Abstract
The recovery of an intact epithelium following lung injury is critical for restoration of lung homeostasis. The initial processes following injury include an acute inflammatory response, recruitment of immune cells, and epithelial cell spreading and migration upon an autologously secreted provisional matrix. Injury causes the release of factors that contribute to repair mechanisms including members of the epidermal growth factor and fibroblast growth factor families (TGF-α, KGF, HGF), chemokines (MCP-1), interleukins (IL-1β, IL-2, IL-4, IL-13), and prostaglandins (PGE2), for example. These factors coordinate processes involving integrins, matrix materials (fibronectin, collagen, laminin), matrix metalloproteinases (MMP-1, MMP-7, MMP-9), focal adhesions, and cytoskeletal structures to promote cell spreading and migration. Several key signaling pathways are important in regulating these processes, including sonic hedgehog, Rho GTPases, MAP kinase pathways, STAT3, and Wnt. Changes in mechanical forces may also affect these pathways. Both localized and distal progenitor stem cells are recruited into the injured area, and proliferation and phenotypic differentiation of these cells leads to recovery of epithelial function. Persistent injury may contribute to the pathology of diseases such as asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis. For example, dysregulated repair processes involving TGF-β and epithelial-mesenchymal transition may lead to fibrosis. This review focuses on the processes of epithelial restitution, the localization and role of epithelial progenitor stem cells, the initiating factors involved in repair, and the signaling pathways involved in these processes.
Keywords: wound healing, alveolar epithelium, airway epithelium, mechanotransduction
injury to the lung epithelium can be caused by bacterial or viral infections, inflammation, allergic reactions (asthma), exposure to xenobiotics (e.g., cigarette smoke), physical trauma (mechanical ventilation), cancer, or pathology of unknown origin (idiopathic fibrosis). Depending on the nature of the injury, lung repair mechanisms are initiated immediately following the insult and include an acute inflammatory response involving cytokine release, immune cell recruitment, and activation of the coagulation cascade (Fig. 1). Injury can occur in both proximal and distal airways, but the initial response of epithelial cells in different regions is similar: nearby progenitor cells migrate and spread to cover denuded surfaces. During the progression of the wound repair process, these localized progenitor cells, as well as newly recruited circulating progenitor cells, proliferate and undergo phenotypic differentiation to reestablish the integrity and functional organization of the epithelial layer. While the early repair processes in response to various types of injury are similar, the response to persistent injury may vary markedly in different regions of the lung due to variations in tissue architecture, matrix properties, level of oxygenation, proximity to the vasculature and/or lymphatics, presence of surfactant or mucous secretions, mechanical properties of underlying parenchyma, and the type of resident progenitor cell in different regions. Such regional differences when coupled with persistent injury may contribute to the pathology of lung diseases. For example, repeated exposure to cigarette smoke elicits a chronic cycle of injury and repair that leads to significant changes in the structure, function, and gene expression of airway and alveolar epithelial cells that contributes to chronic obstructive pulmonary disease (COPD) in a subset of smokers (reviewed in Refs. 24, 63, 125, 129, 132, 158). Others have outlined a process in which a repetitive or recurrent injury to the alveolar-capillary membrane causes loss of basement membrane integrity and activation of fibroblasts/myofibroblasts that results in dysregulated and exaggerated repair processes leading to fibrosis (61, 160, 191). Similarly, there is growing support for the hypothesis that inappropriate repair of airway epithelial cells following repeated exposures to allergens or other environmental toxins drives airway remodeling and hyperresponsiveness in asthma (26, 58, 64, 65, 100). In this review, we describe the different types of progenitor cells that facilitate lung epithelial repair, as well as the processes of spreading, migration, proliferation, and differentiation. We also discuss the major signaling pathways that have been shown to regulate these repair mechanisms in the lung and how interactions among these pathways can lead to dysregulated repair.
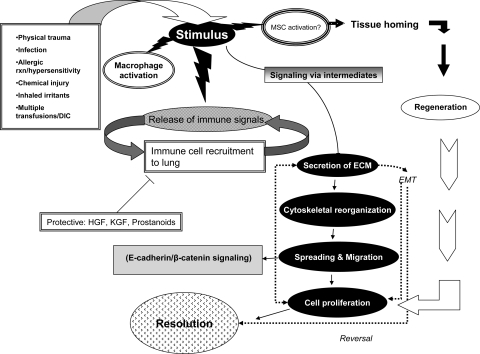
Fig. 1.
Sequence of events in epithelial repair. Following injury, macrophages, neutrophils, and localized stem cells become activated, and growth factors, cytokines, interleukins, and matrix materials are released. Some of these factors recruit other immune cells to the site of injury. Cells adjacent to the site of injury secrete extracellular matrix (ECM) through integrin and growth factor signaling, reorganize their cytoskeleton to facilitate movement, secrete MMPs that degrade cell-matrix attachments, and spread and migrate along the autologously secreted provisional matrix using filopodial and lamellipodial extensions. Both localized and recruited progenitor cells proliferate and continue to secrete ECM. Dysregulated repair may lead to epithelial-mesenchymal transition (EMT) and fibrosis. Resolution occurs by means of cell cycle arrest, apoptosis, disappearance of immune cells, and deactivation of macrophages.
EPITHELIAL RESTITUTION
As described above, epithelial restitution involves spreading and migration of neighboring epithelial cells to cover the denuded area, and this is followed by migration and proliferation of progenitor cells to restore cell numbers and differentiation to restore function (125, 161, 179, 198, 200, 201). In the airways, the first 12–24 h after injury involve cell spreading and migration as the primary repair mechanisms, while proliferation begins by 15–24 h and continues for days to weeks. This has been demonstrated in vivo by examining epithelial wound repair in the guinea pig (36–38, 76) and rat (67) trachea. The recovery of a pseudostratified epithelium may require several weeks (34). In vivo repair of the alveolar epithelium has been more difficult to examine, and most studies have focused on alveolar type II (ATII) cell proliferation as an index of repair (115, 130, 196). Thus, considerably less is known about the early mechanisms of epithelial repair at the alveolar level. However, the progression of spreading, migration, and proliferation has been demonstrated using cultured ATII cells (72). Nevertheless, one of the major challenges in the field is the development of models and methods to examine epithelial repair mechanisms directly in the lung, particularly in the alveoli.
The concept that the initial wound closure of pulmonary epithelial cells occurs primarily by mechanisms involving cell spreading and migration is supported by studies involving other types of epithelium including kidney (MDCK) (45), cornea (25), and gut (101). The mechanisms of epithelial wound healing involve interactions between initiating factors, extracellular matrix (ECM) components, signaling pathways, and structural components, some of which are discussed in more detail below. The dynamic remodeling that occurs during cell spreading and migration relies upon the establishment of directional polarity of the migrating cells and extension of filopodial and lamellipodial protrusions in the direction of migration (71, 80, 88, 139), and RhoA and Rac1 are prominent signaling intermediates in these processes. During cell movement into the wound area, the actin cytoskeleton interacts with focal adhesion complexes and integrins. Integrins associate with ECM components and provide traction for the cell as it pulls forward. The driving force for this mechanism is the contraction of actomyosin complexes. It has been suggested that the actin bundles at the wound edge can serve to distribute forces created by actively moving cells more evenly to nearby cells (45). We previously observed the formation of these prominent actin bundles at the wound edge of both human bronchial epithelial cells (28) and rat ATII cells (29). Adhesion sites provide the traction for the cells during these processes and act as the signaling fulcrum between the ECM and cellular cytoskeleton. Two types of adhesion sites have been described: focal adhesions that connect to actin stress fiber bundles, and focal complexes that associate with actin filament networks and filopodia (154). Focal complexes can become focal adhesions through changes in activity of members of the Rho GTPase family (142).
PROGENITOR CELLS
While eye, gut, and skin epithelia constantly renew by proliferation and migration of stem cells, lung epithelial cells renew slowly and are stimulated to self-renew upon injury (39, 41, 51, 52, 66, 136, 161). Measurements of [3H]thymidine incorporation indicated that less than 1% of mouse bronchiolar epithelial cells entered S-phase in 24 h, and this rate increased >10-fold upon injury (134). Similar measurements of bromodeoxyuridine (BrdU) incorporation in rat lungs indicated labeling indices of 0.7% and 0.3% for alveolar and bronchial epithelial cells, respectively (195). In the airways, non-ciliated epithelial Clara cells of the lung are believed to function as facultative progenitor cells, normally quiescent but capable of returning to proliferation after attrition or injury (161). Such cells, determined by pulse-chase experiments to be proliferating, were devoid of secretory granules and smooth endoplasmic reticulum (39, 41). After replenishment of ciliated cells, the Clara cell is believed to return to quiescence. Redifferentiation occurs days after epithelial injury, reestablishing mucociliary function (39). At the alveolar level, the ATII cell is generally considered to be the facultative progenitor cell for the recovery of both the type I (ATI) and type II pneumocyte pools, with the capability to differentiate into ATI cells following lung injury (105, 106, 163). However, it should be pointed out that the evidence in support of the role of ATII cells as progenitors is based in part on early studies showing increased ATII cell proliferation following injury and the incorporation of 3H-thymidine in ATII cells followed by incorporation into ATI cells (2, 40). Further support has been provided by studies demonstrating that primary cultures of ATII cells are capable of differentiating into ATI-like cells (9, 10, 33, 69, 138). However, new evidence has emerged that rat ATI cells also proliferate, express Oct4 (a pluripotency marker), and show phenotypic plasticity in vitro (54, 127). Kim et al. (74) have described bronchioalveolar stem cells isolated from the bronchoalveolar duct that putatively maintain both Clara cells and alveolar epithelial cells, but others have suggested a clear distinction between these two cell compartments (161). Thus, definitive identification of alveolar progenitor and stem cells remains elusive and is an important challenge in the field.
While the localization and identification of adult epithelial progenitor/stem cells in the lungs is ongoing, several niche populations have been studied (reviewed in Refs. 99, 161, 178) primarily through rodent models of lung injury. Liu and Engelhardt (99) propose five putative stem cell niches in the mouse lung that include an unidentified cell type in the submucosal glands of the proximal trachea (35), basal cells within the intercartilagenous zones of the trachea and bronchi (11, 141, 151), variant Clara cells (Clarav) associated with neuroendocrine cells that form neuroepithelial bodies residing in the bronchioles (66, 135), Clarav cells associated with bronchiolar alveolar duct junctions (52, 74), and ATII cells in the alveoli. Recent reports have suggested other stem cell niches in the lungs that participate in the repair of airway epithelium. In mice after naphthalene wounding, ciliated bronchiolar epithelial cells, previously believed to be terminally differentiated, underwent squamous metaplasia with upregulated expression of transcription factors active during lung embryogenesis (β-catenin, FOXA2, FOXJ1, SOX17, SOX2) growing and spreading underneath Clara cells (119). The authors suggested that ciliated epithelial cells differentiated into both ciliated and non-ciliated cell types to repopulate the airway epithelium. In contrast, Rawlins et al. (130) found through heritable marker lineage-tracing experiments using FOXJ1CreEr2T mice that ciliated cells did not function as progenitors during development or after treatment with naphthalene or sulfur dioxide. However, other studies have demonstrated that ciliated cells morphologically differentiated into mucous-secreting cells in acute asthma or viral infection models (131, 167). Thus, there are still many unanswered questions regarding regional differences in cells responsible for maintenance and repair. Furthermore, the specific identification and localization of stem cell niches in the lung is complicated by the possibility of sequestering microenvironments that may be necessary to maintain epithelia with a relatively slow rate of turnover (161).
In addition to stem cell niches located within the lungs, several groups have provided intriguing evidence suggesting that bone marrow-derived stem cells (BMSC) can engraft into the lungs and differentiate into lung epithelial cells (reviewed in Ref. 86). One in vitro study demonstrated the capability of BMSC to express markers of bronchial epithelial cells and to repair an injured epithelial layer (155), whereas another proposed a model for the correction of the Cl− transport defect in cystic fibrosis patients using BMSC that had differentiated into lung epithelial cells (177). Several groups have suggested that BMSC may be useful in repair mechanisms following lung injury, but the mechanisms of engraftment and differentiation are poorly understood, and the results may depend on the injury model, the route of delivery, and the population of BMSC used. One group suggested that transplanted BMSC differentiated into ATI cells following bleomycin injury in mice (85), but no differentiation was seen in later studies by the same group using an irradiation model; the authors suggested that the earlier results may have been due to an artifact in the staining of the tissue (84). Ortiz and collaborators (114) used an enriched population of BMSC that were depleted of hematopoietic cell types, and demonstrated lung engraftment following bleomycin injury, the presence of donor-derived cells among isolated ATII cells, and reduced bleomycin-induced injury. Liebler et al. (98) showed that human BMSC were retained in the distal portion of the lungs in bleomycin-treated mice, suggesting that these cells could repopulate alveoli. Other groups have demonstrated attenuation of endotoxin-induced lung injury utilizing BMSC in mice (57, 194). A recent study demonstrated that allogeneic human BMSC or media conditioned by these cells was capable of restoring alveolar fluid clearance in isolated perfused human lungs following endotoxin-induced injury (91). Finally, a subpopulation of epithelial-like BMSC from humans and mice was identified that expressed CCSP (Clara cell specific protein) and alveolar type I and II markers, homed to the lung after naphthalene-induced injury, and successfully repopulated the airways of lethally irradiated mice (193). There are thus sources exogenous to the lung functioning as stem cell reservoirs able to repair the lung epithelium. The underlying mechanisms for recruitment and phenotypic differentiation, however, remain to be established.
PATHWAYS OF EPITHELIAL REPAIR
The process of epithelial repair outlined above is complex, involving interactions between multiple initiating factors, soluble ligands, structural elements, signaling pathways, and the mechanical environment. In the sections below, we will examine some of these components and how these interactions occur in bronchial and in alveolar epithelium. Figure 2 illustrates these repair processes in the airways, some specific cell types involved, and their roles, and Table 1 summarizes some of the many factors and pathways that have been shown to be involved in epithelial repair. While this review cannot exhaustively discuss all possible components and interactions, in the following sections we will focus on a limited number for which there is evidence of importance in the lung.
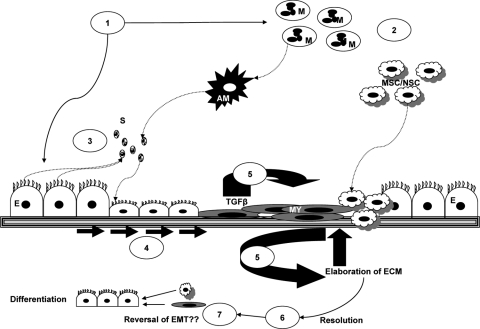
Fig. 2.
Processes involved in epithelial repair in the airways. Injury (1) initiates activation of macrophages (M) to activated macrophages (AM) and the recruitment of immune cells (2), mesenchymal stem cells (MSC), or niche stem cells (NSC). Soluble factors (S) secreted by immune and nonimmune cells (3) act on epithelial cells and fibroblasts, which spread and migrate (4) closing the wound and reestablishing intact barrier function. TGF-β signaling results in trans-differentiation of epithelial cells to myofibroblasts (MY), the EMT, and the initiation of a chronic inflammatory response (5) with further elaboration of ECM. During resolution (6), immune cells disappear from the site of injury, macrophages are deactivated, cells differentiate, and hyperproliferation is reduced through apoptosis, allowing tissue remodeling (7).
Table 1.
Summary of factors and pathways involved in epithelial repair
| Pathway or Factor | Functional Effect | Model System |
|---|---|---|
| Epidermal growth factor family | ||
| EGF, TGF-α, HB-EGF, EGFR | Enhanced epithelial repair via stimulation of mitosis, migration, spreading (72, 95, 96, 143, 171) | Mouse airways in vivo, neonatal 1° rabbit ATII, 1° rat ATII |
| Pulmonary fluid from ALI patients containing TGF-α stimulated wound healing (48) | A549 | |
| EGF-stimulated repair dependent on K+ currents (164) | 1° rat ATII | |
| Migration and EGFR activation decreased in cells from cystic fibrosis patients (165) | 1° HBEC | |
| EGFR | EGFR expression upregulated in asthma biopsies (126) | Human bronchial biopsies |
| EGF, HB-EGF | EGF stimulated migration and HB-EGF stimulated proliferation in asthma healing (77, 126) | GP AEC, 16HBE |
| Fibroblast growth factor family | ||
| HGF | Stimulated mitogenesis (104, 117) | Rat ATII in vitro/in vivo |
| Increased HGF levels in edema fluid from ALI, but not hydrostatic edema, patients (172) | Human ALI or hydrostatic edema patients | |
| Stimulated proliferation & migration (46) | Rat ATII | |
| Differentiation (111) | HBEC | |
| HGF & KGF | Increased levels in BAL of ALI & ARDS patients and increased mRNA & protein levels in cultured fibroblasts of ALI/ARDS patients (128) | Human BAL fluid, human fibroblasts in vitro |
| HGF | Stimulated migration but not proliferation (199) | Nasal HBEC |
| KGF | Stimulated migration, spreading, accelerated repair, mitogenesis (30, 47, 115, 118, 168) | 1° rat ATII, rat ATII in vivo |
| Directed (early) expression in lung epithelium caused pulmonary malformation and embryonic lethality in lung epithelium (153) | Transgenic mice expressing KGF | |
| Protective against hyperoxia, stimulated ATII proliferation (115) | Rats in vivo | |
| Increased cellular adhesion via EGFR activation (5) | 1° rat ATII | |
| Increased wound closure (blocked by inhibition of PKC) and overcame inhibition of repair caused by cyclic mechanical strain (181) | NHBE, Calu 3 | |
| Accelerated repair (53) | Heterotopic syngeneic tracheal transplants (mouse) | |
| Stimulated Clara cell proliferation (42) | Rats in vivo | |
| Rat ATII hyperplasia stimulated by KGF resolved spontaneously by differentiation to type I or II cells or apoptosis (43, 44) | Rats in vivo | |
| Protective against radiation or H2O2 (147, 182) | Calu 3, 16HBE | |
| Restoration of alveolar barrier function by BMSC dependent on release of KGF (92) | Ex vivo perfused human lung and BMSC | |
| HGF-MEK-ERK | Increased proliferation (162) | BEAS-2B, 16HBE |
| HGF-COX-2, Akt, MAPK, β-catenin | Increased proliferation (93) | NHBE |
| FGF-10-Grb2-SOS-Ras/RAF-1-ERK1/2 pathway | Reduced DNA damage caused by H2O2 or mechanical stretch (169, 170) | 1° rat ATII |
| Chemokine receptors | ||
| Chemokine receptor 3 (CCR3) & ligands | Expression increased in asthmatic patients, stimulated wound closure (6) | Bronchial biopsies from asthmatics, BEAS-2B, 16HBE, 1° HBE |
| CCR2 | Protein expressed in wounded monolayer (23) | 1° mouse ATII |
| CCR2 ligand MCP-1 | Stimulated repair in wounded monolayer (23) | 1° mouse ATII |
| Interleukins | ||
| IL-2 via ERKs | Stimulated migration, reduced apoptosis; enhanced by IFN-γ (97) | 1° rat ATII, rats in vivo |
| IL-4 via IRS-1 or IRS-2 but not STAT6 | Stimulated migration and repair (187) | 1° HBEC |
| IL-4 & IL-13 | Decreased migration (3) | Calu 3 |
| IFN-γ | Increased migration (3) | Calu 3 |
| IL-13 via HB-EGF, EGFR activation | Stimulation of proliferation, enhanced repair (4) | 1HAEo−, differentiated HBEC |
| IL-13-TGF-α & EGFR activation | Stimulation of proliferation (8, 171) | NHBE, mouse BE |
| IL-1β | Stimulation of wound closure, blocked by MAPK inhibitor PD-98059 (48, 49) | 1° rat ATII, A549 |
| IL-1β via NF-κB p65 | Acceleration of migration, but not in cells from asthmatic patients (186) | 1° differentiated HBE, 16HBE |
| Eicosanoids | ||
| COX-1, COX-2, PGE2 | Inhibition of COX blocked wound closure; PGE2 enhanced wound closure (144) | 16HBE, NHBE, CTE |
| Coagulation pathway | ||
| Tissue factor, fibrinogen, factor XIIIA | Stimulation of repair (121) | NHBE, 16HBE |
| Integrins | ||
| αv-, β5-, β6-, α5-subunits | Increased expression in migrating cells at wound edge (122) | In vivo xenograft of human bronchial epithelium |
| αvβ3- and β1-integrins | Migration mediated by these integrins (75) | 1° rat ATII |
| α2β1-integrin | Migration on fibronectin mediated by this integrin (75) | 1° rat ATII |
| α2-, α3-, α6-integrins via EGF | Migration and spreading on collagen IV (185) | 16HBE |
| β1-integrin | Subunit required for migration on laminin-1 or −2 (185) | 16HBE |
| αvβ6 and αvβ8-integrins | These integrins activated TGF-β1; TGF-β1 decreased wound closure (112) | NHBE |
| Extracellular matrix and matrix metalloproteinases | ||
| Vitronectin via αv-integrins & inter-α-trypsin inhibitor 1a1 | Stimulated cell adhesion & migration, promotion of wound repair (1) | A549, BEAS-2B |
| MMP-1 | Decreased cell adhesion & stiffness; increased migration on collagen I (123) | A549, 1° rat ATII |
| MMP-7 (matrilysin) | Upregulation correlated with migration and increased wound closure (120) | Wild-type and null MMP-7 mouse AEC cultures |
| Facilitation of E-cadherin ectodomain shedding from injured cells (107) | A549, Calu 3 | |
| Stimulation of cells regulated by TIMP-1 (18) | Matrilysin or TIMP-1 null mouse AEC cultures | |
| Shedding of syndecan-1 facilitated wound closure, stimulation of repair (17) | Wild-type and null MMP-7 mouse AEC cultures | |
| Duox-1-dependent stimulation of migration (184) | NHBE, HBE1 | |
| MMP-9 | Increased MMP-9 protein after arsenic exposure and decreased migration; MMP-9 inhibition enhanced wound repair (113) | 16HBE |
| Plasminogen activator inhibitor-1 | Decreased repair (89) | 1° mouse ATII |
| Decreased repair in asthmatics correlated with increased PAI-1 protein & mRNA (159) | 1° BEC from pediatric asthmatics | |
| Urokinase plasminogen activator & plasmin | Required for efficient wound closure; decreased KGF-stimulated wound repair upon inhibition of PAI-1 activity (102) | 1° rat ATII |
| Signal transduction intermediates | ||
| STAT3 and GP130 | Decreased repair (73) | ATII cells of null STAT3 or GP130 mice, NHBE |
| PTEN | Blocking accelerated wound closure (87) | Differentiated primary HBEC, BEAS-2B |
| PI3K | Inhibition decreased cell migration after injury (31) | 16HBE |
| β-catenin | ||
| GSK3β and β-catenin | Wound closure inhibited by overexpression of GSK3β and enhanced by overexpression of β-catenin (203) | PBEC, 16HBE |
| β-catenin | Regulated cell differentiation and fate during branching morphogenesis (109, 110) | Transgenic mouse expressing activated form of β-catenin |
| Rho GTPases | ||
| RhoA | Inactivated RhoA inhibited wound repair (50) | A549 |
| Decreased RhoA activity stimulated migration (156) | 1° BPE | |
| RhoA or Rac1, Rho kinase | Constitutively active or dominant negative RhoA or Rac1 inhibited wound closure; inhibition of Rho kinase stimulated cell migration (28) | 16HBE |
| CA or DN RhoA | Increased RhoA activity blocked by KGF; CA RhoA inhibited wound closure; DN RhoA stimulated wound closure (30) | 1° ATII isolated from rats ventilated with high tidal volume |
| Rac1 via Tiam 1 | Inhibitor of Tiam 1/decreased Rac1 activity inhibited cell migration (29) | 1° rat ATII, A549 |
| MAP kinase pathways | ||
| p38 MAPK and CXCR3 ligand I-TAC | Inhibitors decreased migration; p38 and JNK rapidly activated near wound edge (188) | 16HBE |
| Mechanical stretch-induced inhibition of cell migration associated with decreased JIP-3-induced JNK phosphorylation (32) | 16HBE | |
| P38 inhibition decreased chemotaxis, inhibition of ERK 1/2 had no effect (152) | 1° HBE, 16HBE | |
| Stimulation of wound repair by VIP blocked by inhibition of ERK 1/2 (56) | 16HBE | |
| Nitric oxide and ERK1/2 | Inhibition of migration by NO via decreased ERK1/2 activation (12) | NHBE |
| MIF and ERK1/2 | MIF stimulated ERK1/2 phosphorylation and proliferation but not in ATI-like cells (103) | 1° mouse ATII, 1° differentiated ATI-like cell |
| Wnt | Aberrent Wnt/β-catenin pathway activation (22) | Human IPF |
| Lung branching morphogenesis regulated by Wnt (27) | Gain and loss of function studies in TOPGAL or Wnt reporter mice | |
| - WISP-1 | Mediates pulmonary fibrosis in mice; upregulated in human IPF (83) | Mouse in vivo, human IPF |
| TGF- β | Stimulation of migration & cytoskeleton reorganization, decreased proliferation in rabbit cells (7) | 1° rabbit TEC |
| - and MMP-2 | Stimulation of wound repair (90) | Lung BE biopsies |
| - and EMT | Induction of EMT in lungs following overexpression of TGFβ (78) | Triple transgenic mouse and adenoviral expression of TGF-β |
| - and EMT, with β-catenin | Induction of EMT (83) | 1° rat ATII |
| Smad3 & transgelin | Stimulation of migration (197) | A549 |
| TGF-β & Smad3 | Acceleration of wound repair, survival promotion (14) | 1° rat ATII |
| TGF-β | Inhibited epithelial sheet migration, increased cell adhesion (157) | BPEC |
| TGF-β and integrins αvβ6 and αvβ8 | Wounding induced activation of TGF-β via these integrins and migration was inhibited by exogenous TGF-β and stimulated by anti-TGF-β or αvβ8 antibodies (112) | NHBE |
| TGF-β and MMP-7 | MMP-7 activated TGF-β, mediated fibrosis and IPF (17) | MMP-7 null mouse cultures, mouse naphthalene injury model, BEAS-2B |
| E-cadherin | Loss correlated with induction of EMT (70) | HBE cancer |
| Shh | Activated during repair and development (183) | Mouse embryonic lung epithelial cells, Ptch heterozygous null, adult mouse BE cells |
| Induced EMT & metastasis in cancer (70) | HBE cancer | |
| Mechanical forces | ||
| Cyclic stretch, compression | Inhibition of cell spreading & migration, stimulation of BrdU incorporation (146, 149) | Calu-3, NHBE, CTE, 1HAEo− |
| - and FAK | Loss of FAK phosphorylation and decreased cell migration (30, 32) | 16HBE, 1° rat ATII |
| - and JIP-3 | Dissociation of JIP-3 from FAK (31) | 16HBE |
| - and JNK | Decreased activation of JNK (31) | 16HBE |
| - and Paxillin, RhoA | Decreased phosphorylation of FAK & paxillin, increased RhoA activity, decreased cell adhesion (30) | 1° ATII from rats ventilated with high tidal volume |
| - and Tiam 1, Rac1 | Decreased wound repair via decreased Rac1 activity (29) | 1° rat ATII |
| - and Cell stiffness | Elastic modulus of cells migrating at wound edge & stiffness varied markedly (175) | 16HBE |
1°, primary; BE, bronchial epithelium; TEC, tracheal epithelial cell; ATII, alveolar epithelial type II; ATI, alveolar epithelial type I; A549, human lung carcinoma A549 cell line; 1° BPE, primary bovine bronchial epithelial cells; 16HBE, human bronchial epithelial cell line (16HBE14o-); 1° HBEC, primary human bronchial epithelial cells; CTE, primary feline airway epithelial cell line; BEAS-2B, human bronchial epithelia cell line; GP, guinea pig; AEC, airway epithelial cell; BEC, bronchial epithelial cell; BMSC, bone marrow-derived mesenchymal stem cells; 1HAEo− cells, human airway epithelial cell line (SV40-transformed); PBEC, primary porcine bronchial epithelial cells; BPE, bovine pulmonary epithelial cells; IPF, idiopathic pulmonary fibrosis; CA, constitutively active; DN, dominant negative.
Growth Factors, Cytokines, and Other Soluble Factors
Injury to lung epithelium and the ensuing inflammatory response causes the release of a wide variety of soluble factors from fibroblasts, endothelial cells, macrophages, and the injured and neighboring epithelial cells, as well as from the underlying matrix and from plasma exudate. The milieu of soluble factors that participate in epithelial repair includes growth factors, cytokines, chemokines, interleukins, prostaglandins, and matrix components. The majority of studies examining these soluble factors have focused on the response of cultured cells, and our understanding of the role of these factors is limited by a lack of precise knowledge about their cellular source and the spatial and temporal regulation of their activity in vivo. It is important to recognize that the conditions regulating the responsiveness of specific cells to these factors have not been clearly elucidated. However, they serve important roles in each of the processes of repair, some of which are described below.
Epidermal growth factor family.
Several members of the epidermal growth factor (EGF) family of growth factors have been shown to participate in epithelial repair. In the airways, expression of EGF, transforming growth factor-α (TGF-α), and the EGF receptor (EGFR) were upregulated within 1–2 days of naphthalene injury in the distal bronchioles of mice, and EGFR expression began to decrease 4–7 days after injury (171). EGF and TGF-α were both shown to stimulate proliferation of cultured neonatal rabbit ATII cells (143), and Leslie and collaborators (95) found that heparin-binding EGF (HB-EGF) was mitogenic for rat ATII cells. Lesur et al. (96) demonstrated that rat ATII cell migration was stimulated by EGF in the absence of commitment to proliferation. Using cultured rat alveolar epithelial monolayers, Kheradmand and colleagues (72) demonstrated that cell spreading was more important than cell migration in wound closure and that both serum and TGF-α significantly increased wound closure following a scratch wound. Soluble TGF-α was found to be present in the pulmonary edema fluid of patients with acute lung injury (ALI) (20), and subsequent studies demonstrated that edema fluid from ALI patients also stimulated the repair of wounded human A549 cell monolayers (48). This group found that cell spreading and motility in a wounded rat ATII monolayer were enhanced by the addition of soluble fibronectin and that wound healing was stimulated by IL-1β via a pathway that was EGF or TGF-α dependent (49). Trinh et al. (164) found that EGF stimulated Katp and KvLQT1 currents and channel expression via the EGFR in an autocrine fashion in primary rat alveolar type II cells. They subsequently found that bronchial epithelial cell migration was significantly lower in cells isolated from cystic fibrosis patients compared with cells from controls and that this decrease correlated with decreased EGFR activation and decreased K+ currents (165). Thus, expression of EGFR and EGF family peptides appears to increase in response to lung injury, and these peptides stimulate proliferation, spreading, and migration of lung epithelial cells. There is still much to learn about the interactions between EGFR and downstream signaling pathways and how these regulate repair.
EGFR in epithelial repair in asthma.
Studies have suggested that EGFR signaling may be altered in asthma and thus affect epithelial repair. EGFR immunoreactivity was found to be significantly higher in the epithelium from biopsies of patients with mild and severe asthma compared with normal subjects (126). These authors and others have demonstrated EGF-stimulated migration of bronchial epithelial cells (77, 126). Although β2-adrenergic agonists are commonly used in the treatment of asthma, one group found that isoproterenol attenuated EGF-stimulated wound closure of bronchial epithelial cells (150). Using an in vitro human bronchial epithelial model (1HAEo- cells), mechanical injury was shown to cause secretion of EGF and HB-EGF, and the release of HB-EGF (but not EGF) was dependent on IL-13, which caused indirect activation of the EGFR (4). IL-13 has also been shown to stimulate proliferation of primary human bronchial epithelial (HBE) cells through mechanisms involving TGF-α (8). Given the important role of IL-13 in the pathogenesis of experimental asthma (55, 192), these findings suggest a potential role for IL-13-dependent HB-EGF secretion and HBE proliferation in dysregulated bronchial epithelial repair in asthma.
Fibroblast growth factor family.
Three members of the fibroblast growth factor (FGF) family play important roles in epithelial repair in the lungs (reviewed in Ref. 180): keratinocyte growth factor (KGF), hepatocyte growth factor (HGF), and FGF-10. HGF levels were shown to be increased in the pulmonary edema fluid from patients with ALI compared with fluid from patients with hydrostatic edema fluid, but KGF levels were unchanged (172). In contrast, it was recently reported that the bronchoalveolar lavage fluid (BAL) of patients with ALI or acute respiratory distress syndrome (ARDS) contained increased levels of both HGF and KGF (128) and that the BAL elicited upregulation of HGF and KGF mRNA and protein in cultured fibroblasts. HGF is a known mitogen for rat ATII cells in vitro (104) and in vivo (117). HGF inhibited the formation of basement membrane and stimulated both proliferation and migration of immortalized ATII cells (46); however, HGF increased cell migration but not proliferation during human nasal epithelial repair in vitro (199), suggesting that epithelial cells from different locations may display differing responses to HGF. Takami et al. (162) reported that HGF stimulated proliferation of a human bronchial epithelial cell line (BEAS-2B) and that the MEK-ERK signaling pathway was necessary but not sufficient for this stimulation. Another group also reported stimulation of human bronchial epithelial cell proliferation by HGF that was dependent on increased cyclooxygenase-2 (COX-2) expression mediated by an Akt-, MAPK-, and β-catenin-dependent pathway (93). In addition, HGF has been shown to be important in the differentiation of cultured human bronchial epithelial cells (111). Thus HGF has been demonstrated to produce pleiotrophic effects mediated by MAPK, eicosanoids, and β-catenin pathway elements.
KGF is protective against injury and accelerates repair.
KGF has a well-documented role in lung development, and targeted expression of KGF in lung epithelium causes extensive pulmonary malformation and embryonic lethality (153). The protective effects of KGF against injury were first reported by Panos et al. (115) using rats exposed to hyperoxia, and subsequent studies have demonstrated protection against several different types of injury (reviewed in Ref. 180). KGF also accelerates repair mechanisms in lung epithelium following injury. As in the case of HGF, KGF is mitogenic for ATII cells in vitro and in vivo (115, 118, 168) and also stimulates ATII cell migration and enhances ATII cell adhesion (30, 47). When administered intratracheally to rats before isolation of alveolar epithelial cells, KGF stimulated wound closure in cultured monolayers primarily by increasing cell spreading and migration at the wound edge, and increased cellular adhesion in an EGFR activation-dependent manner (5). We also found that KGF increased the rate of wound closure of bronchial epithelial cell monolayers by stimulation of cell spreading and migration, and further, that these effects were blocked by inhibition of protein kinase C. KGF treatment also overcame the inhibitory effects of cyclic mechanical stretch on epithelial repair (181). Using a tracheal transplant model in mice, Gomperts and colleagues (53) showed that KGF accelerated the repair of injured tracheal epithelium. These studies clearly demonstrate the beneficial effects of KGF in repair following lung injury. Fehrenbach et al. (42) have suggested that the effects of KGF on proliferation in the airways may be restricted to Clara cells. Hyperplasia of ATII cells stimulated by KGF treatment in rats resolved spontaneously by differentiation of type II to type I cells or by apoptosis (43, 44). FGF-10 shares many similarities with KGF and moreover has been shown to reduce ATII cell DNA damage caused by mechanical stretch or H2O2 through mechanisms involving MAPK activation via the Grb2-SOS-Ras/RAF-1-ERK1/2 pathway (169, 170). While KGF and other members of the FGF family have clear protective roles against injury and may accelerate repair, their potential clinical utility for lung injury is uncertain. As discussed by Ware and Matthay (180), lung injury is generally not anticipated, except potentially in response to chemotherapy or radiation therapy, and so its usefulness for pretreatment may be limited. However, we previously demonstrated that KGF also partially prevented the loss of barrier function of cultured airway epithelial cells when given after exposure to either radiation or hydrogen peroxide (147, 182), and a recent study suggested that restoration of alveolar barrier function by postinjury treatment with allogeneic BMSC was dependent on release of KGF (91). Thus, given the potential restorative capabilities of KGF, it might be useful in the treatment of ALI in which epithelial repair is essential for recovery.
Mediators of inflammation: chemokines, eicosanoids, interleukins.
Other soluble factors that are normally associated with inflammation have also been shown to participate in epithelial repair mechanisms. Expression of the chemokine receptor-3 (CCR3) was found to be increased in bronchial biopsies from asthmatics, and treatment of primary HBE cells and cell lines (BEAS-2B and 16HBE14o-) with CCR3 ligands stimulated wound closure (6). Since repair processes are considered to be compromised in asthmatic airways, the upregulation of CCR3 may be a compensatory mechanism to enhance repair. CCR2 was expressed in isolated mouse ATII cells, and treatment with a CCR2 ligand (monocyte chemoattractant protein-1; MCP-1) stimulated repair in wounded monolayers (23). We previously demonstrated that inhibition of COX-1 or COX-2 essentially blocked wound closure of human and feline airway epithelial cells and that wound closure was restored by addition of PGE2 (144). Sustained effects on wound closure were observed when COX was inhibited only at the beginning of the repair, suggesting that eicosanoids participate in early wound healing events.
Interleukin effects may be context sensitive.
Interleukins have also been shown to participate in repair processes in the lung, but in some cases differing responses have been reported. Inflammatory mediators such as interleukins are increased in asthma and in ALI. IL-1β has been shown to augment the repair of injured rat ATII cells (49) and A549 cells (48), and the MAPK inhibitor PD-98059 blocked this stimulation in ATII cells. White and collaborators (186) found that IL-1β accelerated the migration of an HBE cell line (16HBE14o-) and primary differentiated HBE cells, but this effect was not observed in cells isolated from asthmatics. IL-1β-stimulated migration was also reduced using an siRNA approach to decrease the expression of the p65 component of NF-κB, suggesting that the cells from asthmatics might have compromised NF-κB function that affects either receptor expression or downstream signaling pathways. Proallergic response activators IL-4 and IL-13 were both shown to decrease the migration of Calu-3 human lung epithelial cells, while the innate immune response molecule IFN-γ enhanced migration (3). In contrast, Booth et al. (8) demonstrated that IL-13 induced proliferation of primary HBE cells through mechanisms involving TGF-α and activation of the EGFR (see above and Ref. 8), and Allahverdian et al. (4) showed that IL-13 stimulated HB-EGF release, activation of EGFR, and enhanced the repair of wounded 1HAEo- cells. Also, White and collaborators (187) found that IL-4 stimulated the migration and repair of differentiated primary HBE cells through mechanisms involving insulin receptor substrate (IRS)-1 or IRS-2 but not the transcription factor STAT6 (signal transducer and activator of transcription-6). Differences in responsiveness of Calu3 cells compared with primary cultures or other cell lines may explain the above differences in response to IL-4 and IL-13. Another proinflammatory compound, IL-2, was shown to increase the migration of injured rat ATII cells and reduce apoptosis of these cells through mechanisms dependent on extracellular signal-regulated kinases (ERKs) (97). The effects of IL-2 were enhanced by pretreatment with the innate immune modulator IFN-γ. Coupled with the finding that BALF from asthmatics contained increased levels of IL-2, IL-3, IL-4, IL-5, and granulocyte/macrophage colony-stimulating factor (GM-CSF), but not IFN-γ (140), and that epithelial repair is thought to be dysregulated in asthmatic airways, there remain many questions about the role of inflammatory mediators in epithelial repair.
Matrix, Integrins, and Glycoproteins
During the early stages of epithelial restitution, the processes of cell spreading and migration involve coordinated movement of cells in which adhesion sites between the cells and the substrate are alternately formed and released as the cells contract and advance forward. The matrix beneath the cells undergoes remodeling during the repair process, and components within this matrix interact with cellular integrin receptors to regulate cell migration, proliferation, and differentiation (reviewed recently in Ref. 19). For example, in the early stages of epithelial restitution, cells migrate on a provisional matrix composed of serum fibronectin and fibrinogen. Antibody blocking studies demonstrated the importance of tissue factor, fibrinogen, and factor XIIIA in the repair of normal HBE cells and 16HBE14o- cells (121). Although space limitations preclude an extensive discussion, integrins and matrix enzymes play a major role in epithelial repair, and several are discussed below.
Integrins regulate cell migration.
The adhesion and migration of cells upon the underlying matrix involves cell surface receptors such as integrins that consist of α- and β-subunits, and interactions between matrix components and integrin subunits affect epithelial repair mechanisms in the lung. Pilewsky et al. (122) used an in vivo xenograft model of human bronchial epithelium to demonstrate increased expression of the αv-, β5-, β6-, and α5-integrin subunits in cells migrating at the wound edge. Kim and collaborators (75) used antibody blocking studies to demonstrate that migration of rat ATII cells on fibronectin was partially mediated by αvβ3- and β1-integrins, and that migration on type I collagen was dependent on α2β1-integrin. A similar approach using 16HBE14o- cells showed that EGF-stimulated migration and spreading on collagen IV was blocked by antibodies to α2-, α3-, α6-integrins and that the β1-integrin subunit was required for migration on laminin-1 or laminin-2 (185). Together, these studies demonstrate that cell migration is regulated in part by the identity of the integrin subunits and their interaction with the underlying matrix. Vitronectin is a component of extracellular matrix that promotes cell adhesion and migration through binding to several different αv-integrins. Adair and collaborators (1) found that inter-α-trypsin inhibitor (IaI), found in serum protein, binds to vitronectin and promotes the repair of wounded A549 and BEAS-2B cells. Dystroglycan is a glycosylated extracellular protein that functions as a lectin and was found to increase in expression 12 h after wounding of 1HAEo- cells and to regulate cell migration (189). Integrins may also function as activators of other factors that regulate wound closure. Neurohr et al. (112) found that αvβ6- and αvβ8-integrins were expressed on airway epithelial cells and were capable of activating TGF-β1, which resulted in decreased wound closure. Thus, although several specific integrins have been identified in epithelial repair processes, there are still gaps in our knowledge regarding the overall integration of integrin expression, regulation, and activation.
Matrix metalloproteinases are activated during repair.
The matrix metalloproteinase (MMP) family of enzymes is not normally expressed in healthy tissues (except, importantly, MMP-7), but the expression of these enzymes is upregulated during most repair processes. MMPs are involved in remodeling of extracellular matrix and facilitate cell migration, and several members of this family have been implicated in lung epithelial repair mechanisms. Treatment of A549 cells or primary rat ATII cells with exogenous MMP-1 caused decreased cell adhesion and cell stiffness and increased cell migration on type I collagen (123). The expression of MMP-7, or matrilysin, is known to be upregulated in migrating airway epithelial cells (120), and MMP-7 facilitates E-cadherin ectodomain shedding from injured airway epithelial cells (107). MMP-7-mediated stimulation of airway epithelial cells is negatively regulated by tissue inhibitor of metalloproteinases-1 (TIMP-1) (18), and MMP-7 shedding of syndecan-1 from mouse airway epithelial cells was shown to facilitate wound closure by altering the conformation of the α2β1-integrin (17). MMP-9 has also been shown to stimulate the repair of bronchial epithelial cells in vitro (94), and MMP-9-mediated stimulation of HBE cell migration was shown to be dependent on the activation of dual oxidase 1 (Duox1) (184). In contrast, exposure of 16HBE14o- to arsenic caused increased MMP-9 activity and expression and decreased cell migration, whereas inhibition of MMP-9 enhanced wound repair (113). Another group previously found that plasminogen activator inhibitor-1 (PAI-1), another protein associated with matrix degradation, decreased the repair of ATII cells by binding to vitronectin (89). In support of the role of PAI-1 in epithelial repair, Stevens et al. (159) showed that primary bronchial epithelial cells from pediatric asthmatics exhibited significantly reduced wound repair compared with cells from healthy control subjects, and this correlated with a significant increase in PAI-1 mRNA and protein expression. Interestingly, gene silencing of PAI-1 decreased wound repair of cells from healthy controls, but had little effect on cells from asthmatics. In a study of KGF-stimulated repair of rat ATII cells it was found that wound healing was decreased by inhibition of PAI-1 activity, but the proteolytic activity of urokinase plasminogen activator (uPA) and plasmin were required for efficient wound healing (102).
Although substantial progress has been made in identifying matrix and structural components involved in epithelial repair, many unanswered questions remain. Some of these include fundamental questions in biology such as how cells organize matrix and in turn how matrix regulates the functional activity of different cell types during repair. A question of particular relevance in the lungs is how the physical/mechanical environment interacts with the biochemical environment. For example, diseases such as pulmonary fibrosis and chronic obstructive pulmonary disease (COPD) involve changes in the local mechanical environment of the lung, and the progression of the disease may involve an imbalance between the activation of MMPs and their inhibitors caused by changes in mechanics. Furthermore, potential interactions between biochemical and mechanical signals are important in light of the fact that many in vitro studies involve cells grown on a substrate that is an order of magnitude greater in stiffness than native tissue.
Signal Transduction Intermediates and Pathways
While there has been extensive study of the many factors (growth factors, cytokines, integrins, matrix components, etc.) that are involved in epithelial repair mechanisms, the signal transduction intermediate pathways that are activated by these factors have received less attention. Using a naphthalene injury model in mice, Watkins et al. showed extensive activation of the sonic hedgehog (Shh) signaling pathway during repair of the airway epithelium, and, interestingly, this pattern of activation was similar to that observed during normal airway development (183), prompting discussions of whether repair actually recapitulates ontogeny. Shh signaling has also been implicated in the epithelial-mesenchymal transition (EMT), and this is discussed below. Targeted deletion of either STAT3 or GP130 (glycoprotein 130) from pulmonary epithelial cells resulted in compromised repair of the bronchial epithelium following naphthalene injury (73). This study also showed that in vitro migration of airway epithelial cells was inhibited by expression of dominant-negative STAT3 and was stimulated by expression of wild-type STAT3. Another intermediate in cell signaling that has been shown to affect wound closure is phosphoinositide-3 kinase (PI3-kinase), a known regulator of cell motility. PI3-kinase activity is suppressed by PTEN (phosphatase and tensin homolog deleted on chromosome ten), and inhibition of PTEN, expression of a dominant negative form of PTEN, or siRNA directed against PTEN caused accelerated wound closure of primary human airway epithelial cells (87). Adding to this evidence, we recently showed that inhibition of PI3-kinase or expression of a dominant negative form of PI3-kinase in 16HBE14o- cells caused decreased cell migration following injury (31). These studies highlight the importance of PI3-kinase/PTEN signaling in airway epithelial healing and the need for further study of this pathway. Another key intermediate, β-catenin (discussed in more detail below), is an essential mediator of the classical Wnt signaling pathway, regulating the differentiation of respiratory epithelial cells in vivo (109) and cell fate during branching morphogenesis in the lung (110). In vitro wound closure of bovine bronchial epithelial cells was inhibited by overexpression of glycogen synthase kinase 3β (GSK3β), while overexpression of β-catenin, which is known to be phosphorylated and downregulated by GSK3β, promoted wound closure (203). In this study, scratch wounding was followed by nuclear translocation of β-catenin, which promoted the expression of cyclin D1 (indicative of cell proliferation). As is true in the broader context of wound healing, the spatial localization and activation of these signaling intermediates, both within a particular cell and within the tissue structure, is essential to regulate the repair process. However, our current knowledge of the spatial biology of these processes within the lungs is limited.
Rho GTPases regulate cytoskeletal remodeling and cell contraction.
Cytoskeletal remodeling is important in each of the processes associated with wound healing. Actin remodeling, formation and turnover of adhesion sites, and actomyosin contraction are all regulated by members of the Rho family of small guanosine triphosphatases (GTPases). RhoA is involved in the organization of actin into bundles and stress fibers, the formation of large focal adhesions, and the promotion of actomyosin tension through its action on myosin light chain phosphorylation. Rac1 stimulates the formation of focal complexes and polymerization of actin in lamellipodia, while Cdc42 performs a similar function in filopodia. While these GTPases have been widely studied in different tissues, and the specific roles vary from tissue to tissue, a limited number of studies have examined the role of GTPases in lung epithelial repair. Inactivation of RhoA by the bacterial toxin ExoT caused inhibition of wound repair in A549 cells (50), whereas a decrease in RhoA activity caused by stimulation of protein kinase A stimulated bronchial epithelial cell migration (156). We found that expression of either a constitutively active (CA) or a dominant negative (DN) form of RhoA inhibited wound closure of 16HBE14o- cells, suggesting that normal repair requires an appropriate balance of RhoA activity (28). Similar inhibition was found when wild-type Rac1 was overexpressed or when a dominant negative form of Rac1 was expressed. Inhibition of Rho kinase, the downstream effector of RhoA, stimulated cell migration. In another study we found that RhoA activity was increased in ATII cells isolated from rats following high tidal volume (25 ml/kg) mechanical ventilation, but this increased activity was blocked by treatment with KGF (30). Expression of CA-RhoA in rat ATII cells inhibited wound closure, whereas expression of DN-RhoA stimulated wound closure, in contrast with the results from 16HBE14o- cells in which DN-RhoA caused decreased cell migration. We recently demonstrated that cell migration of rat ATII and A549 cells was inhibited by treatment with an inhibitor of Tiam1, a Rac1-specific guanine exchange factor (29). As discussed above, it is important to recognize that the various activities of these molecules vary with location in the cells. For example, Rac1 may be more active at the leading edge of the migrating cell, whereas RhoA may be more active stabilizing adhesions for traction and generating tension in the more central regions of the cell. Studies that clarify these molecular functions and their localization will be key to understanding migration and wound healing in the lung.
MAP kinase pathways.
Several of the factors that regulate lung epithelial repair utilize the mitogen-activated protein (MAP) kinase pathway. These serine/threonine protein kinases are involved in cell proliferation, differentiation, and migration. White and collaborators (188) showed that migration of primary human airway epithelial cells and 16HBE14o- cells on collagen-IV was decreased when cells were treated with inhibitors of p38 MAPK, JNK, or ERK1/2, and that both p38 and JNK were rapidly activated in cells near the wound edge. We recently showed that mechanical stretch-induced inhibition of cell migration involved a decrease in JIP3-induced JNK phosphorylation (32). Inhibition of p38 MAPK also decreased chemotaxis of primary human airway epithelial cells and 16HBE14o- cells in response to the CXCR3 ligand I-TAC, but inhibition of ERK1/2 had no effect (152). Another group found that stimulation of wound repair in cultured human bronchial epithelial cells by vasoactive intestinal peptide (VIP) was blocked by inhibition of ERK1/2 (56). Bove et al. (12) found that inflammatory levels of nitric oxide inhibited the migration of human bronchial epithelial cells in part because of a decrease in activation of ERK1/2. Interestingly, macrophage migration inhibitory factor (MIF) stimulated rapid and sustained ERK1/2 phosphorylation and proliferation in mouse ATII cells, but failed to do so in differentiated ATI-like cells (103). Thus, the roles for specific members of the MAPK family in epithelial repair appear to vary in different tissues and in response to various activators. As in the case of differences in response to soluble mediators, activation of different MAPK pathways may be dependent on temporal and spatial variables such as integrin/matrix interactions and the presence of inflammatory mediators during repair.
Wnt, TGF-β Signaling, and Epithelial-Mesenchymal Transition
Wnt/β-catenin signaling.
The role of β-catenin-mediated wingless integration (Wnt) signaling is proving to be central to mechanisms of lung healing in fibrosis, cancer, and stem cell maintenance (62, 79, 82, 124, 133, 190). β-catenin signaling stimulates tissue remodeling, cell migration, and wound closure (through Cox-2/PGE2 or MMP) or tissue remodeling and destruction (through MMPs, IL-1, IL-6, IL-8, IL-15, MCP-1, TNF-α, ICAM1 or TGF-β) (124). Wnt binding to cognate Frizzled (Fzd) receptors results in GSK-3β phosphorylation, inactivating GSK-3β, and the cytosolic accumulation of β-catenin, which then translocates to the nucleus and participates in lymphoid enhancer factor/T cell factor-p300 (LEF/TCF-p300)-dependent gene transcription (124, 191). MMPs proteolytically degrade basement membrane, facilitating cell migration, and are key activators of TGF-β. This activation directly or indirectly stimulates many of the proinflammatory cytokines (e.g., IL-1) that participate in inflammation-mediated tissue destruction and elaboration of ECM and thickened hyaline membranes. Wnt/β-catenin signaling has also been shown to regulate the stem cell hierarchy in the renewal of lung epithelium (137). However, a recent study used a targeted knockout strategy in bronchiolar epithelium and found that β-catenin was not required for maintenance and repair following naphthalene-induced airway injury (202). It is important to recognize that Wnt/β-catenin signaling has been documented to vary in different tissues and contexts (stage of development, receptor binding, pathway) (13, 15, 21, 108), and there are still many unresolved questions regarding the role of Wnt/β-catenin both in development and in repair mechanisms.
TGF-β signaling is complex in repair.
Because of its multifunctional roles in development, inflammation, wound repair, cell proliferation, and differentiation, the role of TGF-β in lung epithelial repair mechanisms is both complex and controversial. TGF-β is both a potent immunosuppressor and proinflammatory. TGF-β-induced target genes include connective tissue growth factor (CTGF), α-smooth muscle actin (α-SMA), collagen 1a2 (col1a2), and PAI-2 (serpine 2) (reviewed in Ref. 191). Several of these gene products elaborate connective tissue and α-smooth muscle actin, the expression of which is a hallmark of cells that differentiate into myofibroblasts via the epithelial-mesenchymal transition (EMT, described below). Two early studies suggested that TGF-β stimulated cell migration of cultured rabbit tracheal epithelial cells (7) and 16HBE14o- cells (68), but cell proliferation was reduced in the former study. Another group demonstrated that TGF-β stimulated airway epithelial wound repair in vitro by upregulating MMP-2 and altering the matrix upon which cells migrated (90). Migration was also stimulated by TGF-β in A549 cells and primary mouse ATII cells in a Smad-3- and transgelin-dependent manner (197). TGF-β was also shown to accelerate rat ATII scratch wound repair through Smad-dependent signaling (14). In contrast, Spurzem and collaborators (157) found that TGF-β inhibited epithelial sheet migration of bovine bronchial epithelial cells. In addition, Neurohr et al. (112) showed that wounding of primary airway epithelial cells induced activation of TGF-β through integrins αvβ6 and αvβ8, and that cell migration was inhibited by exogenous TGF-β and stimulated by antibodies against TGF-β or αvβ8. These contrasting effects of TGF-β may be due to differences in cell origin (airway vs. alveolar, transformed vs. primary, human vs. rodent), differences in matrix or integrin expression, or differences in concentration-dependent responses.
Epithelial-mesenchymal transition.
Defining the role of TGF-β in epithelial repair mechanisms is further complicated by its role in EMT (reviewed more extensively in Ref. 191). EMT involves the conversion of differentiated epithelial cells into fibroblasts and myofibroblasts that occurs normally during development, but in aberrant wound repair processes, EMT may lead to fibrosis and scarring. TGF-β has been identified as a “master switch” in the induction of EMT in several tissues including the lung, and, specifically, together with β-catenin, in ATII cells (82). EMT occurs in cancer metastasis and fibrosis and in human idiopathic pulmonary fibrosis (IPF) (81, 82). MMP7 found on the surface of lung epithelial cells (17) is one of several MMPs that activate TGF-β, and is known to be a key mediator of pulmonary fibrosis and IPF. Chen et al. (17) found that MMP7 promoted inflammation early in the injury response (via shedding syndecan-1/KC complexes), enhanced cell migration and wound closure, and later, in adaptive immune responses, facilitated shedding of E-cadherin. EMT is accompanied by loss of E-cadherin (191), which leads to the disassembly of adherens junctions and impaired cell-cell contacts preceding cell migration. The TGF-β inhibitors Smad7 or BMP7 can inhibit EMT (191). Although recent studies have identified several mediators and pathways involved in EMT and its dysregulation, a more complete understanding of these processes and how aberrant repair results in fibrosis and scarring in the lungs is needed.
Mechanical Forces
Both repair mechanisms following injury and normal epithelial turnover occur within an environment and upon a substrate that is undergoing cyclic mechanical deformation. Epithelial repair is a dynamic process involving cell spreading and cell migration in the early stages, and these processes inherently involve biomechanics: cytoskeletal remodeling, adhesion/loss of adhesion, generation of force/relaxation, cell locomotion, etc. Furthermore, as discussed above, the role of interactions between mechanical and biochemical signals in the lung is not well understood. This is particularly important in light of the potential for alterations in repair in diseases that involve changes in lung mechanics such as IPF, asthma, and COPD. In addition, patients with ALI placed on mechanical ventilators may experience elevated levels of mechanical distention that may contribute to injury or affect repair mechanisms. To investigate the latter possibility in vitro, we used cell lines (Calu-3, 1HAEo-) and primary cultures of human bronchial epithelial cells to demonstrate that both cyclic stretch and cyclic compression significantly inhibited wound closure by inhibiting cell spreading and cell migration (148). Inhibition was dependent on the magnitude of mechanical strain, and, interestingly, the extent of inhibition was dependent on the duration of strain during each cycle and not specifically the frequency of stretch. In contrast to the inhibitory effects on spreading and migration, mechanical stretch and compression were shown to stimulate BrdU incorporation in wounded bronchial epithelial cells in culture (146). We later showed that treatment with KGF abrogated the inhibitory effects of mechanical strain on wound repair by promoting spreading and migration (181). More recently, we demonstrated that mechanical stretch of wounded 16HBE14o- cells caused loss of focal adhesion kinase (FAK) phosphorylation, dissociation of JIP-3 (JNK-interacting protein 3) from FAK, and decreased activation of JNK (32). We also found that ATII cells isolated from rats following 2 h of high tidal volume mechanical ventilation (25 ml/kg with 50% oxygen) exhibited decreased phosphorylation of FAK and paxillin, increased RhoA activity, and decreased cell adhesion (30). Wound repair of cultured rat ATII cells was inhibited by adenoviral expression of a kinase-inactive from of FAK (FRNK), whereas repair was stimulated by overexpression of wild-type FAK. We also found that cyclic stretch of rat ATII cells caused decreased wound repair through mechanisms involving decreased Tiam1-mediated Rac1 activity (29). Although it is well-recognized that there are spatial variations in the localization and activity of Rho GTPases, focal adhesion, and other signaling molecules during coordinated cell migration, the relationship between these dynamic processes and the local mechanical properties of the cells are less well understood. Atomic force microscopy was used to demonstrate that the elastic modulus of 16HBE14o- cells varied markedly in cells migrating at the wound edge and that cell stiffness was significantly increased between 10 and 15 μm from the wound edge and significantly decreased within the first 5 μm (175). These results suggest that epithelial repair mechanisms may in part be directed by changes in localized mechanical properties. In addition to inhibitory effects on early processes of epithelial restitution, mechanical forces may also negatively impact epithelial repair in other ways. Several groups have demonstrated that mechanical stretch of cells in culture or high tidal volume in vivo causes ATII cell injury (173, 174), apoptosis (59, 60), and necrosis (16, 166). However, the interplay between mechanical and biochemical signaling during repair processes in vivo has not been extensively studied.
Conclusions and Future Directions
This survey of the current knowledge of mechanisms of lung epithelial repair illustrates some of the complexities of this field and points toward some of the challenges for future research. Clearly there is a need for advancements in methods that can be used to examine epithelial repair mechanisms in vivo. Approaches involving lineage tracking and in situ visualization coupled with transgenic mouse models will be useful in the detailed identification and characterization of resident progenitor and stem cells in discrete, and in some cases sequestered, regions of the lung. These approaches may also be useful for investigating how persistent injury or inflammation can lead to different pathologies depending on the site of injury (airway vs. alveolar). Differences in gene profiles in epithelial cells from patient samples may help us to understand why repeated exposure to cigarette smoke progresses to COPD in some, but not all, smokers. Similar genetic profiling from epithelial cells of asthmatics may provide clues about the importance of chronic epithelial injury and repair in the pathogenesis of asthma. Another major challenge is to determine the source, mechanisms of recruitment and engraftment, and the regulation of differentiation of BMSC. The development of improved methods for the isolation, identification, and characterization of these cells will be required, and it will be important to translate these approaches into studies of human cells. While numerous factors involved in epithelial migration, spreading, and proliferation have been identified from studies of cultured cells, there is a significant need for understanding how these varied and sometimes disparate signals are integrated into a coordinated response. This will require increased understanding of the source of the mediators, the pathways that become activated, the timing of their activation, their spatial targeting, and the factors that regulate their responsiveness. For example, the role of TGF-β in epithelial repair appears to depend on the context and site of the injury, and the factors that lead to dysregulated EMT and fibrosis have not been clearly elucidated. More universal challenges include increasing our understanding of the regulation of matrix and its role in regulating repair, the examination of how signaling pathways vary spatially within cells to regulate migration and spreading, and the determination of how changes in localized mechanical forces and tissue properties alter repair mechanisms. As we have attempted to describe, there have recently been many exciting and important revelations in this field, but many unanswered questions remain, and a comprehensive understanding of epithelial repair in the lungs is still far from complete.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-064981, HL-080417, and HL-094366.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Adair JE, Stober V, Sobhany M, Zhuo L, Roberts JD, Negishi M, Kimata K, Garantziotis S. Inter-α-trypsin inhibitor promotes bronchial epithelial repair after injury through vitronectin binding. J Biol Chem 284: 16922–16930, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson IY, Bowden DH. Derivation of type 1 epithelium from type 2 cells in the developing rat lung. Lab Invest 3 2: 736–745, 1975 [PubMed] [Google Scholar]
- 3.Ahdieh M, Vandenbos T, Youakim A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-gamma. Am J Physiol Cell Physiol 281: C2029–C2038, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Allahverdian S, Harada N, Singhera GK, Knight DA, Dorscheid DR. Secretion of IL-13 by airway epithelial cells enhances epithelial repair via HB-EGF. Am J Respir Cell Mol Biol 38: 153–160, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L163–L169, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Beck LA, Tancowny B, Brummet ME, Asaki SY, Curry SL, Penno MB, Foster M, Bahl A, Stellato C. Functional analysis of the chemokine receptor CCR3 on airway epithelial cells. J Immunol 177: 3344–3354, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Boland S, Boisvieux-Ulrich E, Houcine O, Baeza-Squiban A, Pouchelet M, Schoevaert D, Marano F. TGF beta 1 promotes actin cytoskeleton reorganization and migratory phenotype in epithelial tracheal cells in primary culture. J Cell Sci 109: 2207–2219, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Booth BW, Adler KB, Bonner JC, Tournier F, Martin LD. Interleukin-13 induces proliferation of human airway epithelial cells in vitro via a mechanism mediated by transforming growth factor-alpha. Am J Respir Cell Mol Biol 25: 739–743, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Borok Z, Danto SI, Lubman RL, Cao Y, Williams MC, Crandall ED. Modulation of t1α expression with alveolar epithelial cell phenotype in vitro. Am J Physiol Lung Cell Mol Physiol 275: L155–L164, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Borok Z, Lubman RL, Danto SI, Zhang XL, Zabski SM, King LS, Lee DM, Agre P, Crandall ED. Keratinocyte growth factor modulates alveolar epithelial cell phenotype in vitro: expression of aquaporin 5. Am J Respir Cell Mol Biol 18: 554–561, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 24: 662–670, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Bove PF, Wesley UV, Greul AK, Hristova M, Dostmann WR, van der Vliet A. Nitric oxide promotes airway epithelial wound repair through enhanced activation of MMP-9. Am J Respir Cell Mol Biol 36: 138–146, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bradley RS, Brown AM. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J 9: 1569–1575, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckley S, Shi W, Barsky L, Warburton D. TGF-β signaling promotes survival and repair in rat alveolar epithelial type 2 cells during recovery after hyperoxic injury. Am J Physiol Lung Cell Mol Physiol 294: L739–L748, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Burrus LW, McMahon AP. Biochemical analysis of murine Wnt proteins reveals both shared and distinct properties. Exp Cell Res 220: 363–373, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Cavanaugh KJ, Jr, Margulies SS. Measurement of stretch-induced loss of alveolar epithelial barrier integrity with a novel in vitro method. Am J Physiol Cell Physiol 283: C1801–C1808, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Chen P, Abacherli LE, Nadler ST, Wang Y, Li Q, Parks WC. MMP7 shedding of syndecan-1 facilitates re-epithelialization by affecting alpha(2)beta(1) integrin activation. PloS One 4: e6565, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen P, McGuire JK, Hackman RC, Kim KH, Black RA, Poindexter K, Yan W, Liu P, Chen AJ, Parks WC, Madtes DK. Tissue inhibitor of metalloproteinase-1 moderates airway re-epithelialization by regulating matrilysin activity. Am J Pathol 172: 1256–1270, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Parks WC. Role of matrix metalloproteinases in epithelial migration. J Cell Biochem 108: 1233–1243, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesnutt AN, Kheradmand F, Folkesson HG, Alberts M, Matthay MA. Soluble transforming growth factor-alpha is present in the pulmonary edema fluid of patients with acute lung injury. Chest 111: 652–656, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. J Invest Dermatol 129: 1614–1627, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/beta-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christensen PJ, Du M, Moore B, Morris S, Toews GB, Paine R., 3rd Expression and functional implications of CCR2 expression on murine alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 286: L68–L72, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc 5: 772–777, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Danjo Y, Gipson IK. Actin ‘purse string’ filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J Cell Sci 111: 3323–3332, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Davies DE. The role of the epithelium in airway remodeling in asthma. Proc Am Thorac Soc 6: 678–682, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dean CH, Miller LA, Smith AN, Dufort D, Lang RA, Niswander LA. Canonical Wnt signaling negatively regulates branching morphogenesis of the lung and lacrimal gland. Dev Biol 286: 270–286, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Desai LP, Aryal AM, Ceacareanu B, Hassid A, Waters CM. RhoA and Rac1 are both required for efficient wound closure of airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 287: L1134–L1144, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Desai LP, Chapman KE, Waters CM. Mechanical stretch decreases migration of alveolar epithelial cells through mechanisms involving Rac1 and Tiam1. Am J Physiol Lung Cell Mol Physiol 295: L958–L965, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai LP, Sinclair SE, Chapman KE, Hassid A, Waters CM. High tidal volume mechanical ventilation with hyperoxia alters alveolar type II cell adhesion. Am J Physiol Lung Cell Mol Physiol 293: L769–L778, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Desai LP, White SR, Waters CM. Cyclic mechanical stretch decreases cell migration by inhibiting phosphatidylinositol 3-kinase- and focal adhesion kinase-mediated JNK1 activation. J Biol Chem 285: 4511–4519, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desai LP, White SR, Waters CM. Mechanical stretch decreases FAK phosphorylation and reduces cell migration through loss of JIP3-induced JNK phosphorylation in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L520–L529, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar type II cells with time in culture. Biochim Biophys Acta 846: 155–166, 1985 [DOI] [PubMed] [Google Scholar]
- 34.Dupuit F, Gaillard D, Hinnrasky J, Mongodin E, de Bentzmann S, Copreni E, Puchelle E. Differentiated and functional human airway epithelium regeneration in tracheal xenografts. Am J Physiol Lung Cell Mol Physiol 278: L165–L176, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol 24: 649–652, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Effects of topical budesonide on epithelial restitution in vivo in guinea pig trachea. Thorax 50: 785–792, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. In vivo restitution of airway epithelium. Cell Tissue Res 281: 305–316, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Erjefalt JS, Erjefalt I, Sundler F, Persson CG. Microcirculation-derived factors in airway epithelial repair in vivo. Microvasc Res 48: 161–178, 1994 [DOI] [PubMed] [Google Scholar]
- 39.Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest 38: 648–653, 1978 [PubMed] [Google Scholar]
- 40.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol 22: 142–150, 1975 [DOI] [PubMed] [Google Scholar]
- 41.Evans MJ, Johnson LV, Stephens RJ, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 35: 246–257, 1976 [PubMed] [Google Scholar]
- 42.Fehrenbach H, Fehrenbach A, Pan T, Kasper M, Mason RJ. Keratinocyte growth factor-induced proliferation of rat airway epithelium is restricted to Clara cells in vivo. Eur Respir J 20: 1185–1197, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Fehrenbach H, Kasper M, Koslowski R, Pan T, Schuh D, Muller M, Mason RJ. Alveolar epithelial type II cell apoptosis in vivo during resolution of keratinocyte growth factor-induced hyperplasia in the rat. Histochem Cell Biol 114: 49–61, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Fehrenbach H, Kasper M, Tschernig T, Pan T, Schuh D, Shannon JM, Muller M, Mason RJ. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur Respir J 14: 534–544, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Fenteany G, Janmey PA, Stossel TP. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr Biol 10: 831–838, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Furuyama A, Mochitate K. Hepatocyte growth factor inhibits the formation of the basement membrane of alveolar epithelial cells in vitro. Am J Physiol Lung Cell Mol Physiol 286: L939–L946, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Galiacy S, Planus E, Lepetit H, Fereol S, Laurent V, Ware L, Isabey D, Matthay M, Harf A, d'Ortho MP. Keratinocyte growth factor promotes cell motility during alveolar epithelial repair in vitro. Exp Cell Res 283: 215–229, 2003 [DOI] [PubMed] [Google Scholar]
- 48.Geiser T, Atabai K, Jarreau PH, Ware LB, Pugin J, Matthay MA. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1β-dependent mechanism. Am J Respir Crit Care Med 163: 1384–1388, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Geiser T, Jarreau PH, Atabai K, Matthay MA. Interleukin-1β augments in vitro alveolar epithelial repair. Am J Physiol Lung Cell Mol Physiol 279: L1184–L1190, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Geiser TK, Kazmierczak BI, Garrity-Ryan LK, Matthay MA, Engel JN. Pseudomonas aeruginosa ExoT inhibits in vitro lung epithelial wound repair. Cell Microbiol 3: 223–236, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci USA 106: 9286–9291, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol 161: 173–182, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomperts BN, Belperio JA, Fishbein MC, Keane MP, Burdick MD, Strieter RM. Keratinocyte growth factor improves repair in the injured tracheal epithelium. Am J Respir Cell Mol Biol 37: 48–56, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 297: L1045–L1055, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282: 2261–2263, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guan CX, Cui YR, Zhang M, Bai HB, Khunkhun R, Fang X. Intracellular signaling molecules involved in vasoactive intestinal peptide-mediated wound healing in human bronchial epithelial cells. Peptides 28: 1667–1673, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Gupta N, Su X, Popov B, Lee JW, Serikov V, Matthay MA. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol 179: 1855–1863, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Hackett TL, Knight DA. The role of epithelial injury and repair in the origins of asthma. Curr Opin Allergy Clin Immunol 7: 63–68, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Hammerschmidt S, Kuhn H, Gessner C, Seyfarth HJ, Wirtz H. Stretch-induced alveolar type II cell apoptosis: role of endogenous bradykinin and PI3K-Akt signaling. Am J Respir Cell Mol Biol 37: 699–705, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol 30: 396–402, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Harari S, Caminati A. IPF: new insight on pathogenesis and treatment. Allergy In press [DOI] [PubMed] [Google Scholar]
- 62.He B, Jablons DM. Wnt signaling in stem cells and lung cancer. Ernst Schering Found Symp Proc 5: 27–58, 2006 [DOI] [PubMed] [Google Scholar]
- 63.Henson PM, Vandivier RW, Douglas IS. Cell death, remodeling, and repair in chronic obstructive pulmonary disease? Proc Am Thorac Soc 3: 713–717, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Holgate ST. Epithelium dysfunction in asthma. J Allergy Clin Immunol 120: 1233–1244; quiz 1245–1236, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc 6: 655–659, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 24: 671–681, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Horiba K, Fukuda Y. Synchronous appearance of fibronectin, integrin alpha 5 beta 1, vinculin and actin in epithelial cells and fibroblasts during rat tracheal wound healing. Virchows Arch 425: 425–434, 1994 [DOI] [PubMed] [Google Scholar]
- 68.Howat WJ, Holgate ST, Lackie PM. TGF-β isoform release and activation during in vitro bronchial epithelial wound repair. Am J Physiol Lung Cell Mol Physiol 282: L115–L123, 2002 [DOI] [PubMed] [Google Scholar]
- 69.Isakson BE, Lubman RL, Seedorf GJ, Boitano S. Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am J Physiol Cell Physiol 281: C1291–C1299, 2001 [DOI] [PubMed] [Google Scholar]
- 70.Kato Y, Hirano T, Yoshida K, Yashima K, Akimoto S, Tsuji K, Ohira T, Tsuboi M, Ikeda N, Ebihara Y, Kato H. Frequent loss of E-cadherin and/or catenins in intrabronchial lesions during carcinogenesis of the bronchial epithelium. Lung Cancer 48: 323–330, 2005 [DOI] [PubMed] [Google Scholar]
- 71.Kaverina I, Krylyshkina O, Small JV. Regulation of substrate adhesion dynamics during cell motility. Int J Biochem Cell Biol 34: 746–761, 2002 [DOI] [PubMed] [Google Scholar]
- 72.Kheradmand F, Folkesson HG, Shum L, Derynk R, Pytela R, Matthay MA. Transforming growth factor-α enhances alveolar epithelial cell repair in a new in vitro model. Am J Physiol Lung Cell Mol Physiol 267: L728–L738, 1994 [DOI] [PubMed] [Google Scholar]
- 73.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Muller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol 172: 1542–1554, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 121: 823–835, 2005 [DOI] [PubMed] [Google Scholar]
- 75.Kim HJ, Henke CA, Savik SK, Ingbar DH. Integrin mediation of alveolar epithelial cell migration on fibronectin and type I collagen. Am J Physiol Lung Cell Mol Physiol 273: L134–L141, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Kim JS, McKinnis VS, Adams K, White SR. Proliferation and repair of guinea pig tracheal epithelium after neuropeptide depletion and injury in vivo. Am J Physiol Lung Cell Mol Physiol 273: L1235–L1241, 1997 [DOI] [PubMed] [Google Scholar]
- 77.Kim JS, McKinnis VS, Nawrocki A, White SR. Stimulation of migration and wound repair of guinea-pig airway epithelial cells in response to epidermal growth factor. Am J Respir Cell Mol Biol 18: 66–74, 1998 [DOI] [PubMed] [Google Scholar]
- 78.Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, Sheppard D, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA 103: 13180–13185, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kleber M, Sommer L. Wnt signaling and the regulation of stem cell function. Curr Opin Cell Biol 16: 681–687, 2004 [DOI] [PubMed] [Google Scholar]
- 80.Kole TP, Tseng Y, Jiang I, Katz JL, Wirtz D. Intracellular mechanics of migrating fibroblasts. Mol Biol Cell 16: 328–338, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Konigshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, Eickelberg O. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One 3: e2142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Konigshoff M, Eickelberg O. WNT signaling in lung disease: a failure or a regeneration signal? Am J Respir Cell Mol Biol 42: 21–31, 2010 [DOI] [PubMed] [Google Scholar]
- 83.Konigshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, Gunther A, Eickelberg O. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest 119: 772–787, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 33: 328–334, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 128: 5181–5188, 2001 [DOI] [PubMed] [Google Scholar]
- 86.Krause DS. Bone marrow-derived cells and stem cells in lung repair. Proc Am Thorac Soc 5: 323–327, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lai JP, Dalton JT, Knoell DL. Phosphatase and tensin homologue deleted on chromosome ten (PTEN) as a molecular target in lung epithelial wound repair. Br J Pharmacol 152: 1172–1184, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell 84: 359–369, 1996 [DOI] [PubMed] [Google Scholar]
- 89.Lazar MH, Christensen PJ, Du M, Yu B, Subbotina NM, Hanson KE, Hansen JM, White ES, Simon RH, Sisson TH. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol 31: 672–678, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Lechapt-Zalcman E, Pruliere-Escabasse V, Advenier D, Galiacy S, Charriere-Bertrand C, Coste A, Harf A, d'Ortho MP, Escudier E. Transforming growth factor-β1 increases airway wound repair via MMP-2 upregulation: a new pathway for epithelial wound repair? Am J Physiol Lung Cell Mol Physiol 290: L1277–L1282, 2006 [DOI] [PubMed] [Google Scholar]
- 91.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA 106: 16357–16362, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther 9: 1259–1270, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee YH, Suzuki YJ, Griffin AJ, Day RM. Hepatocyte growth factor regulates cyclooxygenase-2 expression via β-catenin, Akt, and p42/p44 MAPK in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 294: L778–L786, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Legrand C, Polette M, Tournier JM, de Bentzmann S, Huet E, Monteau M, Birembaut P. uPA/plasmin system-mediated MMP-9 activation is implicated in bronchial epithelial cell migration. Exp Cell Res 264: 326–336, 2001 [DOI] [PubMed] [Google Scholar]
- 95.Leslie CC, McCormick-Shannon K, Shannon JM, Garrick B, Damm D, Abraham JA, Mason RJ. Heparin-binding EGF-like growth factor is a mitogen for rat alveolar type II cells. Am J Respir Cell Mol Biol 16: 379–387, 1997 [DOI] [PubMed] [Google Scholar]
- 96.Lesur O, Arsalane K, Lane D. Lung alveolar epithelial cell migration in vitro: modulators and regulation processes. Am J Physiol Lung Cell Mol Physiol 270: L311–L319, 1996 [DOI] [PubMed] [Google Scholar]
- 97.Lesur O, Brisebois M, Thibodeau A, Chagnon F, Lane D, Fullop T. Role of IFN-gamma and IL-2 in rat lung epithelial cell migration and apoptosis after oxidant injury. Am J Physiol Lung Cell Mol Physiol 286: L4–L14, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Liebler JM, Lutzko C, Banfalvi A, Senadheera D, Aghamohammadi N, Crandall ED, Borok Z. Retention of human bone marrow-derived cells in murine lungs following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol 295: L285–L292, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu X, Engelhardt JF. The glandular stem/progenitor cell niche in airway development and repair. Proc Am Thorac Soc 5: 682–688, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lloyd CM, Saglani S. Asthma and allergy: the emerging epithelium. Nat Med 16: 273–274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol 156: 985–996, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Maquerlot F, Galiacy S, Malo M, Guignabert C, Lawrence DA, d'Ortho MP, Barlovatz-Meimon G. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. Am J Pathol 169: 1624–1632, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Marsh LM, Cakarova L, Kwapiszewska G, von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor-induced epithelial repair. Am J Physiol Lung Cell Mol Physiol 296: L442–L452, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Mason RJ, Leslie CC, McCormick-Shannon K, Deterding RR, Nakamura T, Rubin JS, Shannon JM. Hepatocyte growth factor is a growth factor for rat alveolar type II cells. Am J Respir Cell Mol Biol 11: 561–567, 1994 [DOI] [PubMed] [Google Scholar]
- 105.Mason RJ, Williams MC. Type II alveolar cell. Defender of the alveolus. Am Rev Respir Dis 115: 81–91, 1977 [DOI] [PubMed] [Google Scholar]
- 106.Mason RJ, Williams MC, Greenleaf RD, Clements JA. Isolation and properties of type II alveolar cells from rat lung. Am Rev Respir Dis 115: 1015–1026, 1977 [DOI] [PubMed] [Google Scholar]
- 107.McGuire JK, Li Q, Parks WC. Matrilysin (matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 162: 1831–1843, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4: e115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mucenski ML, Nation JM, Thitoff AR, Besnard V, Xu Y, Wert SE, Harada N, Taketo MM, Stahlman MT, Whitsett JA. β-Catenin regulates differentiation of respiratory epithelial cells in vivo. Am J Physiol Lung Cell Mol Physiol 289: L971–L979, 2005 [DOI] [PubMed] [Google Scholar]
- 110.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 278: 40231–40238, 2003 [DOI] [PubMed] [Google Scholar]
- 111.Myerburg MM, Latoche JD, McKenna EE, Stabile LP, Siegfried JS, Feghali-Bostwick CA, Pilewski JM. Hepatocyte growth factor and other fibroblast secretions modulate the phenotype of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 292: L1352–L1360, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Neurohr C, Nishimura SL, Sheppard D. Activation of transforming growth factor-beta by the integrin alphavbeta8 delays epithelial wound closure. Am J Respir Cell Mol Biol 35: 252–259, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olsen CE, Liguori AE, Zong Y, Lantz RC, Burgess JL, Boitano S. Arsenic upregulates MMP-9 and inhibits wound repair in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 295: L293–L302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 100: 8407–8411, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Panos RJ, Bak PM, Simonet WS, Rubin JS, Smith LJ. Intratracheal instillation of keratinocyte growth factor decreases hyperoxia-induced mortality in rats. J Clin Invest 96: 2026–2033, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Panos RJ, Patel R, Bak PM. Intratracheal administration of hepatocyte growth factor/scatter factor stimulates rat alveolar type II cell proliferation in vivo. Am J Respir Cell Mol Biol 15: 574–581, 1996 [DOI] [PubMed] [Google Scholar]
- 118.Panos RJ, Rubin JS, Csaky KG, Aaronson SA, Mason RJ. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest 92: 969–977, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 34: 151–157, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parks WC, Lopez-Boado YS, Wilson CL. Matrilysin in epithelial repair and defense. Chest 120: 36S–41S, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Perrio MJ, Ewen D, Trevethick MA, Salmon GP, Shute JK. Fibrin formation by wounded bronchial epithelial cell layers in vitro is essential for normal epithelial repair and independent of plasma proteins. Clin Exp Allergy 37: 1688–1700, 2007 [DOI] [PubMed] [Google Scholar]
- 122.Pilewski JM, Latoche JD, Arcasoy SM, Albelda SM. Expression of integrin cell adhesion receptors during human airway epithelial repair in vivo. Am J Physiol Lung Cell Mol Physiol 273: L256–L263, 1997 [DOI] [PubMed] [Google Scholar]
- 123.Planus E, Galiacy S, Matthay M, Laurent V, Gavrilovic J, Murphy G, Clerici C, Isabey D, Lafuma C, d'Ortho MP. Role of collagenase in mediating in vitro alveolar epithelial wound repair. J Cell Sci 112: 243–252, 1999 [DOI] [PubMed] [Google Scholar]
- 124.Pongracz JE, Stockley RA. Wnt signalling in lung development and diseases. Respir Res 7: 15, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 726–733, 2006 [DOI] [PubMed] [Google Scholar]
- 126.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 14: 1362–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 127.Qiao R, Yan W, Clavijo C, Mehrian-Shai R, Zhong Q, Kim KJ, Ann D, Crandall ED, Borok Z. Effects of KGF on alveolar epithelial cell transdifferentiation are mediated by JNK signaling. Am J Respir Cell Mol Biol 38: 239–246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Quesnel C, Marchand-Adam S, Fabre A, Marchal-Somme J, Philip I, Lasocki S, Lecon V, Crestani B, Dehoux M. Regulation of hepatocyte growth factor secretion by fibroblasts in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol 294: L334–L343, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Randell SH. Airway epithelial stem cells and the pathophysiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc 3: 718–725, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA 104: 410–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol 162: 2069–2078, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rennard SI, Togo S, Holz O. Cigarette smoke inhibits alveolar repair: a mechanism for the development of emphysema. Proc Am Thorac Soc 3: 703–708, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reya T, Clevers H. Wnt signalling in stem cells and cancer. Nature 434: 843–850, 2005 [DOI] [PubMed] [Google Scholar]
- 134.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 156: 269–278, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 278: L1256–L1263, 2000 [DOI] [PubMed] [Google Scholar]
- 136.Reynolds SD, Reynolds PR, Snyder JC, Whyte F, Paavola KJ, Stripp BR. CCSP regulates cross talk between secretory cells and both ciliated cells and macrophages of the conducting airway. Am J Physiol Lung Cell Mol Physiol 293: L114–L123, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, Mariani T, Di PY, Taketo MM, Stripp BR. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells 26: 1337–1346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 273: L246–L255, 1997 [DOI] [PubMed] [Google Scholar]
- 139.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science 302: 1704–1709, 2003 [DOI] [PubMed] [Google Scholar]
- 140.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 326: 298–304, 1992 [DOI] [PubMed] [Google Scholar]
- 141.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, Randell SH, Hogan BL. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA 106: 12771–12775, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol 9: 640–648, 1999 [DOI] [PubMed] [Google Scholar]
- 143.Ryan RM, Mineo-Kuhn MM, Kramer CM, Finkelstein JN. Growth factors alter neonatal type II alveolar epithelial cell proliferation. Am J Physiol Lung Cell Mol Physiol 266: L17–L22, 1994 [DOI] [PubMed] [Google Scholar]
- 144.Savla U, Appel HJ, Sporn PH, Waters CM. Prostaglandin E(2) regulates wound closure in airway epithelium. Am J Physiol Lung Cell Mol Physiol 280: L421–L431, 2001. [DOI] [PubMed] [Google Scholar]
- 146.Savla U, Olson LE, Waters CM. Mathematical modeling of airway epithelial wound closure during cyclic mechanical strain. J Appl Physiol 96: 566–574, 2004 [DOI] [PubMed] [Google Scholar]
- 147.Savla U, Waters CM. Barrier function of airway epithelium: effects of radiation and protection by keratinocyte growth factor. Radiat Res 150: 195–203, 1998 [PubMed] [Google Scholar]
- 148.Savla U, Waters CM. Mechanical strain inhibits repair of airway epithelium in vitro. Am J Physiol Lung Cell Mol Physiol 274: L883–L892, 1998. [DOI] [PubMed] [Google Scholar]
- 150.Schnackenberg BJ, Jones SM, Pate C, Shank B, Sessions L, Pittman LM, Cornett LE, Kurten RC. The β-agonist isoproterenol attenuates EGF-stimulated wound closure in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 290: L485–L491, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Schoch KG, Lori A, Burns KA, Eldred T, Olsen JC, Randell SH. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol 286: L631–L642, 2004 [DOI] [PubMed] [Google Scholar]
- 152.Shahabuddin S, Ji R, Wang P, Brailoiu E, Dun N, Yang Y, Aksoy MO, Kelsen SG. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol 291: C34–C39, 2006 [DOI] [PubMed] [Google Scholar]
- 153.Simonet WS, DeRose ML, Bucay N, Nguyen HQ, Wert SE, Zhou L, Ulich TR, Thomason A, Danilenko DM, Whitsett JA. Pulmonary malformation in transgenic mice expressing human keratinocyte growth factor in the lung. Proc Natl Acad Sci USA 92: 12461–12465, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Small JV, Kaverina I, Krylyshkina O, Rottner K. Cytoskeleton cross-talk during cell motility. FEBS Lett 452: 96–99, 1999 [DOI] [PubMed] [Google Scholar]
- 155.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 100: 2397–2402, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Spurzem JR, Gupta J, Veys T, Kneifl KR, Rennard SI, Wyatt TA. Activation of protein kinase A accelerates bovine bronchial epithelial cell migration. Am J Physiol Lung Cell Mol Physiol 282: L1108–L1116, 2002 [DOI] [PubMed] [Google Scholar]
- 157.Spurzem JR, Sacco O, Rickard KA, Rennard SI. Transforming growth factor-beta increases adhesion but not migration of bovine bronchial epithelial cells to matrix proteins. J Lab Clin Med 122: 92–102, 1993 [PubMed] [Google Scholar]
- 158.Steiling K, Lenburg ME, Spira A. Airway gene expression in chronic obstructive pulmonary disease. Proc Am Thorac Soc 6: 697–700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stevens PT, Kicic A, Sutanto EN, Knight DA, Stick SM. Dysregulated repair in asthmatic paediatric airway epithelial cells: the role of plasminogen activator inhibitor-1. Clin Exp Allergy 38: 1901–1910, 2008 [DOI] [PubMed] [Google Scholar]
- 160.Strieter RM. What differentiates normal lung repair and fibrosis? Inflammation, resolution of repair, and fibrosis. Proc Am Thorac Soc 5: 305–310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 5: 328–333, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takami K, Takuwa N, Okazaki H, Kobayashi M, Ohtoshi T, Kawasaki S, Dohi M, Yamamoto K, Nakamura T, Tanaka M, Nakahara K, Takuwa Y, Takizawa H. Interferon-gamma inhibits hepatocyte growth factor-stimulated cell proliferation of human bronchial epithelial cells: upregulation of p27(kip1) cyclin-dependent kinase inhibitor. Am J Respir Cell Mol Biol 26: 231–238, 2002 [DOI] [PubMed] [Google Scholar]
- 163.Ten Have-Opbroek AA. Immunological study of lung development in the mouse embryo. II. First appearance of the great alveolar cell, as shown by immunofluorescence microscopy. Dev Biol 69: 408–423, 1979 [DOI] [PubMed] [Google Scholar]
- 164.Trinh NT, Prive A, Kheir L, Bourret JC, Hijazi T, Amraei MG, Noel J, Brochiero E. Involvement of KATP and KvLQT1 K+ channels in EGF-stimulated alveolar epithelial cell repair processes. Am J Physiol Lung Cell Mol Physiol 293: L870–L882, 2007 [DOI] [PubMed] [Google Scholar]
- 165.Trinh NT, Prive A, Maille E, Noel J, Brochiero E. EGF and K+ channel activity control normal and cystic fibrosis bronchial epithelia repair. Am J Physiol Lung Cell Mol Physiol 295: L866–L880, 2008 [DOI] [PubMed] [Google Scholar]
- 166.Tschumperlin DJ, Oswari J, Margulies AS. Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162: 357–362, 2000 [DOI] [PubMed] [Google Scholar]
- 167.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, Spoor MS, You Y, Brody SL, Holtzman MJ. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116: 309–321, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 93: 1298–1306, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Upadhyay D, Bundesmann M, Panduri V, Correa-Meyer E, Kamp DW. Fibroblast growth factor-10 attenuates H2O2-induced alveolar epithelial cell DNA damage: role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol 31: 107–113, 2004 [DOI] [PubMed] [Google Scholar]
- 170.Upadhyay D, Correa-Meyer E, Sznajder JI, Kamp DW. FGF-10 prevents mechanical stretch-induced alveolar epithelial cell DNA damage via MAPK activation. Am J Physiol Lung Cell Mol Physiol 284: L350–L359, 2003 [DOI] [PubMed] [Google Scholar]
- 171.Van Winkle LS, Isaac JM, Plopper CG. Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am J Pathol 151: 443–459, 1997 [PMC free article] [PubMed] [Google Scholar]
- 172.Verghese GM, McCormick-Shannon K, Mason RJ, Matthay MA. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med 158: 386–394, 1998 [DOI] [PubMed] [Google Scholar]
- 173.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Deformation-induced lipid trafficking in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 280: L938–L946, 2001 [DOI] [PubMed] [Google Scholar]
- 174.Vlahakis NE, Schroeder MA, Pagano RE, Hubmayr RD. Role of deformation-induced lipid trafficking in the prevention of plasma membrane stress failure. Am J Respir Crit Care Med 166: 1282–1289, 2002 [DOI] [PubMed] [Google Scholar]
- 175.Wagh AA, Roan E, Chapman KE, Desai LP, Rendon DA, Eckstein EC, Waters CM. Localized elasticity measured in epithelial cells migrating at a wound edge using atomic force microscopy. Am J Physiol Lung Cell Mol Physiol 295: L54–L60, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr, Spees JL, Bertucci D, Peister A, Weiss DJ, Valentine VG, Prockop DJ, Kolls JK. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA 102: 186–191, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Warburton D, Perin L, Defilippo R, Bellusci S, Shi W, Driscoll B. Stem/progenitor cells in lung development, injury repair, and regeneration. Proc Am Thorac Soc 5: 703–706, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000 [DOI] [PubMed] [Google Scholar]
- 180.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol 282: L924–L940, 2002 [DOI] [PubMed] [Google Scholar]
- 181.Waters CM, Savla U. Keratinocyte growth factor accelerates wound closure in airway epithelium during cyclic mechanical strain. J Cell Physiol 181: 424–432, 1999 [DOI] [PubMed] [Google Scholar]
- 182.Waters CM, Savla U, Panos RJ. KGF prevents hydrogen peroxide-induced increases in airway epithelial cell permeability. Am J Physiol Lung Cell Mol Physiol 272: L681–L689, 1997 [DOI] [PubMed] [Google Scholar]
- 183.Watkins DN, Berman DM, Burkholder SG, Wang B, Beachy PA, Baylin SB. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 422: 313–317, 2003 [DOI] [PubMed] [Google Scholar]
- 184.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282: 3213–3220, 2007 [DOI] [PubMed] [Google Scholar]
- 185.White SR, Dorscheid DR, Rabe KF, Wojcik KR, Hamann KJ. Role of very late adhesion integrins in mediating repair of human airway epithelial cell monolayers after mechanical injury. Am J Respir Cell Mol Biol 20: 787–796, 1999 [DOI] [PubMed] [Google Scholar]
- 186.White SR, Fischer BM, Marroquin BA, Stern R. Interleukin-1β mediates human airway epithelial cell migration via NF-κB. Am J Physiol Lung Cell Mol Physiol 295: L1018–L1027, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.White SR, Martin LD, Abe MK, Marroquin BA, Stern R, Fu X. Insulin receptor substrate-1/2 mediates IL-4-induced migration of human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 297: L164–L173, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.White SR, Tse R, Marroquin BA. Stress-activated protein kinases mediate cell migration in human airway epithelial cells. Am J Respir Cell Mol Biol 32: 301–310, 2005 [DOI] [PubMed] [Google Scholar]
- 189.White SR, Wojcik KR, Gruenert D, Sun S, Dorscheid DR. Airway epithelial cell wound repair mediated by alpha-dystroglycan. Am J Respir Cell Mol Biol 24: 179–186, 2001 [DOI] [PubMed] [Google Scholar]
- 190.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 423: 448–452, 2003 [DOI] [PubMed] [Google Scholar]
- 191.Willis BC, Borok Z. TGF-β-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol 293: L525–L534, 2007 [DOI] [PubMed] [Google Scholar]
- 192.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 282: 2258–2261, 1998 [DOI] [PubMed] [Google Scholar]
- 193.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest 119: 336–348, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Xu J, Woods CR, Mora AL, Joodi R, Brigham KL, Iyer S, Rojas M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am J Physiol Lung Cell Mol Physiol 293: L131–L141, 2007 [DOI] [PubMed] [Google Scholar]
- 195.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol 279: L1146–L1158, 2000 [DOI] [PubMed] [Google Scholar]
- 196.Yee M, Vitiello PF, Roper JM, Staversky RJ, Wright TW, McGrath-Morrow SA, Maniscalco WM, Finkelstein JN, O'Reilly MA. Type II epithelial cells are critical target for hyperoxia-mediated impairment of postnatal lung development. Am J Physiol Lung Cell Mol Physiol 291: L1101–L1111, 2006 [DOI] [PubMed] [Google Scholar]
- 197.Yu H, Konigshoff M, Jayachandran A, Handley D, Seeger W, Kaminski N, Eickelberg O. Transgelin is a direct target of TGF-beta/Smad3-dependent epithelial cell migration in lung fibrosis. FASEB J 22: 1778–1789, 2008 [DOI] [PubMed] [Google Scholar]
- 198.Zahm JM, Chevillard M, Puchelle E. Wound repair of human surface respiratory epithelium. Am J Respir Cell Mol Biol 5: 242–248, 1991 [DOI] [PubMed] [Google Scholar]
- 199.Zahm JM, Debordeaux C, Raby B, Klossek JM, Bonnet N, Puchelle E. Motogenic effect of recombinant HGF on airway epithelial cells during the in vitro wound repair of the respiratory epithelium. J Cell Physiol 185: 447–453, 2000 [DOI] [PubMed] [Google Scholar]
- 200.Zahm JM, Kaplan H, Herard AL, Doriot F, Pierrot D, Somelette P, Puchelle E. Cell migration and proliferation during the in vitro wound repair of the respiratory epithelium. Cell Motil Cytoskel 37: 33–43, 1997 [DOI] [PubMed] [Google Scholar]
- 201.Zahm JM, Pierrot D, Chevillard M, Puchelle E. Dynamics of cell movement during the wound repair of human surface respiratory epithelium. Biorheology 29: 459–465, 1992 [DOI] [PubMed] [Google Scholar]
- 202.Zemke AC, Teisanu RM, Giangreco A, Drake JA, Brockway BL, Reynolds SD, Stripp BR. β-Catenin is not necessary for maintenance or repair of the bronchiolar epithelium. Am J Respir Cell Mol Biol 41: 535–543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu M, Tian D, Li J, Ma Y, Wang Y, Wu R. Glycogen synthase kinase 3beta and beta-catenin are involved in the injury and repair of bronchial epithelial cells induced by scratching. Exp Mol Pathol 83: 30–38, 2007. [DOI] [PubMed] [Google Scholar]