Abstract
Validity of biobank studies on hormone associated cancers depend on the extent the sample preservation is affecting the hormone measurements. We investigated the effect of long-term storage (up to 22 years) on immunoassay measurements of three groups of hormones and associated proteins: sex-steroids [estradiol, progesterone, testosterone, dihydroepiandrosterone sulphate (DHEAS), sex hormone-binding globulin (SHBG)], pregnancy-specific hormones [human chorionic gonadotropin (hCG), placental growth hormone (pGH), alpha-fetoprotein (AFP)], and insulin-like growth factor (IGF) family hormones exploiting the world largest serum bank, the Finnish Maternity Cohort (FMC). Hormones of interest were analyzed in a random sample of 154 Finnish women in the median age (29.5 years, range 25 to 34 years) of their first pregnancy with serum samples drawn during the first trimester. All hormone measurements were performed using commercial enzyme-linked- or radio-immunoassays. Storage time did not correlate with serum levels of testosterone, DHEAS, hCG, pGH and total IGFBP-1. It had a weak or moderate negative correlation with serum levels of progesterone (Spearman’s ranked correlation coefficient (rs)=− 0.36), IGF-I (rs=−0.23) and IGF binding protein (BP)-3 (rs=−0.38), and weak positive correlation with estradiol (rs=0.23), SHBG (rs=0.16), AFP (rs=0.20) and non-phosphorylated IGF binding protein (BP)-1 (rs=0.27). The variation of all hormone levels studied followed the kinetics reported for early pregnancy. Bench-lag time (the time between sample collection and freezing for storage) did not materially affect the serum hormone levels. In conclusion, the stored FMC serum samples can be used to study hormone-disease associations, but close matching for storage time and gestational day are necessary design components of all related biobank studies.
Introduction
Biological samples collected from large population based cohorts before the diagnosis of a chronic disease allow accumulation of sizeable numbers of diseased cases nested in the cohort. Analysis of the cases’ samples and those of appropriately matched controls can provide essential information about etiopathogenesis of the disease [1], [2]. In the Nordic countries there are several exceptionally large and long-standing cohorts with biological samples stored at ≤−20°C [3], [4]. Although these biobanks are a unique resource, long-term storage of the samples may deteriorate quality of the samples. This can result in attenuation of the biomarker-disease risk estimates and/or misclassification of exposures among the cases and the controls [5].
Pregnancy is characterized by dynamic changes in the levels of circulating steroid and protein hormones. Studies on disease associations of endogenous hormone exposure during pregnancy have, however, been hampered by unknown validity of hormone and associated protein measurements in biological samples stored for a long time. No statistically significant differences were found in distribution of IGF-I and IGFBP-3 levels between newly collected and serum samples stored at −80°C for up to 9 years [6]. Several authors have reported either stability [1], [7], [8] or some degradation over time [9–11] of estradiol and/or testosterone levels in serum samples stored at the very low or low (−20°C) temperature. On the other hand, one study reported apparently normal progesterone levels in sera stored at −20°C for up to 7 years [12], while in another study progesterone levels decreased up to 40% over 3 years of cryopreservation at −80°C [1].
The Finnish Maternity Cohort (FMC) [4] is to the best of our knowledge the world’s largest serum bank with 1470000 samples from 750000 pregnant women. In our studies on hormones and subsequent risk of cancer nested within the FMC we assume that the storage of the serum samples could have affected the hormone levels and may confound the relative risk assessment. Thus, we investigated the effect of storage on the serum levels of three groups of hormones and associated proteins: sex-steroid, pregnancy-specific hormones, and insulin-like growth factor related hormones.
Material and methods
Finnish Maternity Cohort
The FMC contains serum samples from almost all pregnant Finnish women (~98%) since 1983. Following an informed consent the blood samples collected at the municipal Maternity Care Units from women during the first trimester of pregnancy for screening of congenital infections: Treponema pallidum, human immunodeficiency virus and hepatitis B virus. At the prenatal serology laboratory of the Finnish National Public Health Institute in Oulu sera are separated by centrifugation from the blood samples. Following the screening, remaining sera with 1 to 3 mL in volume are stored unthawn in a centralized, closely monitored facility at −25°C in a polypropylene cryo-vials (Nunc GmbH&Co, Wiesbaden, Germany).
In addition, personal identification number, and data on reproductive history (number of pregnancies and deliveries), place of residence at the time of sample collection, date of sample collection at Maternity Care Units, date of sample processing in Oulu and date of expected delivery are registered in the FMC data files. The identification and access of the individuals from the FMC is not allowed by the Finnish law but according to the informed consent the samples can be used for epidemiological studies.
Study subjects
Hormones of interest were analyzed in 14 random samples collected at every other year from 1984 to 2004 of altogether 154 women during the first trimester of their first pregnancy. Mean and median ages of the women at sample withdrawal were 29.2 and 29.5 years (range 25 to 34 years).
Gestational day (GD) was defined as the time between the first day of the last menstruation and sample withdrawal of the subjects, and calculated using the formula: 280 – (date of expected delivery – date of blood sampling at Maternity Care Units). The mean GD for the study population was 77.7±26.7 days (range 42 to 222 days).
Bench-lag time (BLT) was defined as the time between sample collection and freezing for storage. The mean BLT for the study population was 3.5±2.2 days (range 1 to 14 days). Forty one percent of the samples had a BLT 1 to 2 days, 35% a BLT 3 to 4 days and 24% a BLT greater than 4 days.
Sample collection and handling
All the 154 previously unthawn serum samples included for this study were retrieved from the FMC storage for the first time. No hemolysis or visual signs of freeze-drying were observed in any sample. A total volume of 370 μL was aliquoted from each sample and sent to the Department of Medical Bioscience, University of Umeå, Sweden for the hormone and protein measurements.
Laboratory methods
Quantification of serum hormones and associated binding-proteins were performed in the same serial of analyses using commercial competitive or non-competitive enzyme-linked or radio-immunoassays at the Clinical Chemistry Laboratory, Department of Medical Biosciences, Umeå University, Umeå, Sweden. Specifications of the hormone assays and intra- and inter-assay coefficients of variation (CV) obtained from the manufacturers are given in Table I.
Table 1.
Assay methods used.
| Range of coefficient of variation (%) | |||
|---|---|---|---|
| Analyte | Method | Intra assay | Inter assay |
| Estradiol | ALPCO Diagnostics Estradiol EIA | 7.5–12.2 | 8.2–12.1 |
| Progesterone | R&D Systems’ Progesterone EIA | 4.9–7.6 | 2.7–8.3 |
| Testosterone | DPC IMMULITE 2000 Total testosterone Chemiluminescent EIA | 5.1–16.3 | 7.2–24.3 |
| DHEAS | DPC IMMULITE 2000 DHEA-S04 Chemiluminescent EIA | 4.9–9.8 | 7.9–13 |
| SHBG | DPC IMMULITE 2000 SHBG Chemiluminescent EIA | 0.03–4.2 | 4.0–6.6 |
| hCG | DPC hCG Coat-A-Count IRMA | 2.6–5.8 | 4.6–7.0 |
| AFP | DPC IMMULITE 2000 AFP Chemiluminescent ELA | 2.1–6.3 | 4.5–12 |
| hPGH | BIOCODE-HYCEL hPGH IRMA | 3.0–5.5 | 5.0–7.9 |
| IGF-1 | DSL IGF-1 ELISA (DSL-10-2800) | 4.5–8.6 | 3.3–6.8 |
| IGFBP-3 | DSL IGFBP-3 ELISA (DSL-10-6600) | 7.3–9.6 | 8.2–11.4 |
| total IGFBP-1 | DSL Total IGFBP-1 ELISA (DSL-10-7800) | 1.7–4.6 | 6.2–7.6 |
| np IGFBP-1 | Medix Biochemica IGFBP-1 IEMA | 4.4–5.4 | 4.4–5.9 |
Note: EIA-enzyme immunoassay; IRMA-immunoradiometric assay; ELISA-enzyme-linked immunosorbent assay; IEMA-immunoenzymetric assay.
Statistical methods
All measured hormone and other protein levels were ln-transformed to reduce departures from the normal distribution. Descriptive statistics were calculated for each variable separately. Box-plots were drawn for the hormone levels and storage time. Spearman’s partial ranked correlation coefficient (rs) was used to test for correlation between the serum hormone levels and 1) serum storage time, 2) BLT or 3) GD at serum sampling. Multivariate regression analysis was used to evaluate on continuous scale the effect of BLT on hormone levels. Additionally, a nonparametric local regression model with smoothing parameters set from 0.1 to 1 was fitted to describe the effect of GD on variation of hormone levels. The best smoothing parameter was chosen for every hormone according to the smallest AICc1 statistics for all the smoothing parameters that were specified in the model statement. Analyses were done by using (GLM), (CORR) and (LOESS) procedures of the SAS program version 9.1.
Results
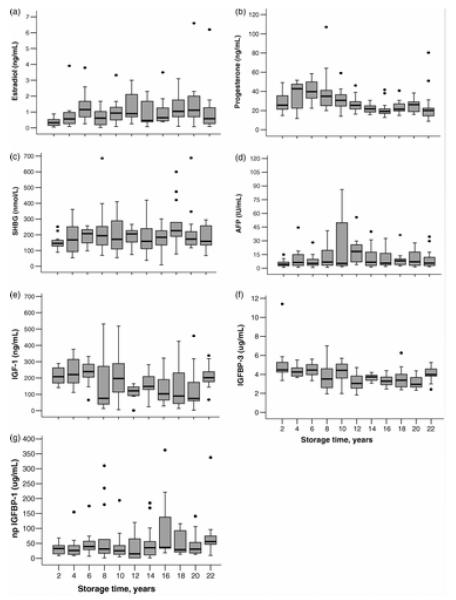
When comparing to the reference serum levels of pregnant women, the studied hormone and associated protein levels remained within clinically normal range with the exception of relatively low estradiol levels. There is no consistent pattern in that some hormones increase whereas others decrease with storage time (Figure 1). However, Spearman’s ranked correlation analysis with the effects of BLT, GD and maternal age controlled, showed a decrease by storage time for serum levels of progesterone (rs=−0.36), IGF-I (rs=−0.23) and IGFBP-3 (rs=−0.38), whereas an increase by storage time was found for estradiol (rs=0.23), SHBG (rs=0.16), and AFP (rs=0.20) levels, and serum levels of non-phosphorylated (np) IGF binding protein (BP)-1 (rs=0.27). No correlation was observed between the storage time and serum levels of testosterone, DHEAS, hCG, pGH and total IGFBP-1 (data not shown).
Figure 1.
Variation of hormone levels by storage time in FMC biobank samples. a) estradiol (p <0.006)*, b) progesterone (p<0.0001), c) SHBG (p<0.04), d) AFP (p<0.017), e) IGF-1 (p<0.007), f) IGFBP-3 (p<0.0001), g) np IGFBP-1 (p<0.002). *P-values (Spearman’s ranked correlation analysis).
Levels of np IGFBP-1, but no other serum hormone and binding-protein levels, decreased with increasing length of the BLT on a continuous scale in the GLM analysis (data not shown). The Spearman’s ranked correlation did not show statistically significant correlations between BLT and serum hormone levels including that of np IGFBP-1.
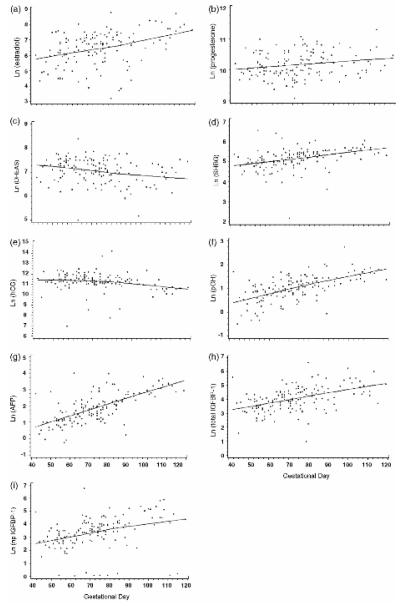
Samples from individuals with GD between 40 and 120 days (145 women) were used for the analysis of the impact of GD. The regression model analysis revealed that the hormone levels by GD followed the dynamics of the pregnancy (Figure 2). With the effect of BLT and maternal age controlled, serum levels of estradiol (rs=0.42), progesterone (rs=0.21), SHBG (rs=0.54), pGH (rs=0.66), AFP (rs=0.7), total IGFBP-1 (rs=0.54), np IGFBP-1 (rs=0.49) increased with increasing GD, while levels of DHEAS (rs=−0.25) and hCG (rs=−0.37) levels decreased with increasing GD.
Figure 2.
Variation of hormone levels by gestational day during early pregnancy in FMC biobank samples.*,† a) estradiol (p<0.0001)‡, b) progesterone (p<0.01), c) DHEAS (p<0.002), d) SHBG (p<0.0001), e) hCG (p<0.0001), f) pGH (p<0.0001), g) AFP (p<0.0001), h) total IGFBP-1 (p<0.0001), i) np IGFBP-1 (p<0.0001). *Hormone levels presented on logarithmic scale. † Figures fitted using the local regression model. ‡P-values (Spearman’s ranked correlation analysis).
Discussion
This study was conducted to investigate the impact of long-term storage of serum samples obtained from pregnant women on the measurement of three groups of hormones and associated proteins: sex-steroid, pregnancy-specific hormones, and insulin-like growth factor related hormones. This kind of information is crucial in epidemiological studies on the association of pregnancy associated hormones and subsequent risk of cancer nested within maternity cohorts.
No consistent pattern of level variation with storage time was found for any of the hormones or associated binding-proteins. Some hormone and protein levels decreased and some increased with increasing storage time. However, the changes did not exceed the inter assay variations of the enzyme-immunological tests, and the levels remained within the clinically normal range. Some fluctuation of the hormone levels by storage time and GD could be due to individual characteristics of the pregnant women.
Overall stability of sex-steroid and other pregnancy-related hormones and associated binding-proteins or causes of their degradation in stored serum/plasma samples are not well established. Reasons for the observed variation remain speculative, and can be caused by a number of confounding factors. The decrease that has previously been reported in progesterone levels after long-term storage at −70°C was suggested to be related with molecular modification or by interference of the cryotube material used in low temperature storage [1]. On the other hand, apparently normal progesterone levels in sera stored at −20°C for up to 7 years have been reported [12]. However, comparing storage at −20°C with storage at −70°C may not be acceptable for the sex steroid hormones [11], [13]. For example, dissociation of SHBP from estradiol and testosterone over time may decrease measurable nonbound levels of these hormones [13]. On the other hand, changes in IGF-1 and IGFBP-3 levels in stored serum sample have been suggested to be due to potential post-sampling proteolysis [14]. The effect of storage time on measurement of specific hormone might vary according to the assay method. Different antibodies may bind to different parts of peptide hormones and this could affect the performance in old samples where, for example, terminal amino acids might be lost. Thus, matching for storage time, a common design component of nested case-control studies is needed also to control for the observed variations in the hormones and hormone measurements studied.
No substantial effect of the length of BLT on the sex-steroid and pregnancy-specific hormone and binding-protein levels could be found. Decreasing levels of serum npIGFBP-1 with increasing length of the BLT on a continuous scale in the GLM analysis could be due to chance. However, in nested case-control studies researchers should be aware of potential hormone measurement variability by BLT. Elevated level of hCG has been found with BLT of 2 days [15], but several studies have shown an increase in testosterone level with a delay in sample processing [16–18]. On the other hand delayed processing of up to 3 days does not seem to alter levels of estrogens and most androgens [16–20].
The variation of hormone levels by GD followed the dynamics of the pregnancy and were in agreement with what was expected based on available clinical data [21]. However, trimester-specific reference intervals for several hormones are difficult to establish due to lack of specificity in enzyme-immunoassay techniques [22]. The lack of expected drop in hCG levels by GD probably reflects the relatively early GD at serum sample withdrawal. Thus, in nested case-control studies considering pregnancy-related hormones the data on GD at sample withdrawal should be taken into account in matching.
In conclusion, the samples of the FMC, preserved for up to 22 years at −25°C can be used to study hormone-disease associations. The quality of the samples stored at the FMC is always assured by the following procedures: (a) serum is obtained by a standardized procedure and all aliquots are prepared in the same way, (b) samples are aliquoted into appropriate airtight tubes to prevent freeze-drying effect with long-term storage, (c) storage temperature is monitored regularly, (d) the time between sample collection and freezing for long-term storage is kept as shorter as possible. In the future, as a part of quality assurance of the studies based on FMC material, samples of a particular research will be validated for analyte measurements (hormones, antibodies, trace elements) to assure study quality.
Acknowledgements
We thank Annika Uimonen and Stig Karlsson for their excellent technical assistance in the conduct of the study. The study was supported by US National Institutes of Health research grants CA120061 and CA114329. There is no conflict of interest.
References
- 1.Bolelli G, Muti P, Micheli A, Sciajno R, Franceschetti F, Krogh V, et al. Validity for epidemiological studies of long-term cryoconservation of steroid and protein hormones in serum and plasma. Cancer Epidemiol Biomarkers Prev. 1995;4:509–13. [PubMed] [Google Scholar]
- 2.Doll R, Peto R. The causes of cancer: Quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–308. [PubMed] [Google Scholar]
- 3.Jellum E, Andersen A, Lund-Larsen P, Theodorsen L, Ørjasæter H. Experience of the Janus Serum Bank in Norway. Environ Health Perspect. 1995;3:85–8. doi: 10.1289/ehp.95103s385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pukkala E, Andersen A, Berglund G, Gislefoss R, Gudnason V, Hallmans G, et al. Nordic biological specimen banks as basis for studies of cancer causes and control – more than 2 million sample donors, 25 million persons years and 100000 prospective cancers. Acta Oncol. 2007;46:286–307. doi: 10.1080/02841860701203545. [DOI] [PubMed] [Google Scholar]
- 5.Landi MT, Caporaso N. Sample collection, processing and storage. IARC Sci Publ. 1997;142:223–36. [PubMed] [Google Scholar]
- 6.Ito Y, Nakachi K, Imai K, Hashimoto S, Watanabe Y, Inaba Y, et al. Stability of frozen serum levels of insulin-like growth factor-I, insulin-like growth factor-II, insulin-like growth factor binding protein-3, transforming growth factor beta, soluble Fas, and superoxide dismutase activity for the JACC study. J Epidemiol. 2005;15:S67–S73. doi: 10.2188/jea.15.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson BE, Berstein L, Ross RK, Depue RH, Judd HL. The early in utero oestrogen and testosterone environment of blacks and whites: Potential effects on male offspring. Br J Cancer. 1988;57:216–8. doi: 10.1038/bjc.1988.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garland FC, Friedlander NJ, Barrett-Connor E, Khaw KT. Sex hormones and postmenopausal breast cancer: A prospective study in an adult community. Am J Epidemiol. 1992;135:1220–30. doi: 10.1093/oxfordjournals.aje.a116228. [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, Gutai JP, Kuller LH, Dai WS. Usefulness of steroid hormone levels in predicting coronary artery disease in men. Am J Cardiol. 1987;60:771–7. doi: 10.1016/0002-9149(87)91021-6. [DOI] [PubMed] [Google Scholar]
- 10.Phillips GB, Yano K, Stemmermann GN. Decrease in serum estradiol values with storage. N Engl J Med. 1984;311:1635. doi: 10.1056/NEJM198412203112514. [DOI] [PubMed] [Google Scholar]
- 11.Phillips GB, Yano K, Stemmermann GN. Serum sex hormone levels and myocardial infarction in the Honolulu Heart Program. Pitfalls in prospective studies on sex hormones. J Clin Epidemiol. 1988;41:1151–6. doi: 10.1016/0895-4356(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 12.Key TJ, Pike MC, Baron JA, Moore JW, Wang DY, Thomas BS, et al. Cigarette smoking and steroids hormones in women. J Steroid Biochem Mol Biol. 1991;4A:529–34. doi: 10.1016/0960-0760(91)90247-3. [DOI] [PubMed] [Google Scholar]
- 13.Langley MS, Hammond GL, Bardsley A, Sellwood RA, Anderson DC. Serum steroid binding proteins and the bioavailability of estradiol in relation to breast disease. J Natl Cancer Inst. 1985;75:823–9. doi: 10.1093/jnci/75.5.823. [DOI] [PubMed] [Google Scholar]
- 14.Khosravi J, Diamandi A, Bodani U, Khaja N, Krishna RG. Pitfalls of immunoassay and sample for IGF-I: Comparison of different assay methodologies using various fresh and stored serum samples. Clin Biochem. 2005;38:659–66. doi: 10.1016/j.clinbiochem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Masse J, Summers A, Cherian G, Forest JC. Transportation of maternal serum specimens for screening for chromosomal aneuploidies: Effect of seasonal variation, distance, and freezing on the stability of the biological markers. Clin Biochem. 2000;33:273–7. doi: 10.1016/s0009-9120(00)00076-x. [DOI] [PubMed] [Google Scholar]
- 16.Kristal AR, King IB, Albanes D, Pollak MN, Stanzyk FZ, Santella RM, et al. Centralized blood processing for the selenium and vitamin E cancer prevention trial: Effects of delayed processing on carotenoids, tocopherols, insulin-like growth factor-I, insulin-like growth factor binding protein 3, steroid hormones, and lymphocyteviability. Cancer Epidemiol Biomarkers Prev. 2005;14:727–30. doi: 10.1158/1055-9965.EPI-04-0596. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson SE, London SJ, Chute CG, Barbieri RL, Jones L, Kaplan LA, et al. Effect of transport conditions on the stability of biochemical markers in blood. Clin Chem. 1989;35:2313–6. [PubMed] [Google Scholar]
- 18.Key T, Oakes S, Davey G, Moore J, Edmond LM, McLoone UJ, et al. Stability of vitamins A, C, E, carotenoids, lipids, and testosterone in whole blood stored at 4 degrees C for 6 and 24 hours before separation of serum and plasma. Cancer Epidemiol Biomarkers Prev. 1996;5:811–4. [PubMed] [Google Scholar]
- 19.Taieb J, Benattar C, Birr AS, Lindenbaum A, Frydman R, Olivennes F. Delayed assessment of serum and whole blood estradiol, progesterone, follicle-stimulating hormone, and luteinizing hormone kept at room temperature or refrigerated. Fertil Steril. 2000;74:1053–4. doi: 10.1016/s0015-0282(00)01546-6. [DOI] [PubMed] [Google Scholar]
- 20.Ellis MJ, Livesey JH, Evans MJ. Hormone stability in human whole blood. Clin Biochem. 2003;36:109–12. doi: 10.1016/s0009-9120(02)00440-x. [DOI] [PubMed] [Google Scholar]
- 21.Speroff L, Glass RH, Kase NG. Clinical gynecologic endocrinology and infertility. 5th ed Lippincott Williams & Wilkins, Baltimore; Maryland: 1994. [Google Scholar]
- 22.Soldin OP, Guo T, Weiderpass E, Tractenberg RE, Hilakivi-Clarke L, Soldin SJ. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Am S Reprod Med. 2005;84:701–10. doi: 10.1016/j.fertnstert.2005.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]