Significantly different from LETO-SED at respective age (P < 0.05). ASMs, acid-soluble metabolites.
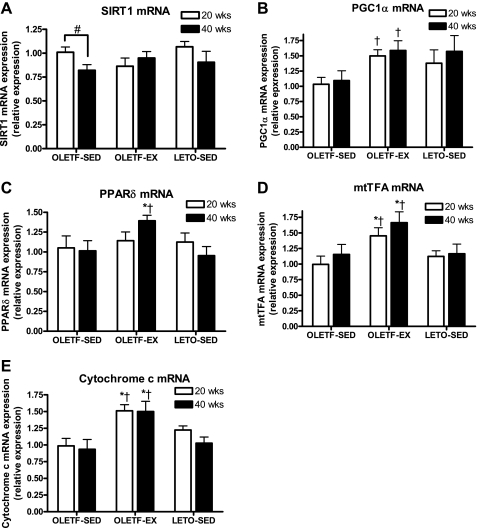
Markers of mitochondrial protein content and genes controlling MitoFAOX.
We measured potential regulating factors of MitoFAOX as well as indices of mitochondrial protein content. SIRT1 mRNA did not differ among groups regardless of age but was significantly reduced from 20 to 40 wk in the OLETF-SED animals (Fig. 4A). PGC-1α mRNA was significantly higher at 20 and 40 wk in the OLETF-EX rats compared with OLETF-SED (Fig. 4B). PPARδ mRNA expression did not differ among groups at 20 wk but was significantly elevated at 40 wk in the OLETF-EX compared with OLETF-SED and LETO-SED animals (Fig. 4C). Both cytochrome c and mtTFA mRNA were significantly elevated in the exercising OLETFs compared with sedentary OLETFs and LETOs at 20 and 40 wk of age (Fig. 4, D and E).
Fig. 4.
Changes in silent mating type information regulation 2 homolog 1 (SIRT1; A), peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-1α; B), PPARδ (C), mitochondrial transcription factor A (mtTFA; D), and cytochrome c (E) mRNA expression in the RG muscle. Values are means ± SE (n = 6–8). #Significant difference between 20- and 40-wk values within respective animal group (P < 0.01). †Significantly different from OLETF-SED at respective age (P < 0.05). *Significantly different from LETO-SED at respective age (P < 0.05).
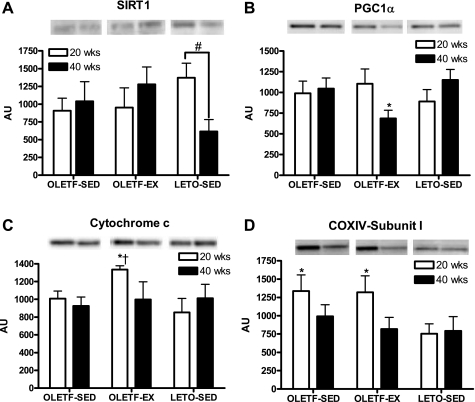
Despite elevated gene expression of several of the mitochondrial markers in the OLETF-EX group, only cytochrome c protein content was significantly elevated in the OLETF-EX compared with both sedentary groups at 20 wk of age (Fig. 5C). In addition, at 20 wk, mitochondrial-encoded COX-IV-subunit I protein content was significantly elevated in the RG of OLETF-SED and OLETF-EX animals compared with LETO-SED animals (Fig. 5D). However, by 40 wk, COX-IV content was reduced and no longer significantly different among groups. SIRT1 protein content did not differ among groups regardless of age but did drop significantly by 50–60% in the LETO-SED animals from 20 to 40 wk. In 20-wk-old animals, PGC-1α protein content did not differ among groups. By 40 wk, PGC-1α protein content was reduced in the OLETF-EX rats (P = 0.06) to a level significantly lower than that of LETO-SED (P < 0.05). AMPKα and PPARδ protein content did not differ among groups regardless of age (data not shown).
Fig. 5.
Changes in silent SIRT1 (A), PGC-1α (B), cytochrome c (C), and cytochrome c oxidase IV (COX-IV)-subunit I protein content (D) in the RG muscle. Values are means ± SE (n = 5–8). #Significant difference between 20- and 40-wk values within respective animal group (P < 0.05). †Significantly different from OLETF-SED at respective age (P < 0.05). *Significantly different from LETO-SED at respective age (P < 0.05). AU, arbitrary units.
DISCUSSION
Our design allowed a direct comparison of skeletal muscle mitochondrial changes in OLETF-SED rats to two different groups of rats that maintained insulin sensitivity: nonobese sedentary controls (LETO-SED) and OLETF rats that performed daily physical activity (OLETF-EX). During the transition from insulin resistance to type 2 diabetes, OLETF-SED animals did not display significant alterations in skeletal muscle mitochondrial measurements when compared with LETO-SED animals, except for a loss of β-HAD activity. These findings suggest that skeletal muscle mitochondrial dysfunction may not play a role in the transition from insulin resistance to type 2 diabetes, but rather, a constant caloric overload and expanding adiposity may be the primary driver in this hyperphagic animal model. In contrast, significant differences in mitochondrial measurements between the OLETF-SED and OLETF-EX rats were revealed most prominently at 40 wk. Maintenance of insulin sensitivity in the OLETF-EX rats was paired with elevated complete MitoFAOX, elevated β-HAD and citrate synthase activity, and increased markers of mitochondrial gene expression compared with OLETF-SED animals. Whether these exercise-associated changes in mitochondrial measurements are an obligatory feature or merely associated with the maintenance of insulin sensitivity remains unknown. Regardless, daily physical activity and higher energy expenditure completely protected against insulin resistance in the hyperphagic OLETF rat.
The role of skeletal muscle mitochondrial dysfunction in the development of insulin resistance and type 2 diabetes is highly debated (11). High-fat diets are commonly used to induce insulin resistance but can also trigger increased mitochondrial content and fatty acid oxidation (8), contradicting the hypothesis that impaired mitochondrial lipid metabolism is a cause of insulin resistance. In the OLETF-SED rats, excessive consumption of normal chow (16% of energy from fat) led to elevated circulating FFAs, which putatively increased mitochondrial enzyme activities and MitoFAOX in muscle to levels witnessed in OLETF-EX rats at 20 wk, both of which were higher than in LETO-SED. Thus, sedentary-hypercaloric conditions paired with insulin resistance evoked similar changes in mitochondrial measurements, as did daily physical activity and prevention of insulin resistance at 20 wk. It was only at 40 wk that the OLETF-EX rats displayed higher measurements of mitochondrial function, with the most prominent differences being revealed for complete vs. incomplete MitoFAOX.
High-fat feeding has been shown to induce glucose intolerance in association with high rates of incomplete fatty acid oxidation (flux through β-oxidation is higher than flux through the TCA cycle), resulting in the intramuscular accumulation of lipid metabolites (15). In the same study, exercise training enabled a coordinated regulation of β-oxidative flux, TCA cycle activity, and enhanced skeletal muscle mitochondrial capacity to completely oxidize fatty acids, which coincided with lower metabolite accumulation and improved glucose tolerance (15). In addition, prevention of mitochondrial fatty acid entry during high-lipid conditions protects against insulin resistance in part through reduced accumulation of lipid metabolites (17). In support of these findings, complete (to CO2) MitoFAOX was ∼25% higher in the exercising OLETF rats compared with LETO and OLETF sedentary animals at 40 wk of age. The maintenance of complete MitoFAOX occurred in conjunction with the conservation of glycemic control. Whether complete degradation of fatty acids in the isolated mitochondria in the OLETF-EX rats plays a primary role in maintaining insulin sensitivity or is merely an associated factor remains unanswered, but one could speculate a metabolic benefit by decreasing fat storage and less production of incomplete metabolites.
The overall importance of MitoFAOX in the development of insulin resistance is complicated by the drawbacks of assessing lipid metabolism with in vitro maximal capacity methods. As stated previously (11), it is unlikely that the mitochondrial capacity is ever reached in skeletal muscle of sedentary rats during fasting or fed conditions. It also is unlikely that voluntary wheel running evoked a maximal capacity response in the mitochondria of the OLETF-EX, especially at later time points, because daily running distances began to fall off. Therefore, future studies that measure lipid metabolism with indirect calorimetry or with tracers at the whole body level, rather than just in vitro capacity means, are needed to elucidate the true role of MitoFAOX in the development of insulin resistance. Furthermore, the importance of whole body measurements of fatty acid oxidation has recently been reinforced by Hoehn et al. (10), showing that raising fatty acid oxidation through pharmacological or genomic methods without raising total energy expenditure does not positively affect adiposity or insulin sensitivity.
PGC-1α is known to coactivate multiple mitochondrial transcription factors, including PPARδ (35), leading to the upregulation in fatty acid oxidation, in part through increased PGC-1α protein stability by the protein deacetylase SIRT1 and through mtTFA activation, a key component in transcription of multiple oxidative genes, including cytochrome c (6). In the sedentary OLETF and LETO animals, the gene expression and protein content of many of these factors did not differ between groups and remained largely unchanged from 20 to 40 wk despite a large reduction in total MitoFAOX, suggesting the lack of a primary role in skeletal muscle MitoFAOX. However, the one exception was mitochondrial-encoded COX-IV-subunit I protein content, which was significantly higher in OLETF-SED and OLETF-EX compared with LETO-SED at 20 wk and dropped to the LETO-SED levels by 40 wk. The elevation and subsequent reduction corresponded with the reductions seen in total MitoFAOX in both the OLETF-EX and OLETF-SED groups from 20 to 40 wk.
Previous exercise training studies have demonstrated increases in skeletal muscle fatty acid oxidation (1, 16), PGC-1α mRNA content (23), mitochondrial size and number (7, 12), and normalization of skeletal muscle mitochondrial content in patients with type 2 diabetes (33). Using daily voluntary wheel running to prevent type 2 diabetes in the current study, OLETF-EX rats had ∼50% higher skeletal muscle PGC-1α, mtTFA, and cytochrome c mRNA levels at 20 and 40 wk in the OLETF-EX compared with OLETF-SED rats. However, despite elevated gene expression, only the protein content of total cytochrome c was higher at 20 wk in the OLETF-EX rat. This suggests that either posttranscriptional modifications are affecting protein translation or stability in the skeletal muscle of the hyperphagic OLETF-EX animals or the decline/reduced intensity in daily running distance may be playing a significant role.
Increases in oxidative stress are also linked to mitochondrial dysfunction and insulin resistance. We have previously shown that 16 wk of daily voluntary exercise in the OLETF rat was associated with lower levels of lipid peroxidation without significant changes in intramyocellular lipids (21). In the current report, OLETF-SED rats exhibited increases in SOD and catalase activity from 20 to 40 wk when the health of the animal deteriorated, changes not witnessed in OLETF-EX and LETO-SED rats. These are likely responses to increased production of reactive oxygen species in the skeletal muscle of OLETF-SED animals and suggest that increased oxidative stress may be involved in the disease progression but likely do not mediate the changes observed in mitochondrial measurements in this report.
In conclusion, under sedentary conditions, skeletal muscle mitochondrial dysfunction does not appear to be associated with the transition from insulin resistance to type 2 diabetes in the hyperphagic OLETF rat. Rather, expanding adiposity and hyperlipidemia may play a more primary role. However, chronic prevention of type 2 diabetes through increased daily physical activity in hyperphagic conditions is associated with the maintenance or enhancement of skeletal muscle mitochondrial enzyme activity and fatty acid oxidation. Future studies are needed to elucidate whether the mitochondrial changes evoked by daily physical activity are obligatory or merely associated with the maintenance of insulin sensitivity.
GRANTS
This work was partially supported by the College of Veterinary Medicine and Department of Internal Medicine at the University of Missouri and National Institutes of Health Grants HL-36088 (M. H. Laughlin) and F32-DK-83182 (R. S. Rector). This work also was supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO, and a career development grant from the Veterans Health Administration (J. P. Thyfault).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The OLETF and LETO rats were a generous gift of the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). We thank Suzie Ridenhour and Craig Meers for excellent technical assistance with this work and Whitney Collins and Aaron Bunker for help with animal husbandry. We also thank the Diabetes Diagnostics Laboratory at the University of Missouri for help with the Hb A1c measurements.
REFERENCES
- 1.Baldwin KM, Klinkerfuss GH, Terjung RL, Mole PA, Holloszy JO. Respiratory capacity of white, red, and intermediate muscle: adaptative response to exercise. Am J Physiol 222: 373–378, 1972 [DOI] [PubMed] [Google Scholar]
- 2.Bass A, Brdiczka D, Eyer P, Hofer S, Pette D. Metabolic differentiation of distinct muscle types at the level of enzymatic organization. Eur J Biochem 10: 198–206, 1969 [DOI] [PubMed] [Google Scholar]
- 3.Benton CR, Han XX, Febbraio M, Graham TE, Bonen A. Inverse relationship between PGC-1α protein expression and triacylglycerol accumulation in rodent skeletal muscle. J Appl Physiol 100: 377–383, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J 18: 1144–1146, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Booth FW, Laye MJ, Lees SJ, Rector RS, Thyfault JP. Reduced physical activity and risk of chronic disease: the biology behind the consequences. Eur J Appl Physiol 102: 381–390, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med 37: 737–763, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gollnick PD, King DW. Effect of exercise and training on mitochondria of rat skeletal muscle. Am J Physiol 216: 1502–1509, 1969 [DOI] [PubMed] [Google Scholar]
- 8.Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci USA 105: 7815–7820, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes 50: 817–823, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Hoehn KL, Turner N, Swarbrick MM, Wilks D, Preston E, Phua Y, Joshi H, Furler SM, Larance M, Hegarty BD, Leslie SJ, Pickford R, Hoy AJ, Kraegen EW, James DE, Cooney GJ. Acute or chronic upregulation of mitochondrial fatty acid oxidation has no net effect on whole-body energy expenditure or adiposity. Cell Metab 11: 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holloszy JO. Skeletal muscle “mitochondrial deficiency” does not mediate insulin resistance. Am J Clin Nutr 89: 463S–466S, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Hoppeler H, Lüthi P, Claassen H, Weibel ER, Howald H. The ultrastructure of the normal human skeletal muscle. A morphometric analysis on untrained men, women and well-trained orienteers. Pflugers Arch 344: 217–232, 1973 [DOI] [PubMed] [Google Scholar]
- 13.Iossa S, Mollica MP, Lionetti L, Crescenzo R, Botta M, Liverini G. Skeletal muscle oxidative capacity in rats fed high-fat diet. Int J Obes Relat Metab Disord 26: 65–72, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Itani SI, Zhou Q, Pories WJ, MacDonald KG, Dohm GL. Involvement of protein kinase C in human skeletal muscle insulin resistance and obesity. Diabetes 49: 1353–1358, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Koves TR, Li P, An J, Akimoto T, Slentz D, Ilkayeva O, Dohm GL, Yan Z, Newgard CB, Muoio DM. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem 280: 33588–33598, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Koves TR, Noland RC, Bates AL, Henes ST, Muoio DM, Cortright RN. Subsarcolemmal and intermyofibrillar mitochondria play distinct roles in regulating skeletal muscle fatty acid metabolism. Am J Physiol Cell Physiol 288: C1074–C1082, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab 7: 45–56, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol 106: 161–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstråle M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267–273, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 104: 708–715, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA 100: 8466–8471, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 546: 851–858, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rector RS, Thyfault JP, Morris RT, Laye MJ, Borengasser SJ, Booth FW, Ibdah JA. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. Am J Physiol Gastrointest Liver Physiol 294: G619–G626, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest 118: 2992–3002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shima K, Shi K, Sano T, Iwami T, Mizuno A, Noma Y. Is exercise training effective in preventing diabetes mellitus in the Otsuka-Long-Evans-Tokushima fatty rat, a model of spontaneous non-insulin-dependent diabetes mellitus? Metabolism 42: 971–977, 1993 [DOI] [PubMed] [Google Scholar]
- 28.Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes 54: 1926–1933, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Srere PA. Citrate synthase. Meth Enzymol 13: 3–5, 1969 [Google Scholar]
- 30.Tai MM. A mathematical model for the determination of total area under glucose tolerance and other metabolic curves. Diabetes Care 17: 152–154, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol 292: C729–C739, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Thyfault JP, Rector RS, Uptergrove GM, Borengasser SJ, Morris EM, Wei Y, Laye MJ, Burant CF, Qi NR, Ridenhour SE, Koch LG, Britton SL, Ibdah JA. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. J Physiol 587: 1805–1816, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toledo FG, Menshikova EV, Ritov VB, Azuma K, Radikova Z, DeLany J, Kelley DE. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes 56: 2142–2147, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Turcotte LP, Swenberger JR, Zavitz Tucker M, Yee AJ. Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats. Diabetes 50: 1389–1396, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Wang YX, Lee CH, Tiep S, Yu RT, Ham J, Kang H, Evans RM. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell 113: 159–170, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature 414: 782–787, 2001. [DOI] [PubMed] [Google Scholar]