Abstract
Mechanisms for receptor-mediated anthrax toxin internalization and delivery to the cytosol are well understood. However, far less is known about the fate followed by anthrax toxin receptors prior and after cell exposure to the toxin. We report that Anthrax Toxin Receptor 1/Tumor Endothelial Marker 8 (TEM8) localized at steady state in Rab11a-positive and transferrin receptor-containing recycling endosomes. TEM8 followed a slow constitutive recycling route of ∼30 minutes as determined by pulsed surface biotinylation and chase experiments. A Rab11a dominant negative mutant and Myosin Vb tail expression impaired TEM8 recycling by sequestering TEM8 in intracellular compartments. Sequestration of TEM8 in intracellular compartments with monensin coincided with increased TEM8 association with a multi-protein complex isolated with antibodies against transferrin receptor. Addition of the cell-binding component of anthrax toxin, Protective Antigen, reduced TEM8 half-life from seven to three hours, without preventing receptor recycling. Pharmacological and molecular perturbation of recycling endosome function using monensin, dominant negative Rab11a, or myosin Vb tail, reduced PA-binding efficiency and TEM8-dependent cell spreading on PA-coated surfaces without affecting toxin delivery to the cytosol. These results indicate that the intracellular fate of TEM8 differentially affect its cell adhesion and cell intoxication functions.
Keywords: TEM8, anthrax toxin, Rab11, Transferrin Receptor, cell spreading
Introduction
Anthrax Toxin Receptor 1/Tumor Endothelial Marker 8 (ANTXR1/TEM8) mediates cell internalization of the Bacillus anthracis toxin. This toxin comprises the cell-binding component Protective Antigen (PA), and two enzymes, the metalloprotease Lethal Factor (LF) and/or an adenylate cyclase Edema Factor (EF). Cell surface receptors bind the toxin and re-localize to lipid-rafts where they undergo endocytosis via a mechanism dependent on clathrin-coated vesicles and on TEM8 ubiquitination by the E3 ligase Cbl [1]. Toxin-receptor complexes are transported to the early endosome, where the acidic pH causes the toxin complex to dissociate from the receptor [1, 2]. PA dissociates as an heptamer, which inserts into the endosomal membrane to form a pore, facilitating EF and LF passage into the cytosol (reviewed in [3]). Once in the cytosol, these enzymatic components increase cAMP levels and trigger proteolytic cleavage of the amino terminus of several MAPK kinases. These later events are essential effectors of anthrax pathophysiology [4-6].
In contrast to the detailed studies of the trafficking pathway followed by receptor-toxin complexes, little is known about the fate that TEM8 follows in the absence of the toxin or after toxin dissociates from the receptor in endosomes. TEM8 could be targeted for degradation in lysosomes or could recycle back to the surface. Presumably, recycling mechanisms would regulate the availability of receptors for toxin binding at the cell surface, modulating toxicity as well as other still largely unexplored toxin-independent physiological activities of this cell surface molecule.
TEM8 functions independent of toxin biology have been greatly illuminated by the structure of this receptor as well as by cell and animal studies, which suggest a role of TEM8 in cell-matrix interactions. TEM8's extracellular domain comprises a Von Willebrand type A domain including a MIDAS motif, structurally and functionally homologous to the I domain of several integrins. This domain mediates binding to PA [7]. We have previously described that TEM8 homology to adhesion molecules in the extracellular domain further extends to the cytoplasmic domain. TEM8 cytosolic domain engages the actin cytoskeleton and is necessary for receptor mediated cell spreading when a ligand (PA) is presented to cells as a surface-coating substrate [8]. Consistent with a role in cell-matrix interactions, TEM8-null mice exhibit the phenotype of extracellular matrix accumulation in several tissues [9]. TEM8 putative function in cell adhesion to matrices is of potential relevance for tumor-associated endothelial cells, which uniquely express high levels of TEM8 [9, 10]. For this reason, TEM8 is currently considered as a candidate molecule for selective delivery of antitumoral agents to tumor vasculature [11]. Thus, regulation of TEM8 cell-surface levels by receptor delivery to or retrieval from the plasma membrane could modulate TEM8 toxin-independent functions, such as cell adhesion and/or metabolism of extracellular matrices.
In the current work, we found a pool of TEM8 receptors that resides constitutively in the endocytic pathway. This intracellular TEM8 pool is in equilibrium with receptors residing at the cell surface. Endosomal TEM8 trafficked through recycling endosomes via a Rab11a- and myosin Vb-dependent mechanism, similar to transferrin receptor. Perturbation of this TEM8 recycling mechanism selectively impaired receptor-mediated cell spreading on surfaces coated with TEM8 ligands, without affecting anthrax toxin-dependent cell intoxication. Our findings indicate that TEM8 cell adhesion and cell intoxication functions are differentially regulated by the endocytic fate followed by the receptor.
Materials and Methods
Reagents and antibodies
Rat anti HA (Roche), rabbit polyclonal HA (Bethyl Lab), Synaptophysin, Rab4, EGFR, N-terminus MEK (Millipore), C-terminus MEK (Santa Cruz), transferrin receptor, Rab11a (Zymed), Syntaxin 13 (Stress Gene), beta actin, (Sigma), anti FITC, 488-labeled human transferrin, Alexa-labeled secondary antibodies (Invitrogen). The Lamp1 antibody was developed by J.T. August and was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. DSP, EZ-Link Sulfo-NHS-Biotin, Neutroavidin-agarose beads (Thermo Scientific), anti-rabbit and anti-mouse secondary antibody conjugated Dynal beads (Invitrogen). Defined Quality PA, FITC-labeled 63kDa PA and Lethal Factor were purchased from List Biologicals Laboratory. HA tagged TEM8 in the pIRESHyg2 was generously provided by Dr. S. Leppla [12]. GFP tagged Rab11 and Myosin Vb tail constructs were a gift from L. Volpicelli-Daley [13].
Cell lines & Transfections
Hek293 cells (adherent cell strain) were obtained from QBiogene and cultivated in 10%FBS DME, 100U/ml penicillin and 100μg/ml streptomycin in 10%CO2. PC12 cells were cultivated in DME 10%Horse Serum, 5%FBS, 100U/ml penicillin and 100μg/ml streptomycin in 10%CO2. B16F10 melanoma cells (ATCC) were grown in DME 10%FBS, 100U/ml penicillin, 100μg/ml streptomycin, 1mMGlutamine and in 5%CO2. CT26 cells (ATCC) were grown in RPMI 10%FBS. Hek293 and PC12 cells permanently expressing TEM8 were obtained after transfection with Lipofectamine 2000 (Invitrogen) and selection with 0.1mg/ml Hygromycin and were used only until passage 10.
Immunofluorescence
Cells were plated on glass coverslips, washed with PBS-CM and fixed for 20 min with 4% PFA in PBS on ice-water bath and then processed as described before [8]. All scale bars represent 10um. To measure co-localization Images were processed and analyzed using MetaMorph software version 3.0 (Molecular Devices, Sunnyvale, CA). Each image was thresholded to similar levels and co-localization was determined as the percentage of pixel area overlap for each endosomal marker over TEM8 [14].
Subcellular fractionation and gradient separation
Intracellular organelles were fractionated as described before [15, 16]. Briefly, early passage PC12 cells permanently expressing TEM8 were homogenized using a cell-cracker. After clearing unbroken cells and large membranes by 8 min centrifugation at 1,000 × g, 500μg of S1 supernatant was loaded on a 10 to 45% sucrose gradient that was separated by centrifugation at 148,000×g for 60 min in a SW55 rotor. Alternatively, S1 supernatant was centrifuged at 27,000× g for 45 minutes to generate a P2 pellet and a S2 supernatant. The fractions collected were analyzed by western blot.
Protein cross-linking
Cells were placed on an ice-water bath and incubated with PBS-CM without or with 1mM DSP (Thermo Scientific) for 2h. After the 2h incubation, the non-reactive DSP groups were quenched by adding 1M Tris Buffer pH 7.5 to a 50mM final concentration. After 3 washes for 10 minutes with PBS-CM, the cells were lysed with 0.5% TX-100 Buffer A (10mM Hepes pH 7.4, 150mM NaCl, 1mM EGTA, 0.1mM MgCl2 [17, 18]. Protein complexes were immunoprecipitated using antibody pre-bound magnetic beads. Two hundred micrograms of protein was used for transferrin receptor co-immunoprecipitation, while 500μg of protein was used for control co-immunoprecipitations (anti-Lamp1 and no-antibody). Precipitated complexes and 10μg of lysates (input) were separated by electrophoresis and analyzed by western blot with the indicated antibodies.
Receptor half-life
Cells were labeled overnight with 100μCi/ml [S35]-methionine (MP Biomedicals) in methionine-deficient DME supplemented with 10% dialyzed FBS (Invitrogen). Labeled TEM8 and TFR were immunoprecipitated at the indicated time after the radioactive media was washed and complete media, containing cold methionine, was restituted with or without 1μg/ml PA to start the chase. Each precipitation was done in duplicate in three independent experiments. For quantification, the fluorograms were scanned to determine the relative intensity of each band using NIH Image. The intensity at time 0 received a 100%.
Surface biotinylation
To label cell surface proteins at steady state, cell monolayer was incubated with 0.5mM EZ-Link Sulfo-NHS-Biotin (Thermo Scientific) in PBS-CM in a water-ice bath for 30 minutes. Excess reagent was quenched for 10 minutes with 50mM NH4Cl prepared in PBS-CM. For chase experiments, after three washes to eliminate free biotin, the cells were either kept at 4°C, or shifted to 16°C or 37°C for the indicated time, with or without 1μg/ml PA. Cells were then returned at 4°C and incubated twice for 20 min with 50mM Glutathione, 90mM NaCl, 0.1mM CaCl2, 1mM MgCl2 adjusted with 60mM NaOH to pH7 and 1% BSA. After 3 PBS washes, cells were solubilized in 100μl RIPA Buffer, 400μl 0.1% TX-100 Buffer A supplemented with antiproteases. Samples were sonicated and 200μgr of protein were incubated with 30μl of Neutroavidin beads (Thermo) to precipitate biotin labeled proteins. To label and detect receptors exposed at the cell surface during cell spreading, biotin was added to the spreading media, which was HBSS supplemented with 0.2% glucose and 1mM MnCl2. After 1 h at 37°C to allow for cell spreading, non-reacted biotin was quenched and washed with PBS-CM. Biotinylated proteins were separated as before.
PA binding assay
One hundred thousand control or TEM8 expressing Hek293 cells were seeded on 24 well plates. Triplicates were incubated for 1h at 4°C or at 22°C with 0.5μg/ml FITC labeled 63kDa PA (total binding). Some wells had 10μg/ml PA added to determine unspecific binding. After 3 washes with 1mM MgCl2, 0.1mM CaCl2 Hank's, cells were lysed in Buffer A and fluorescence was determined using a Synergy HT plate reader (BioTek). Specific binding was determined by subtracting unspecific binding to total binding values. Cell number was corrected after measuring protein concentration in each well.
MAPKK degradation and spreading on PA and Matrigel
Lethal toxin dependent N-terminus cleavage of MAPKK and cell spreading assays were performed essentially as before [8]. However, to measure spreading area and simultaneously score cells for GFP expression level, the actin cytoskeleton was stained with Rhodamine conjugated Phalloidin (Invitrogen). For quantification, spreading area was measured using Metamorph (Molecular Devices) and each cell in images taken at random, was cataloged as no GFP-expressing or GFP positive when showing a defined GFP-positive perinuclear compartment but no cytosolic staining. A box graph was generated using Kaleidagraph Software.
RT-PCR
Endogenous TEM8, TEM8 variant 1, CMG2 and GAPDH were amplified from cDNA prepared from B16F10 and CT-26 cells using the following primers: mouse GAPDH 5′aactttggcattgtggaagg3′ and 5′acacattgggggtaggaaca3′, human and mouse TEM8 5′ctgcaccactggaatgaaatc3′ and 5′tgtctcctcctggcagaactt3′, TEM8 variant 1 5′gagaaaagggctccacaga3″ and 5′acccacaaggcatcgagttttc3′ human- and mouse CMG2 5′ctttcattgtgttttcttctcaagcaac3′ and 5′gttttcaagcctcctgctttctgaat3′. TEM8 oligos amplified a product when cDNA from TEM8 transfected Hek293 cells was used as a template. No bands were amplified by TEM8 or CMG2 oligos when using cDNA from non-transfected HEK 293 cells or when reverse transcriptase was omitted (not shown). The identity of the amplified product was confirmed by sequencing.
Statistical analysis
Samples were compared and significance was determined using a t-test assuming a two tailed distribution of samples of unequal variance.
Results
A pool of TEM8 resides in intracellular compartments
Previously published work localizes TEM8 at the plasma membrane. TEM8 endosomal localization has been regarded as a toxin-induced event triggered after binding to this receptor [19]. Our current studies indicate that at steady state and in the absence of toxin, a pool of TEM8 resides in intracellular vesicular compartments. Our studies use expressed human HA-tagged full length TEM8 (isoform or variant 1) due to the lack of antibodies to reliably detect low levels of endogenous TEM8 in cultured cells. The presence of TEM8 in intracellular compartments is unlikely to be an artifact of overexpression as we observed intracellular pools irrespective of the expression level of transfected TEM8. Moreover, this observation was recapitulated in diverse cell types, including primary cultured cells such as human microvascular endothelial cells and mouse skin fibroblasts (not shown), as well as transformed cells lines, including PC12 and Hek293 cells (Figs. 1 and 2).
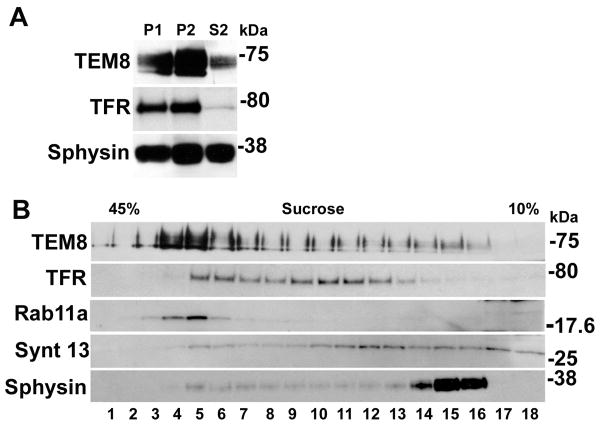
Figure 1.
TEM8 distributes in intracellular compartments. A. PC12 cell homogenates were fractionated by differential centrifugation and fractions analyzed by Western Blot. Subcellular fractions P1 (whole cells and plasma membrane), P2 (organelles and light membranes) and S2 (small vesicles and cytosol) were characterized with antibodies against transferrin receptor (TFR), a marker of recycling endosomes and synaptophysin (sphysin), a marker of small vesicles. B PC12 cell low speed supernatants (S1) were sedimented in sucrose velocity gradients and fractions analyzed by Western Blot with antibodies against TEM8, and the recycling endosome markers TFR, Rab11a, syntaxin13 (Synt 13). Heavier fractions are depicted to the left of the gradient (1 of 2 experiments is shown).
Figure 2.
TEM8 co-localizes with endosome markers and associates to transferrin receptor-containing protein complexes. A Immunofluorescence staining for TEM8 (HA) demonstrating co-localization with Alexa 488 labeled human transferrin (TF), with TFR- and Rab11a- but not Rab4-positive intracellular compartments. To label intracellular compartments with transferrin, serum deprived Hek298 cells were incubated with 50 μg/ml Alexa 488-labeled human transferrin for 1h prior to fixation. Size bar is 10μm. B Quantification of co-localization of the endosomal markers TF, TFR, Rab11 and Rab4 with TEM8. The number of analyzed cells is indicated at the bottom of the bar. P value was determined with a t-test C Detection of TEM8-associated to multiprotein complexes containing transferrin receptor after chemical cross-linking using DSP in TEM8 expressing Hek293 cells. Cells were treated in the absence (odd lanes) or presence of DSP (even lanes). Detergent extracts were immunoprecipitated with magnetic beads alone (lanes 1-2) or beads decorated with TFR antibodies (lanes 3-4). Immune complexes were analyzed by immunoblot to detect TEM8, TFR and actin. Inputs represent 5%. 1 of 8 independent experiments is shown. D Co-immunoprecipitation of TEM8 with transferrin receptor after chemical cross-linking in Hek293 cells treated with vehicle or with 2μM monensin for the indicated time. The experimental design is similar to C, except that cells were treated in the absence or presence of monensin (2μM) at 37°C before incubation with DMSO vehicle control (DSP- lanes) or DSP (DSP + lanes). 1 of 3 experiments is shown.
To identify the compartments where TEM8 resides, we performed subcellular fractionation. We expressed HA-tagged human TEM8 in PC12 cells for which we have developed a well-characterized procedure for organelle fractionation [15, 16, 20]. TEM8 expression in this cell type conferred sensitivity to anthrax toxin measured by N-terminus cleavage of MAPKK (not shown), thus confirming that expressed TEM8 was competent for receptor mediated toxin internalization and delivery to the cytosol in this cell type. At steady state, TEM8 cofractionated with transferrin receptor (TFR), in a P2 membrane fraction, which is essentially devoid of plasma membrane and endoplasmic reticulum. These later membranes remain in P1 pellets [15, 16, 20]. The distribution of TEM8 in P2 fractions was further analyzed by sucrose velocity sedimentation of low-speed S1 supernatant, which comprises the contents present on P2 fractions plus small microvesicles and cytosolic components contained in S2 [15, 20, 21]. TEM8-positive organelles distributed along the sucrose gradient with a prominent peak that overlaps with markers of recycling endosomes, predominantly Rab11a and transferrin receptor (TFR), and to a lesser extent, syntaxin 13 a marker of early and recycling endosomes (Fig. 1B, fractions 4-6) [22]. A small pool of TEM8 minimally co-migrated with synaptophysin, a marker for microvesicles in PC12 cells (Fig. 1B, fractions 14 to 16). Thus, these results suggest that in PC12 cells, TEM8 resides constitutively in endosomes.
TEM8 coexists with TFR and Rab11 in recycling endosomes
TEM8 co-sedimentation with TFR and Rab11-positive organelles in sucrose gradients predicts TEM8 localization in TFR and Rab11-positive organelles. To test this hypothesis, we performed double-labeling immunofluorescence experiments in TEM8-HA expressing Hek293 cells labeled with various markers of recycling endosomes. These cells recapitulate toxin-dependent intoxication only in the presence of transfected receptor and undergo TEM8-dependent cell spreading on PA-coated surfaces [8]. Immunofluorescence analysis showed that TEM8 was present in functionally defined endosomes after continuous loading of cells with Alexa 488-labeled transferrin and co-localized with endogenous TFR and Rab11a, but not with Rab4 (Fig. 2A). Quantitative analysis of co-localization yielded a similar percentage of co-localization between TEM8 and either TF or TFR. The degree of co-localization of Rab11 with TEM8 was much smaller, however it was double of that observed with endogenous Rab4 and statistically different (Fig. 2B).
Because the great extent of co-localization between TEM8 and TFR, we tested whether both molecules are in sufficient proximity to be detected as a complex. Thus, we asked next whether TEM8 associates with TFR containing multi-protein complexes using a cross-linking strategy to stabilize labile protein interactions followed by purification by immunoprecipitation of TFR. We selected dithiobis-(succinimidylpropionate) (DSP), a homobifunctional cell-permeable cross-linker with a 12Å spacer arm. This reagent contains a disulfide bond that allows cleavage of cross-linked products for gel electrophoresis separation and western blot analysis of the proteins present in the complex. Furthermore, we employed non-saturating in vitro DSP incubation conditions, previously shown to stabilize known cargo molecules with the adaptor complex AP-3 into high molecular weight complexes without including non-related proteins [18]. We isolated TFR containing protein complexes by immunoprecipitation and resolved bead bound material by SDS-PAGE. No co-precipitation was detected in the absence of DSP (Fig. 2C, odd lanes) or when antibodies were omitted. The selectivity of these interactions was determined by the absence of β-actin in the immunoprecipitates and of any protein when primary antibodies were omitted (Fig. 2C). Similarly, immunoprecipitation of another abundant membrane protein of late endosomes-lysosomes, LAMP1, neither isolated TEM8 nor TFR in the cross-linked complexes, excluding spurious interactions between membrane proteins (Fig. 5C). TEM8 and TFR co-localization suggest that this complex is formed in intracellular compartments. This model predicts that interference with the exit of membrane proteins from recycling endosomes would increase association between TEM8 and TFR proteins. Monensin is a cell-permeable ionophore that dissipates the pH gradient of intracellular organelles such as endosomes, thus preventing receptor recycling back to the cell surface [23-25]. We analyzed TEM8 co-precipitation with TFR after DSP cross-linking in cells treated in the absence or presence of monensin. Monensin treatment increased TEM8 co-precipitation with TFR after 1 hour of drug treatment (Fig. 2D, compare lanes 2 and 4). Together with our previous findings, these results indicate that TEM8 and TFR reside in a complex in the recycling pathway. Moreover, they suggest that TEM8 could follow a similar trafficking route and mechanism as TFR, constitutively recycling between the plasma membrane and endosomes.
Figure 5.
PA addition does not affect TEM8 recycling and induces receptor degradation. A TEM8-expressing Hek293 cells were surface biotinylated at 4°C with 0.5 mM EZ-Link Sulfo-NHS-Biotin. Unbound biotin was washed and cells were shifted for the indicated times to 37°C in the absence (PA-) or presence (PA+) of 1μg/ml PA. Cells were incubated with GSH to remove remaining surface biotin, washed and lysed at 4°C. Biotinylated proteins were isolated with streptavidin conjugated agarose beads and bound complexes resolved by SDS-PAGE and analyzed by Western Blot with antibodies against TEM8 (1 of 2 experiments). B Graphic representation of the quantification of the experiment shown in A. C TEM8 expressing Hek293 cells were treated or not with 1μg/ml PA for 30min. Cell were washed and treated at 4°C with DMSO vehicle control (DSP- lanes) or with DSP (DSP+ lanes). Detergent extracts were immunoprecipitated with magnetic beads decorated with LAMP1 antibodies or beads decorated with TFR antibodies. Immune complexes were analyzed by immunoblot to detect TEM8, TFR and LAMP1. Inputs represent 5%. 1 of 3 independent experiments is shown. D TEM8 expressing Hek293 cells were labeled overnight with 100μCi/ml [S35]-methionine and chased in media containing cold methionine either in the absence or presence of 1μg/ml PA for the indicated times. Chase was ended by cell lysis and TEM8 and TFR were immunoprecipitated. Radiolabeled immunocomplexes were resolved by SDS-PAGE and radioactive bands detected by fluorography.1 of 3 independent experiments is shown.
TEM8 recycles between recycling endosomes and the cell surface and this is dependent on Rab11 GTPase
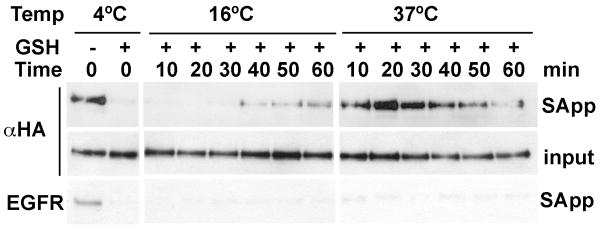
To test whether TEM8 receptors recycle between intracellular compartments and the plasma membrane, we followed the fate of surface biotinylated proteins with a disulfide-bonded biotinylation reagent. This reagent is cleaved by the addition of the cell impermeable reducing agent glutathione allowing the distinction of surface from internalized molecules. The later become protected from the reducing agent. Cells were labeled with reducible sulfo-NHS-SS-Biotin at 4°C. After washing the excess of biotin reagent, cells were warmed up to either 16°C, temperature at which endocytosis slows down and receptor accumulates in sorting endosomes [25] or to 37°C, temperature at which receptors follow the normal trafficking route. At different time after temperature shift, cells were returned to 4°C and washed twice with 50μM glutathione to release surface accessible biotin. Cells were lysed and biotinylated proteins were isolated by precipitation using streptavidin-conjugated agarose beads. Bead-bound complexes were analyzed by western blot for TEM8. Glutathione treatment stripped biotin label quantitatively from the surface, because no detectable biotinylated TEM8 was found in cells continuously maintained and treated with glutathione at 4°C (Fig. 3). At 16°C, the rate of internalization is slow, thus most TEM8 remained at the surface and accessible to glutathione, however a small pool of TEM8 became protected in intracellular compartments after 40 min. In contrast, when cells were shifted to 37°C, TEM8 receptors were progressively internalized and completely protected from glutathione within 25 ± 5.7 min (average ± SD, average of 4 independent transfections). The amount of glutathione-inaccessible biotinylated TEM8 decreased progressively afterwards, reaching its lowest level at 52.5 ± 9.5 min, when all the labeled receptors have become accessible to GSH. Total TEM8 levels detected in cell lysates remained constant, indicating that receptor degradation is negligible during this time frame. These results indicate that internalized TEM8 returns to the cell surface and that this cycle occurs in ∼30 min, time at which the internalized biotinylated receptors start to become accessible to GSH at the cell surface again. Importantly, these experiments show that the receptor is endocytosed constitutively in the absence of ligand, in contrast to EGFR, which in the absence of ligand, remains at the cell surface accessible to glutathione (Fig. 3).
Figure 3.
Recycling of cell surface labeled TEM8. Cell surface proteins in TEM8 expressing Hek293 cells were surface labeled by biotinylation at 4°C with 0.5 mM EZ-Link Sulfo-NHS-Biotin. Protein trafficking was allowed by incubating the cells at 16°C or 37°C for the indicated times. Cells were returned to 4°C to remove cell surface biotin by two GSH washes (GSH+ lanes) or were maintained in buffer in the absence of GSH (GSH- lanes). Proteins remaining biotinylated were recovered by streaptavidin-agarose beads precipitation from cell detergent lysates. Proteins associated to streptavidin beads were analyzed by western blot and probed for HA and epidermal growth factor receptor (EGFR).
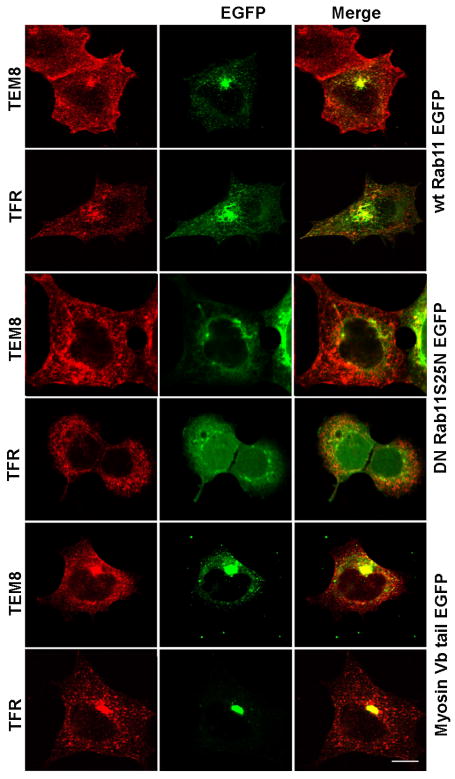
Cells posses a fast (∼5min) Rab4-dependent and a slow (∼15-30min) Rab11-dependent mechanism for protein recycling to the plasma membrane [26]. Recycling kinetics estimated from our biotinylation assays and the poor co-localization with Rab4 (Fig. 2B) are consistent with TEM8 recycling via a slow mechanism. Therefore, we tested whether Rab11 is involved in TEM8 endosomal recycling. Overexpression of wild type (wt) or mutants versions of Rab11 reduce the exit rate of reporter molecules from recycling endosomes, causing accumulation of these molecules in a perinuclear compartment [27]. The function of this recycling endosome is also disrupted by the expression of the myosin Vb tail, which binds and interferes with the GTPase activity of Rab11a, Rab11b and Rab25 leading to the accumulation of receptors in recycling endosomes [28]. TEM8 expressing Hek293 cells were co-transfected with EGFP tagged versions of wild type Rab11a, the dominant negative Rab11a mutant S25N or myosin Vb tail. The subcellular distribution of TEM8 was compared to TFR, known to traffic by Rab11- and myosin Vb-dependent mechanisms [25, 27, 28]. Immunofluorescence staining revealed perinuclear co-localization of TEM8 and TFR with wild type EGFP-Rab11a, consistent with localization in recycling endosomes (Fig.4). Expression of Rab11a S25N mutant resulted in a dispersed appearance of the perinuclear recycling endosome, TFR-positive compartments as well as TEM8 containing vesicles, consistent with previously described phenotypes [13]. Furthermore, myosin Vb tail expression caused a prominent accumulation of TEM8 and TFR in a EGFP myosin Vb-positive perinuclear compartment, phenotype consistent with entrapment of receptors in the recycling endosome (Fig.4). Thus, these studies lead us to conclude that similar to TFR, TEM8 recycles between the plasma membrane and the perinuclear recycling endosomes by a Rab11a GTPase and myosin Vb-regulated mechanism.
Figure 4.
TEM8 and transferrin receptor distribution is altered by agents that perturb recycling endosomes. TEM8 expressing Hek293 cells were transfected with wild type EGFP-Rab11a, dominant negative EGFP-Rab11S25N, or a dominant negative EGFP-Myosin Vb tail construct. Fixed cells were double stained with antibodies against GFP and either HA to detect TEM8 or transferrin receptor. Size bar is 10μm.
Anthrax Protective Antigen does not affect TEM8 recycling
Protective antigen (PA) binding to TEM8 changes the trafficking route of cell surface receptors, inducing receptor de-palmitoylation, localization to lipid raft domains in the plasma membrane and ubiquitination leading to receptor internalization [2]. Thus, we tested next whether the presence of PA, a TEM8 ligand, would alter TEM8 endocytic recycling. Cell surface proteins of Hek293 cells expressing TEM8 were biotinylated at 4°C as before. The cells were shifted to 37°C in the absence or presence of 1μg/ml PA and after the indicated time, returned to 4°C and washed with 50μM GSH. Biotinylated proteins were recovered from cell lysates by streptavidin agarose precipitation and the precipitated proteins were analyzed by western blot. PA addition reduced TEM8 internalization rate, with maximal receptor accumulation occurring at 30 minutes (Fig. 5A-B). Afterwards, biotinylated TEM8 returned to the surface and became accessible to the GSH wash (Fig. 5A-B). Consistent with this result, we also found that TEM8 was still present in cross-linked protein complexes containing transferrin receptor after 30 min of PA addition (Fig. 5C). No receptor co-precipitated with anti LAMP-1 antibodies, a lysosome membrane protein abundant in late endosomes used as a control (Fig. 5C). Furthermore, no differences in TEM8/TFR co-localization were detected after 1hr PA incubations (data not shown). Because many receptors are downregulated by ligand-induced degradation, we examined next whether PA changes the receptor half-life. We measured TEM8 half-life in pulse-chase experiments and immunoprecipitation from [S35] methionine-labeled cells (Fig. 5D). TEM8 receptor half-life was 7 ± 1h (average ± standard deviation, 3 independent experiments) and PA decreased receptor half-life to 4.3 ± 1.4h. This effect was selective, because under these conditions, TFR half-life was not modified by PA treatment. After 1h, PA increased TEM8 localization in late endosomes as evidenced by immunoflurescence staining using anti Lamp-1 or syntaxin 8 antibodies (Supplementary Figure 1). Collectively, these experiments indicate that PA does not prevent TEM8 recycling at early times after its internalization, yet it targets TEM8 to late endosomal compartments for degradation at later times.
TEM8 recycling in the presence of its ligand PA, predicts increased total PA loading at early time points as a result of continuous exposure of new, unoccupied receptors at the cell surface. We measured specific PA binding at 4°C, which represents receptors at the cell surface at a given time and at 22°C, which is permissive for endocytosis and recycling. After one hour at 22°C cells bound twice as much FITC labeled PA, than at 4°C (Fig. 6A). Increased PA binding was sensitive to monensin, but not to cycloheximide treatment, excluding a role of newly synthesized TEM8 in the increased levels of PA binding observed at 22°C (Fig. 6B). Monensin-dependent inhibition of PA binding was not due to pH-dependent changes of FITC signal, because cells are solubilized prior to fluorescence emission determination. Intracellular PA accumulation predicts that the ligand dissociates from the receptor before sorting into the recycling pathway. Thus, we incubated cells expressing two inhibitors blocking the exit of proteins from the recycling endosome, Rab11a S25N or myosin Vb tail with FITC labeled PA to test whether the ligand is directed to the recycling endosome at some point. To distinguish both fluorescent tags, FITC was stained with rabbit anti-FITC antibody and Alexa 568-conjugated secondary antibody. Antibody binding to this moiety quenches the green fluorescence and allows the visualization of PA in the red fluorescence channel. After 1h FITC-PA incubation at 37°C, immunofluorescence staining for FITC, showed that cells expressing either Rab11a S25N or myosin Vb tail bound and internalized PA (Fig.6C). However, PA was not detected in Rab11 or myosin Vb tail-positive compartments. This result is consistent with: 1) the previously reported mechanism for dissociation of PA from TEM8 in early endosomes occurring before TEM8 sorting to a recycling endosome [29], and 2) the notion that PA and TEM8 follow different endosomal trafficking routes after dissociation.
Figure 6.
Receptor recycling leads to increased PA accumulation, but not to ligand targeting to recycling endosomes. A Specific binding of FITC labeled PA63 to non-transfected or TEM8 expressing Hek293 cells at 4°C or 22°C for 60 min. B FITC-PA63 binding to TEM8 expressing Hek293 cells untreated or pre-incubated for 15 min with 2μM monensin or for 60 min with 10μg/ml with cycloheximide respectively (1 of 4 experiments). C Immunoflurescence staining with anti FITC and Alexa 555 secondary antibody in TEM8-Hek293 cells transfected with the indicated EGFP constructs. Cell were fixed after a 1h incubation with 1μg/ml FITC-PA63 at 37°C. One of two experiments is shown. Size bar is 10μm.
Disruption of TEM8 recycling affects TEM8-dependent cell spreading but not toxin internalization and translocation
To assess the role of Rab11a- myosin Vb-dependent recycling mechanisms upon TEM8 function, we explored whether disruption of TEM8 recycling affect either TEM8-dependent anthrax toxin internalization and/or cell adhesion [8]. We reasoned that because of divergent trafficking routes followed by receptor and toxin in early endosomes, interference with receptor recycling would reduce cell surface ligand binding capability, yet without disrupting toxin translocation into the cytosol. We studied anthrax toxin translocation into the cytosol analyzing N-terminus cleavage of MAPKK (MEK-1) by lethal factor. Expression of myosin Vb tail did not alter the rate of N-terminus cleavage of MAPKK (MEK-1) in TEM8 expressing Hek293 cells (Fig. 7A). This result is consistent with our finding that PA reaches intracellular compartments despite disruption of recycling endosomes (Fig. 6C). Since recycling of receptors to the plasma membrane is essential for cell spreading and cell migration processes [30], we hypothesized that reduction of TEM8 transport to the cell surface would reduce TEM8-mediated cell spreading. Thus, we tested the effects of disrupting receptor recycling by the expression of myosin Vb tail on TEM8-mediated cell spreading on PA-coated surfaces. We found that Hek293 cell spreading on PA-coated dishes was significantly reduced in cells expressing myosin Vb tail (Fig. 7B). The effects of Myosin Vb tail expression on cell spreading on this substrate was selective as myosin Vb tail had no effect when cells were plated on Matrigel, a basement membrane-like protein mixture engaging multiple adhesion molecules (mean spreading area control= 114,263 ± 6,906 vs. myosin Vb expressing cells= 83,122± 5,776 (Fig. 7C)). GFP expression had no effect on cell spreading on either substrate. These results indicate that the pool of recycling TEM8 receptors is necessary for cell spreading.
Figure 7.
Myosin Vb tail does not affect lethal toxin delivery to the cytosol, but interferes with TEM8-mediated cell spreading on PA coated surfaces. A Control or TEM8 expressing Hek293 either transfected with GFP or EGFP-Myosin Vb tail were incubated in the absence or presence of Lethal Toxin (1ug/ml PA, 0.2ug/ml Lethal Factor) for the indicated time. Cell intoxication was assessed by Western Blot analysis for N-terminus MEK-1 cleavage. B EGFP-Myosin Vb tail transfected TEM8-Hek293 cells were plated on PA or Matrigel (MA) coated glass coverslips and stained with Alexa labeled phalloidin to score for spreading area and GFP expression in the same cell. Size bar is 10μm. C Box plot graph shows the spreading area distribution of 30 cells from each indicated condition. The middle line in each condition represents the mean, the boxes represent the distribution of 50% of the cells, the lines represent the maximum and minimum values and the white dots represent outliers as determined by the graphing software. p value was determined using a t-test. D TEM8 expressing Hek293 cells either transfected with GFP or EGFP-Myosin Vb tail were plated on 0.1%BSA or 10μg/ml PA coated dishes in media containing 0.5 mM EZ-Link Sulfo-NHS-Biotin for 60 minutes at 37°C. Cells were washed and detergent soluble extracts were incubated with streptavidin conjugated agarose beads and bound complexes resolved by SDS-PAGE. Biotinylated proteins were analyzed by Western Blot using antibodies against TEM8 and EGF receptor (EGFR).
A model whereby TEM8 present in recycling endosomes contributes to the cell surface pool engaged in cell adhesion mechanisms, predicts that TEM8 levels will increase at the cell surface when cells are plated on substrata coated with TEM8 ligand. To test this prediction we plated EGFP or Myosin Vb EGFP transfected cells on BSA- or PA-coated dishes in the presence of biotinylation reagent. After incubation for 1h at 37°C for spreading to occur, biotinylation was stopped and the free biotin was washed. The amount of biotinylated TEM8 was measured by streptavidin precipitation and western blot analysis of the precipitate for TEM8. As predicted, the surface content of TEM8 was substantially increased in cells plated on PA-coated surfaces when compared to cells plated on dishes decorated with a substrate non-permissive for cell adhesion, bovine serum albumin (BSA) (Figure 7D, compare lanes 1 and 3). Importantly, TEM8 surface levels increase induced by PA coated surface was abolished by the expression of myosin Vb tail (Figure 7D, compare lanes 3 and 4). This result is consistent with TEM8 entrapment in intracellular compartments by Myosin Vb tail. EGFR, an unrelated cell surface molecule, did not show any difference in biotinylation levels as a result of Myosin Vb tail expression or cell spreading on either substrate. These results evidence a selective increase in receptor exposure at the cell surface during cell spreading on a TEM8 ligand.
Our experiments follow the behavior of transfected HA tagged TEM8 due to the unavailable TEM8 antibodies. To confirm our previous findings, we tested whether Myosin Vb tail would disrupt the cell adhesion function of endogenously expressed TEM8. It has been previously reported that B16F10 melanoma cells express TEM8 [31]. We confirmed this result by RT-PCR of total RNA extracted from B16F10 melanoma cells using TEM8 specific primers (Fig. 8A). Moreover, we found that B16F10 cells do not express CMG2, a second anthrax toxin receptor highly related to TEM8 [32] (Fig. 8A). In contrast, we found mRNA encoding both CMG2 and TEM8 in the undifferentiated colon carcinoma-derived CT26 murine tumor cell line (Fig. 8A). B16F10 melanoma cells readily undergo cell spreading on PA coated dishes as well as on Matrigel (Fig. 8B), reproducing our findings in Hek293 cells expressing HA-tagged TEM8 (Fig. 7B-D). Expression of EGFP in B16F10 melanoma cells did not affect cell spreading when compared to non-transfected cells. In contrast, Myosin Vb tail expression significantly reduced cell spreading of B16F10 melanoma cells seeded on PA, but not on Matrigel (Fig. 8C). Thus, these results indicate that endogenous TEM8 is regulated by Myosin Vb in the same fashion as transfected TEM8. Thus, endogenous TEM8 is mobilized from recycling endosomes to the cell surface for receptor-mediated cell spreading.
Figure 8.
A. B16F10 Melanoma cells express endogenous TEM8. The expression of TEM8 (all isoforms and variant 1) but not CMG2 (all variants) was detected by RT-PCR in B16F10 cells and CT26 colon carcinoma cells. B Phase contrast microphotography of B16F10 cells spreading on PA or Matrigel coated dishes after 1h of plating. C Box plot depiction of the spreading area of B16F10 cells expressing either EGFP or Myosin Vb tail and non-transfected plated on BSA, PA or Matrigel coated-dishes. Thirty randomly selected cells were analyzed per condition.
Discussion
Here we show that TEM8 resides constitutively in transferrin receptor- and Rab11a-positive recycling endosomes. Consistent with this subcellular localization, TEM8 recycling was perturbed by monensin as well as expression of Rab11a- and Myosin Vb-function perturbing mutants. TEM8 residence in endosomal compartments in the absence of the toxin cell binding component, PA, predicts the existence of a mechanism mediating constitutive internalization of cell surface TEM8. Such a mechanism would be different to the previously described ligand-induced receptor internalization via lipid rafts. Lipid raft-dependent internalization occurs only when PA binds to its receptor, induces receptor clustering and forms a heptamer or when TEM8 receptor palmitoylation is prevented [1, 2]. Internalization via lipid rafts requires TEM8 ubiquitination [2] and leads to PA [1] and receptor degradation (this work). In this context, we speculate that TEM8 constitutive endocytosis is likely to involve internalization of palmitoylated receptors by a mechanism distinct from lipid-rafts and ubiquitination. A precedent for such a mechanism is MUC1, a mucin-like transmembrane glycoprotein, which is dependent on palmitoylation for recycling [33]. Alternatively, Hek293 and other cell lines examined could express an endogenous ligand eliciting TEM8 constant endocytosis and recycling. One precedent for differential traffic induced by ligand is TGFα and EGF binding to EGFR. TGFα binding to EGFR is more acid labile than EGF binding [34]. Therefore, as the pH decreases during ligand-receptor complex progression along the endocytic pathway, TGFα-bound EGF receptors become hypo-ubiquitinated and diverted to a recycling pathway, while EGF binding promotes receptor degradation [35]. As a consequence TGFα is much more mitogenic than EGF [36]. A similar differential ligand-induced endosomal trafficking has been described for keratinocyte growth factor receptor as well [37].
We found that along the endocytic pathway, TEM8 associates to a TFR containing multi-protein complex detected by chemical cross-linking. No association was found when primary antibody was omitted or when antibodies for LAMP-1, a transmembrane protein present in late endosomes and lysosomes were used. The association is also selective, as we did not detect actin, the most abundant cytosolic protein. TFR and TEM8 association to a common multimolecular complex is supported by co-immunolocalization in intracellular compartments and increased complex formation when protein exit from endosomes is prevented with monensin. Consistently, TFR and TEM8 receptors accumulated in a perinuclear compartment when myosin Vb tail was used to sequester proteins in the recycling endosome. We are inclined to exclude the formation of this complex in early endosomes, because cross-linking with TFR did not increase when receptor internalization and traffic to the early endosome is induced with PA. Upon internalization, PA presumably still bound to the receptor, is rapidly transported to TFR containing Rab5-positive endosomes [19]. Taken together, our results point to association of TEM8 to TFR containing complexes as occurring in the recycling endosome or in vesicles exiting the recycling endosome in route to the plasma membrane.
Using cell surface biotinylation, we measured that a full cycle for transit of TEM8 to and from the plasma membrane takes approximately 30 minutes. This timing is consistent with the receptors following the “slow” recycling route, reported for several Rab11-dependent recycling molecules such as β1 integrin [38] and transferrin basolateral recycling in epithelial cells [39]. TEM8 traffic through the Rab11 positive endosome is supported by co-localization with endogenous Rab11, GFP tagged Rab11 and disruption of recycling endosomes using Rab11 S25N and myosin Vb tail. Rab11 S25N neither binds to myosin V nor other rab11 effectors [13, 28]. Myosins V (a and b) are actin-based motors that move vesicles towards the cell periphery. Thus, the myosin Vb tail functions as a powerful dominant negative for the recycling pathway inducing intracellular sequestration of membrane proteins recycling through this compartment. As a consequence, Myosin Vb tail expression inhibited cell spreading on PA-coated substrata by both Hek293 cells exogenously expressing TEM8 or B16F10 melanoma cells endogenously carrying this receptor.
Although the endogenous ligand and the physiological function of TEM8 remain unknown, endosomal residence and recycling of this molecule has implications for regulatory and signaling mechanism relevant to cell adhesion and tumorigenesis. Endosomal recycling, together with endocytosis, regulate receptor desensitization by clearing occupied receptors and returning them unoccupied to the cell surface competent to bind new ligand [40, 41]. We found this to be the case for TEM8, as cells incubated with monensin or at temperatures non permissive for recycling accumulated less PA. TEM8 recycling could mediate Collagen I and Collagen VI clearance, as these molecules accumulate in the extracellular matrix of TEM8-deficient animals [9]. Receptor recycling offers an escape to proteolytic degradation [42] and provides a new context for putative signal transduction mechanisms engaged by TEM8, such as the demonstrated for growth factor receptors and adhesion molecules [43-45].
Receptor recycling mechanisms generate vectorial signaling and delivery of adhesion receptors essential for cell motility [46]. Vectorial delivery of cell adhesion molecules and receptors to the plasma membrane is essential for cell locomotion and chemotaxis to ensure the rapid exposure of new receptors at the leading edge at a rate otherwise incompatible with the synthesis of new cell surface molecules [47]. Cell spreading is considered a model for membrane and cytoskeletal dynamics occurring during cell locomotion. In this model, receptor number and plasma membrane is increased by abrupt exocytosis from recycling compartments [48-50]. Integrins are constantly endocytosed and recycled back to the membrane during cell spreading and migration [30, 51, 52]. For integrin α5β1 in particular, specific signaling from Rab11 endosomes has been demonstrated to promote directional migration and RhoA activation [45].
Consistently with these requirements for cell spreading, Myosin Vb dependent recycling lead to increased exposure at the cell surface and was necessary to mediate TEM8-dependent cell spreading on PA coated substrates. These findings together with TEM8 linkage to the actin cytoskeleton predict a role for this receptor in modulating cell migration.
Supplementary Material
Supplementary Figure 1: PA increases TEM8 localization in late-endosomes. TEM8 expressing Hek293 cells were incubated in the absence or presence of 1μg/ml PA for 1h at 37°C. TEM8 localization was determined by immunofluorescence microscopy using anti HA antibodies to detect TEM8 and either anti LAMP-1 or anti syntaxin 8 as late endosomal markers. Size bar=10μm.
Acknowledgments
This work was supported by grants from the National Institutes of Health to E.W. (CA127136) and V.F. (NS42599 and GM077569).
Abbreviations
- PA
Protective Antigen
- HA
hemagglutinin
- EGFP
Enhanced Green Fluorescent Protein
- MAPKK
Mitogen-Activated Kinase Kinase
- MEK
Mitogen-activated Extracellular signal regulated Kinase
- DME
Dulbecco's Modified Eagle's Medium
- EGF
Epidermal Growth Factor
- PFA
Paraformaldehyde
- PBS
Phosphate Buffered Saline
- BSA
bovine serum albumin
- FBS
fetal bovine serum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrami L, Liu S, Cosson P, Leppla SH, van der Goot FG. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J Cell Biol. 2003;160:321–328. doi: 10.1083/jcb.200211018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrami L, Leppla SH, van der Goot FG. Receptor palmitoylation and ubiquitination regulate anthrax toxin endocytosis. J Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 4.Leppla SH. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982;79:3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duesbery NS, Webb CP, Leppla SH, Gordon VM, Klimpel KR, Copeland TD, Ahn NG, Oskarsson MK, Fukasawa K, Paull KD, Vande Woude GF. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 1998;280:734–737. doi: 10.1126/science.280.5364.734. [DOI] [PubMed] [Google Scholar]
- 6.Frankel AE, Kuo SR, Dostal D, Watson L, Duesbery NS, Cheng CP, Cheng HJ, Leppla SH. Pathophysiology of anthrax. Front Biosci. 2009;14:4516–4524. doi: 10.2741/3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley KA, Mogridge J, Jonah G, Rainey A, Batty S, Young JA. Binding of anthrax toxin to its receptor is similar to alpha integrin-ligand interactions. J Biol Chem. 2003;278:49342–49347. doi: 10.1074/jbc.M307900200. [DOI] [PubMed] [Google Scholar]
- 8.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- 9.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Wang H, Currie BM, Molinolo A, Leung HJ, Moayeri M, Basile JR, Alfano RW, Gutkind JS, Frankel AE, Bugge TH, Leppla SH. Matrix metalloproteinase-activated anthrax lethal toxin demonstrates high potency in targeting tumor vasculature. J Biol Chem. 2008;283:529–540. doi: 10.1074/jbc.M707419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu S, Leppla SH. Cell surface tumor endothelium marker 8 cytoplasmic tail-independent anthrax toxin binding, proteolytic processing, oligomer formation, and internalization. J Biol Chem. 2003;278:5227–5234. doi: 10.1074/jbc.M210321200. [DOI] [PubMed] [Google Scholar]
- 13.Volpicelli LA, Lah JJ, Fang G, Goldenring JR, Levey AI. Rab11a and myosin Vb regulate recycling of the M4 muscarinic acetylcholine receptor. J Neurosci. 2002;22:9776–9784. doi: 10.1523/JNEUROSCI.22-22-09776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newell-Litwa K, Salazar G, Smith Y, Faundez V. Roles of BLOC-1 and adaptor protein-3 complexes in cargo sorting to synaptic vesicles. Mol Biol Cell. 2009;20:1441–1453. doi: 10.1091/mbc.E08-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clift-O'Grady L, Desnos C, Lichtenstein Y, Faundez V, Horng JT, Kelly RB. Reconstitution of synaptic vesicle biogenesis from PC12 cell membranes. Methods. 1998;16:150–159. doi: 10.1006/meth.1998.0662. [DOI] [PubMed] [Google Scholar]
- 16.Salazar G, Love R, Werner E, Doucette MM, Cheng S, Levey A, Faundez V. The zinc transporter ZnT3 interacts with AP-3 and it is preferentially targeted to a distinct synaptic vesicle subpopulation. Mol Biol Cell. 2004;15:575–587. doi: 10.1091/mbc.E03-06-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craige B, Salazar G, Faundez V. Phosphatidylinositol-4-kinase type II alpha contains an AP-3-sorting motif and a kinase domain that are both required for endosome traffic. Mol Biol Cell. 2008;19:1415–1426. doi: 10.1091/mbc.E07-12-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem. 2009;284:1790–1802. doi: 10.1074/jbc.M805991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrami L, Lindsay M, Parton RG, Leppla SH, van der Goot FG. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J Cell Biol. 2004;166:645–651. doi: 10.1083/jcb.200312072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein Y, Desnos C, Faundez V, Kelly RB, Clift-O'Grady L. Vesiculation and sorting from PC12-derived endosomes in vitro. Proc Natl Acad Sci U S A. 1998;95:11223–11228. doi: 10.1073/pnas.95.19.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Wit H, Lichtenstein Y, Geuze HJ, Kelly RB, van der Sluijs P, Klumperman J. Synaptic vesicles form by budding from tubular extensions of sorting endosomes in PC12 cells. Mol Biol Cell. 1999;10:4163–4176. doi: 10.1091/mbc.10.12.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basu SK, Goldstein JL, Anderson RG, Brown MS. Monensin interrupts the recycling of low density lipoprotein receptors in human fibroblasts. Cell. 1981;24:493–502. doi: 10.1016/0092-8674(81)90340-8. [DOI] [PubMed] [Google Scholar]
- 24.Stein BS, Bensch KG, Sussman HH. Complete inhibition of transferrin recycling by monensin in K562 cells. J Biol Chem. 1984;259:14762–14772. [PubMed] [Google Scholar]
- 25.Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci U S A. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 27.Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG. Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol. 1996;135:913–924. doi: 10.1083/jcb.135.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Provance DW, Jr, Mercer JA, Bahler M, Goldenring JR. Myosin vb is associated with plasma membrane recycling systems. Mol Biol Cell. 2001;12:1843–1857. doi: 10.1091/mbc.12.6.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scobie HM, Marlett JM, Rainey GJ, Lacy DB, Collier RJ, Young JA. Anthrax toxin receptor 2 determinants that dictate the pH threshold of toxin pore formation. PLoS One. 2007;2:e329. doi: 10.1371/journal.pone.0000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caswell PT, Norman JC. Integrin trafficking and the control of cell migration. Traffic. 2006;7:14–21. doi: 10.1111/j.1600-0854.2005.00362.x. [DOI] [PubMed] [Google Scholar]
- 31.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 32.Scobie HM, Rainey GJ, Bradley KA, Young JA. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc Natl Acad Sci U S A. 2003;100:5170–5174. doi: 10.1073/pnas.0431098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kinlough CL, McMahan RJ, Poland PA, Bruns JB, Harkleroad KL, Stremple RJ, Kashlan OB, Weixel KM, Weisz OA, Hughey RP. Recycling of MUC1 is dependent on its palmitoylation. J Biol Chem. 2006;281:12112–12122. doi: 10.1074/jbc.M512996200. [DOI] [PubMed] [Google Scholar]
- 34.Ebner R, Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991;2:599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roepstorff K, Grandal MV, Henriksen L, Knudsen SL, Lerdrup M, Grovdal L, Willumsen BM, van Deurs B. Differential effects of EGFR ligands on endocytic sorting of the receptor. Traffic. 2009;10:1115–1127. doi: 10.1111/j.1600-0854.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belleudi F, Leone L, Nobili V, Raffa S, Francescangeli F, Maggio M, Morrone S, Marchese C, Torrisi MR. Keratinocyte growth factor receptor ligands target the receptor to different intracellular pathways. Traffic. 2007;8:1854–1872. doi: 10.1111/j.1600-0854.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- 38.Powelka AM, Sun J, Li J, Gao M, Shaw LM, Sonnenberg A, Hsu VW. Stimulation-dependent recycling of integrin beta1 regulated by ARF6 and Rab11. Traffic. 2004;5:20–36. doi: 10.1111/j.1600-0854.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- 39.Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Odley A, Hahn HS, Lynch RA, Marreez Y, Osinska H, Robbins J, Dorn GW., 2nd Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling. Proc Natl Acad Sci U S A. 2004;101:7082–7087. doi: 10.1073/pnas.0308335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 42.Gage RM, Kim KA, Cao TT, von Zastrow M. A transplantable sorting signal that is sufficient to mediate rapid recycling of G protein-coupled receptors. J Biol Chem. 2001;276:44712–44720. doi: 10.1074/jbc.M107417200. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Pennock S, Chen X, Wang Z. Endosomal signaling of epidermal growth factor receptor stimulates signal transduction pathways leading to cell survival. Mol Cell Biol. 2002;22:7279–7290. doi: 10.1128/MCB.22.20.7279-7290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, Wood TL. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem. 2007;282:22513–22524. doi: 10.1074/jbc.M704309200. [DOI] [PubMed] [Google Scholar]
- 45.White DP, Caswell PT, Norman JC. alpha v beta3 and alpha5beta1 integrin recycling pathways dictate downstream Rho kinase signaling to regulate persistent cell migration. J Cell Biol. 2007;177:515–525. doi: 10.1083/jcb.200609004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jekely G, Sung HH, Luque CM, Rorth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Bretscher MS, Aguado-Velasco C. Membrane traffic during cell locomotion. Curr Opin Cell Biol. 1998;10:537–541. doi: 10.1016/s0955-0674(98)80070-7. [DOI] [PubMed] [Google Scholar]
- 48.Gauthier NC, Rossier OM, Mathur A, Hone JC, Sheetz MP. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell. 2009;20:3261–3272. doi: 10.1091/mbc.E09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci U S A. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jovic M, Naslavsky N, Rapaport D, Horowitz M, Caplan S. EHD1 regulates beta1 integrin endosomal transport: effects on focal adhesions, cell spreading and migration. J Cell Sci. 2007;120:802–814. doi: 10.1242/jcs.03383. [DOI] [PubMed] [Google Scholar]
- 51.Jones MC, Caswell PT, Norman JC. Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol. 2006;18:549–557. doi: 10.1016/j.ceb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 52.Pellinen T, Ivaska J. Integrin traffic. J Cell Sci. 2006;119:3723–3731. doi: 10.1242/jcs.03216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: PA increases TEM8 localization in late-endosomes. TEM8 expressing Hek293 cells were incubated in the absence or presence of 1μg/ml PA for 1h at 37°C. TEM8 localization was determined by immunofluorescence microscopy using anti HA antibodies to detect TEM8 and either anti LAMP-1 or anti syntaxin 8 as late endosomal markers. Size bar=10μm.