Abstract
Background
Previously we reported that insulin-like growth factor (IGF)-I during early pregnancy is positively associated with maternal risk of breast cancer. To explore this association further we designed a new study limited to women who donated a blood sample during their first full-term pregnancy.
Methods
A case-control study was nested within the Northern Sweden Maternity Cohort (NSMC) in which repository since 1975, serum specimens remaining after early pregnancy screening for infectious diseases had been preserved. Study subjects were selected among women who donated a blood sample during the full-term pregnancy that led to the birth of their first child. 244 women with invasive breast cancer were eligible. Two controls, matching the index case for age and date at blood donation were selected (n=453). IGF-I was measured in serum samples on an Immulite 2000 analyzer. Conditional logistic regression was used to estimate odds ratios and 95% confidence intervals.
Results
A significant positive association of breast cancer with IGF-I was observed, with OR of 1.73 (95 % CI: 1.14–2.63) for the top tertile, p < 0.009. Sub-group analyses indicated stronger effect of IGF-I in women ≤ age 25 and > age 30 than in women age 25–30 at index pregnancy. A stronger association of IGF-I with risk was also observed in cases diagnosed within 15 years of blood donation: OR 2.46 (95 % CI: 1.02–5.91).
Conclusions
The results of the study add further evidence for an adverse effect of elevated IGF-I concentrations during early reproductive life on risk of breast cancer.
Keywords: insulin-like growth factor (IGF)-I, breast cancer, pregnancy, nested case-control study
Introduction
Previously we reported the results of a case-control study, nested within the Northern Sweden Maternity Cohort (NSMC), in which insulin-like growth factor (IGF)-I concentrations measured mostly during the first trimester of pregnancy were related to risk of breast cancer [1]. Consistent with observations in non-pregnant women [2, 3], a direct association of moderate strength of breast cancer with IGF-I was observed. Subgroup analyses showed that the association was substantially stronger in women who donated blood during their first pregnancy (primiparous) and that the effect of IGF-I may be stronger at younger age at index pregnancy, but the sample size was small with equal number of primi- and multiparous women, precluding meaningful separation of the effects of parity and age at blood donation [1].
To further explore the role of IGF-I during early reproductive life on risk of breast cancer and to examine the effect of maternal age on this association, we designed a new study, limited to women who donated a blood sample during their first pregnancy ending with a live- or still-birth. Building on the experience acquired during our preliminary study, we were able to increase the efficiency of the new study by applying more stringent eligibility and matching criteria.
Materials and methods
Study population
The NSMC, based at the University Hospital in Umeå (Sweden), was established in 1975 with the purpose of preserving for research purposes serum samples from pregnant women tested for systemic infections. The NSMC participants are residents of one of the four northernmost counties of Sweden (total population ~800 000) who have attended a maternity health care clinic in the region during pregnancy. Blood samples are drawn mostly during the final weeks of the first trimester of pregnancy (weeks 6–18), and are periodically shipped frozen to a central repository at Umeå University Hospital, where they are analyzed for systemic infections and stored at −20 °C. The NSMC contains over 110 000 first-trimester serum samples from approximately 83 000 women.
We designed a nested case-control study limited to women who provided a blood sample during a singleton full-term pregnancy (FTP, ≥ 259 days) which led to the birth of their first live- or stillborn child. Women whose blood had been drawn after day 120 of pregnancy, were age 40 or older at the time of blood donation, had used hormonal medications to become pregnant or during pregnancy, or had previously been diagnosed with any cancer (except non-melanoma skin cancer) were also excluded. Further, women whose samples were drawn after January 1, 1988 were considered ineligible for the study as at that time the protocol for sample treatment was changed, which compromised hormonal measurements in those specimens as reported previously [4].
Potential cases were women diagnosed for the first time with invasive epithelial breast cancer at least one year after their entry into the cohort and up to June 08, 2007. The cases were identified through record linkages with the Swedish Cancer Registry using the unique 10-digit personal identification number assigned to every person born or legally resident in Sweden. The registration of newly detected cancers in Sweden is based on compulsory reports from all physicians in public or private hospitals and the completeness of cancer registration is considered close to 100%.
Because on-file information in the NSMC is limited to personal identity number, names, consecutive serial number of sampling, and place of sampling, identification of eligible cases and controls required a multi-step approach, including linkages with the Swedish Birth Registry and retrieval of medical records of a pool of eligible subjects. The Swedish Birth Registry is based on data from standardized medical records used in all Swedish maternal care and delivery units since 1973 [5]. More than 99% of all births in Sweden are registered [6]. In general, the quality of the information is considered high, although individual variables have variable completeness, as procedures to collect information have changed over the years.
At the beginning of the study a limited linkage with the Swedish Birth Registry was carried out and information on maternal parity, dates of deliveries and gestational age at birth for all singleton births was retrieved. The initial study file was limited to women who donated a blood sample during the singleton pregnancy which led to the birth of their first live- or still-born child (primiparous pregnancies), whose pregnancy was ≥ 37 gestational weeks (data were available for all subjects) and who were younger than age 41 at the date of birth of their first child. The file was linked with the Swedish Cancer Registry to retrieve information on cancer occurrence. Women with in situ breast cancer or any invasive cancer diagnosis (with the exception of non-melanoma skin cancer) prior to their first FTP were excluded. A total of 315 potentially eligible cases were identified, of which 58 (18%) were included in our previous report. 291 (92%) of the eligible cases had an available blood sample and were included in the current study.
For each case, potential controls were selected at random among cohort members alive and free of in situ breast cancer or any invasive cancer (with the exception of non-melanoma skin cancer) at the time of diagnosis of the case and matching the case on age at blood sampling (± 6 months), and date of blood sampling (± 3 months). Lists of up to 14 potential controls were drawn for each case. New controls were selected for the cases included in our initial report to satisfy the more stringent selection protocol applied in the current study. For each case and 4 of her potential controls a full copy of the maternity and delivery records was requested from one of the 10 hospitals in the region. If the medical record of a subject was not found in the local hospital according to the registered place of residence, requests were sent to one or two of the nearest hospitals. Of the requested 1504 records (291 cases and 1213 controls), 1359 (90%) were obtained (for 257 cases and 1102 controls). Records were first abstracted on the information necessary to establish for gestational age at blood donation - date of blood drawing and date of last menstrual period. If information on time at blood draw was missing, or gestational age at blood draw was exceeding day 120, or there was no biological specimen available for analysis (controls only), the subject was considered ineligible for the study (6 cases and 120 controls). If available, two controls fulfilling the eligibility and matching criteria were selected for each case (1:2 matching). If there were no eligible controls for a given case among the first 4 subjects identified as potential controls, the records of 4 additional potential control subjects were requested (necessary for 14 cases). Among eligible subjects, for those belonging to a matched set with a case and at least one control, the remainder of the information from the medical record was abstracted, with duplicate entries for dates of sampling and last menstrual period. Additionally, sixteen subjects (4 cases) were excluded because of hormone treatment during index pregnancy and 3 cases for whom no eligible control could be identified. In total, 244 cases and 453 controls were included in the study.
Smoking during pregnancy has been shown to influence hormone levels and could also be related to subsequent risk of maternal breast cancer. To ensure that the information on smoking corresponded to the date of blood collection and to ascertain the quality of medical records data on maternal smoking a cotinine measurement was conducted.
Laboratory analysis
Technicians who were unaware of the case-control status of the specimens, performed the IGF-I and cotinine measurements in the laboratory of Clinical Chemistry, University Hospital of Umeå. Individually matched case and control serum samples were always included in the same batch. To control the quality of the measurements, a pool of serum was created at the beginning of the study and 2 aliquots, undistinguishable from the case-control samples were randomly inserted within each batch. IGF-I was quantified by immunometric and cotinine by solid-phase competitive chemiluminescence assays on an Immulite 2000 Siemens analyzer. According to manufacturer instructions, women with serum cotinine levels equal or greater than 25 ng/mL were considered smokers. The intra- and inter-batch coefficients of variation estimated from analyses of laboratory controls with 74 ng/mL IGF-I were 4.3% and 5.6%. The respective numbers based on the blinded quality control specimens were 10.9% and 3.9%. The intra- and inter-batch coefficients of variation for the cotinine analyses were 5.5% and 9.9% at a concentration of 18 ng/mL and 5.6% and 8.5% at a concentration of 60 ng/mL, respectively.
Statistical analysis
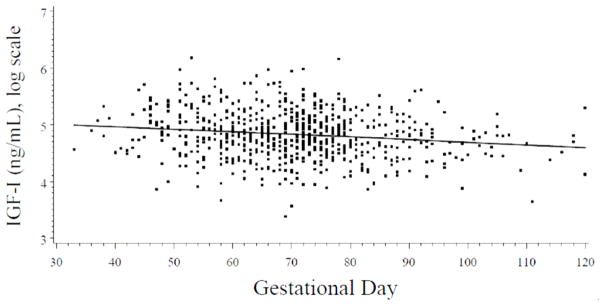
IGF-I levels are relatively constant during early pregnancy[6, 7] and in our data there was only a weak association of IGF-I with gestational day(r = −0.14, p=0.0002). Ideally, optimal control for hormonal variations with gestational age can be achieved by matching on this factor. However, exact matching would have imposed unwarranted constraints for control selection and would have not been feasible even in a much larger cohort with gestational age data available on file. To account for hormone variation with gestational day at blood donation we applied the statistical approach initially described by Richardsonet al [8]. In brief, the mean curve of IGF-I variation during pregnancy was estimated on the basis of all available IGF-I data using local linear regression [9], a nonparametric smoothing technique that employs weighted regression and uses varying subsets of the data to estimate the curve at each point (Figure 1 here). Prior to analysis, original IGF-I levels were natural log-transformed to limit heteroscedasticity. Concentrations for each woman were characterized as the difference (residual) between her assay value and the estimated mean IGF-I value determined for the day of gestation on which the blood sample was donated. In the remainder of this paper, the term ‘IGF-I concentrations’ refers to residuals of IGF-I. No outliers, defined as IGF-I concentrations exceeding 3 times the interquartile range, were identified.
Figure 1.
Distribution of log insulin-like growth factor (IGF)-I values by gestational day for all study subjects. The IGF-I levels decrease weakly with gestational age (r = − 0.14, p=0.0002), which is consistent with literature that little variation of IGF-I concentrations during early pregnancy [6,7]. The solid line shows the progression of IGF-I during pregnancy, estimated by local linear regression.
Mean IGF-I concentrations by case-control status or other characteristics of interest were compared by mixed-effects regression models. Pearson correlation coefficients were used to relate IGF-I concentrations to maternal and child characteristics of interest (e.g., age, height, weight). Kappa statistics was computed to compare maternal smoking at baseline as reported by the mother and according to cotinine concentrations.
The conditional logistic regression model, which is appropriate for matched data, was used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) associated with increasing IGF-I concentrations. Subjects were classified in tertiles using the frequency distribution of the controls. Tests for trend were computed by treating the IGF-I tertiles as an ordered categorical variable. Likelihood ratio tests were used to assess statistical significance. Additionally, analyses with IGF-I on the continuous scale were also performed. IGF-I - breast cancer associations were explored within strata of age at blood sampling (by median and < age 25, 25–30, ≥ 30), age at cancer diagnosis (by median), as well as by lag time (by median and less or ≥ 15 years). Tests of heterogeneity between the ORs in different subgroups were based on chi-square statistics, calculated as the deviations of logistic regression coefficients observed in each of the subgroups, relative to the overall regression coefficient [10]. None of the tested covariates influenced the point estimates substantially (<8%) and thus were not included in the final model. All tests of statistical significance were two-sided and considered significant if the p-values were < 0.05. SAS version 9.1 (SAS Institute, Inc.) was used for the analyses.
The study was approved annually by the Ethics Committees of the University of Umeå and New York University School of Medicine.
Results
Descriptive characteristics of the study population are presented in Table 1 (Table 1 here). Mean age at sampling was 27 years for both cases and controls. For the majority of study subjects (81%), blood was drawn between the 8th and the 14th gestational week. Mean gestational day was 70.7 days for cases and 70.2 days for controls. Mean age at diagnosis was 45.6 years (range: 25.5–63.8 years), 76% of the cases being diagnosed before age 50. Mean lag time between blood donation and cancer diagnosis was 18.6 years (range: 2.7–30.5 years). On average, cases had fewer children by index date than controls (2.0 vs. 2.2, p < 0.05), but all other maternal and child characteristics studied were similar in both groups. IGF-I concentrations were inversely correlated with maternal age (r=0.11, p <0.001), but no other significant correlations/subgroup differences of IGF-I with maternal or child characteristics were observed. A Kappa statistic of 0.84 indicated an excellent correspondence between smoking reported in the medical record and assessed by cotinine measurement in maternal blood.
Table 1.
Selected characteristics of study subjects, mean (std) or n (percentage).
| Characteristics | Cases (244) | Controls (453) | P-value* |
|---|---|---|---|
| Maternal age at sampling (years) | 27.0 (4.2) | 26.9 (4.1) | 0.76 |
| Gestational day | 70.7 (14.5) | 70.2 (14.2) | 0. 64 |
| Age at diagnosis (years) | 45.6 (6.5) | - | |
| Lag time (years) | 18.6 (6.1) | - | |
| Primi-gravida | 201 (82%) | 356 (77%) | 0.23 |
| Maternal weight at enrolment (kg) | 61.2 (8.3) | 61.6 (9.5) | 0.61 |
| Maternal height at enrolment (cm) | 165.0 (5.6) | 165.3 (5.7) | 0.46 |
| Mothers reporting smoking at enrolment# | 75 (31%) | 121 (27%) | 0.26 |
| Cotinine ≥ 25 ng/mL | 89 (37%) | 147 (33%) | 0.28 |
| Child sex (boy) | 135 (55%) | 237 (52%) | 0.45 |
| Child birth weight (g) | 3,481 (443) | 3,447 (460) | 0.32 |
| Child birth height (cm) | 50.4 (2.2) | 50.4 (2.0) | 0.83 |
| Placental weight (g)** | 575 (125) | 580 (121) | 0.64 |
| Parity by index date | 0.10 | ||
| 1 | 59 (24%) | 88 (19%) | |
| 2 | 129 (53%) | 230 (51%) | |
| >2 | 56 (23%) | 135 (30%) |
Chi-square test for categorical variables and mixed-effects regression models for continuous variables;
237 cases and 439 controls with data on smoking status at enrolment;
208 cases and 391 controls with data on placental weight.
IGF-I concentrations were higher in cases than in controls (0.04 vs. −0.02, p < 0.03, corresponding to IGF-I concentrations centered at gestational day 70 of 140.55 and 132.96 ng/mL, respectively). Table 2 summarizes the results from conditional logistic regression analyses (Table 2 here). Overall, breast cancer risk significantly increased with elevated IGF-I concentrations, with women in the top tertile of IGF-I concentrations having 73% higher risk than women with IGF-I in the bottom tertile. Analyses excluding cases included in our initial report (n=58) showed very similar risk estimates: OR = 1.21 for the second vs. lowest tertile, 95% CI: 0.75–1.93; and OR = 1.60 for the top vs. lowest tertile, 95 % CI: 1.00–2.57, p < 0.05.
Table 2.
| 1 | 2 | 3 | P-trend | OR cont. | P-ct | |
|---|---|---|---|---|---|---|
| All women | ||||||
| All women | 1.00 | 1.26 (0.83–1.92) | 1.73 (1.14–2.63) | 0.009 | 1.59 (1.05–2.42) | 0.029 |
| (cases/controls) | 64/151 | 78/151 | 102/151 | |||
| By subgroups of age at sampling** | ||||||
| < 25 years | 1.00 | 1.99 (0.88–4.52) | 2.69 (1.23–5.91) | 0.014 | 2.13 (1.00–4.54) | 0.050 |
| (cases/controls) | 16/50 | 28/50 | 38/51 | |||
| 25–30 years | 1.00 | 0.68 (0.35–1.30) | 1.16 (0.65–2.08) | 0.547 | 1.22 (0.67–2.21) | 0.512 |
| (cases/controls) | 38/69 | 26/69 | 45/70 | |||
| ≥ 30 years | 1.00 | 2.22 (0.93–5.28) | 1.78 (0.67–4.67) | 0.232 | 1.99 (0.74–5.35) | 0.174 |
| (cases/controls) | 10/31 | 24/31 | 19/32 | |||
| By median age at diagnosis** | ||||||
| < 45.9 years | 1.00 | 1.18 (0.64–2.20) | 1.64 (0.94–2.84) | 0.072 | 1.98 (1.12–3.49) | 0.018 |
| (cases/controls) | 31/75 | 37/75 | 53/76 | |||
| ≥ 45.9 years | 1.00 | 1.17 (0.66–2.07) | 1.61 (0.88–2.96) | 0.122 | 1.22 (0.65–2.27) | 0.533 |
| (cases/controls) | 35/75 | 39/76 | 49/76 | |||
| By lag time period** | ||||||
| <15 years | 1.00 | 1.30 (0.52–3.29) | 2.46 (1.02–5.91) | 0.038 | 2.24 (0.98–5.14) | 0.057 |
| (cases/controls) | 12/34 | 15/34 | 29/34 | |||
| ≥15 years | 1.00 | 1.21 (0.76–1.93) | 1.52 (0.94–2.43) | 0.084 | 1.41 (0.87–2.30) | 0.163 |
| (cases/controls) | 52/116 | 63/118 | 73/117 | |||
Tertiles were based on the frequency distribution of controls;
Continuous analysis represent risk for a change in one unit of IGF-I residuals;
none of the heterogeneity tests reached statistical significance.
Subgroup analyses by median age at sampling (26.5 years) indicated a substantially stronger effect of IGF-I concentrations in the younger (OR = 2.03 for top vs. lowest tertile; 95% CI: 1.13–3.64, p = 0.017) than in the older subgroup (OR = 1.34 for top vs. lowest tertile; 95% CI: 0.74–2.41), although the test for heterogeneity was not significant. More detailed analyses in age subgroups showed that elevated IGF-I is associated with increased risk of breast cancer in women either younger than 25 or age 30 and older at blood donation, but not in those age 25 to 30 at blood donation (Table 2), although there was no indication for statistical heterogeneity.
Analyses by median age at diagnosis indicated a stronger effect in women with relatively early diagnosis of breast cancer (Table 2). Analyses by pre-defined categories of lag-time showed a stronger effect in women diagnosed relatively soon after blood donation (< 15 years) versus those diagnosed 15 years or later. The estimates were particularly high for women diagnosed within 10 years of blood donation, but number of such cases was very small (n=25, data not shown). None of the heterogeneity tests was significant. Sensitivity analyses limited to subjects with gestational age < 98 days (strictly first trimester) or on original scale of IGF-I concentrations yielded similar or higher risk estimates to those in the whole study (data not shown). None of potential confounders considered [maternal height, weight, smoking at blood donation (based separately on cotinine concentrations and questionnaire information), baby’s height, weight, gender, placental weight or parity by index date (cancer diagnosis)] altered risk estimates substantially (all < 8 % change) and were not included in the final models.
Discussion
The current study confirms our previous observation that elevated IGF-I during early pregnancy is associated with increased risk of maternal breast cancer. Women with IGF-I concentrations in the top tertile had 73% higher risk in comparison with women with IGF-I in the bottom tertile. A number of epidemiological studies in non-pregnant women have also shown a direct association of breast cancer with IGF-I [2], believed to be mediated by the strong mitogenic and antiapoptotic effects of the hormone on both normal breast epithelial and tumor cells [11].
The major strengths of this study are its prospective design, important to minimize reverse-causation bias, and the inclusion of a fairly large number of very young women, typically not enrolled in most on-going cohorts. A unique feature of the current investigation is that it included women who donated blood during the first part of the full-term pregnancy ending with the birth of their first child and thus allowed to explore the effect of IGF-I exposure prior to maturation and differentiation of the glands induced by a pregnancy completed to term. Cases and controls were tightly matched for age and date at blood donation and detailed data on additional important covariates were obtained through linkages with the high quality national Swedish registries and by retrieval of individual medical records following a standardized procedure. Thus, we were able to fully account for the effect of parity, age at first pregnancy and pregnancy duration on study results. The high quality and reliability of the retrieved information was confirmed by the excellent correspondence between recorded data on maternal smoking and cotinine measurements in serum.
Study limitations include the long-term storage of the serum specimens at relatively high temperature (−20 °C) and the change in protocol for sample preservation which affected the quality of the hormonal measurements and led to the exclusion of potentially eligible cases, who donated blood after January 1, 1988. However, in samples donated before 1988, storage time was not associated with IGF-I concentrations (r= − 0.04). Mean IGF-I concentrations of the 58 cases, part of our previous report [1], which were measured by a different radio-immuno assay, were very similar (140.6 vs. 136.6 ug/L, p = 0.53). As per study design, the vast majority of the cases (76%) were diagnosed before age 50, thus our findings concern mostly breast cancer occurring before menopause. It is known that the prevalence of important characteristics of breast cancer (e.g. estrogen, progesterone, or HER2 receptor expression) and risk factors, including pregnancy, differ by age (menopausal status) at diagnosis [12, 13]. Finally, a fair amount of sub-group analyses were conducted and these results should be considered merely as explorative.
The importance of IGF-signaling in mammary gland development has been clearly demonstrated in a wealth of experimental work, showing that normal development of the gland during puberty is possible only in the combined presence of IGF-I and estradiol [14–17]. IGF-I stimulates directly the development of terminal end buds (the undifferentiated structural elements which represent the active growth centers of mammary parenchyma), ductal growth and branching [16, 18]. The role of IGF-I during pregnancy is less studied, although IGF-I has been shown to be essential for the initial lobular-alveolar changes in the glands and for posttranscriptional regulation of milk protein expression during alveolar development [16, 17].
Circulating IGF-I during the first half of the pregnancy is fairly constant and very similar to the concentrations measured before the index pregnancy or in age-matched non-pregnant women [6, 7, 19]. During that period IGF-I synthesis is mostly under the control of pituitary GH [20, 21] and could be considered as representative of non-pregnant exposure. A direct association of breast cancer with elevated IGF-I in this population of young women, and particularly in those below age 25 is in line with the hypothesis postulating that immature glands, that have not yet undergone a differentiation cycle associated with completion of a pregnancy, are most vulnerable to the mitogenic and antiapoptotic hormonal effects. This parallels observations of stronger adverse effect of radiation before age 20–25 than later in life [22, 23] or of the greater potency of carcinogens to induce tumors in young versus age matched-parous or older animals [24, 25]. The well-established association of breast cancer risk with height, a correlate of GH/IGF-I exposure during puberty, is also believed to be largely mediated by IGF-I and to reflect effects of the hormone on the still undifferentiated breast [4].
In this study we also observed a somewhat stronger effects of IGF-I in women with early diagnosis, with relatively short lag-time between blood donation and cancer manifestation, and, in women above age 30 at sampling. This risk pattern is reminiscent of the so called ‘transient’ increase in risk following a childbirth, which in uniparous women peaks about 5 to 7 years after pregnancy and trails for at least 10–15 years after childbirth [26]. One of the major mechanisms believed to underlie the transient increase in breast cancer is the massive hormonal exposure during pregnancy that would stimulate the growth and progression of cells that have undergone early stages of malignant transformation to overt cancers [27]. IGF-I involvement is in the ‘transient increase in risk’ is plausible, given its strong mitogenic and antiapoptotic effects. In addition, experiments with IGF-transgenic mice have shown that IGF-I induced decrease in apoptosis interferes in the normal post-partum remodelling of the gland [28, 29]. Increased IGF-I signalling during breast involution, a process associated with activation of pro-inflammatory and wound healing mechanisms [30], may facilitate tumor cell dissemination and also could influence risk of developing breast cancer. Finally, high exposure to elevated IGF-I particularly during pregnancy, on the background of high estrogens and progesterone, could also be important in disease pathogenesis.
In summary, the results of this study add further evidence for an adverse effect of elevated IGF-I concentrations during early reproductive life on risk of breast cancer. Further research into the role of IGF-I on breast development and maturation during pregnancy and in post-partum involution of the glands in humans is of interest.
Acknowledgments
This work was supported by the US National Cancer Institute [CA114329 to P.T., and CA120061 to A.L.]. The authors are indebted to Yelena Afanasyeva, Anne Marie Ahren, Soren Holmgren, Ritu Andersson and Lena Selbrand for their excellent technical assistance in the conduct of the study.
References
- 1.Lukanova A, Toniolo P, Zeleniuch-Jacquotte A, et al. Insulin-like growth factor I in pregnancy and maternal risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2489–2493. doi: 10.1158/1055-9965.EPI-06-0625. [DOI] [PubMed] [Google Scholar]
- 2.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 3.Fletcher O, Gibson L, Johnson N, et al. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14:2–19. [PubMed] [Google Scholar]
- 4.Lukanova A, Andersson R, Wulff M, et al. Human chorionic gonadotropin and alpha-fetoprotein concentrations in pregnancy and maternal risk of breast cancer: a nested case-control study. Am J Epidemiol. 2008;168:1284–1291. doi: 10.1093/aje/kwn254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cnattingius S, Ericson A, Gunnarskog J, Kallen B. A quality study of a medical birth registry. Scand J Soc Med. 1990;18:143–148. doi: 10.1177/140349489001800209. [DOI] [PubMed] [Google Scholar]
- 6.Monaghan JM, Godber IM, Lawson N, et al. Longitudinal changes of insulin-like growth factors and their binding proteins throughout normal pregnancy. Ann Clin Biochem. 2004;41:220–226. doi: 10.1258/000456304323019596. [DOI] [PubMed] [Google Scholar]
- 7.Black AJ, Topping J, Durham B, Farquharson RG, Fraser WD. A detailed assessment of alterations in bone turnover, calcium homeostasis, and bone density in normal pregnancy. J Bone Miner Res. 2000;15:557–563. doi: 10.1359/jbmr.2000.15.3.557. [DOI] [PubMed] [Google Scholar]
- 8.Richardson BE, Hulka BS, Peck JL, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998;148:719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 9.Cleveland WS, Loader C. Smoothing by local regression: priciples and methods. In: Schimek MG, editor. Statistical Theory and Computational Aspects of Smoothing. 1. Springer; New York: 1996. pp. 113–120. [Google Scholar]
- 10.Whitehead A, Whitehead J. A general parametric approach to the meta-analysis of randomized clinical trials. Stat Med. 1991;10:1665–1677. doi: 10.1002/sim.4780101105. [DOI] [PubMed] [Google Scholar]
- 11.Pollak MN. Endocrine effects of IGF-I on normal and transformed breast epithelial cells: potential relevance to strategies for breast cancer treatment and prevention. Breast Cancer Res Treat. 1998;47:209–217. doi: 10.1023/a:1005950916707. [DOI] [PubMed] [Google Scholar]
- 12.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clavel-Chapelon F. Differential effects of reproductive factors on the risk of pre- and postmenopausal breast cancer. Results from a large cohort of French women. Br J Cancer. 2002;86:723–727. doi: 10.1038/sj.bjc.6600124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones RA, Moorehead RA. The impact of transgenic IGF-IR overexpression on mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia. 2008;13:407–413. doi: 10.1007/s10911-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 15.Rowzee AM, Lazzarino DA, Rota L, Sun Z, Wood TL. IGF ligand and receptor regulation of mammary development. J Mammary Gland Biol Neoplasia. 2008;13:361–370. doi: 10.1007/s10911-008-9102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kleinberg DL, Wood TL, Furth PA, Lee AV. Growth hormone and insulin-like growth factor-I in the transition from normal mammary development to preneoplastic mammary lesions. Endocr Rev. 2009;30:51–74. doi: 10.1210/er.2008-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loladze AV, Stull MA, Rowzee AM, et al. Epithelial-specific and stage-specific functions of insulin-like growth factor-I during postnatal mammary development. Endocrinology. 2006;147:5412–5423. doi: 10.1210/en.2006-0427. [DOI] [PubMed] [Google Scholar]
- 18.Bonnette SG, Hadsell DL. Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology. 2001;142:4937–4945. doi: 10.1210/endo.142.11.8500. [DOI] [PubMed] [Google Scholar]
- 19.Hall K, Enberg G, Hellem E, et al. Somatomedin levels in pregnancy: longitudinal study in healthy subjects and patients with growth hormone deficiency. J Clin Endocrinol Metab. 1984;59:587–594. doi: 10.1210/jcem-59-4-587. [DOI] [PubMed] [Google Scholar]
- 20.Lacroix MC, Guibourdenche J, Frendo JL, Muller F, Evain-Brion D. Human placental growth hormone--a review. Placenta. 2002;23(Suppl A):S87–S94. doi: 10.1053/plac.2002.0811. [DOI] [PubMed] [Google Scholar]
- 21.Eriksson L, Frankenne F, Eden S, Hennen G, von Schoultz B. Growth hormone 24-h serum profiles during pregnancy--lack of pulsatility for the secretion of the placental variant. Br J Obstet Gynaecol. 1989;96:949–953. doi: 10.1111/j.1471-0528.1989.tb03352.x. [DOI] [PubMed] [Google Scholar]
- 22.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin’s disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 23.Ronckers CM, Erdmann CA, Land CE. Radiation and breast cancer: a review of current evidence. Breast Cancer Res. 2005;7:21–32. doi: 10.1186/bcr970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grubbs CJ, Peckham JC, Cato KD. Mammary carcinogenesis in rats in relation to age at time of N-nitroso-N-methylurea administration. J Natl Cancer Inst. 1983;70:209–212. [PubMed] [Google Scholar]
- 25.Russo IH, Russo J. Role of hormones in mammary cancer initiation and progression. J Mammary Gland Biol Neoplasia. 1998;3:49–61. doi: 10.1023/a:1018770218022. [DOI] [PubMed] [Google Scholar]
- 26.Liu Q, Wuu J, Lambe M, Hsieh SF, Ekbom A, Hsieh CC. Transient increase in breast cancer risk after giving birth: postpartum period with the highest risk (Sweden) Cancer Cause Control. 2002;13:299–305. doi: 10.1023/a:1015287208222. [DOI] [PubMed] [Google Scholar]
- 27.Pathak DR. Dual effect of first full term pregnancy on breast cancer risk: empirical evidence and postulated underlying biology. Cancer Cause Control. 2002;13:295–298. doi: 10.1023/a:1015282916368. [DOI] [PubMed] [Google Scholar]
- 28.Wood TL, Richert MM, Stull MA, Allar MA. The insulin-like growth factors (IGFs) and IGF binding proteins in postnatal development of murine mammary glands. J Mammary Gland Biol Neoplasia. 2000;5:31–42. doi: 10.1023/a:1009511131541. [DOI] [PubMed] [Google Scholar]
- 29.Neuenschwander S, Schwartz A, Wood TL, Roberts CT, Jr, Hennighausen L, Leroith D. Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J Clin Invest. 1996;97:2225–2232. doi: 10.1172/JCI118663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat Rev Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]