Abstract
Mechanical forces are critical for fetal lung development. Using surfactant protein C (SP-C) as a marker, we previously showed that stretch-induced fetal type II cell differentiation is mediated via the ERK pathway. Caveolin-1, a major component of the plasma membrane microdomains, is important as a signaling protein in blood vessels exposed to shear stress. Its potential role in mechanotransduction during fetal lung development is unknown. Caveolin-1 is a marker of type I epithelial cell phenotype. In this study, using immunocytochemistry, Western blotting, and immunogold electron microscopy, we first demonstrated the presence of caveolin-1 in embryonic day 19 (E19) rat fetal type II epithelial cells. By detergent-free purification of lipid raft-rich membrane fractions and fluorescence immunocytochemistry, we found that mechanical stretch translocates caveolin-1 from the plasma membrane to the cytoplasm. Disruption of the lipid rafts with cholesterol-chelating agents further increased stretch-induced ERK activation and SP-C gene expression compared with stretch samples without disruptors. Similar results were obtained when caveolin-1 gene was knocked down by small interference RNA. In contrast, adenovirus overexpression of the wild-type caveolin-1 or delivery of caveolin-1 scaffolding domain peptide inside the cells decreased stretch-induced ERK phosphorylation and SP-C mRNA expression. In conclusion, our data suggest that caveolin-1 is present in E19 fetal type II epithelial cells. Caveolin-1 is translocated from the plasma membrane to the cytoplasm by mechanical stretch and functions as an inhibitory protein in stretch-induced type II cell differentiation via the ERK pathway.
Keywords: caveolin, lung development, surfactant protein C
mechanical forces generated in utero by repetitive breathing-like movements and by fluid distension are critical for normal fetal lung development (12). We and others have identified several receptors and signaling proteins that modulate fetal lung development (17, 30, 31, 38, 40, 41). Specifically, using surfactant protein (SP) C (SP-C) as a marker, we found that stretch-induced fetal type II cell differentiation is mediated via the ERK pathway (31). However, the precise molecular and cellular mechanisms by which lung cells sense mechanical stimuli to influence lung development are not fully understood.
Lipid rafts and caveolae are localized regions of the plasma membrane that contain high concentrations of cholesterol and glycosphingolipids (15). These microdomains play important roles in cell signaling, cell adhesion, and membrane traffic (26, 33, 34). Caveolins are a family of proteins present in the microdomains. Caveolin-1 and caveolin-2 are expressed in most cell types, whereas caveolin-3 is primarily present in muscle cells (36). Caveolin-1 is important to maintain the invaginated structure of the caveola. Caveolin-1 was initially thought to be exclusive of the caveolae; however, further studies demonstrated the presence of caveolin outside the caveolae regions of the plasma membrane. In addition, caveolin-1 has also been found in the plasma membrane of cells that lack caveolae (11) and as a soluble protein in multiple cellular compartments (18). The interactions of caveolin-1 with other molecules support a role for this protein in lipid transport, membrane traffic, and cell signaling (18).
There is direct evidence for the participation of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels (43). Caveolin-1 has been shown to regulate several signaling pathways in endothelial cells (1, 8, 27), vascular smooth muscle cells (14, 32), and mesangial cells (45) exposed to mechanical stress. However, the potential role of caveolin-1 in mechanotransduction during fetal lung development is unknown.
Caveolin-1 is weakly and inconsistently expressed in adult type II cells (3, 9, 22) and is a well-known marker of the type I epithelial cell phenotype (5, 42). On the other hand, caveolin-1 and caveolin-2 are abundantly expressed in the developing lung parenchyma and also within the epithelial cells that line the developing bronchioles in embryonic day 16 (E16) mouse embryos (6). These data suggest that there might be a difference in expression of caveolin in the epithelium between the fetal lung and the adult lung, but no information is available.
Therefore, the objectives of this study were to investigate whether caveolin-1 is found in fetal type II epithelial cells and to assess its potential role as a signaling protein in fetal lung development. We found that caveolin-1 is localized not only in the plasma membrane, but also in the cytoplasm and nucleus of fetal type II cells, and participates in stretch-induced ERK activation and SP-C mRNA expression, suggesting a potential role in mechanotransduction during fetal lung development.
MATERIALS AND METHODS
Cell isolation and mechanical stretch.
Animal experiments were approved by and performed in compliance with the Lifespan Institutional Animal Care and Use Committee (Providence, RI). Fetal lungs were obtained at embryonic day 19 (E19) from timed-pregnant Sprague-Dawley rats (Charles River, Wilmington, MA) after intraperitoneal administration of pentobarbital sodium. Type II cells were isolated as previously described (30). Briefly, after collagenase digestion, cell suspensions were sequentially filtered through 100-, 30-, and 20-μm nylon meshes using screen cups (Sigma). Clumped nonfiltered cells from the 30- and 20-μm nylon meshes were collected after several washes with DMEM to facilitate the filtration of nonepithelial cells. Further type II cell purification was achieved by incubation of the cells in 75-cm2 flasks for 30 min. Nonadherent cells were collected and plated on Bioflex multiwell plates (Flexcell International, Hillsborough, NC) precoated with laminin-1 (2 μg/cm2). The purity of the cells was determined to be 90 ± 5% by microscopic analysis of epithelial cell morphology and immunostaining for SP-C (16, 31). Monolayers were maintained overnight in serum-free DMEM and then mounted in a strain unit (model FX-4000, Flexcell International). Cells were ∼80% confluent immediately before experiments. An equibiaxial cyclical strain regimen of 5% was applied at intervals of 60 cycles/min for different lengths of time. This regimen was chosen to simulate fetal breathing movements experienced by type II epithelial cells during fetal lung development and was shown to promote type II cell differentiation (28). Cells grown on nonstretched membranes were treated in an identical manner and served as controls.
Immunogold electron microscopy.
E19 type II epithelial cells cultured on Bioflex membranes were fixed briefly (30 min) with 1% glutaraldehyde in 0.1 M phosphate buffer at 4°C. Cells were detached from the membranes, pelleted, and fixed for 3 h at 4°C. Pellets were washed with buffer and then treated with 0.1 M glycine at 4°C for 20 min to quench free aldehyde groups. Samples were rinsed with distilled water and stained with 2% uranyl acetate for 1 h at room temperature in darkness. Samples were then passed through 30% and 50% ethanol and briefly rinsed in 78% ethanol (4 × 20 min), all at 4°C. Samples were then incubated in a mixture of 78% ethanol and LR White resin for 1 h, infiltrated with LR White acrylic resin for 2 days at 4°C, and then polymerized overnight at 49°C. Ultrathin (70–80 nm) sections were prepared and retrieved onto unsupported 300-mesh nickel grids, which had been treated with Coat-Quick “G” (Electron Microscopy Sciences). In a moist chamber, grid-mounted sections were immersed in droplets of blocking solution containing 0.5% BSA and 3% normal goat serum in PBS for 30 min at room temperature. Grids were transferred to droplets of anti-caveolin-1 antibody (BD Transduction Laboratories) at a 1:50 dilution in PBS-BSA and incubated overnight at 4°C. Negative control included grids in which the primary antibody was omitted. After rinses in PBS-BSA, grids were immersed in droplets of goat anti-rabbit IgG antibody coupled to 10-nm colloidal gold (Aurion) at a 1:30 dilution for 2 h at room temperature. Grids were then rinsed in buffer followed by distilled water and contrasted in 2% aqueous uranyl acetate for 5 min. Sections were examined using a Morgagni 268 transmission electron microscope, and images were collected with an AMT Advantage 542 charge-coupled device camera system.
Simplified method for the preparation of detergent-free lipid rafts.
Six Bioflex plates per experimental condition containing subconfluent E19 type II epithelial cells were washed and scraped into base buffer [20 mM Tris·HCl (pH 7.8) and 250 mM sucrose] containing 1 mM CaCl2 and 1 mM MgCl2. After centrifugation, pellets were resuspended in base buffer containing CaCl2, MgCl2, and protease inhibitors. The cells were then lysed and centrifuged twice, and the resulting postnuclear supernatants were added to an equal volume (2 ml) of base buffer containing 50% OptiPrep and placed in the bottom of a 12-ml centrifuge tube. An 8-ml gradient of 0–20% OptiPrep in base buffer was added on top of the lysate. Gradients were centrifuged for 90 min at 52,000 g using an SW-41 rotor in a Beckman ultracentrifuge. Seventeen 1-ml fractions were collected, and 100-μl aliquots of each fraction were subjected to SDS-PAGE and immunoblotting for caveolin-1 (20).
Fluorescence microscopy.
After experiments, cultured cells from control and stretch conditions were fixed in 4% paraformaldehyde for 20 min at room temperature. Silastic membranes were cut from the Bioflex plates and mounted on glass slides, and cells were permeabilized in 0.1% Triton X-100 in PBS for 10 min at room temperature. Samples were then incubated in blocking buffer (1× Tris-buffered saline, 0.1% Tween 20, and 2% normal goat serum in PBS) for 1 h at room temperature and incubated with primary antibody to caveolin-1 (Cell Signaling, Beverly, MA) at 1:250 dilution in blocking buffer at 4°C overnight (negative controls were incubated in blocking buffer without the primary antibody). Cells were washed and incubated in Alexa Fluor 488 goat anti-rabbit secondary antibody at 1:500 dilution (Molecular Probes) in the same buffer solution for 1 h at room temperature. Monolayers were then washed, mounted, and analyzed by confocal fluorescence microscopy. For double staining of caveolin-1 and SP-C, freshly isolated E19 cells were first incubated in 5 mM EDTA for 10 min at 37°C to facilitate separation of the cells and then placed on Cytospin slide chambers (Thermo Scientific) and centrifuged at 1,000 rpm for 5 min. Slides were immediately fixed in 95% ethanol-5% glacial acetic acid for 2 min and rinsed in PBS (3 × 5 min). Cells were incubated with primary antibody to caveolin-1 (BD Transduction Laboratories) at 1:50 dilution in blocking buffer at 4°C overnight, washed, and incubated with Alexa Fluor 488 secondary antibody (Invitrogen) at 1:200 dilution in blocking buffer for 1 h. After they were washed, permeabilized, and incubated in blocking solution as described above, slides were incubated with primary antibody to SP-C (Santa Cruz Biotechnology) at 1:30 dilution in blocking buffer at 4°C overnight and then in Cy3 goat anti-rabbit secondary antibody (Jackson ImmunoResearch) at 1:500 dilution. Slides were washed and mounted for confocal microscopy analysis.
Assessment of ERK activation.
After experiments, cell monolayers were lysed with ice-cold RIPA buffer [150 mM NaCl, 100 mM Tris base (pH 7.5), 1% deoxycholate, 0.1% SDS, 1% Triton X-100, 3.5 mM Na3VO4, 2 mM PMSF, 50 mM NaF, and 100 mM sodium pyrophosphate] with protease inhibitors. Lysates were centrifuged, and total protein contents were determined by the bicinchoninic acid method. Protein samples were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). Membranes were incubated for 1 h at room temperature in blocking buffer [25 mM Tris·Cl (pH 8.0), 1.25 mM NaCl, and 0.1% Tween 20 with 5% nonfat dry milk] and then incubated with anti-phosphorylated ERK½ antibody (Cell Signaling) for 1 h at room temperature. Then membranes were washed, and secondary antibody (donkey anti-rabbit horseradish peroxidase diluted 1:2,000 in blocking buffer) was added for 1 h at room temperature. Immunoreactive phosphorylated ERK was detected by enhanced chemiluminescence (Amersham, Arlington Heights, IL). To control for protein loading, membranes were stripped and reprobed with antibody to total ERK (Cell Signaling).
Northern blot analysis.
Total cellular RNA was isolated using a single-step method previously described (29). Briefly, RNA was denatured at 65°C for 5 min and fractionated by 1.4% agarose and 2.2 M formaldehyde gel electrophoresis. RNA was blotted, transferred to GeneScreen (NEN, Boston, MA) nylon membranes, and immobilized by UV cross-linking. The SP-C probe was synthesized from linearized recombinant phagemid template using in vitro transcription (Promega, Madison, WI), T7 RNA polymerase, and [α-32P]UTP (Amersham). Blots were hybridized with these probes and washed in 0.5× saline-sodium citrate-1% SDS. 18S rRNA fluorescence bands were used to control for differences in sample loading and RNA integrity. Blots were exposed to X-ray film with intensifying screens at −80°C.
Real-time PCR.
Total RNA was isolated as described above and purified further using the Turbo DNA-Free kit (Ambion). One microgram of total RNA was reverse-transcribed into cDNA using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's instructions. Predesigned TaqMan SP-C (catalog no. Rn01466216_g1) primer was purchased from Assays-on-Demand Gene Expression Products (Applied Biosystems). To amplify the cDNA by quantitative RT-PCR, 2 μl of the resulting cDNA were added to a mixture of 20 μl of TaqMan Gene Expression Master Mix (Applied Biosystems) and Assays-on-Demand Gene Expression Assay Mix containing forward and reverse primers and TaqMan labeled probe (Applied Biosystems). Standard curves were generated for each primer set and the housekeeping gene 18S rRNA. Linear regression revealed efficiencies between 96% and 99%. Therefore, fold expressions of stretched samples relative to controls were calculated using the threshold cycle (ΔΔCT) method for relative quantification, as previously described (40). Samples were normalized to the 18S rRNA. No differences in relative quantification values for 18S were found between control and strain samples. The reactions were performed in a 7500 Fast real-time PCR system (Applied Biosystems). All assays were performed in triplicate.
Small interference RNA transfection by electroporation.
Fetal lung cells were transiently transfected using Nucleofector technology (Amaxa Biosystems). Freshly isolated E19 type II epithelial cells were plated on T75 flasks overnight. On the following day, cells were harvested by trypsinization, and aliquots of 2 × 106 cells in RPMI with 10% FBS were centrifuged at 100 g for 10 min; supernatants were discarded, and cell pellets were resuspended in 100 μl of basic Nucleofector solution (Primary Mammalian Epithelial Cells Protocol, Amaxa). Samples were mixed individually with different concentrations (1–4 μM) of siGENOME nontargeting siRNA Pool 2, siGENOME SMART Pool rat caveolin-1 (both from Dharmacon, Thermo Scientific), or the siRNA directed against the positive control GAPDH (Ambion, Applied Biosystems), transferred into the appropriate cuvettes, and subjected to electrical pulses using the Nucleofactor II apparatus (Amaxa Biosystems). Samples containing no siRNA were otherwise treated in an identical manner and served as negative controls (pulse only). On the basis of previous studies from our laboratory (40), a T-13 program from Amaxa Biosystems was selected. After electroporation, samples were immediately transferred into Eppendorf tubes containing prewarmed RPMI plus 10% FBS and incubated at 37°C for 10 min. Cells were then transferred into Bioflex plates precoated with laminin-1 and left undisturbed for 24 h in a culture incubator. On the next day, monolayers were exposed to mechanical strain for different periods of time. Control conditions for each experiment included no transfection, electrical pulse only, nontargeted siRNA (negative control), and GAPDH siRNA (positive control). In addition, for each set of samples, it was confirmed by Western blot that caveolin-1 protein was, in fact, inhibited by siRNA.
Adenoviral infection.
The premade recombinant adenoviruses encoding human wild-type caveolin-1 and caveolin-2 genes were obtained from a commercial source (Capital Biosciences). Adenovirus encoding green fluorescent protein (GFP; a kind gift from Dr. Jisu Li, Brown University) was used as negative control. Adenoviruses were amplified in HEK 293 cells, harvested, and purified with the Adeno-X virus purification kit (Clontech, Mountainview, CA) according to the manufacturer's recommendations. Adenoviral titers were determined using the Adeno-X rapid titer kit (Clontech) and expressed as multiplicity of infection. Subconfluent E19 monolayers were infected with adenoviruses at different multiplicities of infection in medium containing serum-free DMEM plus 1.2 mM EGTA (pH 7.4). After incubation for 90 min at 37°C, the virus-containing medium was removed and fresh serum-free DMEM was added.
Caveolin-1 scaffolding domain peptides.
Peptides, corresponding to the putative scaffolding domain of caveolin-1 or the scrambled control peptide, were synthesized as previously described (2) by the W. M. Keck Biotechnology Resource Laboratory (Yale University). Peptides were synthesized with rhodamine at the NH2 terminus to demonstrate cell internalization.
Statistical analysis.
Values are means ± SE from at least three experiments, with different animals used for each experiment. Data were analyzed with ANOVA followed by post hoc tests, and Instat 3.0 (GraphPad Software, San Diego, CA) was used for statistical analysis; P < 0.05 was considered statistically significant.
RESULTS
Caveolin-1 is present in E19 fetal type II epithelial cells.
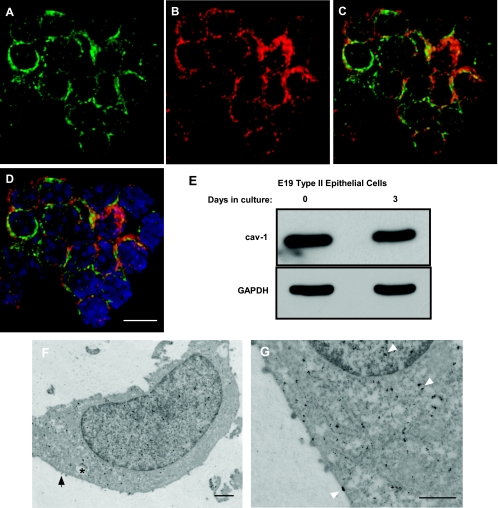
To assess the potential role of caveolin-1 as a signaling protein, we studied first whether caveolin-1 is present in E19 fetal type II epithelial cells. Figure 1, A–D, shows that a high percentage (85%) of freshly isolated cells that are positive for SP-C also stained for caveolin-1. In addition, Western blot experiments reveal the presence of caveolin-1 in suspended, freshly isolated E19 type II cells and in cells after 3 days in culture (Fig. 1E). By immunogold electron microscopy, caveolin-1 was detected not only in the plasma membrane, but also in the cytoplasm and nucleus, of cultured fetal type II epithelial cells (Fig. 1, F and G). Together, these results demonstrate the presence of caveolin-1 in freshly isolated and cultured E19 fetal type II epithelial cells.
Fig. 1.
Caveolin-1 is present in fetal type II epithelial cells. Freshly isolated embryonic day 19 (E19) type II cells were processed as described in materials and methods and stained for caveolin-1 (A) and surfactant protein C (SP-C, B). C: merged image. D: nuclear staining with 4′,6′-diamidino-2-phenylindole (blue). Note high nuclear-to-cytoplasm ratio of unseeded, freshly isolated type II cells. Scale bar, 10 μm. E: proteins from freshly isolated E19 type II epithelial cells or after 3 days in culture were collected and processed to detect the presence of caveolin-1 (cav-1). Blots were stripped and reprobed with GAPDH to control for protein loading. Blots are representative of 2 independent experiments. F and G: E19 type II cells were processed to investigate the presence of caveolin-1 protein by immunogold electron microscopy. A type II cell with dense inclusion (∗) and microvilli (arrow) is shown. Caveolin-1 is present in the cell membrane, cytoplasm, and nucleus (arrowheads in G). Scale bars, 500 nm.
Mechanical stretch translocates caveolin-1 from the plasma membrane to the cytoplasm.
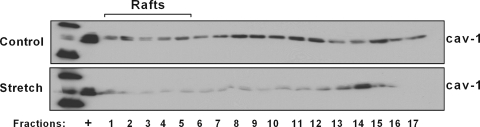
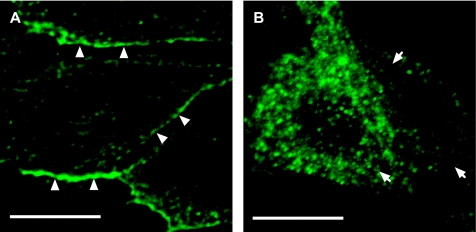
Caveolin-1 participates in mechanotransduction of endothelial cells (8). Stretch also translocates caveolins outside the caveolae in vascular smooth muscle cells (14). To investigate whether mechanical stretch affects caveolin-1 localization, E19 fetal type II epithelial cells were exposed to 5% cyclic stretch for 15 min. With use of a simplified method for the preparation of detergent-free lipid rafts (20), Fig. 2 shows a significant decrease of caveolin-1 protein distribution in the lipid rafts and heavy (nonbuoyant) fractions of the plasma membrane after mechanical stretch compared with unstretched samples. In addition, immunocytochemistry studies demonstrate that, under nonstretch conditions, caveolin-1 is localized preferentially in the plasma membrane. After 15 min of cyclic stretch, caveolin-1 staining shows a diffuse localization in the cytoplasm (Fig. 3). These data, along with Fig. 2, suggest that mechanical stretch translocates caveolin-1 from the plasma membrane into the intracellular spaces.
Fig. 2.
Mechanical stretch translocates caveolin-1 outside the plasma membrane. E19 type II cells were exposed to 5% cyclic stretch for 15 min; unstretched samples were used as controls. Cells were lysed, and the lipid raft and nonraft membrane fractions were separated as described in materials and methods. Equal volumes from each fraction were analyzed by SDS-PAGE followed by Western blotting for caveolin-1. +, Positive control for caveolin-1 protein. Blot is representative of 3 independent experiments.
Fig. 3.
Effect of mechanical stretch on cellular localization of caveolin-1. Confocal fluorescence immunocytochemistry images show that, under control conditions, caveolin-1 is detected mostly in the plasma membrane (arrowheads in A). In contrast, after 15 min of mechanical strain, caveolin-1 portrays a more diffuse pattern, located mostly inside the cytoplasm (arrows in B). Scale bars, 10 μm.
Lipid raft disruption increases strain-induced ERK phosphorylation and SP-C mRNA expression.
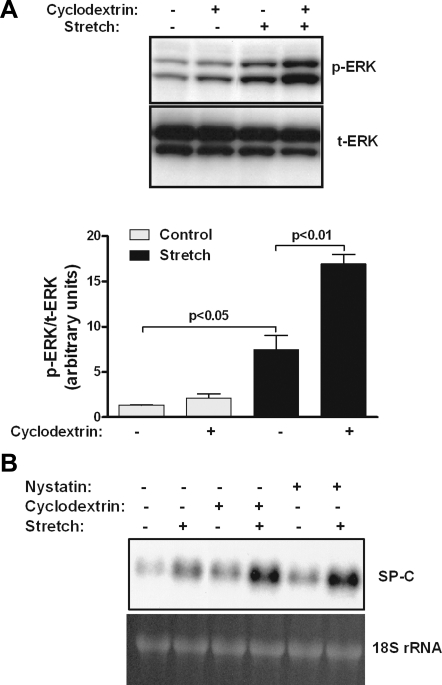
Previous studies from our laboratory showed that stretch-induced upregulation of SP-C (a marker of type II cell differentiation) is mediated via the ERK pathway (31). To investigate the potential role of caveolin as a signaling protein in mechanotransduction of fetal type II epithelial cells, lipid rafts were disrupted using cholesterol-chelating agents, such as methyl-β-cyclodextrin and nystatin. E19 type II cell monolayers were preincubated with cyclodextrin and then exposed to 5% cyclic stretch for 15 min. As expected, mechanical stretch activated ERK by fivefold compared with control samples (Fig. 4A). The addition of cyclodextrin did not significantly affect ERK stimulation under control conditions; however, after 15 min of stretch, ERK phosphorylation was further stimulated by 2.5-fold compared with stretched samples without disruptor. Similar results were obtained on stretch-induced SP-C mRNA expression in cells incubated with cyclodextrin or nystatin (Fig. 4B). These experiments indicate that disruption of lipid rafts produces additional stimulation of strain-induced ERK phosphorylation and SP-C mRNA expression. However, these studies are unable to distinguish whether these effects are due to the loss of cholesterol from the plasma membrane, decrease of caveolin from the lipid rafts, or increase of nonraft caveolin.
Fig. 4.
Effects of lipid raft inhibitors on ERK phosphorylation and SP-C mRNA expression. A: E19 monolayers were incubated (or not) with the lipid raft disruptor cyclodextrin (10 mmol/l) and then exposed to 5% cyclic stretch for 15 min. Proteins were collected and processed to detect ERK activation [phosphorylated ERK (p-ERK)] by Western blotting. Blots were stripped and reprobed with total ERK (t-ERK) to control for protein loading. Blots represent results from 3 separate experiments. B: E19 type II cells were plated on Silastic membranes and maintained in culture overnight. On the next day, monolayers were preincubated (or not) with a cholesterol-chelating agent [methyl-β-cyclodextrin (10 mmol/l) or nystatin (1 μg/ml)] for 1 h and then subjected to 5% cyclic strain for 16 h. SP-C mRNA expression was analyzed by Northern blotting. Top blot represents results from 2 independent experiments. Bottom blot shows 18S rRNA to control for sample loading and RNA integrity.
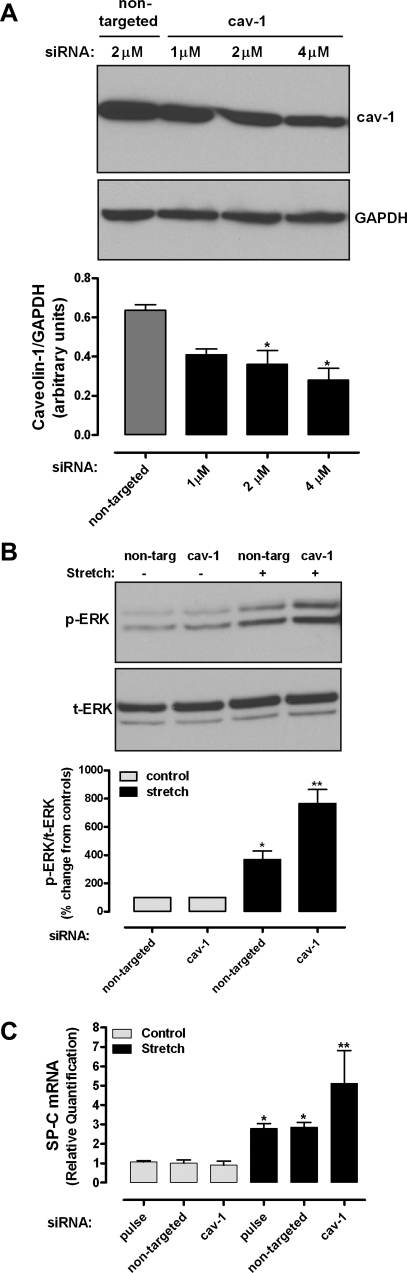
Downregulation of caveolin-1 increases stretch-induced ERK phosphorylation and SP-C mRNA expression.
To investigate further the role of caveolin-1 in mechanotransduction, caveolin-1 was knocked down by siRNA. E19 type II cells were transfected by electroporation as described in materials and methods. Control experiments showed 30%, 45%, and 55% reduction of caveolin-1 protein abundance after transfection of caveolin-1 siRNA at 1.5, 2, and 4 μM, respectively, compared with nontargeted siRNA (Fig. 5A). We then studied the effects of caveolin-1 inhibition on ERK phosphorylation and SP-C mRNA expression. Figure 5B shows that, in samples transfected with nontargeted siRNA, mechanical stretch increased ERK phosphorylation by 3.7-fold. Knockdown of caveolin-1 stimulated further stretch-induced ERK phosphorylation by twofold compared with nontargeted stretch samples. Similarly, mechanical stretch increased SP-C mRNA expression by 2.8-fold in negative control samples (electrical pulse only) or in samples transfected with nontargeted siRNA. In samples transfected with caveolin-1 siRNA, stretch further increased SP-C mRNA by approximately twofold. Taken together, these data suggest that caveolin-1 functions as an inhibitor of stretch-induced ERK phosphorylation and SP-C gene expression.
Fig. 5.
Effects of caveolin-1 small interference RNA (siRNA) on ERK phosphorylation and SP-C mRNA expression. A: E19 type II cells were transfected by electroporation, as described in materials and methods, with nontargeted siRNA or caveolin-1 siRNA at different concentrations. Two days later, proteins were collected and processed by Western blotting to detect caveolin-1 abundance. Blots were reprobed with GAPDH to control for protein loading. Blots represent results from 3 independent experiments. *P < 0.05 vs. nontargeted (Dunnett's test). B: E19 cells were transfected with nontargeted or caveolin-1 siRNA (2 μM) and then exposed to 5% cyclic stretch for 15 min. Proteins were collected and processed to detect ERK phosphorylation by Western blotting. Blots represent results from 3 independent experiments. Values, expressed as percent change from controls normalized to 100, are from 3 independent experiments. *P < 0.05 vs. nontargeted control; **P < 0.001 vs. nontargeted stretch. C: E19 type II cells were transfected by electroporation with nothing (pulse only), nontargeted siRNA, or caveolin-1 siRNA (2 μM). Total RNA was reverse-transcribed, and the cDNA product for SP-C was analyzed by quantitative RT-PCR using the ΔΔCT method for relative quantification. *P < 0.001 vs. respective controls; **P < 0.01 vs. pulse or nontargeted siRNA stretch (n = 4).
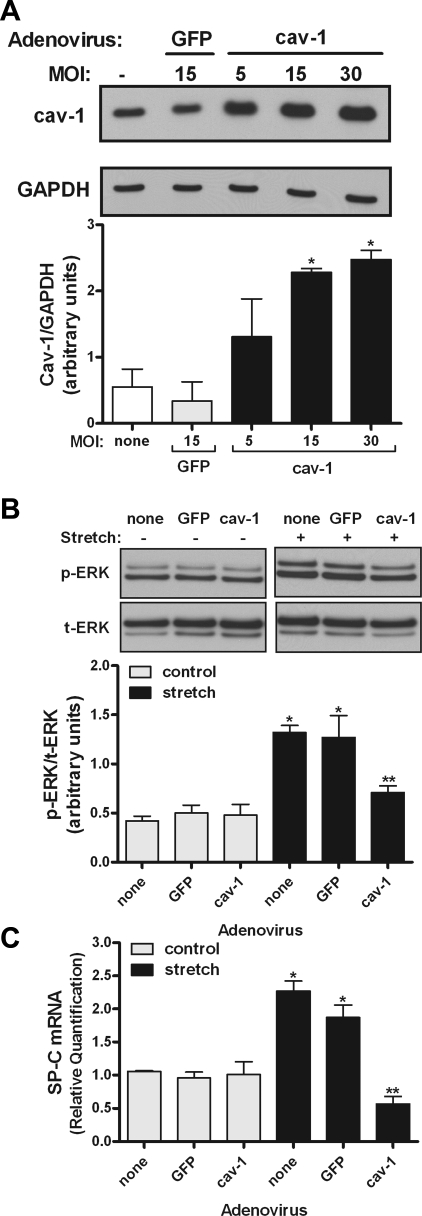
Overexpression of caveolin-1 or delivery of caveolin-1 scaffolding domain peptide decreases stretch-induced ERK activation and SP-C mRNA expression.
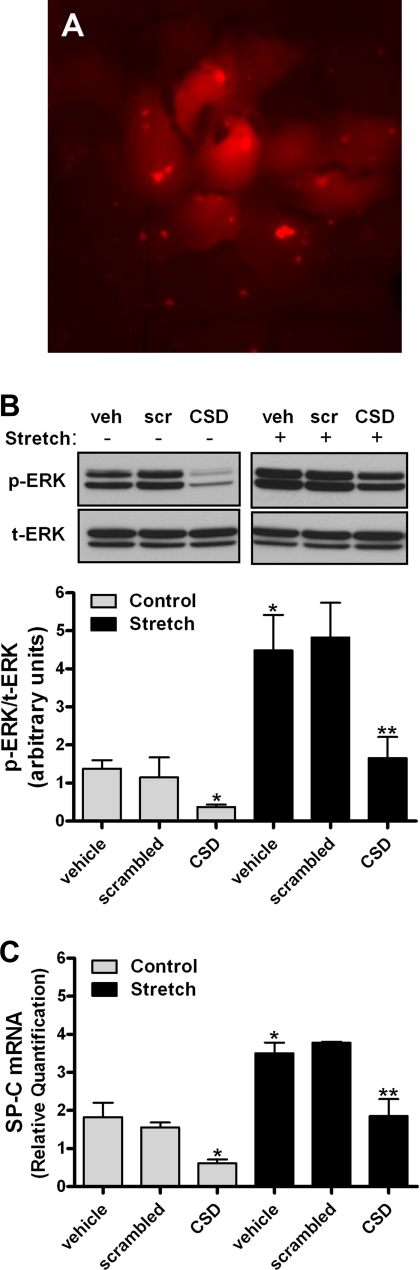
To support these previous findings, wild-type caveolin-1 gene was overexpressed using adenovirus vectors. Figure 6A demonstrates that adenovirus infection increased caveolin-1 protein. Figure 6B shows that mechanical stretch increased ERK phosphorylation by threefold in noninfected cells or in cells infected with the negative control GFP. In samples infected with caveolin-1 gene, ERK phosphorylation decreased by 50% compared with stretched samples without adenoviruses. Similarly, adenovirus infection with caveolin-1 significantly decreased SP-C mRNA by 75% compared with stretched samples without adenovirus or adenovirus expressing GFP. To more specifically address whether translocation of caveolin-1 from the plasma membrane into the cytoplasm by stretch negatively regulates stretch-induced differentiation, caveolin-1 scaffolding domain (CSD) peptide was delivered inside the cells, as previously described (2). Figure 7A demonstrates internalization of the peptide into type II cells after 6 h of incubation. ERK phosphorylation and SP-C mRNA expression decreased 50–75% under control and stretch conditions in E19 monolayers incubated with CDS compared with vehicle or scrambled peptide (Fig. 7, B and C). These data, along with the results from Figs. 5 and 6, support a negative role of caveolin-1 in stretch-induced type II cell differentiation via the ERK pathway.
Fig. 6.
Effect of adenovirus overexpressing wild-type caveolin-1 on ERK phosphorylation and SP-C mRNA expression. A: E19 type II cells were infected with adenovirus expressing wild-type caveolin-1 at different multiplicity of infection (MOI) or negative control green fluorescent protein (GFP). Two days later, proteins were collected and processed by Western blotting to detect caveolin-1 abundance. Blots were reprobed with GAPDH to control for protein loading. Blots represent results from 3 independent experiments. *P < 0.05 vs. none or GFP (Tukey's multiple comparisons test). B: E19 cells were infected with 15 MOI of adenovirus expressing caveolin-1 and then exposed to 5% cyclic stretch for 15 min. Proteins were collected and processed to detect ERK phosphorylation by Western blotting. Blots represent results from 3 independent experiments. *P < 0.001 vs. matched controls; **P < 0.05 vs. stretch none or adenovirus GFP (n = 3). C: E19 type II cells were infected with nothing (none) or adenoviruses expressing GFP (negative control) or caveolin-1 (all at 15 MOI). RNA was extracted and samples were processed by quantitative RT-PCR to detect SP-C mRNA expression. *P < 0.05 vs. matched controls; **P < 0.01 vs. stretch none or adenovirus GFP (n = 4).
Fig. 7.
Caveolin-1 scaffolding domain (CSD) inhibits ERK phosphorylation and SP-C gene expression. A: fluorescence immunocytochemistry images demonstrating uptake of the rhodamine-labeled CSD peptide by type II cells after 6 h of incubation (1 μM). Original magnification ×20. B and C: E19 monolayers were incubated for 6 h with vehicle (veh) or scrambled (scr) peptide or CSD peptide (both at 1 μM) and then exposed to 5% cyclic stretch for 15 min (B) or 16 h (C). Proteins and RNA were collected and processed to detect ERK activation or SP-C gene expression. *P < 0.05 vs. control vehicle or scrambled; **P < 0.05 vs. stretch vehicle or scrambled (n = 4). Blots in B represent results from 4 independent experiments.
DISCUSSION
The main findings of this study are that caveolin-1 is present and has a role in mechanotransduction of E19 fetal type II epithelial cells. Caveolin-1 is translocated from the plasma membrane to the cytoplasm by mechanical stretch and functions as an inhibitory protein in stretch-induced ERK phosphorylation and SP-C mRNA expression.
Caveolin-1 is a well-known marker of type I epithelial cell phenotype (5, 42). No caveolin-1 (3) or low levels of caveolin-1 (9, 22) have been previously detected in adult type II epithelial cells. Furthermore, caveolin-1 expression is induced when primary type II cells are cultured on plastic substrates for several days (3). Therefore, to account for a potential dedifferentiation of type II cells into type I phenotype in our experimental system and to show the presence of caveolin-1 in fetal type II cells, caveolin-1 protein abundance was investigated in freshly isolated cells by immunocytochemistry and Western blotting. Our results from Fig. 1, A–D, demonstrate the presence of caveolin-1 in 85% of cells that also stained positive for SP-C, indicating that caveolin-1 is present in E19 type II epithelial cells. In addition, immunogold electron microscopy analysis revealed the presence of caveolin-1 in the plasma membrane, cytoplasm, and nucleus of E19 type II epithelial cells. Other studies showed that caveolin-1 is not only an integral membrane protein but also a soluble protein present in multiple cellular compartments (4, 18). Additionally, our Western blot findings suggest that cultured E19 cells still retain the type II cell phenotype, given that the amount of caveolin-1 did not change significantly after 3 days in culture compared with freshly isolated cells (Fig. 1E). Together, these studies point to some phenotypic differences between fetal and adult type II cells. Although we cannot completely rule out the contribution of minor contamination with other cell types during the isolation process and some degree of dedifferentiation after days in culture for some of these results, we postulate that fetal type II cells have a less defined “terminal” phenotype than differentiated adult type II cells. This hypothesis is supported by previous studies in developing lungs demonstrating the presence of “intermediate” cells possessing type II and type I phenotypes (7, 21).
By lipid raft-rich membrane fractions and fluorescence immunocytochemistry, we found that mechanical stretch translocates caveolin-1 from the plasma membrane into the cytoplasm. With use of a simplified method for the preparation of the lipid rafts (20), caveolin-1 was also detected in the nonraft fractions of the plasma membrane. An explanation for these observations could be an incomplete disruption of the association of the rafts with the actin cytoskeleton during the lysis procedure, resulting in the generation of relatively heavy raft membranes (20). However, these results are also consistent with the detection of caveolin in regions of the plasma membrane separate from the lipid rafts and caveolae microdomains (11). In either case, our results clearly demonstrate a decrease of caveolin-1 in the plasma membrane induced by mechanical stretch. Past studies have also shown translocation of caveolin-1 from the membrane to the cytoplasm after shear stress (37), cholesterol oxidation (35), heat shock, or hyperosmotic stress (13). In contrast, other investigations found that caveolin-1 is translocated to noncaveolar membrane sites in response to cyclic stretch (14). Differences in cell type, stimulus applied, and role in tissues can account for these results; however, all these studies clearly demonstrate that caveolin-1 localization is influenced by mechanical forces.
Previous studies from our laboratory showed that stretch-induced fetal type II cell differentiation (using SP-C as a marker) is mediated via the ERK pathway (31). Therefore, we investigated how lipid rafts in general, and caveolin-1 in particular, affect stretch-induced fetal type II cell differentiation. We observed that disruption of the lipid rafts with cholesterol-chelating agents further enhanced stretch-induced ERK phosphorylation and SP-C mRNA expression compared with stretch samples without disruptors. Contradictory results have been found on ERK pathway activation by cholesterol-chelating agents. In some studies, depletion of cholesterol was found to inhibit stress-induced ERK activation (23–24, 44); in other investigations, the opposite was found (10, 14). It has been suggested that disruption of lipid rafts with cholesterol-chelating agents decreases caveolin present in the caveolae and increases noncaveolar caveolin (14). Therefore, our results would be consistent with the CSD hypothesis, in which caveolin-1 inside the lipid rafts inhibits cell signaling. Upon release by disruption of the lipid rafts, caveolin would allow interaction with other signaling proteins and stimulate the ERK pathway and SP-C gene expression. However, the findings that signal transduction is altered when lipid rafts are disrupted by depletion of cholesterol may also suggest that cholesterol-rich domains participate in the control of cell signaling (25, 26, 39). In fact, it has been proposed that lipid rafts themselves form a scaffold that organizes multiple signaling molecules, and the integrity of this lipid scaffold depends on cholesterol (18, 19). One good example is the interaction between the platelet-derived growth factor receptor and multiple signaling molecules in caveolae, in which cholesterol is required for coupling of the platelet-derived growth factor receptor with its normal substrates (19). A function for caveolin-1 would be to maintain the lipid scaffold by removing oxidized cholesterol and replacing it with new cholesterol (15).
To further clarify the role of caveolin in mechanotransduction, caveolin-1 expression in fetal type II cells was altered using siRNA and adenovirus methods. Our results are consistent with the hypothesis that caveolin-1 is a negative regulator of differentiation of fetal type II epithelial cells exposed to stretch, given that downregulation of caveolin-1 increased stretch-induced ERK phosphorylation and SP-C gene expression. Opposite results were observed when caveolin-1 was overexpressed using adenovirus vectors. However, these investigations are unable to distinguish whether this inhibitory effect of caveolin occurs at the plasma membrane level, as a soluble protein, or both. To elucidate this issue, E19 type II cell monolayers were incubated with CSD peptide. The CSD, a yuxtamembrane region of the caveolin-1 protein, plays a critical role in signal transduction by binding to multiple signaling molecules (26, 33, 34). In general, interactions with CSD suppress the activity of binding proteins (18). Consistent with these observations, our findings show that delivery of CSD peptide inside the cells inhibits ERK activation and SP-C gene expression (Fig. 7). Although the exact mechanism for this inhibitory effect is unknown, we speculate that translocation of caveolin-1 outside the plasma membrane by mechanical stretch would allow the CSD to interact and inhibit ERK phosphorylation and, eventually, SP-C gene expression.
In summary, our data demonstrate the presence of caveolin-1 in freshly isolated and cultured E19 fetal type II epithelial cells. Caveolin-1 is translocated from the plasma membrane to the cytoplasm by mechanical stretch and functions as an inhibitory protein in stretch-induced type II cell differentiation via the ERK pathway. These studies provide additional information on how mechanical forces may influence fetal lung development in general and fetal type II cell differentiation in particular.
GRANTS
This work was supported by National Institute of Child Health and Human Development Grant HD-052670 (J. Sanchez-Esteban).
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Brenda Vecchio for manuscript preparation, Carol Ayala for technical assistance with the electron microscopy experiments, and Virginia Hovanesian for assistance with confocal microscopy analysis.
REFERENCES
- 1.Boyd NL, Park H, Yi H, Boo YC, Sorescu GP, Sykes M, Jo H. Chronic shear induces caveolae formation and alters ERK and Akt responses in endothelial cells. Am J Physiol Heart Circ Physiol 285: H1113–H1122, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Campbell L, Hollins AJ, Al-Eid A, Newman GR, von Ruhland C, Gumbleton M. Caveolin-1 expression and caveolae biogenesis during cell transdifferentiation in lung alveolar epithelial primary cultures. Biochem Biophys Res Commun 262: 744–751, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Chretien A, Piront N, Delaive E, Demazy C, Ninane N, Toussaint O. Increased abundance of cytoplasmic and nuclear caveolin 1 in human diploid fibroblasts in H2O2-induced premature senescence and interplay with p38αMAPK. FEBS Lett 582: 1685–1692, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Dahlin K, Mager EM, Allen L, Tigue Z, Goodglick L, Wadehra M, Dobbs L. Identification of genes differentially expressed in rat alveolar type I cells. Am J Respir Cell Mol Biol 31: 309–316, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31). FEBS Lett 429: 330–336, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Flecknoe S, Harding R, Maritz G, Hooper SB. Increased lung expansion alters the proportions of type I and type II alveolar epithelial cells in fetal sheep. Am J Physiol Lung Cell Mol Physiol 278: L1180–L1185, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Frank PG, Lisanti MP. Role of caveolin-1 in the regulation of the vascular shear stress response. J Clin Invest 116: 1222–1225, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs S, Hollins AJ, Laue M, Schaefer UF, Roemer K, Gumbleton M, Lehr CM. Differentiation of human alveolar epithelial cells in primary culture: morphological characterization and synthesis of caveolin-1 and surfactant protein-C. Cell Tissue Res 311: 31–45, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Furuchi T, Anderson RG. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal-related kinase (ERK). J Biol Chem 273: 21099–21104, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol 17: 51–57, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Hooper SB, Wallace MJ. Role of physical, endocrine and growth factors in lung development. In: The Lung: Development, Aging, and the Environment, edited by Harding R, Pinkerton KE, Plopper CG. London: Elsevier Academic, 2004, p. 131–148 [Google Scholar]
- 13.Kang YS, Ko YG, Seo JS. Caveolin internalization by heat shock or hyperosmotic shock. Exp Cell Res 255: 221–228, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Kawabe J, Okumura S, Lee MC, Sadoshima J, Ishikawa Y. Translocation of caveolin regulates stretch-induced ERK activity in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 286: H1845–H1852, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Laude AJ, Prior IA. Plasma membrane microdomains: organization, function and trafficking. Mol Membr Biol 21: 193–205, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee HS, Wang Y, Maciejewski BS, Esho K, Fulton C, Sharma S, Sanchez-Esteban J. Interleukin-10 protects cultured fetal rat type II epithelial cells from injury induced by mechanical stretch. Am J Physiol Lung Cell Mol Physiol 294: L225–L232, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Liu M, Post M. Mechanochemical signal transduction in the fetal lung. J Appl Physiol 89: 2078–2084, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem 277: 41295–41298, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Liu P, Wang P, Michaely P, Zhu M, Anderson RG. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J Biol Chem 275: 31648–31654, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Macdonald JL, Pike LJ. A simplified method for the preparation of detergent-free lipid rafts. J Lipid Res 46: 1061–1067, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Mercurio AR, Rhodin JA. An electron microscopic study on the type I pneumocyte in the cat: differentiation. Am J Anat 146: 255–271, 1976 [DOI] [PubMed] [Google Scholar]
- 22.Newman GR, Campbell L, von Ruhland C, Jasani B, Gumbleton M. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium: implications for alveolar epithelial type I cell function. Cell Tissue Res 295: 111–120, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Park H, Go YM, Darji R, Choi JW, Lisanti MP, Maland MC, Jo H. Caveolin-1 regulates shear stress-dependent activation of extracellular signal-regulated kinase. Am J Physiol Heart Circ Physiol 278: H1285–H1293, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Park H, Go YM, John PL, Maland MC, Lisanti MP, Abrahamson DR, Jo H. Plasma membrane cholesterol is a key molecule in shear stress-dependent activation of extracellular signal-regulated kinase. J Biol Chem 273: 32304–32311, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Pike LJ. Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta 1746: 260–273, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res 44: 655–667, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Rizzo V, Morton C, DePaola N, Schnitzer JE, Davies PF. Recruitment of endothelial caveolae into mechanotransduction pathways by flow conditioning in vitro. Am J Physiol Heart Circ Physiol 285: H1720–H1729, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Esteban J, Cicchiello LA, Wang Y, Tsai SW, Williams LK, Torday JS, Rubin LP. Mechanical stretch promotes alveolar epithelial type II cell differentiation. J Appl Physiol 91: 589–595, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Esteban J, Tsai SW, Sang J, Qin J, Torday JS, Rubin LP. Effects of mechanical forces on lung-specific gene expression. Am J Med Sci 316: 200–204, 1998 [PubMed] [Google Scholar]
- 30.Sanchez-Esteban J, Wang Y, Filardo EJ, Rubin LP, Ingber DE. Integrins β1, α6, and α3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol 290: L343–L350, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Esteban J, Wang Y, Gruppuso PA, Rubin LP. Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am J Respir Cell Mol Biol 30: 76–83, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Sedding DG, Hermsen J, Seay U, Eickelberg O, Kummer W, Schwencke C, Strasser RH, Tillmanns H, Braun-Dullaeus RC. Caveolin-1 facilitates mechanosensitive protein kinase B (Akt) signaling in vitro and in vivo. Circ Res 96: 635–642, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1: 31–39, 2000 [DOI] [PubMed] [Google Scholar]
- 34.Smart EJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, Okamoto T, Lisanti MP. Caveolins, liquid-ordered domains, and signal transduction. Mol Cell Biol 19: 7289–7304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smart EJ, Ying YS, Conrad PA, Anderson RG. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol 127: 1185–1197, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stan RV. Structure of caveolae. Biochim Biophys Acta 1746: 334–348, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Sun RJ, Muller S, Stoltz JF, Wang X. Shear stress induces caveolin-1 translocation in cultured endothelial cells. Eur Biophys J 30: 605–611, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Torday JS, Rehan VK. Stretch-stimulated surfactant synthesis is coordinated by the paracrine actions of PTHrP and leptin. Am J Physiol Lung Cell Mol Physiol 283: L130–L135, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M, Hilenski L, Santanam N, Becker PL, Ma Y, Griendling KK, Alexander RW. Cholesterol depletion inhibits epidermal growth factor receptor transactivation by angiotensin II in vascular smooth muscle cells: role of cholesterol-rich microdomains and focal adhesions in angiotensin II signaling. J Biol Chem 276: 48269–48275, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Maciejewski BS, Lee N, Silbert O, McKnight NL, Frangos JA, Sanchez-Esteban J. Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am J Physiol Lung Cell Mol Physiol 291: L820–L827, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Maciejewski BS, Soto-Reyes D, Lee HS, Warburton D, Sanchez-Esteban J. Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-α. J Physiol 587: 1739–1753, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol 65: 669–695, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Yu J, Bergaya S, Murata T, Alp IF, Bauer MP, Lin MI, Drab M, Kurzchalia TV, Stan RV, Sessa WC. Direct evidence for the role of caveolin-1 and caveolae in mechanotransduction and remodeling of blood vessels. J Clin Invest 116: 1284–1291, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeidan A, Broman J, Hellstrand P, Sward K. Cholesterol dependence of vascular ERK½ activation and growth in response to stretch: role of endothelin-1. Arterioscler Thromb Vasc Biol 23: 1528–1534, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Zhang B, Peng F, Wu D, Ingram AJ, Gao B, Krepinsky JC. Caveolin-1 phosphorylation is required for stretch-induced EGFR and Akt activation in mesangial cells. Cell Signal 19: 1690–1700, 2007 [DOI] [PubMed] [Google Scholar]