Abstract
Glucagon-like peptide-1 (GLP-1)-based therapies for diabetes have aroused interest because of their effects on insulin secretion and glycemic control. However, a mechanistic model enabling quantitation of pancreatic response to GLP-1 has never been developed. To develop such a model we studied 88 healthy individuals (age 26.3 ± 0.6 yr, BMI 24.9 ± 0.4 kg/m2) by use of a hyperglycemic clamp. A variable infusion maintained glucose concentrations at 150 mg/dl for 240 min. At 120 min, an intravenous infusion of GLP-1 was started (0.75 pmol·kg−1·min−1 from 120–180 min, 1.5 pmol·kg−1·min−1 from 181–240 min). Consequently, plasma C-peptide concentration rose from 1,852.0 ± 62.8 pmol/l at 120 min to 4,272.2 ± 176.4 pmol/l at 180 min and to 6,995.8 ± 323.5 pmol/l at 240 min. Four models of GLP-1 action on insulin secretion were considered. All models share the common assumption that insulin secretion is made up of two components, one proportional to glucose rate of change through dynamic responsivity, Φd, and one proportional to glucose through static responsivity, Φs, but differing by modality of GLP-1 control. The model that best fit C-peptide data assumes that above-basal insulin secretion depends linearly on GLP-1 concentration and its rate of change. An index (Π) measuring the percentage increase of secretion due to GLP-1 is derived. Before GLP-1 infusion, Φd = 245.7 ± 15.6 10−9 and Φs = 25.2 ± 1.4 10−9 min−1. Under GLP-1 stimulus, Π = 12.6 ± 0.71% per pmol/l, meaning that an increase of 5 pmol/l in peripheral GLP-1 concentrations induces an ∼60% increase in over-basal insulin secretion.
Keywords: glucagon-like peptide-1, incretins, C-peptide, hyperglycemic clamp, β-cell responsivity, parameter estimation
glucagon-like peptide-1 (GLP-1) is produced by the enteroendocrine L cells of the intestinal mucosa and is released into the portal circulation in response to meal ingestion (2). It arises from the posttranslational processing of proglucagon by prohormone convertase-1 (PC-1) in the enteroendocrine L cells of the intestinal mucosa (16). GLP-1 enhances insulin secretion and inhibits glucagon release in a glucose-dependent manner (17), prompting the development of GLP-1-based therapies for the treatment of diabetes (11). However, infused GLP-1 is rapidly inactivated by the widely distributed enzyme dipeptidyl peptidase-4 (DPP-4), which removes the two NH2-terminal amino acids, consequently requiring constant infusion to maintain its effects on insulin secretion. GLP-1-based therapy for type 2 diabetes has required the development of GLP-1 receptor agonists that resist the action of DPP-4 (21) or compounds that inhibit DPP-4, thereby raising endogenous concentrations of active GLP-1 (10). It has previously been suggested that genetic differences may explain some of the variation to GLP-1 response in prior studies (25).
Several studies are available on GLP-1 action on insulin secretion both at the cellular (13, 14, 18) and at the whole body levels (1, 2, 10, 15, 17, 19); however, none of them has ever aimed to mechanistically model GLP-1 action on insulin secretion. In fact, quantitating the effect of GLP-1 on insulin secretion is not straightforward, given that GLP-1 delays gastric emptying (26) and is a glucose-dependent secretagogue. Consequently, measuring the response to an oral meal challenge has required the use of modeling techniques to account for changes in insulin secretion in response to changing glucose concentrations. Changes in gastric emptying alter the rate of portal appearance of meal-derived glucose and consequently need to be accounted for in such situations. These considerations have often led to the use of a hyperglycemic clamp to quantitate response to GLP-1 infusion. However, peripheral insulin concentrations do not represent insulin secretion but rather the sum of pancreatic insulin secretion, hepatic insulin extraction, and distribution into the circulating compartment. Thus, more accurate measurements of insulin secretion are based on deconvolution (12, 24–27) from C-peptide concentrations, given that both are secreted in an equimolar fashion and C-peptide is not extracted by the liver. However, to mechanistically describe pancreatic insulin secretion and derive indexes of β-cell function, one can use the C-peptide minimal model (4), which allows exploration of the influence of glucose concentrations on insulin secretion, after a glucose perturbation. Furthermore, infusion of GLP-1 adds a second secretagogue that influences insulin secretion in concert with hyperglycemia. Developing a modification of the C-peptide minimal model that allows quantitation of the effect of hyperglycemia and GLP-1 on β-cell secretion would therefore be an important tool with which to explore the basis for interindividual differences in insulin secretion in response to GLP-1.
We therefore studied a group of healthy subjects by use of a hyperglycemic clamp and two infusion rates of GLP-1 (half-maximal and maximal). We subsequently used glucose, GLP-1, and C-peptide data to develop a model to describe these data and quantitate the percentage increase of secretion due to GLP-1.
RESEARCH DESIGN AND METHODS
Subjects
After approval from the Mayo Institutional Review Board, 88 subjects with an overnight fasting glucose <5.3 mmol/l gave written informed consent to participate in the study. All subjects were in good health, were at a stable weight, and did not engage in regular vigorous exercise. Subjects (52 females and 36 males) were <40 yr of age (average 26.3 ± 0.6 yr) at the time of participation and had no history of impaired fasting glucose or diabetes or of prior therapy with antidiabetic medication. Subjects had a BMI between 19 and 40 kg/m2 (average 24.9 ± 0.4 kg/m2) and no history of chronic disease or prior abdominal surgery. All subjects were instructed to follow a weight maintenance diet containing ∼55% carbohydrate, 30% fat, and 15% protein for the period of study. Body composition was measured using dual-energy X-ray absorptiometry (DPX scanner; Lunar, Madison, WI).
Experimental Design
Subjects were admitted to the CRU at 1700 on the evening prior to the study. Subsequently, they consumed a standard 10 cal/kg meal (55% carbohydrate, 30% fat, and 15% protein), after which they fasted overnight. At 0600 (−60 min) a forearm vein was cannulated with an 18-gauge needle to allow infusions to be performed. An 18-gauge cannula was inserted retrogradely into a vein of the dorsum of the contralateral hand. This was placed in a heated Plexiglas box maintained at 55°C to allow sampling of arterialized venous blood. At time 0 min a primed (0.1 g/kg over 4 min), continuous infusion of 50% dextrose was initiated. The dextrose infusion rate was varied to maintain peripheral glucose concentrations at ∼150 mg/dl (Fig. 1). At 120 min, GLP-1(7–36) amide (Bachem, San Diego, CA) was infused (15 pmol/kg lean body mass over 10 min and then at a rate of 0.75 pmol·kg−1·min−1). At 180 min, the infusion rate was increased to 1.5 pmol·kg−1·min−1. Prior experience with infusion of GLP-1 at a fixed rate (1.2 pmol·kg−1·min−1) has suggested that such an infusion requires 20–30 min to reach adequate concentrations of GLP-1 (32). Given the short duration of infusion, we used a higher infusion rate for the first 10 min to rapidly achieve elevated GLP-1 concentrations. We did not use a GLP-1 bolus over 2–3 min, in order to avoid potential nausea and vomiting associated with a rapid, sudden rise of GLP-1 concentrations. Blood was collected at frequent intervals over the duration of the study (240 min).
Fig. 1.
Average glucose infusion rate used to maintain glucose level at 150 mg/dl, despite change in GLP-1 induced C-peptide concentrations. Error bars represent standard error.
Analytic Techniques
Plasma samples were placed on ice, centrifuged at 4°C, separated, and stored at −20°C until assayed. Glucose concentrations were measured using a glucose oxidase method (Yellow Springs Instruments, Yellow Springs, OH). Plasma GLP-1, glucagon, and C-peptide were measured by RIA using reagents supplied by Linco Research (St. Louis, MO). Sample tubes utilized for measurement of active GLP-1 had 100 μM of DPP-4 inhibitor (Linco Research, St. Louis, MO) added.
C-peptide Minimal Model
The model used to describe C-peptide secretion before GLP-1 infusion is the C-peptide minimal model originally proposed in Ref. 28 for graded up-and-down glucose infusion and then employed also during an oral test (4). Briefly, the pancreatic secretion (SR) is linked to plasma C-peptide concentration by the two-compartment model of C-peptide kinetics originally proposed by Eaton et al. (12):
| (1) |
where CP1 and CP2 (pmol/l) are C-peptide concentrations in the accessible and peripheral compartments, respectively, and k01, k12, and k21 (min−1) are C-peptide kinetic parameters fixed to standard values (30) to ensure numerical identification of the overall model. The model also assumes that SR is made up of a basal (SRb), a static (SRs), and a dynamic (SRd) component:
| (2) |
SRs is assumed equal to the provision of releasable insulin to β-cells, controlled by glucose concentration above a threshold level h:
| (3) |
Basically, the static secretion component is proportional, through parameter Φs, to delayed glucose concentration above a threshold h, with a time delay equal to 1/α. Φs is the static β-cell responsivity to glucose and measures the ability of glucose to stimulate SRs.SRd represents the secretion of insulin from the promptly releasable pool and is proportional to the rate of increase of glucose:
| (4) |
where Φd is the dynamic β-cell responsivity to glucose and measures the ability of glucose rate of change to stimulate SRd.
The over-basal insulin secretion (ΔSR) is thus
| (5) |
GLP-1 Minimal Models
Since several reports in the literature indicate that GLP-1 acts only on above-basal insulin secretion while it has no effect on basal secretion, (2, 13, 17), we assumed that GLP-1 potentiates only above-basal insulin secretion. Four models of increasing complexity of GLP-1 action have been tested.
Model 1.
Model 1 assumes a proportional action of GLP-1 on over-basal insulin secretion:
| (6) |
with ΔSR(t) the glucose-dependent secretion rate before GLP-1 infusion, ΔSRglp1(t) the glucose-dependent secretion rate after GLP-1 infusion, GLP1(t) the over-basal hormone concentration, and a model parameter.
Model 2.
Model 2 assumes a proportional plus derivative action of GLP-1 on ΔSR :
| (7) |
with a and b model parameters representing the percentage increase of ΔSR due to the GLP-1 and GLP-1 rate of change, respectively.
Model 3.
Our data show that the relationship between GLP-1 and C-peptide concentrations is likely to be nonlinear (see results, Plasma C-peptide and GLP-1 concentration). Thus, model 3 is proposed, which assumes a nonlinear (Michaelis-Menten) action of GLP-1 on ΔSR:
| (8) |
with c and d model parameters representing, respectively, the maximum percentage increase of ΔSR due to GLP-1 and the value of the above-basal GLP-1 concentration at which the half-maximum percentage increase is obtained.
Model 4.
Finally, model 4 assumes a nonlinear and derivative action of GLP-1 on ΔSR:
| (9) |
with b, c, and d defined as above.
The GLP-1 Potentiation Index
It is useful to quantify the ability of GLP-1 to control insulin secretion through a potentiation index (Π). The index is defined as the ratio between the average percent increase in over-basal insulin secretion and average GLP-1 plasma concentration. With this definition, Π (% per pmol/l) can be derived for all the above models. However, since model 2 was selected as the most parsimonious (see results), we report the derivation of Π only for model 2:
| (10) |
with AUC denoting the area under the curve and GLP1max the peak value of over-basal GLP-1 concentration.
Parameter Estimation and Model Comparison
The GLP-1 models have been incorporated into the C-peptide model to describe SR as function of glucose and GLP-1 concentrations. All models were numerically identified on C-peptide data by nonlinear least squares (6, 7), as implemented in SAAM II [Simulation Analysis and modelling software (3)]. Error of C-peptide data was assumed to be independent, Gaussian, and with zero mean and known variance (29). Glucose and GLP-1 concentrations and, for models 2 and 4, GLP-1 rate of change are the model-forcing functions, assumed to be known without error. Since cost and blood volume considerations limited the number of GLP-1 data points available, we calculated the derivative on a virtually continuous signal reconstructed by stochastic deconvolution (9).
Models were compared on the basis of the following criteria (see Ref. 7 for details): ability to describe the data (weighted residual square sum, WRSS), precision of parameter estimates (expressed as CV), model parsimony (Akaike information criterion, AIC), and residual independence (Anderson run test). Moreover, since more complex models collapsed into simpler models when one or more parameters were estimated to be ≅0, we also considered as a criterion the percentage of identifications for which all parameters were nonzero.
Statistical Analysis
Data are presented as means ± SE, if not differently indicated. Two-sample comparisons were done by Wilcoxon signed rank test (significance level set to 5%). Pearson's correlation was used to evaluate univariate correlation.
RESULTS
Plasma C-Peptide and GLP-1 Concentration
Basal plasma C-peptide concentration was 578.3 ± 23.1 pmol/l; at 120 min, before the intravenous infusion of GLP-1 was started, C-peptide concentration was 1,852.0 ± 62.8 pmol/l, due to the rise in glucose concentration, and plasma GLP-1 was 4.4 ± 0.8 pmol/l. After the GLP-1 infusion, plasma GLP-1 concentration increased to 23.9 ± 1.6 pmol/l at 180 min and to 42.2 ± 2.1 pmol/l at 240 min (all differences were statistically significant, P < 0.0001), and consequently C-peptide concentration rose to 4,272.2 ± 176.4 and 6,995.8 ± 323.5 at 180 and 240 min, respectively (all differences were statistically significant, P < 0.0001) (Fig. 2). The ratios between above-basal C-peptide and above-basal GLP-1 concentrations were 266.4 ± 19.8 and 196.6 ± 11.1 at 180 and 240 min, respectively, and the difference was statistically significant (P < 0.0001). As already mentioned, this result supports the hypothesis that GLP-1 acts nonlinearly on C-peptide secretion.
Fig. 2.
Average plasma glucose (top left), insulin (top right) C-peptide (bottom left), and GLP-1 concentrations (bottom right). Error bars represent standard error.
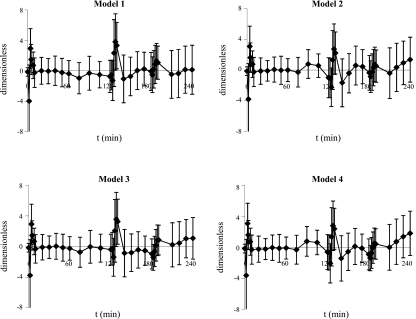
Model Comparison
Table 1 shows the criteria used for model selection. All the tested models fit the data well as confirmed by the Run Test, which supported randomness of residuals in 70% of the subjects. Time courses of weighted residuals obtained with the four models are shown in Fig. 3. Model 4 provided, on average, the best fit. As expected, increasing the complexity of the model (and the number of parameters), worsens the precision of parameter estimates (increased CV), but the parsimony criterion indicates model 4 as the most parsimonious (lowest AIC). On the other hand, model 4 collapsed into simpler models in most cases: e.g., to model 3 in 11 subjects, since parameter b was zero, to model 2 in 43 subjects, since parameter d was very high, and even to model 1 in 17 subjects, since both changes in parameters b and d occurred. Even if in the remaining 21 subjects model 4 was superior to the other models, we are inclined to select model 2 as the best model, since it is the most parsimonious to adequately fit the data in most cases. In addition (see below), it provides estimates of the potentiation index only modestly different from that of model 4, which is the most complex among the proposed models.
Table 1.
Comparison of GLP-1 action models
| Residual Independence (Run Test) | Data Fit (WRSS) | Precision (CV) | Parsimony Criterion (AIC) | No. Parameters | %Nonzero Parameters | |
|---|---|---|---|---|---|---|
| Model 1 | 68% | 192 | 7% | 194 | 1 | 100% |
| Model 2 | 75% | 145 | 13% | 149 | 2 | 77% |
| Model 3 | 70% | 163 | 28% | 167 | 2 | 44% |
| Model 4 | 71% | 109 | 28% | 115 | 3 | 19% |
Fig. 3.
Average weighted residuals obtained with models 1, 2, 3, and 4. Error bars represent standard deviation.
Model Indexes
model parameters are reported in Table 2 together with their precision.
Table 2.
Estimates of model parameters
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| α [min−1] | 0.036 ± 0.002 | 0.042 ± 0.002 | 0.038 ± 0.002 | 0.043 ± 0.002 |
| (10) | (10) | (10) | (10) | |
| h [mg/dl] | 90.15 ± 1.20 | 90.25 ± 1.11 | 93.25 ± 1.17 | 91.84 ± 1.07 |
| (4) | (4) | (4) | (4) | |
| Φd [10−9] | 249.51 ± 16.18 | 245.7 ± 15.6 | 253.35 ± 16.28 | 246.72 ± 15.60 |
| (5) | (5) | (5) | (5) | |
| Φs [10−9·min−1] | 29.9 ± 2.00 | 25.25 ± 1.4 | 29.75 ± 1.87 | 25.77 ± 1.50 |
| (8) | (8) | (8) | (8) | |
| a [l/pmol] | 0.11 ± 0.01 | 0.12 ± 0.01 | ||
| (7) | (6) | |||
| b [l·min/pmol] | 0.62 ± 0.06 | 0.56 ± 0.06 | ||
| (21) | (25) | |||
| c [dimensionless] | 6.40 ± 0.93 | 4.09 ± 0.86 | ||
| (16) | (11) | |||
| d [pmol/l] | 60.73 ± 9.89 | 54.75 ± 10.76 | ||
| (36) | (33) |
Values are means ± SE. Numbers in parentheses represent CV%.
Before GLP-1 infusion, β-cell responsivity indexes were: Φs = 25.2 ± 1.4 × 10−9 min−1 and Φd = 245.7 ± 15.6 × 10−9. Under GLP-1 stimulus, the potentiation index estimated with model 2 was Π = 12.6 ± 0.7% per pmol/l. This means that, if peripheral active GLP-1 concentration increases of 5 pmol/l, as occurs after a meal, over-basal insulin secretion is increased by ∼60%.
Note that if potentiation index were estimated with more complex models, similar results would have been obtained; e.g., with model 4, Π = 15.5 ± 1.1% per pmol/l (correlation with model 2 index: R = 0.95 P < 0.001). This confirms that model 2, despite being less accurate than model 4 in some cases, still provides good estimates of the efficiency of the GLP-1 control on over-basal insulin secretion.
DISCUSSION
GLP-1 is a potent insulin secretagogue that stimulates insulin secretion in a glucose-dependent manner. When infused in supraphysiological concentrations, it delays gastric emptying and promotes satiety (8), leading to weight loss (33). Since these actions are useful in the treatment of type 2 diabetes, GLP-1-based therapy for type 2 diabetes has been developed for clinical use in humans. The role of such therapies in the treatment algorithm for type 2 diabetes is as yet uncertain; consequently, neither DPP-4 inhibitors nor GLP-1 receptor agonists are used as first-line therapy.
However, there has been some suggestion that there is interindividual heterogeneity in response to GLP-1, raising the possibility that some individuals may benefit more from such therapy than others earlier in the disease (25, 31). Given the potential expense and side effects from such intervention, it would be reasonable to develop a quantitative measure of GLP-1-induced insulin secretion that can be used to examine differences in individual response to this hormone. Several studies are available on GLP-1 action on insulin secretion (1, 2, 10, 13–15, 17–19), however, to the best of our knowledge, none of them has ever aimed to mechanistically model GLP-1 action on β-cells. For instance, other investigators have previously utilized a hyperglycemic clamp to measure insulin secretion from deconvoluted C-peptide data. However, such a methodology does not take into account potential changes in glucose concentration during the hyperglycemic clamp or the changing GLP-1 concentrations prevailing during the experiment. The only model that indirectly accounts for a potentiation due to incretin is the one proposed by Mari et al. (20). It introduced a potentiating factor, which modulates the dose-response relation between insulin secretion and plasma glucose to better fit the C-peptide data, but it did not explicitly describe the incretin effect. Other studies (e.g., Ref. 1) also found a correlation between GLP-1 and the potentiating factor or utilized the model to assess different hormone responses in the morning vs. the afternoon (19). Another method used to assess incretin potentiation was that employed in Ref. 5. There the β-cell responsivities to glucose, Φs, and Φd were calculated for both oral and intravenous matched glucose infusions, using the C-peptide minimal model originally proposed by Toffolo et al. for graded up-and-down glucose infusion (28) and then employed during an oral test (4). The difference between oral and intravenous indexes provided an indirect measure of the incretin effect; there was an ∼60% difference between the two indexes, implying that the incretin effect is responsible for such potentiation.
However, in none of the above studies was there an attempt to mechanistically describe GLP-1 action on insulin secretion.
The novelty of the present study is thus the development and validation against experimental data of a model of GLP-1 action on insulin secretion that, in contrast to other methods, allows simultaneous estimation of both β-cell responsivity to glucose and of the ability of GLP-1 to enhance secretion. This model is a potential tool to quantitate between-subject variation in response to GLP-1. To this end, 88 healthy individuals were studied. They underwent a hyperglycemic clamp with an exogenous infusion of GLP-1 (Fig. 1), which brought hormone concentration first to physiological and then to supraphysiological concentrations (Fig. 2).
Four models have been tested. All of them are based on the common assumption that insulin secretion is made up of a basal component; a dynamic component, proportional to glucose rate of change through the dynamic responsivity index Φd (10−9); and a static index, proportional to glucose through the static responsivity Φs (10−9 min−1) (4). Moreover, since it is known that GLP-1 enhances insulin secretion in a glucose-dependent manner (2, 13, 17), all of the tested models assume that GLP-1 concentration modulates the above-basal insulin secretion. Models differ in the modalities with which GLP-1 controls insulin secretion, e.g., linear or nonlinear, static or derivative control.
We found that model 2 was the most parsimonious (Table 1). In fact, it better fit the C-peptide data and provided precise parameter estimates in the largest number of subjects. It provides a potentiation index, Π = 12.6 ± 0.7% per pmol/l, measuring the ability of GLP-1 to promote above-basal insulin secretion. To appreciate the meaning of this index, consider that, during hyperglycemic conditions (∼150 mg/dl), an increase of 5 pmol/l in peripheral GLP-1 concentrations, similar to that occurring after a meal, is predicted to induce a 63% increase in glucose-stimulated insulin secretion. This finding is comparable with the results reported in Ref. 5 for an OGTT, although the levels of GLP-1 were not reported. Furthermore, we also identified the original C-peptide model separately in the intervals 0–120 min (no incretin stimulation) and 120–240 min (incretin stimulation) and estimated two different Φs, i.e., Φs0–120 and Φs120–240. Φs120–240 was 326% higher on average than Φs0–120, due to an average increase in GLP-1 of 29 pmol/l. This is in agreement with Ref. 5 if one assumes that an average 5 pmol/l increase of GLP-1 is likely to occur during an oral test.
As observed in results, model selection criteria would have indicated model 4 as the most parsimonious. However, model 4 reduces to model 3 in 11 subjects, since parameter b was zero, to model 2 in 43 subjects, since parameter d was very high, and to model 1 in 17 subjects, since both changes in parameters b and d occurred. One can thus speculate that model 4 is the most general model that is able to predict the C-peptide concentration in very challenging conditions, such as during a hyperglycemic clamp with GLP-1 at physiological and supraphysiological concentration, whereas in most subjects or different experimental conditions, e.g., during a meal, a simpler model (but derived from model 4) may be sufficient; e.g., model 2 is a better candidate when plasma GLP-1 excursions are smaller; thus, the use of a nonlinear model may be not necessary. Of note, the potentiation index provided by model 2 is virtually identical to that obtained with model 4 (Π = 12.6 ± 0.7% vs. 15.5 ± 1.1% per pmol/l, R = 0.95, P < 0.001). This supports that, even though simpler, model 2 is robust enough to adequately quantify the action of GLP-1 on glucose-dependent insulin secretion.
Finally, it is interesting to note that the model describing the glucose-dependent insulin secretion (4, 28) is linear in both Φd and Φs; i.e., doubling Φd or Φs results in doubling SRd or SRs, respectively. Therefore, all the models described in the section GLP-1 Minimal Models can be rewritten in terms of GLP-1 action of β-cell responsivity indexes; i.e., Π can be defined as the percentage increase of Φs and Φd due to GLP-1. This means that GLP-1 produces the same potentiation on both the dynamic and the static phases. The assumption that both Φd and Φs are similarly modulated by GLP-1 relies on the evidence provided in Ref. 5, where the percentage increases of Φd and Φs due to incretins were similar (58 and 63%, respectively). The adopted protocol design does not allow us to verify this assumption. In fact, when GLP-1 is infused, i.e., from t = 120 to 240 min, glucose is approximately constant and the dynamic phase of insulin secretion is virtually absent. This makes it impossible to separately assess GLP-1 potentiation on the dynamic phase (Φd). As a matter of fact, four additional models, similar in structure to models 1, 2 3 and 4, respectively, but assuming GLP-1 action on static phase (Φs) only, performed similarly to their counterparts. However, we believe that, in light of the applicability of the model to different protocols, the GLP-1 action on dynamic insulin secretion has to be included in the model.
In conclusion, a model of GLP-1 action of insulin secretion in nondiabetic subjects has been proposed. The model has been tested on data obtained from a hyperglycemic clamp with exogenous infusion of GLP-1. Further studies are needed to assess its validity during different protocols, e.g., meal or OGTT, and its applicability in type 2 diabetic patients.
GRANTS
We acknowledge the support of the Mayo Clinic CTSA Grant RR-24150 and Minnesota Obesity Center Grant DK-50456. A. Vella and C. Cobelli are supported by DK-78646 and R. A. Rizza by DK-29953.
DISCLOSURES
One or more authors is employed by and/or has a total financial interest worth more than $10,000 (from consultancy honoraria/expert testimony/corporate grants/patents pending/royalties/other) and/or has a 5% equity in an entity related to the subject matter discussed in the paper. A. Vella has received research grants from Merck and has consulted for Sanofi Aventis and CPEX Pharmaceuticals.
REFERENCES
- 1.Ahrén B, Holst JJ, Mari A. Characterization of GLP-1 effects on beta-cell function after meal ingestion in humans. Diabetes Care 26: 2860– 2864, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP (Review). Gastroenterology 132: 2131– 2157, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Barrett PH, Bell BM, Cobelli C, Golde H, Schumitzky A, Vicini P, Foster D. SAAM II: Simulation, Analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism 47: 484– 492, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Breda E, Cavaghan MK, Toffolo G, Polonsky KS, Cobelli C. Oral glucose tolerance test minimal model indexes of beta-cell function and insulin sensitivity. Diabetes 50: 150– 158, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Campioni M, Toffolo G, Shuster LT, Service FJ, Rizza RA, Cobelli C. Incretin effect potentiates β-cell responsivity to glucose as well as to its rate of change: OGTT and matched intravenous study. Am J Physiol Endocrinol Metab 292: E54– E60, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Carson ER, Cobelli C, Finkelstein L. The Mathematical modeling of Endocrine-Metabolic Systems. Model Formulation, Identification and Validation New York: Wiley, 1983 [Google Scholar]
- 7.Cobelli C, Foster D, Toffolo G. Tracer Kinetics in Biomedical Research: from Data to Model Kluwer Academic/Plenum, New York, 2000 [Google Scholar]
- 8.Delgado-Aros S, Kim DY, Burton DD, Thomforde GM, Stephens D, Brinkmann BH, Vella A, Camilleri M. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol 282: G424– G431, 2002 [DOI] [PubMed] [Google Scholar]
- 9.De Nicolao G, Sparacino G, Cobelli C. Nonparametric input estimation in physiological systems: problems, methods, and case studies. Automatica 33: 851– 870, 1997 [Google Scholar]
- 10.Drucker DJ. Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes. Expert Opin Investig Drugs 12: 87– 100, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 368: 1696– 1705, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Eaton RP, Allen RC, Schade DS, Erickson KM, Standefer J. Prehepatic insulin production in man: kinetic analysis using peripheral connecting peptide behavior. J Clin Endocrinol Metab 51: 520– 528, 1980 [DOI] [PubMed] [Google Scholar]
- 13.Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144: 5149– 5158, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Fujimoto W, Miki T, Ogura T, Zhang M, Seino Y, Satin LS, Nakaya H, Seino S. Niflumic acid-sensitive ion channels play an important role in the induction of glucose-stimulated insulin secretion by cyclic AMP in mice. Diabetologia 52: 863– 872, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Højberg PV, Zander M, Vilsbøll T, Knop FK, Krarup T, Vølund A, Holst JJ, Madsbad S. Near normalisation of blood glucose improves the potentiating effect of GLP-1 on glucose-induced insulin secretion in patients with type 2 diabetes. Diabetologia 51: 632– 640, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Holst JJ, Bersani M, Johnsen AH, Kofod H, Hartmann B, Orskov C. Proglucagon processing in porcine and human pancreas. J Biol Chem 269: 18827– 18833, 1994 [PubMed] [Google Scholar]
- 17.Holst JJ, Toft-Nielsen MB, Orskov C, Nauck M, Willms B. On the effects of glucagon-like peptide-1 on blood glucose regulation in normal and diabetic subjects. Ann NY Acad Sci 805: 729– 736, 1996 [DOI] [PubMed] [Google Scholar]
- 18.Kwan EP, Gao X, Leung YM, Gaisano HY. Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to-granule fusions in rat islet beta cells. Pancreas 35: e45– e54, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Lindgren O, Mari A, Deacon CF, Carr RD, Winzell MS, Vikman J, Ahrén B. Differential islet and incretin hormone responses in morning versus afternoon after standardized meal in healthy men. J Clin Endocrinol Metab 94: 2887– 2892, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of β-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 283: E1159– E1166, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Nauck MA, Meier JJ. Glucagon-like peptide 1 and its derivatives in the treatment of diabetes. Regul Pept 128: 135– 148, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Pillonetto G, Sparacino G, Cobelli C. Reconstructing insulin secretion rate after a glucose stimulus by an improved stochastic deconvolution method. IEEE Trans Biomed Eng 48: 1352– 1354, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin: Pitfalls and limitations. Diabetes 33: 486– 494, 1984 [DOI] [PubMed] [Google Scholar]
- 24.Polonsky KS, Licinio-Paixao J, Given BD, Pugh W, Rue P, Galloway J, Karrison T, Frank B. Use of biosynthetic human C-peptide in the measurement of insulin secretion rates in normal volunteers and type I diabetic patients. J Clin Invest 51: 98– 105, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schafer SA, Tschritter O, Machicao F, Thamer C, Stefan N, Gallwitz B, Holst JJ, Dekker JM, t Hart LM, Nijpels G, van Haeften TW, Haring HU, Fritsche A. Impaired glucagon-like peptide-1-induced insulin secretion in carriers of transcription factor 7-like 2 (TCF7L2) gene polymorphisms. Diabetologia 50: 2443– 2450, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schirra J, Wank U, Arnold R, Goke B, Katschinski M. Effects of glucagon-like peptide-1(7–36)amide on motility and sensation of the proximal stomach in humans. Gut 50: 341– 348, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparacino G, Cobelli C. A stochastic deconvolution method to reconstruct insulin secretion rate after a glucose stimulus. IEEE Trans Biomed Eng 43: 512– 529, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Toffolo G, Breda Cavaghan MK E, Ehrmann DA, Polonsky KS, Cobelli C. Quantitative indexes of β-cell function during graded up-and-down glucose infusion from C-peptide minimal models. Am J Physiol Endocrinol Metab 280: E2– E10, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Toffolo G, Campioni M, Basu R, Rizza RA, Cobelli C. A minimal model of insulin secretion and kinetics to assess hepatic insulin extraction. Am J Physiol Endocrinol Metab 290: E169– E176, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 41: 368– 377, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Vella A, Camilleri M. Pharmacogenetics: potential role in the treatment of diabetes and obesity. Expert Opin Pharmacother 9: 1109– 1119, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vella A, Shah P, Basu R, Basu A, Holst JJ, Rizza RA. Effect of glucagon-like peptide 1(7–36) amide on glucose effectiveness and insulin action in people with type 2 diabetes. Diabetes 49: 611– 617, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 359: 824– 830, 2002. [DOI] [PubMed] [Google Scholar]