Abstract
Zinc is an essential element that facilitates coordination of immune activation during the host response to infection. We recently reported that zinc deficiency increases systemic inflammation, vital organ damage, and mortality in a small animal model of sepsis. To investigate potential mechanisms that cause these phenomena, we used the same animal model and observed that zinc deficiency increases bacterial burden and enhances NF-κB activity in vital organs including the lung. We conducted further studies in the lung to determine the overall impact of zinc deficiency. At the molecular level, NF-κB p65 DNA-binding activity was enhanced by zinc deficiency in response to polymicrobial sepsis. Furthermore, expression of the NF-κB-targeted genes IL-1β, TNFα, ICAM-1, and the acute phase response gene SAA1/2 were elevated by zinc deficiency. Unexpectedly, the amount of NF-κB p65 mRNA and protein was increased in the lung including alveolar epithelia of zinc-deficient mice. These events occurred with a significant and concomitant increase in caspase-3 activity within 24 h of sepsis onset in zinc-deficient mice relative to control group. Short-term zinc supplementation reversed these effects. Reconstitution of zinc deficiency in lung epithelial cultures resulted in similar findings in response to TNFα. Taken together, zinc deficiency systemically enhances the spread of infection and NF-κB activation in vivo in response to polymicrobial sepsis, leading to enhanced inflammation, lung injury, and, as reported previously, mortality. Zinc supplementation immediately before initiation of sepsis reversed these effects thereby supporting the plausibility of future studies that explore zinc supplementation strategies to prevent sepsis-mediated morbidity and mortality.
Keywords: NF-κB pathway, lung, inflammation
sepsis is a frequently occurring serious medical condition caused by infection leading to systemic activation of the host inflammatory response and tissue injury. Subsequent failure of vital organs, including the lung, is the leading cause of morbidity and mortality in sepsis patients (35). Severe sepsis and mortality are strongly associated with overactivation of the inflammatory response (34, 45). Increased expression of cytokines including IL-1β, TNFα, and IL-6 has been reported to be associated with an increased risk of lung and other vital organ failure in sepsis along with a poorer prognosis (22).
Zinc deficiency has been identified as a significant public health problem contributing to ∼800,000 deaths worldwide annually (18) and is one of the underlying causes for infections including pneumonia, diarrhea, and malaria among children <5 yr old (9). Zinc supplementation has been shown to prevent the incidence of pneumonia and diarrhea in children (7). Zinc deficiency in the United States is underestimated and predicted to affect millions (6, 48). Individuals that are most prone to zinc deficiency include the elderly, those with chronic illness, and alcoholics (15, 33, 36, 48). Perhaps not by coincidence, these subjects are also at higher risk of developing severe infection and sepsis. To begin to understand the potential impact of zinc deficiency on sepsis, we studied a small animal cecal ligation and puncture (CLP) model and observed that moderate zinc deficiency, as a consequence of restricted dietary intake, resulted in a significant increase in bacterial burden, systemic inflammation, tissue injury, and mortality. Furthermore, acute zinc supplementation in zinc-deficient mice decreased the host immune response and significantly improved survival, demonstrating that zinc plays an important role in the host response to sepsis (27).
NF-κB is centrally involved in activation of both the host immune and acute phase response by coordinating the expression of a variety of genes (21). Briefly, following pathogen recognition by pattern recognition receptors such as Toll-like receptors, IKK (IκB kinase)-dependent complexes are activated, leading to degradation of IκB and liberation of primarily p65- and c-Rel-containing dimers, which, in turn, translocate into the nucleus to initiate gene transcription (28). The NF-κB pathway is rapidly activated in humans in response to sepsis (2), as demonstrated by nuclear accumulation in alveolar macrophages (32, 37) and peripheral blood mononuclear cells (2, 5). Whereas its activation is vital in triggering the innate immune response (40), elevated and prolonged NF-κB activation is associated with increased acute lung injury, compromised tissue oxygenation, and subsequent dysfunction of other vital organs in sepsis subjects (5). Relative to NF-κB signaling, zinc has the potential to provoke opposite effects that can either activate (11, 17, 49) or inhibit (46, 47, 50) NF-κB-mediated transactivation. These contradictory observations are largely based on relevant cell culture models, which lead us to question what the cumulative impact of nutritional zinc deficiency would be in a clinically relevant animal model of sepsis.
Based on our previous observation that zinc deficiency increased the proinflammatory response and mortality in response to sepsis (27), we hypothesized that zinc deficiency increases susceptibility to bacterial infection and augments NF-κB activity during the early stages of CLP-induced polymicrobial sepsis, thereby predisposing individuals to dysregulation of bacterial clearance and the proinflammatory response, culminating in increased tissue injury. Our focus in this investigation was placed on the lung knowing that it is typically the first vital organ adversely affected by overwhelming inflammation during the early stages of sepsis.
MATERIALS AND METHODS
Animal Studies
Establishing a mouse model of zinc deficiency.
Ten-week-old adult male C57BL/6 mice (∼25 g; Harlan Sprague-Dawley, Indianapolis, IN) with fully developed lungs were randomly placed on a zinc-deficient diet [1 part per million (ppm); TD.85419; Harlan Teklad] or a matched control diet (50 ppm; TD.85420) for 3 wk, a time sufficient to establish subacute zinc deficiency without requiring pair feeding. A zinc-free environment was carefully maintained using deionized water in zinc-free containers and stainless steel cages along with daily cage changes. Plasma zinc concentrations and tissue metallothionein levels were evaluated in the lung and liver to confirm that animals achieved a zinc-deficient state (27). As previously reported, zinc-deficient diets consistently resulted in an ∼2.5-fold decrease in plasma zinc levels as measured by atomic absorption spectroscopy. An additional group received a zinc-fortified diet (100 ppm; TD.07129) for 3 days following an 18-day zinc-deficient regimen (27). Plasma zinc levels consistently returned to normal by the end of the 3-day supplementation regime. Importantly, we reassessed zinc levels in an identical fashion in the repeated studies and recorded similar results (data not shown).
CLP.
At the end of the dietary regime, mice were subjected to CLP via laparotomy under general anesthesia (1% isoflurane) as described previously (10, 23) with slight modifications. Briefly, through an upper midline abdominal incision, the cecum was delivered, ligated with a silk suture 1 cm from the tip, and doubly punctured with a 21-gauge needle. After puncture, the cecum was gently squeezed to extrude fecal content and returned to the abdominal cavity. The laparotomy was then closed. This model reproducibly results in ∼30% mortality within 7 days in our hands. It is important to note that we previously reported that sham surgery (delivery of the cecum without ligation and puncture) had no effect on indices of inflammation, vital organ injury, and mortality so was not further evaluated in this investigation. Animal studies were conducted in accordance with prior approval by The Ohio State University Institutional Animal Care and Use Committee. Blood, lung, liver, spleen, and intestine were collected at 6 or 24 h after CLP. Lung tissue was processed as follows. Immediately after death, the thoracic cavity was opened, and the lung vasculature was lavaged with saline. The left ventricle was cut open to allow perfusion through the right ventricle with 30 ml of PBS. One half of the lung was excised and submitted to RNA or protein extraction. The other half was prepared for fixation by inflation with PBS-buffered formaldehyde at a constant pressure equal to 25 cmH2O, which allows the homogenous expansion of the lung parenchyma. Lung tissue was then embedded in paraffin.
Bacterial Colony Counts
Blood was collected at 24 h after CLP and immediately plated on blood agar plates (TSA II, Trypticase Soy Agar with 10% Sheep Blood; Becton Dickinson) in 5 serial 10-fold dilutions. Following overnight incubation, bacterial colonies were enumerated and compared among different treatment groups.
Imaging and Luciferase Measurements
Transgenic mice (Caliper Life Sciences, Hopkinton, MA) that systemically express luciferase under the control of an NF-κB-responsive promoter were used (8) to determine real-time in vivo imaging of NF-κB activity in intact animals and vital organs. Imaging of transgenic mice was performed with an ultrasensitive camera consisting of an image intensifier coupled to a charge-coupled device (CCD; Xenogen IVIS Imaging System; Xenogen, Alameda, CA). Transgenic mice were administrated different zinc diets and then subjected to CLP as previously described. Whole body imaging was conducted at 4 and 8 h post-CLP. Before imaging, mice were anesthetized under isoflurane. d-luciferin (100 mg/kg; Caliper Life Sciences) dissolved in 200 μl PBS, pH 7.8, was injected intraperitoneally. Grayscale images were obtained for reference. Luminescence emitted from each animal was integrated for 10 min starting 2 min after d-luciferin injection. Following that, individual vital organs were excised from the mice and then placed on six-well plates and immediately imaged. The pseudocolored images represent light intensity. All images were processed with Igor Pro 4.09A and Xenogen Living Image 2.50 software programs (Xenogen).
Evaluation of NF-κB DNA-Binding Activity
The DNA-binding activity of NF-κB in mouse lung tissue lysate was quantified by the TransAM NF-κB p65 transcription factor assay kit using an ELISA-based format (Active Motif North America, Carlsbad, CA). Mouse lung extracts were incubated in 96-well plates coated with the immobilized oligonucleotide (5′-AGTTGAGGGGACTTTCCCAGGC-3′) containing a consensus (5′-GGGACTTTCC-3′) binding site for the p65 subunit of NF-κB. NF-κB binding to the target oligonucleotide was detected by incubation with primary antibody (Ab) specific for the activated form of p65, visualized by anti-IgG horseradish peroxidase conjugate and developing solution, and quantified at 450 nm with a reference wavelength of 655 nm. The background binding, obtained by incubation with a two-nucleotide mutant oligonucleotide (5′-AGTTGAGGCCACTTTCCCAGGC-3′) was subtracted from the value for binding to the consensus DNA sequence.
Real-Time RT-PCR
Total RNA was isolated from lung tissue or cell culture using TRIzol reagent (Invitrogen, Carlsbad, CA). First-strand cDNA synthesis was performed by using ThermoScript RT-PCR System (Invitrogen). Real-time PCR was performed with the 7900HT Fast Real-Time PCR system (Applied Biosystems) using SYBR Green reagents. All the analysis involving mouse lung tissue was normalized against the average cycle threshold number of mouse GAPDH and cyclophilin genes. Studies involving human lung epithelial cell culture were normalized against a human GAPDH probe. The sequences of all the PCR primers are available on request.
Immunohistochemistry and Immunofluorescent Staining of Mouse Lung Tissue
Lung tissue slides were deparaffinized and rehydrated, and then antigen retrieval was performed. Following permeabilization with 1% Triton X-100 and blocking with 2% goat serum in PBS, the sections were incubated with primary rabbit anti-p65 Ab (1:200; Rockland, Gilbertsville, PA; provided by Dr. D. Guttridge, The Ohio State University), which detects total NF-κB p65 protein, and then incubated with the secondary Ab, followed by counterstaining with hematoxylin and microscopic evaluation. For immunofluorescence staining, sections of tissues or cells were incubated with the primary rabbit anti-p65 Ab followed by the secondary Ab (Alexa Fluor 488 goat anti-rabbit Ab; Invitrogen). Nuclear DNA was detected with 4′,6′-diamidino-2-phenylindole (DAPI) or propidium iodide (PI). Rhodamine-labeled Ricinus communis agglutinin I (RCA), a marker for type I lung epithelial cells, was also used for colocalization imaging studies. Before inspection, slides were mounted with Citifluor antifadent mounting medium (AF1; Electron Microscopy Science) and then examined using a disk scanning confocal microscope (Olympus BX61). p65 Protein levels in the lung were quantified using histogram analysis in Adobe Photoshop CS2 (Adobe Systems). Brown pixels, representing positive staining for NF-κB p65 protein, were enumerated.
Cell Culture, Transfection, and Luciferase Assay
The human lung epithelial cell lines A549 and BEAS-2B [American Type Culture Collection (ATCC), Manassas, VA] were routinely maintained in DMEM supplemented with 10% FBS, 1% sodium pyruvate, 0.1 mg/ml streptomycin, and 100 IU/ml penicillin at 37°C in a 5% CO2-humidified atmosphere. Primary human lung epithelial cells (hLECs) were isolated after enzymatic dissociation from trachea, bronchi, and bronchioles of adult donor lungs, seeded onto collagen-coated, semipermeable membranes (0.6 cm2; Millicell HA; Millipore, Bedford, MA), and grown as fully differentiated, polarized, monolayer cells at an air-liquid interface, as previously described (4, 25). Transient transfection was performed with Lipofectamine 2000 (Invitrogen) in A549 and BEAS-2B cells seeded onto 48-well plates. For each well, the plasmid-Lipofectamine complexes were formed by incubating 0.3 μg of NF-κB 3×κB-luc reporter plasmid [provided by Dr. D. Guttridge, The Ohio State University, as previously described (16)], 0.1 μg of promoter-linked Renilla luciferase vector (pRL-TK) plasmid, and 1 μl of Lipofectamine at room temperature for 20 min in a total volume of 100 μl of serum-free DMEM. The DNA-Lipofectamine complexes were then diluted with 100 μl of DMEM and applied at 200 μl per well. After 12 h of incubation, the transfection medium was replaced with DMEM containing 10% FBS with the appropriate solvent or reagents. Cell lysis was performed 6 h after transfection and then subjected to a luciferase reporter gene assay using the Dual-Glo Luciferase Assay System (Promega, Madison, WI).
Cytokine Analysis
Multiplex quantification of cytokine and chemokine levels was conducted in hLEC supernatants obtained 24 h after exposure to either TNFα alone (10 ng/ml) or in combination with the zinc chelating agent N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; 20 μM) or zinc sulfate (5 μM). The concentration of each cytokine was analyzed from a 50-μl sample volume using a custom-made Bio-Plex Cytokine singleplex panel that includes IL-6 and ICAM-1 using the Bio-Plex 200 Analysis System (Bio-Rad, Hercules, CA).
SAA Determination
Approximately 0.5 ml of whole blood was collected via cardiac puncture using a syringe with a 25-gauge × 5/8 in. needle. Serum was separated by centrifugation at 1,000 g for 10 min at 4°C. Quantification of serum amyloid A (SAA) levels was determined by ELISA according to the manufacturer's recommendations (Invitrogen BioSource).
Measurement of Caspase-3 Activity
Caspase activity was measured with 7-amino-4-trifluoromethyl coumarin (AFC). For all AFC preparations, the presence of active caspase-3 in lung cell lysates was determined by an AFC assay using specific fluorosubstrates. Lysates were incubated with cyto-buffer (10% glycerol, 50 mM PIPES, pH 7.0, and 1 mM EDTA) containing 1 mM DTT and 20 μM Asp-Glu-Val-Asp (DEVD)-AFC or Ile-Glu(OMe)-Thr-Asp(OMe)-AFC (Enzyme Systems Products). The release of free AFC was determined using a fluorometer (400-nm excitation and 505-nm emission; Cytofluor 4000; Perseptive, Framingham, MA).
Statistical Analysis
All data are presented as means ± SD. Statistical comparisons among different groups were performed using ANOVA. Systemic bacterial burden between all groups was evaluated by ANOVA followed by Tukey honestly significant difference test. Significance was assumed at a P value of <0.05.
RESULTS
Zinc Deficiency Increases Bacterial Burden and NF-κB Activity in Response to Sepsis In Vivo
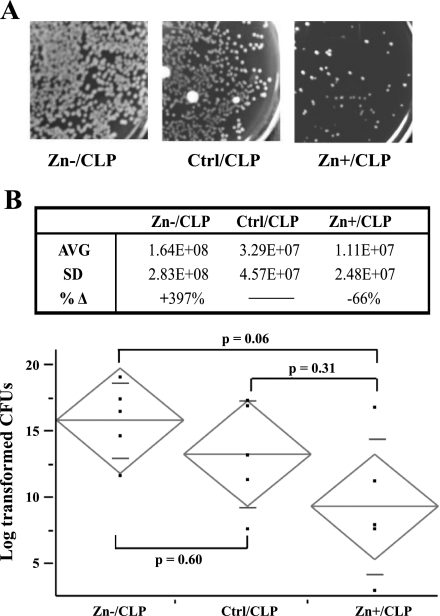
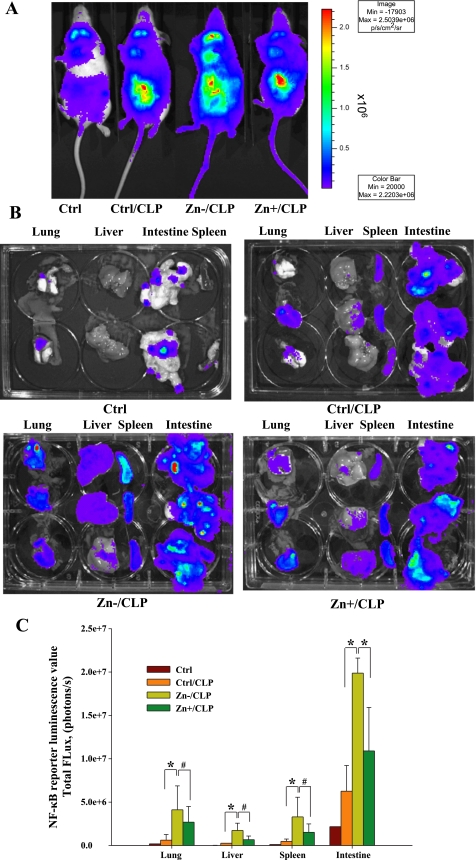
As previously described, mice were randomized into 3 groups and received a normal diet, a zinc-deficient diet, or a zinc-deficient diet followed by a short repletion period. At 24 h post-CLP, mice were killed; blood samples were obtained, plated on blood agar plates, and then enumerated for bacterial colony counts the following day. As shown in Fig. 1, zinc-deficient (Zn−/CLP) mice consistently exhibited higher bacterial counts within their bloodstream compared with zinc-sufficient (Ctrl/CLP) mice. Short-term zinc supplementation (Zn+/CLP) lowered the bacterial counts compared with the Zn−/CLP group. No bacteria were found in animals placed only on dietary modification without CLP (data not shown). Although the comparison between Zn−/CLP and Ctrl/CLP mice did not achieve statistical significance due to interindividual variability within treatment groups, the bacterial counts in the Zn−/CLP mice were all substantially elevated. Next, BALB/c NF-κB luciferase transgenic mice were employed to monitor NF-κB activation in vivo during the early stages of CLP-induced polymicrobial sepsis. Briefly, a total of 11 transgenic mice were randomized into 3 groups and received a normal diet (n = 5), a zinc-deficient diet (n = 3), or a zinc-deficient diet followed by a short repletion period (n = 3) identical to that described for use with C57BL/6 mice (materials and methods). Following the dietary period, a combined total of 9 mice were subjected to receive CLP. At 8 h after CLP treatment, the mice received an intraperitoneal injection of d-luciferin and then were placed posteriorly in a light-sealed chamber and imaged. Initially, we observed a low level of luminescence restricted primarily within the neck region of noninfected control mice consistent with a previous report by an independent group (8). In direct comparison, a very intense signal was detected within the abdominal region in the CLP-treated mice. In addition, luminescence was also observed within the thoracic region. The luminescence in the neck was associated with lymph nodes, the thoracic signal originated from thymus and lung, and the abdominal signal coming from the intestine, presumably originating from the site of CLP. The Zn−/CLP mice consistently had increased bioluminescence in the abdominal and thoracic cavity compared with the other treatment groups as shown by representative images (Fig. 2A). To directly quantify the signal within vital organs, the lung, liver, spleen, and intestine were excised immediately following whole body imaging, and luminescence was measured (Fig. 2B). Quantitative analysis of each vital organ from each animal consistently demonstrated that Zn−/CLP mice had a significant increase in NF-κB-dependent luminescence across all organs studied compared with Ctrl/CLP mice (Fig. 2C). Furthermore, we observed that short-term oral zinc repletion (Zn+/CLP) reduced NF-κB-dependent luminescence in all vital organs studied. Taken together, these findings demonstrate that zinc has a systemic impact on the NF-κB-mediated innate immune response during the early stages of polymicrobial infection.
Fig. 1.
Zinc deficiency increases bacterial burden following cecal ligation and puncture (CLP). Whole blood was obtained 24 h following CLP treatment, plated on blood agar following serial dilutions, and then incubated overnight and enumerated the following day. A: representative pictures of plates from the zinc-deficient (Zn−/CLP), zinc-sufficient (Ctrl/CLP), and zinc-supplemented (Zn+/CLP) treatment groups are shown. B, top: total bacterial counts were obtained from all animals in each treatment group. Average (AVG) and standard deviation (SD) are displayed in this table (n = 5 mice in each treatment group). %Δ Represents the % difference between the zinc-deficient (Zn−) or zinc-supplemented (Zn+) compared with the baseline control zinc diet (Ctrl). B, bottom: bacterial counts were log transformed to obtain a normal distribution and then displayed graphically (long horizontal line in the diamond = group mean, and short horizontal lines in the diamond = SD). The differences between all groups were assessed by ANOVA (P = 0.07) followed by the Tukey honestly significant difference test to look for trends between each group, P values are shown. No bacteria were present in animals placed on normal or zinc-modified diets (data not shown). This figure is representative of 2 separate experiments. cfu, Colony-forming units.
Fig. 2.
Zinc deficiency increases NF-κB activation systemically following CLP. A: a representative whole body image obtained from 1 mouse within each treatment group is shown and includes the untreated control diet (Ctrl) group along with analysis of mice 8 h after CLP in conjunction with 3 different zinc diets, including the control diet (Ctrl/CLP), a zinc-deficient diet (Zn−/CLP), or a zinc-deficient diet followed by acute zinc supplementation (Zn+/CLP) (n = 2 for untreated mice, n = 3 per dietary groups involving CLP treatment). The anesthetized mice were placed in a light-sealed chamber connected to the charge-coupled device (CCD) camera for image analysis. Luminescence emitted from each animal was integrated for 10 min starting 2 min after d-luciferin injection. B: vital organs including the liver, lung, spleen, and intestine were excised from each animal and immediately subjected to quantitative bioluminescent imaging. The composite images of each tissue from each animal are presented after color scale adjustment (the scale for A and B is identical). C: metric analysis of total photon flux of pooled data for each tissue from each treatment group (*P < 0.05, #not statistically significant).
Zinc Deficiency Increases NF-κB Activity in the Lung
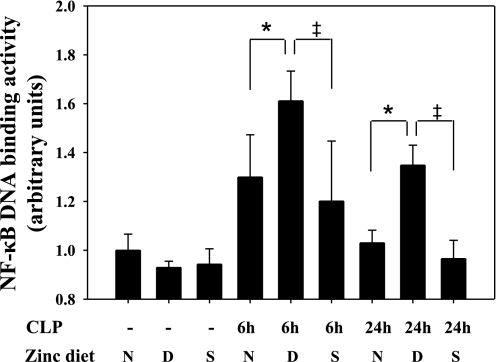
Since the lung is the first vital organ adversely affected at the onset of sepsis, and the extent of acute lung injury directly correlates with mortality (1), we next determined whether zinc deficiency causes more extensive inflammation and injury in the lung through enhancement of NF-κB activity. To test this, we first determined whether lung homogenates obtained from C57BL/6 adult mice (randomized and treated as mentioned in materials and methods) possessed different levels of active NF-κB p65 using the TransAM DNA-binding assay. Lung tissue lysates were obtained from mice on normal and modified zinc diets at 6 and 24 h after CLP treatment. Zn−/CLP mice exhibited increased NF-κB p65 DNA-binding activity in the lung (Fig. 3). Consistent with our previous observation, a significant increase in NF-κB activation was apparent very early (at 6 h) in the lung, suggesting that zinc deficiency likely accelerates the time course of sepsis-initiated NF-κB activation in key vital organs distal to the original site of infection (cecum). Zinc supplementation suppressed the extent of NF-κB activation in the lung at all time points studied.
Fig. 3.
Zinc deficiency increases NF-κB DNA-binding activity in vivo in the lung following CLP. DNA-binding activity of the transcriptional factor NF-κB p65 was quantified in mouse lung tissue obtained from adult male C57BL/6 mice placed on zinc-modified diets at 6 and 24 h following CLP. Values (means ± SD) were normalized according to cellular protein content. Data are representative of 3 separate experiments (n = 5 animals per treatment group: N, zinc-normal diet; D, zinc-deficient diet; S, short-term zinc supplementation diet after a zinc-deficient regimen). *Statistically significant difference between Zn−/CLP and Ctrl/CLP, P < 0.05; ‡statistically significant difference between Zn+/CLP and Zn−/CLP, P < 0.05.
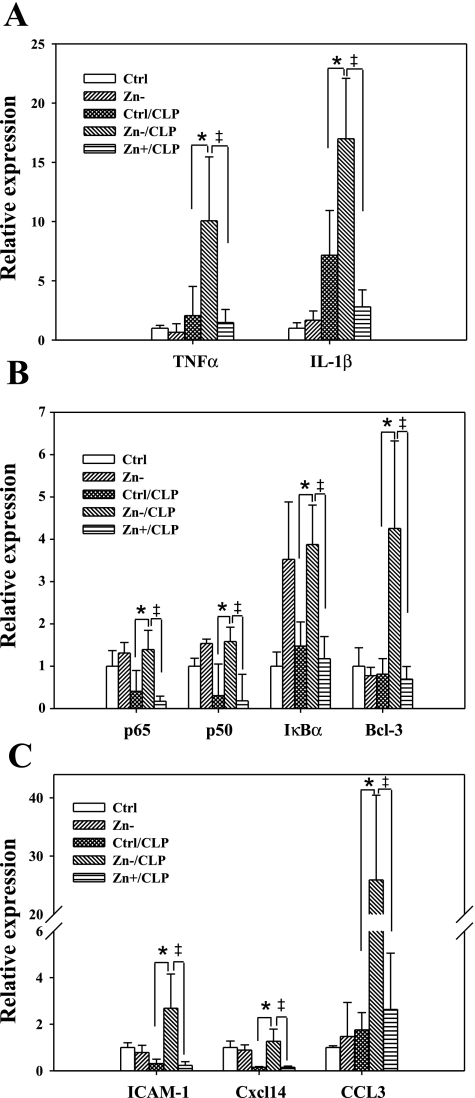
NF-κB initiates the host innate immune response (40) and, in doing so, activates the expression of many genes including but not limited to cytokines, chemokines, acute phase response proteins, as well as NF-κB family members. Based on this, we observed that the expression of NF-κB-regulated genes was increased in the lung of septic mice within 24 h (Fig. 4). The transcript levels of cytokines including IL-1β and TNFα, factors that further enhance NF-κB activation via a positive feedback loop, were increased in Zn−/CLP mice. A similar effect was observed with NF-κB/Rel family members including p50 (NFKB1) and IκB family members including IκBα (NFKBIA) (14, 20, 41). In addition, we observed an increase in the expression of the chemokines CXCL14 and CCL3 as well as ICAM-1 (CD54), all of which are involved in leukocyte recruitment during inflammation (34). The expression of all the evaluated genes was enhanced in response to zinc deficiency (Zn−/CLP) and decreased by zinc supplementation (Zn+/CLP). This is also consistent with the results of p65 DNA-binding activity, thereby demonstrating that in the context of CLP-induced sepsis, zinc deficiency enhances immune activation in the lung.
Fig. 4.
Zinc deficiency modulates NF-κB-mediated related gene expression in the lung following sepsis. C57BL/6 black mice were subjected to modified diets for 3 wk and then subjected to CLP. Mouse lungs were lavaged and perfused with saline at 24 h following CLP. Total RNA was isolated, and the expression of NF-κB-related genes was determined by real-time PCR. The genes included: the cytokines IL-1β and TNFα (A); NF-κB family p65, p50, IκBα, and IκB family member B cell lymphoma 3 (Bcl-3) (B); and chemokines CCL3 and CXCL14 and the cell adhesion molecule ICAM-1 (C). n = 5 lungs per treatment group; *Zn−/CLP vs. Ctrl/CLP, P < 0.05; ‡Zn+/CLP vs. Zn−/CLP, P < 0.05.
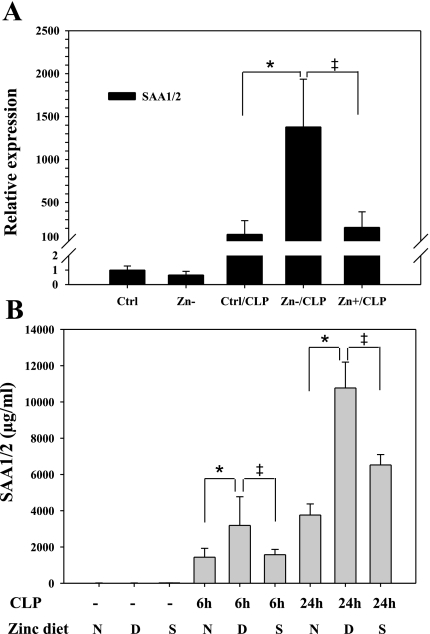
The NF-κB pathway is also centrally involved in activation of the acute phase response following infection (12, 29). SAA1/2 is the most highly expressed acute phase response protein and has recently been reported to play an integral role in pathogen recognition and immune activation (19), so we determined whether SAA expression was modulated in the lung by zinc nutritional status in response to sepsis. As shown, SAA1/2 gene expression was significantly induced in lung tissue following CLP treatment and further enhanced by zinc deficiency (Fig. 5A). Consistent with this finding, circulating plasma SAA1/2 levels were also increased (Fig. 5B). Collectively, these data demonstrate that zinc status also modulates the acute phase response to sepsis by increasing SAA1/2 expression, a known target gene of NF-κB (29).
Fig. 5.
Zinc deficiency enhances the acute phase response in response to sepsis. A: the mRNA levels of serum amyloid A 1/2 (SAA1/2) were measured by real-time PCR (n = 5 animals per treatment group). B: blood was obtained from the same animals at 6 or 24 h following CLP, and SAA levels were measured by ELISA. n = 5 per group; *Zn−/CLP vs. Ctrl/CLP, P < 0.05; ‡Zn+/CLP vs. Zn−/CLP, P < 0.05.
Zinc Deficiency Increases NF-κB p65 in Lung Parenchyma
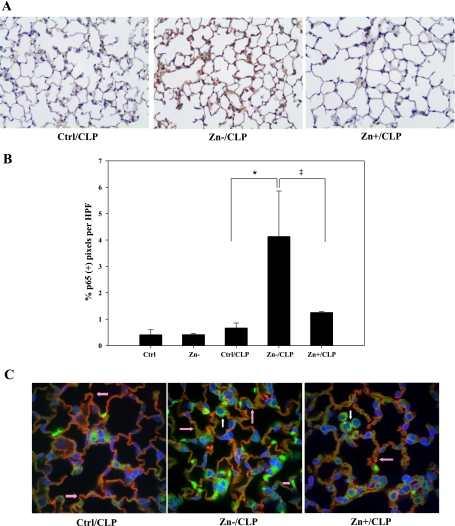
Consistent with mRNA data, immunostaining revealed that the Zn−/CLP treatment group had a significant increase in NF-κB p65 total protein, which was dispersed throughout the entire lung compared with other groups (Fig. 6A). Quantitative analysis of lung tissue confirmed a statistically significant increase in p65 protein (Fig. 6B). To further determine the localization of p65 expression in the lung, immunofluorescent confocal microscopy of similar specimens was conducted. As shown, p65 (green) was most abundant in zinc-deficient animals following CLP and was localized in both nuclear and cytoplasmic compartments of lung cells (Fig. 6C). A significant portion of p65-positive cells were alveolar lung epithelia, as determined by colocalization of p65 in cells staining positive for RCA (red), an alveolar epithelial type I cell-specific marker. We also observed that a portion of positive cells were nonparenchymal and presumably macrophages based on morphological appearance and anatomic location. These results provide evidence that NF-κB p65 expression is significantly increased within the lung as a consequence of zinc deficiency and thereby amplifies innate immune activation during the early stages of sepsis.
Fig. 6.
Increased expression of NF-κB p65 in the lung in response to zinc deficiency and CLP was found. Mouse lungs were inflated and fixed with formaldehyde immediately following lavage and perfusion. A: immunostaining was performed to detect p65 protein (magnification, ×200). Data are representative of a minimum of 5 mice per treatment group. B: 10 random images of each lung specimen were captured and analyzed. The number of brown pixels staining positive for total p65 protein was counted and compared between each treatment group (n = 5 per group; *Zn−/CLP vs. Ctrl/CLP, P < 0.05; ‡Zn+/CLP vs. Zn−/CLP, P < 0.05). C: immunofluorescent staining for p65 was also conducted in conjunction with confocal analysis to identify the cellular location of p65 (green) within lung parenchymal tissue. Rhodamine-labeled Ricinus communis agglutinin (RCA), a lectin-based stain for type I alveolar epithelial cell-specific RCA (red), as well as the nuclear stain 4′,6′-diamidino-2-phenylindole (DAPI; blue) were used to clarify the location, identity, and extent of p65-positive cells. Pink arrows designate p65-positive type I alveolar epithelia, whereas white arrows designate alveolar macrophages. HPF, high-powered field.
Differential Effect of Zinc on NF-κB Activity in Human Lung Epithelia
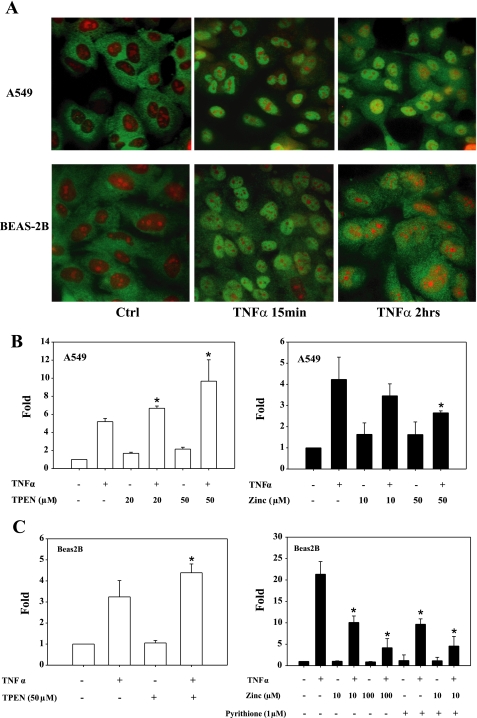
Having observed that zinc nutritional status modulates NF-κB expression and activity in the lung in vivo, we determined whether zinc modulates NF-κB activity in two human lung epithelial cell lines. As expected, we observed that TNFα triggered the rapid mobilization of endogenous NF-κB (p65) into the nucleus in both A549 and BEAS-2B cells (green fluorescence) within 15 min (Fig. 7A). In a similar manner, TNFα treatment induced NF-κB-dependent luciferase expression (3×κB-luc) in both cell lines (Fig. 7, B and C). Consistent with in vivo observations and the work of others (26, 43), prior treatment with the zinc-specific chelator TPEN (31) enhanced NF-κB-mediated luciferase expression in both cell lines. The addition of zinc alone or in combination with the ionophore pyrithione inhibited NF-κB-driven luciferase expression.
Fig. 7.
Zinc modulates NF-κB activity in human lung epithelia. A: TNFα (10 ng/ml) exposure lead to the rapid translocation of NF-κB into the nucleus of human lung epithelial cell lines A549 and BEAS-2B cells as determined by immunofluorescent detection of p65 protein (green). Propidium iodide was used to counterstain nuclei (red). B and C: zinc modulates NF-κB transactivation of a luciferase reporter gene (3×κB-luc) in A549 and BEAS-2B cells. Both cultures were transfected in triplicate with an NF-κB reporter plasmid (3×κB-luc). N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN; 20 or 50 μM × 1 h) was then added to deplete intracellular zinc. Cells were then exposed to TNFα (10 ng/ml) for 6 h. Zinc inhibition study was done in combination with pyrithione before addition of TNFα (10 ng/ml) for 1 h (*P < 0.05 compared with the group of TNFα treatment only).
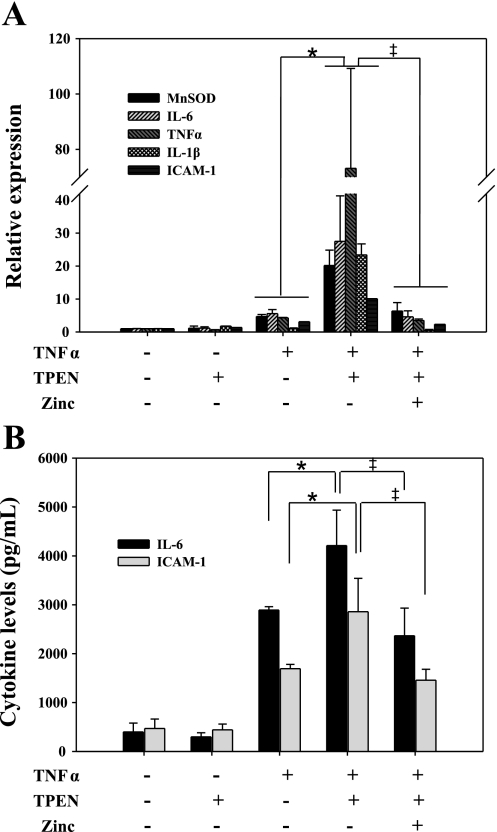
Confirmatory experiments were performed using primary hLECs. Chelation of intracellular zinc with TPEN before TNFα stimulation enhanced the expression of NF-κB regulatory genes including IL-6, TNFα, manganese superoxide dismutase (MnSOD), IL-1β, and ICAM-1 compared with cultures treated with TNFα alone (Fig. 8). We also observed a similar increase in IL-6 and ICAM-1 protein levels in supernatants. In contrast, zinc supplementation following TPEN treatment suppressed the expression of the same NF-κB-driven genes and corresponding proteins. These findings support our previous observations and demonstrate that zinc deficiency enhances NF-κB activity in human lung epithelia, in response to a cytokine that appears shortly after the onset of infection, and that zinc supplementation downregulates the immune response.
Fig. 8.
Zinc deficiency enhances the expression of NF-κB-responsive genes in primary human lung epithelial cells. A: primary human lung epithelial cells (hLECs) were cultured onto collagen-coated, semipermeable membranes. TPEN (20 μM) or zinc (5 μM) were used to modify zinc status as previously described. Cells were then stimulated with TNFα (10 ng/ml) for 6 h to activate the NF-κB pathway. Total RNA was isolated, and the expression of NF-κB target genes was measured. B: in addition, culture supernatants were obtained and measured to determine IL-6 and ICAM-1 concentrations (total donor number: n = 3; *Zn−/CLP vs. Ctrl/CLP, P < 0.05; ‡Zn+/CLP vs. Zn−/CLP, P < 0.05). MnSOD, manganese superoxide dismutase.
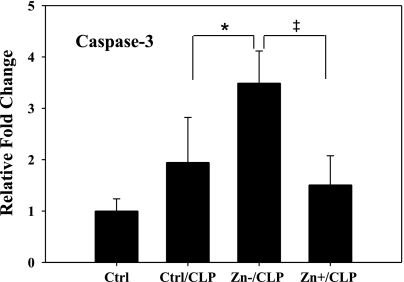
Zinc Deficiency Increases Caspase Activity
Previously, we (27) reported a marked increase in lung injury and the presence of TdT-mediated dUTP nick end labeling (TUNEL)-positive cells, consistent with vital organ dysfunction in Zn−/CLP animals. Based on this, the activity of caspase-3, an enzyme responsible for the execution phase of apoptosis, was determined in lung tissue in response to sepsis and zinc status. Zinc deficiency in conjunction with sepsis resulted in a significant increase in caspase-3 activity in lung tissue compared with other treatment groups (Fig. 9).
Fig. 9.
Zinc deficiency increases caspase-3 activity in the lung in response to CLP. Identical lung specimens as described previously were processed and subjected to analysis for caspase-3 activity at 24 h after CLP in response to zinc deficiency as well as supplementation (n = 5 per group; *Zn−/CLP vs. Ctrl/CLP, P < 0.05; ‡Zn+/CLP vs. Zn−/CLP, P < 0.05).
DISCUSSION
In this investigation, we observed that zinc deficiency increases bacterial burden and enhances NF-κB activity in vivo thereby causing exaggeration of both the innate immune and acute phase response. Short-term zinc repletion before the onset of sepsis, with an oral regimen previously shown to normalize plasma zinc levels (27), significantly reduced the inflammatory response. This is the first report to demonstrate the effects of zinc deficiency on the innate host immune response in relation to the NF-κB pathway in a model of polymicrobial sepsis. In addition, evidence is provided to suggest that the adverse consequences of zinc deficiency are contributed to by dysregulation of the immune response by parenchymal cells within the lung. This is consistent with previous reports demonstrating that zinc deficiency increased shock and liver injury following endotoxin administration (38) and that zinc supplementation abrogates NF-κB activation in an endotoxin-induced inflammation model in mice, thereby reducing TNFα production and subsequent liver injury (50).
Zinc is an essential dietary nutrient that mediates many physiological processes. Knowing that moderate zinc deficiency is highly prevalent in populations that are also susceptible to sepsis, we pursued studies in a conventional animal model of sepsis in which we could establish a clinically relevant degree of zinc deficiency (27). This CLP model mimics many of the clinical features of sepsis in humans. The animals develop progressive polymicrobial bacteremia, cytokine, and chemokine induction, fever, and a hypermetabolic state (23). Consistent with previous studies, we observed that zinc deficiency resulted in a decrease in bacterial clearance within the first 24 h of sepsis onset (24). As shown by real-time in vivo imaging, the septic mice initiated an inflammatory response in the abdominal region shortly following surgery that rapidly disseminated to the thoracic cavity (Fig. 2A). Related to this, IL-1β and TNFα transcript levels in the lung were elevated as early as 2 h following CLP treatment showing that immune activation rapidly spreads to vital organs. When the Zn−/CLP treatment group was compared with Ctrl/CLP animals, the magnitude and rate of NF-κB activation were both significantly increased. The function of zinc as an immunomodulator is controversial since it has been identified as both an activator (11, 17, 49) and repressor (46, 47, 50) of NF-κB. Based on our findings, we conclude that physiological reconstitution of zinc represses immune activation, whereas a lack of zinc, in the setting of severe infection, leads to a systemic increase in NF-κB activation. Knowing that multilayered controls exist to keep NF-κB signaling in check, such as the autoregulatory feedback loop that involves the ubiquitin-editing protein A20, it is plausible that control over this or similar signaling axes may have been compromised by zinc deficiency (44). Interestingly, we observed an increase in A20 expression at 24 h following sepsis in the Zn−/CLP group, which, at face value, is counterintuitive to our findings. Furthermore, we did not observe any differences in the expression of Toll family receptor members (data not shown), suggesting that changes in innate immune activation were not a consequence of pathogen-associated molecular pattern (PAMP) recognition upstream of NF-κB activation. Whether our observations are due to changes in zinc-mediated signal activation or due to a lack of NF-κB suppression remains to be determined.
In the context of sepsis, it is well-accepted that excessive inflammation causes organ dysfunction. Relative to our findings, the acute respiratory distress syndrome (ARDS), a pathological condition induced by sepsis that adversely affects the lungs (1), occurs, in part, due to activation of the NF-κB pathway (3). In support of this, it has been shown that NF-κB binding activity in nuclear extracts from peripheral blood mononuclear cells of septic patients distinguished survivors from nonsurvivors (5, 11, 32). Specifically, increased and prolonged NF-κB activation correlated with increased morbidity and mortality. Consistent with this, we observed an increase in NF-κB activity in the lung of Zn−/CLP mice. Further inspection revealed that this occurred in both immune cells, presumably monocyte/macrophages and parenchymal cells. At the same time, we observed a significant increase in perivascular edema, consistent with epithelial damage, TUNEL-positive cells (27), and caspase-3 activity (Fig. 9), all of which are consistent with a pattern of acute lung injury as that observed in ARDS. Furthermore, we consistently observed an increase in the expression of IκBα and IκB family member B cell lymphoma 3 (Bcl-3), cytokines (TNFα, IL-1β), chemokines (CCL3, CXCL14), adhesion molecules (ICAM-1), and acute phase response genes (SAA1/2) in the lung. Taken together, these findings suggest that local dysregulation of NF-κB-mediated signaling, as a consequence of zinc deficiency, increases the incidence of lung injury, thereby contributing to a worse prognosis. How this occurs despite a concomitant increase in the expression of counterregulatory factors (IκBα and Bcl-3) remains unclear and requires further investigation.
We consistently observed a reduction of NF-κB activity following short-term zinc supplementation in septic mice simultaneously with increased survival and normalization of lung histology (27). This raises the question whether zinc supplementation in humans in a clinically relevant context (e.g., after the onset of sepsis) will provide benefit by preventing vital organ injury. A major limitation of our current investigation is that zinc supplementation is administered before sepsis, a scenario that would be difficult to recapitulate in a clinical setting unless large populations were screened for zinc deficiency, an impractical scenario at the present time. However, given the projected high incidence of zinc deficiency a priori in sepsis patients, further studies that explore zinc supplementation after the onset of sepsis are warranted. This hypothesis is supported by previous animal studies demonstrating that systemic zinc administration during or shortly after the onset of infection decreases mortality (39, 42). It is important to identify that, in these studies, animals were zinc-sufficient before infection and that supraphysiological zinc doses obviated in any benefit and may have increased mortality. Clearly, it will be important to determine whether the therapeutic advantages of zinc administration outweigh risk and, if so, identify which patients will benefit most from zinc supplementation.
Hypozincemia is one of the first systemic changes observed in sepsis that currently is believed to be a consequence of zinc redistribution from the blood compartment into the liver, and perhaps other vital organs (30), to protect the host against pathogen invasion. Gaetke et al. (13) observed an acute decrease of serum zinc levels in response to systemic LPS administration to healthy adult volunteers, which is similar to what we observed in septic mice on control diets (data not shown). A concomitant increase in the expression of the acute phase response protein SAA1/2 was also observed. Considering that the expression of SAA1/2 was further increased in the lung of Zn−/CLP mice compared with normal dietary counterparts (Fig. 5), we conclude that nutritional zinc status also has a profound impact on the acute phase response in vivo.
In summary, this investigation builds on our previous work and others showing that zinc deficiency is detrimental to the host through dysregulation of the initial host response to systemic infection by enhancing inflammation, organ damage, and mortality. Based on our observations using animal and cell culture models, we consistently demonstrate that zinc deficiency results in excessive activation of the NF-κB pathway in a sepsis model and that the resulting consequences are augmentation of the innate immune and acute phase response systemically and within the lung. These effects were largely reversed following zinc supplementation, which normalized inflammatory balance through reduction of NF-κB signaling. Based on these observations, future studies are proposed to explore the incidence of zinc deficiency in “at-risk” subjects as well as rational therapeutic strategies aimed at providing zinc supplementation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R01-HL-086981-01 (D. L. Knoell).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Special thanks go to Dr. Timothy Eubanks for assistance in data interpretation and presentation and Drs. Elliott Crouser, Emily Ho, and Janet King for helpful discussions involving animal studies.
REFERENCES
- 1.Abraham E, Matthay MA, Dinarello CA, Vincent JL, Cohen J, Opal SM, Glauser M, Parsons P, Fisher CJ, Jr, Repine JE. Consensus conference definitions for sepsis, septic shock, acute lung injury, and acute respiratory distress syndrome: time for a reevaluation. Crit Care Med 28: 232–235, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Singer M. Mechanisms of sepsis-induced organ dysfunction. Crit Care Med 35: 2408–2416, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Balibrea JL, Arias-Diaz J. Acute respiratory distress syndrome in the septic surgical patient. World J Surg 27: 1275–1284, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bao S, Knoell DL. Zinc modulates airway epithelium susceptibility to death receptor-mediated apoptosis. Am J Physiol Lung Cell Mol Physiol 290: L433–L441, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Bohrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Mannel D, Bottiger BW, Stern DM, Waldherr R, Saeger HD, Ziegler R, Bierhaus A, Martin E, Nawroth PP. Role of NFkappaB in the mortality of sepsis. J Clin Invest 100: 972–985, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briefel RR, Bialostosky K, Kennedy-Stephenson J, McDowell MA, Ervin RB, Wright JD. Zinc intake of the U.S. population: findings from the third National Health and Nutrition Examination Survey, 1988–1994. J Nutr 130: 1367S–1373S, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener-West M, Faruque AS, Black RE. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low-income population in Bangladesh: randomised controlled trial. Lancet 366: 999–1004, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Carlsen H, Moskaug JO, Fromm SH, Blomhoff R. In vivo imaging of NF-kappa B activity. J Immunol 168: 1441–1446, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Caulfield LE, de Onis M, Blossner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80: 193–198, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Crouser ED, Julian MW, Huff JE, Struck J, Cook CH. Carbamoyl phosphate synthase-1: a marker of mitochondrial damage and depletion in the liver during sepsis. Crit Care Med 34: 2439–2446, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Driessen C, Hirv K, Rink L, Kirchner H. Induction of cytokines by zinc ions in human peripheral blood mononuclear cells and separated monocytes. Lymphokine Cytokine Res 13: 15–20, 1994 [PubMed] [Google Scholar]
- 12.Edbrooke MR, Foldi J, Cheshire JK, Li F, Faulkes DJ, Woo P. Constitutive and NF-kappa B-like proteins in the regulation of the serum amyloid A gene by interleukin 1. Cytokine 3: 380–388, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Gaetke LM, McClain CJ, Talwalkar RT, Shedlofsky SI. Effects of endotoxin on zinc metabolism in human volunteers. Am J Physiol Endocrinol Metab 272: E952–E956, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Ge B, Li O, Wilder P, Rizzino A, McKeithan TW. NF-kappa B regulates BCL3 transcription in T lymphocytes through an intronic enhancer. J Immunol 171: 4210–4218, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Goode HF, Penn ND, Kelleher J, Walker BE. Evidence of cellular zinc depletion in hospitalized but not in healthy elderly subjects. Age Ageing 20: 345–348, 1991 [DOI] [PubMed] [Google Scholar]
- 16.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol 19: 5785–5799, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haase H, Ober-Blobaum JL, Engelhardt G, Hebel S, Heit A, Heine H, Rink L. Zinc signals are essential for lipopolysaccharide-induced signal transduction in monocytes. J Immunol 181: 6491–6502, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr 137: 1101–1105, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Hari-Dass R, Shah C, Meyer DJ, Raynes JG. Serum amyloid A protein binds to outer membrane protein A of gram-negative bacteria. J Biol Chem 280: 18562–18567, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Haskill S, Beg AA, Tompkins SM, Morris JS, Yurochko AD, Sampson-Johannes A, Mondal K, Ralph P, Baldwin AS., Jr Characterization of an immediate-early gene induced in adherent monocytes that encodes I kappa B-like activity. Cell 65: 1281–1289, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell 132: 344–362, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 348: 138–150, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock 24, Suppl 1: 52–57, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Ibs KH, Rink L. Zinc-altered immune function. J Nutr 133: 1452S–1456S, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188: 115–137, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Kim CH, Kim JH, Moon SJ, Chung KC, Hsu CY, Seo JT, Ahn YS. Pyrithione, a zinc ionophore, inhibits NF-kappaB activation. Biochem Biophys Res Commun 259: 505–509, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Knoell DL, Julian MW, Bao S, Besecker B, Macre JE, Leikauf GD, DiSilvestro RA, Crouser ED. Zinc deficiency increases organ damage and mortality in a murine model of polymicrobial sepsis. Crit Care Med 37: 1380–1388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Li X, Liao WS. Cooperative effects of C/EBP-like and NF kappa B-like binding sites on rat serum amyloid A1 gene expression in liver cells. Nucleic Acids Res 20: 4765–4772, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liuzzi JP, Lichten LA, Rivera S, Blanchard RK, Aydemir TB, Knutson MD, Ganz T, Cousins RJ. Interleukin-6 regulates the zinc transporter Zip14 in liver and contributes to the hypozincemia of the acute-phase response. Proc Natl Acad Sci USA 102: 6843–6848, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCabe MJ, Jr, Jiang SA, Orrenius S. Chelation of intracellular zinc triggers apoptosis in mature thymocytes. Lab Invest 69: 101–110, 1993 [PubMed] [Google Scholar]
- 32.Moine P, McIntyre R, Schwartz MD, Kaneko D, Shenkar R, Le Tulzo Y, Moore EE, Abraham E. NF-kappaB regulatory mechanisms in alveolar macrophages from patients with acute respiratory distress syndrome. Shock 13: 85–91, 2000 [DOI] [PubMed] [Google Scholar]
- 33.O'Brien JM, Jr, Lu B, Ali NA, Martin GS, Aberegg SK, Marsh CB, Lemeshow S, Douglas IS. Alcohol dependence is independently associated with sepsis, septic shock, and hospital mortality among adult intensive care unit patients. Crit Care Med 35: 345–350, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Peters W, Ernst JD. Mechanisms of cell recruitment in the immune response to Mycobacterium tuberculosis. Microbes Infect 5: 151–158, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol 8: 776–787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schmuck A, Roussel AM, Arnaud J, Ducros V, Favier A, Franco A. Analyzed dietary intakes, plasma concentrations of zinc, copper, and selenium, and related antioxidant enzyme activities in hospitalized elderly women. J Am Coll Nutr 15: 462–468, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Schwartz MD, Moore EE, Moore FA, Shenkar R, Moine P, Haenel JB, Abraham E. Nuclear factor-kappa B is activated in alveolar macrophages from patients with acute respiratory distress syndrome. Crit Care Med 24: 1285–1292, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Shea-Budgell M, Dojka M, Nimmo M, Lee D, Xu Z. Marginal zinc deficiency increased the susceptibility to acute lipopolysaccharide-induced liver injury in rats. Exp Biol Med (Maywood) 231: 553–558, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Sobocinski PZ, Powanda MC, Canterbury WJ, Machotka SV, Walker RI, Snyder SL. Role of zinc in the abatement of hepatocellular damage and mortality incidence in endotoxemic rats. Infect Immun 15: 950–957, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest 107: 7–11, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ten RM, Paya CV, Israel N, Le Bail O, Mattei MG, Virelizier JL, Kourilsky P, Israel A. The characterization of the promoter of the gene encoding the p50 subunit of NF-kappa B indicates that it participates in its own regulation. EMBO J 11: 195–203, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tocco-Bradley R, Kluger MJ. Zinc concentration and survival in rats infected with Salmonella typhimurium. Infect Immun 45: 332–338, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uzzo RG, Crispen PL, Golovine K, Makhov P, Horwitz EM, Kolenko VM. Diverse effects of zinc on NF-kappaB and AP-1 transcription factors: implications for prostate cancer progression. Carcinogenesis 27: 1980–1990, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol 30: 383–391, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Vincent JL. Metabolic support in sepsis and multiple organ failure: more questions than answers. Crit Care Med 35: S436–S440, 2007 [DOI] [PubMed] [Google Scholar]
- 46.von Bulow V, Dubben S, Engelhardt G, Hebel S, Plumakers B, Heine H, Rink L, Haase H. Zinc-dependent suppression of TNF-alpha production is mediated by protein kinase A-induced inhibition of Raf-1, I kappa B kinase beta, and NF-kappa B. J Immunol 179: 4180–4186, 2007 [DOI] [PubMed] [Google Scholar]
- 47.von Bulow V, Rink L, Haase H. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF-alpha and IL-1 beta production in monocytes by elevation of guanosine 3′,5′-cyclic monophosphate. J Immunol 175: 4697–4705, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Walsh CT, Sandstead HH, Prasad AS, Newberne PM, Fraker PJ. Zinc: health effects and research priorities for the 1990s. Environ Health Perspect 102, Suppl 2: 5–46, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wellinghausen N, Driessen C, Rink L. Stimulation of human peripheral blood mononuclear cells by zinc and related cations. Cytokine 8: 767–771, 1996 [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z, Wang L, Song Z, Saari JT, McClain CJ, Kang YJ. Abrogation of nuclear factor-kappaB activation is involved in zinc inhibition of lipopolysaccharide-induced tumor necrosis factor-alpha production and liver injury. Am J Pathol 164: 1547–1556, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]