Abstract
As environmental temperatures rise, plants seek help from their core molecular mechanisms to adapt. One molecule that comes to the rescue, regulating gene expression, is the chromatin protein H2A.Z.
Unlike many other organisms, plants are sessile and — as relocating to more favourable conditions is not an option — they must be able to adapt to environmental changes. Consequently, plants are highly attuned to their environment and can respond rapidly to change in order to optimize their growth and reproduction. One environmental factor that significantly affects plant development is ambient temperature and fluctuations thereof. Although much attention has been focused on how plants react to temperature changes, no clear mechanisms have emerged for how temperature is perceived or how such signals are transduced and integrated to evoke the proper response. In a paper published in Cell, Kumar and Wigge1 shed light on these questions by showing that a specialized type of histone protein, H2A.Z, is a kingpin in the response of the model plant Arabidopsis to increasing temperature.
As the ambient temperature rises from 12 °C to 27 °C, the expression levels of thousands of genes are adjusted to help the plant adapt. One such gene, the expression of which increases in a linear fashion within this temperature range, is Hsp70. Assuming that the increased Hsp70 expression represents a downstream output of the temperature response, Kumar and Wigge performed a clever genetic screen to identify regulators of the coordinated changes in gene expression that accompany rising temperature.
The authors mutated plants carrying an Hsp70 reporter gene and screened for those that showed abnormal temperature sensitivity, expressing high levels of Hsp70 even at low temperatures. The screen led them to the gene encoding ARP6 — a protein that has several roles in Arabidopsis development through its activity in the SWR1 chromatin-remodelling complex2–4.
Chromatin is a protein–DNA fibre consisting of repeating nucleosome units. Each nucleosome is an octamer, comprised of two copies of each of the four core histone proteins (H2A, H2B, H3 and H4) with about 150 base pairs of DNA wrapped around it. This repeating structure serves not just to compact the DNA into the tiny space of the nucleus; it also has been co-opted to regulate gene expression by virtue of its ability to selectively expose or hide DNA sequences from DNA-binding proteins, which directly regulate gene expression.
In addition to the canonical histones, specialized variants of H3 and H2A also exist in all eukaryotes (organisms such as animals, plants and yeast). One H2A variant is H2A.Z, which imparts special properties on the nucleosomes it inhabits: it allows more complex mechanisms of gene regulation. H2A.Z is generally incorporated into nucleosomes just downstream of the spot where the enzyme RNA polymerase II begins transcription of a gene. The SWR1 complex catalyses this incorporation through a reaction in which H2A.Z replaces H2A within a nucleosome5. In the absence of ARP6, the histone exchange activity of SWR1 is abolished, suggesting that H2A.Z might mediate changes in gene expression associated with temperature responsive.
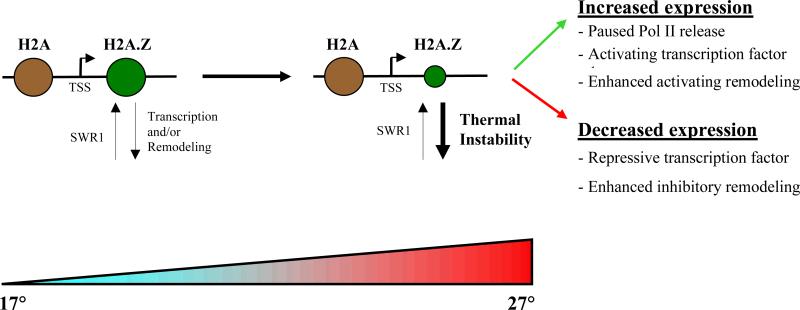
Kumar and Wigge1 next shifted Arabidopsis plants grown at 17 °C to 27°C, and found that the expression of several thousand genes increases, while a similar number of genes show significantly reduced expression. The authors found that, when grown at 17°C, plants deficient in the ARP6 gene show a similar pattern of gene expression as normal plants grown at 27°C. This observation indicates that ARP6 — and therefore H2A.Z — normally suppress geneexpression changes that accompany increases in temperature. In support of this conclusion, Kumar and Wigge found that, in normal plants, the amount of H2A.Z associated with each gene decreases with increasing temperature. Intriguingly, this finding was true both for genes that increase in expression at higher temperatures and those that show decreased expression. So by associating with genes at 17 °C, H2A.Z nucleosomes serve to maintain the expression of temperature-responsive genes at constant levels. At higher temperatures, however, these nucleosomes appear to be destabilized and so are lost from chromatin, allowing increased or reduced expression of genes as needed for growth at high temperatures (Fig. 1).
Figure 1. Thermal regulation of gene expression by the H2A.Z protein.
a, At lower temperatures, an H2A.Z-containing nucleosome is appropriately positioned just downstream of the transcriptional start site (TSS) of many genes. The average occupancy of this nucleosomal protein on a given gene reflects the balance between its deposition by the SWR1 chromatin-remodelling complex and its eviction driven by the RNA polymerase II (Pol II) enzyme or the action of chromatin-remodelling enzymes [sentence OK?]. The presence of H2A.Z-containing nucleosomes might help to maintain gene expression at a level appropriate to lower ambient temperature. b, As temperatures rise, the thermal instability of H2A.Z pushes the equilibrium towards its loss; nucleosomes containing only the H2A histone protein, however, remain unaffected. Depletion or modification of H2A.Z-carrying nucleosomes with rising temperature might allow gene-specific regulatory mechanisms to increase or decrease the expression of specific gene as needed to adapt the plant to the higher temperatures. Thus, through its unique thermal instability, H2A.Z might act as a gatekeeper of the temperature response.
Why H2A.Z has been retained as a minor variant in virtually all eukaryotes, and yet is non-essential in some, has long puzzled scientists. By examining published data, Kumar and Wigge found that gene expression changes in yeast H2A.Z mutants correlate significantly with gene expression changes in response to temperature. This result suggests that H2A.Z-mediated gating of the response to increased ambient temperature is an ancestral mechanism.
Indeed, thermoregulation provides an attractive function to explain the mystery of H2A.Z conservation throughout evolution, insofar as the ancestors of all organisms must have required some general mechanisms to regulate metabolism for efficient responses to changes in temperature. By depositing H2A.Z over the first few nucleosomes downstream of the transcriptional start sites of essentially all genes, eukaryotes can regulate each gene independently through locally altering the amounts, modification state or dynamics of H2A.Z. The modulation of H2A.Z properties would provide genes with a flexible gate that differentially impedes RNA Polymerase II, which must disrupt nucleosomes to gain direct access to DNA and transcribe it into RNA.
Recent evidence6 that ATP-dependent nucleosome remodellers involved in gene regulation also recognize H2A.Z nucleosomes as being different provides additional mechanistic possibilities to explain how H2A.Z nucleosomes might mediate thermoregulation, as well as other processes, such as stabilization of Polycomb-group protein interaction with DNA7. Kumar and Wigge's work therefore opens the door to studies of the specific molecular mechanisms underlying the responses that follow H2A.Z destabilization. It also underscores the importance of chromatin differentiation as a mechanism to evoke specialized transcriptional responses.
References
- 1.Kumar SV, Wigge PA. Cell. 2010;140:136–147. doi: 10.1016/j.cell.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Choi K, et al. Development. 2007;134:1931–1941. doi: 10.1242/dev.001891. [DOI] [PubMed] [Google Scholar]
- 3.Deal RB, Topp CN, McKinney EC, Meagher RB. Plant Cell. 2007;19:74–83. doi: 10.1105/tpc.106.048447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Trillo M, et al. Development. 2006;133:1241–1252. doi: 10.1242/dev.02301. [DOI] [PubMed] [Google Scholar]
- 5.Mizuguchi G, et al. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- 6.Goldman JA, Garlick JD, Kingston RE. J. Biol. Chem. 2009 doi: 10.1074/jbc.M109.072348. doi: 10.1074/jbc.M109.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Creyghton MP, et al. Cell. 2008;135:649–661. doi: 10.1016/j.cell.2008.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]