Abstract
Acute partial compression of the fetal ductus arteriosus (DA) results in an initial abrupt increase in pulmonary blood flow (PBF), which is followed by a significant reduction in PBF to baseline values over the ensuing 2–4 h. We have previously demonstrated that this potent vasoconstricting response is due, in part, to an endothelin-1 (ET-1)-mediated decrease in nitric oxide synthase (NOS) activity. In addition, in vitro data demonstrate that ET-1 increases superoxide levels in pulmonary arterial smooth muscle cells and that oxidative stress alters NOS activity. Therefore, the objectives of this study were to determine the potential role of superoxide in the alterations of hemodynamics and NOS activity following acute ductal constriction in the late-gestation fetal lamb. Eighteen anesthetized near-term fetal lambs were instrumented, and a lung biopsy was performed. After a 48-h recovery, acute constriction of the DA was performed by inflating a vascular occluder. Polyethylene glycol-superoxide dismutase (PEG-SOD; 1,000–1,500 units/kg, n = 7) or PEG-alone (vehicle control group, n = 5) was injected into the pulmonary artery before ductal constriction. Six animals had a sham operation. In PEG-alone-treated lambs, acute ductal constriction rapidly decreased pulmonary vascular resistance (PVR) by 88%. However, by 4 h, PVR returned to preconstriction baseline. This vasoconstriction was associated with an increase in lung superoxide levels (82%), a decrease in total NOS activity (50%), and an increase in P-eNOS-Thr495 (52%) (P < 0.05). PEG-SOD prevented the increase of superoxide after ductal constriction, attenuated the vasoconstriction, preserved NOS activity, and increased P-eNOS Ser1177 (307%, P < 0.05). Sham procedure induced no changes. These data suggest that an acute decrease in NOS activity that is mediated, in part, by increased superoxide levels, and alterations in the phosphorylation status of the endothelial NOS isoform, underlie the pulmonary vascular response to acute ductal constriction.
Keywords: ductus arteriosus, pulmonary circulation
increases in fetal pulmonary arterial pressure induced by mechanical constriction of the ductus arteriosus induce an acute increase in pulmonary blood flow that is followed by active vasoconstriction (1). This so-called “myogenic response,” which returns pulmonary blood flow to preconstriction values within 2–4 h, may represent an adaptive response of the fetal pulmonary vasculature to maintain the normal low flow state (24). However, chronic ductal constriction results in pulmonary vascular remodeling and many of the pathophysiological features of persistent pulmonary hypertension of the newborn (2, 16, 31). In fact, fetal ductal constriction secondary to maternal use of COX inhibitors, such as indomethacin, has been associated with persistent pulmonary hypertension of the newborn (26). Therefore, understanding the mechanisms that mediate changes in pulmonary blood flow following ductal constriction has important clinical implications.
Increasing evidence demonstrates that factors produced by the pulmonary vascular endothelium regulate normal fetal pulmonary vascular tone, as well as the dynamic changes in pulmonary vascular tone following both acute and chronic constriction of the ductus arteriosus. For example, both acute and chronic ductal constriction are associated with decreased nitric oxide (NO) activity and increased endothelin-1 (ET-1)-mediated vasoconstriction (6, 13, 17, 22, 27). In addition, further studies suggest that this decrease in NO activity mediates, in part, the hemodynamic response following acute ductal constriction and are independent of changes in gene expression and dependent on ET-1 activity (12, 17, 23). Moreover, increasing data demonstrate that changes in the redox environment may alter NOS activity by several mechanisms including changes in the nitration and phosphorylation status of the enzyme (5). Last, ET-1 has been demonstrated to increase superoxide levels in fetal pulmonary arterial endothelial cells (19). However, the potential role of these superoxide-NO interactions in mediating the acute changes in fetal pulmonary blood flow following ductal constriction has not been investigated.
Therefore, the objective of this study was to determine the role of superoxide, NO, and their interactions in regulating the dynamic changes in fetal pulmonary blood flow following acute mechanical constriction of the ductus arteriosus. We hypothesized that acute mechanical constriction of the ductus arteriosus would result in increased superoxide production, and superoxide-dependent decreases in NOS activity, with a net result of active pulmonary vasoconstriction. To investigate this hypothesis, we determined superoxide levels, NOS activity, and posttranslational modifications of NOS, before and 4 h after mechanical ductal constriction in late-gestation fetal lambs. To determine potential superoxide-NO interactions following ductal constriction, the same factors were studied in an additional group of fetal lambs that were pretreated with polyethylene glycol-superoxide dismutase (PEG-SOD), a superoxide scavenger. Last, to isolate changes related to the experimental protocol, we studied the same factors in an additional group of fetal lambs that underwent a sham procedure without ductal constriction.
MATERIALS AND METHODS
Surgical preparation.
Eighteen mixed-breed Western pregnant ewes (132–140 days' gestation, term = 145 days) were operated on under sterile conditions with the use of local (2% lidocaine hydrochloride) and intravenous anesthesia (0.002 mg·kg−1·min−1 diazepam and 0.3 mg·kg−1·min−1 ketamine hydrochloride). Fetal anesthesia consisted of local anesthesia with 2% lidocaine hydrochloride and 15 mg/kg im ketamine hydrochloride. Through a uterine incision, the fetal forelimb was exposed. Polyvinyl catheters were inserted into the fetal pedal artery and vein and were advanced to the aorta and the inferior vena cava, respectively. A left lateral thoracotomy was performed in the fourth intercostal space. The pericardium was incised along the main pulmonary trunk. Teflon cannulas attached to polyvinyl catheters were inserted into the proximal main pulmonary trunk, left pulmonary artery, and the left atrium. An ultrasonic flow transducer (Transonic Systems, Ithaca, NY) was placed around the left pulmonary artery. The ductus arteriosus was dissected free and infiltrated with 10% formalin to prevent ductal constriction during manipulation. A vascular occluder was then placed around the ductus arteriosus, but left uninflated. A side-biting vascular clamp was utilized to isolate peripheral lung tissue from the right upper lobe, and the incision was cauterized. Approximately 300 mg of peripheral lung were obtained for the biopsy to determine NOS activity, endothelial nitric oxide synthase (eNOS) protein levels, and superoxide levels. The thoracotomy incision was then closed in layers. Warm saline was instilled to replace the lost amniotic fluid, and the uterine incision was closed. A polyvinyl catheter was placed in the amniotic cavity. The catheters were filled with PBS containing 1,000 U/ml heparin sodium, plugged, and brought to the skin along with the transducer cables, where they were protected in a pouch secured to the ewe's flank. After recovery from anesthesia, the ewe was returned to the cage. Antibiotics (1 million units of penicillin G procaine and 100 mg of gentamicin sulfate) were administered intravenously to the ewe and into the amniotic cavity during surgery and daily thereafter. Buprenorphine (0.01 mg/kg im) was administered for postoperative analgesia. All protocols were approved by the Committee of Animal Research at the University of California, San Francisco.
Experimental protocol.
After a 24-h recovery, the ewe was placed in a study cart with free access to food and water. The fetal catheters were connected to transducers, and 60 min were allowed for stabilization. PEG diluted in 5 ml of normal saline (n = 5, vehicle control) or polyethylene glycol-conjugated superoxide dismutase (n = 7, PEG-SOD) was then delivered through the pulmonary artery catheter. The dose of PEG-SOD (1,000–1, 500 U/kg) was based on previous studies that demonstrate a sustained significant increase in plasma SOD activity (8, 25). Thirty minutes after the dose, baseline measurements of the hemodynamic variables (pulmonary and systemic arterial pressure, left pulmonary blood flow, left atrial pressure, and amniotic cavity pressure) and systemic arterial blood gases and pH were measured (preconstriction).
In 12 of the fetal lambs, the vascular occluder placed around the ductus arteriosus was then inflated with normal saline, to increase mean pulmonary arterial pressure by 15–20 mmHg. The hemodynamic variables were monitored continuously, and systemic arterial blood gases were sampled intermittently. The occluder was occasionally adjusted to maintain the increase in mean pulmonary arterial pressure. This was required approximately once per animal, and there were no differences in the need for occluder manipulations between the two study groups. After 4 h, a repeat cesarean section was then performed, and a peripheral fetal lung biopsy was performed as described above.
To ensure that potential changes demonstrated resulted from ductal constriction, and not from other aspects of the protocol, six of the vehicle-treated fetal lambs underwent the exact protocol without inflation of the vascular occluder (sham operated).
At the end of the protocol, the fetus and ewe were killed with a lethal injection of pentobarbital sodium followed by bilateral thoracotomy as described in the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Fetal weight was then obtained.
Measurements.
Pulmonary and systemic arterial and right and left atrial pressures were measured using Sorenson Neonatal Transducers (Abbott Critical Care Systems, N. Chicago, IL). Mean pressures were obtained by electrical integration. Heart rate was measured by a cardiotachometer triggered from the phasic systemic arterial pressure pulse wave. Left pulmonary blood flow was measured on an ultrasonic flow meter (Transonic Systems, Ithaca, NY). All hemodynamic variables were measured continuously utilizing the Gould Ponemah Physiology Platform (version 4.2) and Acquisition Interface (model ACG-16; Gould, Cleveland, OH) and recorded with a Dell Inspiron 5160 computer (Dell, Round Rock, TX). Blood gases and pH were measured on a Radiometer ABL5 pH/blood gas analyzer (Copenhagen, Denmark). Hemoglobin concentration and oxygen saturation were measured by a cooximeter (model 682; Instrumentation Laboratory, Lexington, MA). Pulmonary vascular resistance was calculated using standard formulas. Body temperature was monitored continuously with a rectal temperature probe.
Assay for NOS activity.
NOS activity was determined using the conversion of 3H-l-arginine to 3H-l-citrulline as described by Bush et al. (7). Briefly, peripheral lung tissues were homogenized in NOS assay buffer (50 mM Tris·HCl, pH 7.5, containing 0.1 mM EDTA and 0.1 mM EGTA) with a protease inhibitor cocktail. Enzyme reactions were carried out at 37°C in the presence of total lung protein extracts (500 μg), 1 mM NADPH, 14 μM tetrahydrobiopterin, 100 μM FAD, 1 mM MgCl2, 5 μM unlabeled l-arginine, 15 nM 3H-l-arginine, calmodulin (25 units), and 5 mM calcium to produce conditions that drive the reaction at maximal velocity. Duplicate assays were run in the presence of the NOS inhibitor l-NAME to detect nonspecific production of 3H-citrulline. This value was then subtracted to obtain the final activity value. Assays were incubated for 60 min at 37°C such that no more than 20% of the 3H-arginine was metabolized, to insure that the substrate was not limiting. The reactions were stopped by the addition of iced stop buffer (20 mM sodium acetate, pH 5, 1 mM l-citrulline, 2 mM EDTA, and 0.2 mM EGTA) and then applied to columns containing 1 ml of Dowex AG50W-X8 resin, Na+ form, preequilibrated with 1 N NaOH. 3H-l-citrulline was then quantitated by scintillation counting. All activities were normalized to the amount of protein in each lysate.
Superoxide quantification.
Superoxide levels in lung tissue taken from shunted lambs were estimated by electronic paramagnetic resonance (EPR) assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl (CMH) in the presence and absence of PEG-SOD, as we have previously described (32, 33).
Approximately 0.1 g of tissue was sectioned from fresh-frozen biopsies of lung tissue and immediately immersed, while still frozen, in 200 μl of EPR buffer [PBS supplemented with 5 μM diethyldithiocarbamate (DETC, Sigma-Aldrich) and 25 μM desferrioxamine (Def MOS, Sigma-Aldrich)]. To determine the specificity of detected EPR signal, additional sample groups were immersed in EPR buffer supplemented with 100 U/ml membrane-permeable form of SOD (PEG-SOD). All samples were incubated for 30 min on ice and then homogenized for 30 s with a VWR PowerMAX AHS 200 tissue homogenizer. Following incubation, samples were analyzed for protein content using Bradford analysis (Bio-Rad). Sample volumes were then adjusted with EPR buffer and 25 mg/ml CMH to achieve equal protein content and a final CMH concentration of 5 mg/ml. Samples were further incubated for 60 min on ice and then centrifuged at 14,000 g for 15 min at room temperature. Thirty-five microliters of supernatant from each sample was loaded into a 50-μl capillary tube and analyzed with a MiniScope MS200 ESR (Magnettech, Berlin, Germany) at a microwave power of 40 mW, modulation amplitude of 3,000 mG, and modulation frequency of 100 kHz, with a magnetic strength of 333.95-3339.94 mT. Resulting EPR spectra were analyzed using ANALYSIS v.2.02 software (Magnettech) whereby the EPR maximum and minimum spectral amplitudes for the CM·superoxide spin-trap product waveform were quantified. Experimental groups were then compared for differences in amplitude using statistical analysis.
Preparation of protein extracts and Western blot analysis.
Lung protein extracts were prepared by homogenizing peripheral lung tissues in Triton lysis buffer (50 mM Tris·HCl, pH 7.6, 0.5% Triton X-100, 20% glycerol) containing a protease inhibitor cocktail [P8340 (5 ml) was used to prevent protein degradation; P5726 (5 ml) was also used in the phospho-eNOS Western blots to prevent phosphate group cleavage, Sigma-Aldrich]. Extracts were then clarified by centrifugation (15,000 g × 10 min at 4°C). Supernatant fractions were then assayed for protein concentration using the Bradford reagent (Bio-Rad, Richmond, CA) and used for Western blot analysis. Western blot analysis was performed as previously described (4, 15, 30). Briefly, protein extracts (25 μg) were separated on 4–12% denaturing polyacrylamide gels and transferred to a nitrocellulose membrane (Bio-Rad). The membranes were blocked with 5% nonfat dry milk in TBS containing 0.1% Tween and exposed to the primary antibody. The primary antibodies used for immunoblotting were anti-phospho Thr495 eNOS, anti-phospho Ser1177 eNOS, and anti-eNOS (all at 1:1,000, Cell Signaling Technology). After blocking, the membranes were washed with TBS containing 0.1% Tween and then incubated with the appropriate secondary antibody coupled to horseradish peroxidase, washed again with TBST, and the protein bands visualized using chemiluminescence (SuperSignal West Femto Maximum Sensitivity Substrate Kit; Pierce, Rockford, IL) and a Kodak 440CF image station (New Haven, CT). To normalize for protein loading, blots were reblocked and then reprobed with the housekeeping protein, β-actin.
Immunoprecipitation-Western blot analysis for eNOS nitration.
We determined the level of eNOS protein nitration utilizing a immunoprecipitation-Western blot technique as we have previously described (30). Frozen lung tissue was homogenized in 3× volume per tissue weight of immunoprecipitation (IP) buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 10 mM MgCl2, 1 mM EDTA, 2% glycerol supplemented with protease inhibitors), centrifuged at 14,000 rpm at 4°C for 10 min, the supernatant collected, and the protein concentration quantified by the Bio-Rad DC Protein Assay (Bio-Rad Laboratories, Hercules, CA). To 1,000 μg of total protein, 1 μg of anti-eNOS antibody was added, the volume was brought to 1 ml with immunoprecipitation buffer, and the mixture was nutated at 4°C overnight. To precipitate the bound eNOS, 10 μl of protein G-Agarose (EMD/Calbiochem) was added, and the samples nutated for 1 h at 4°C. To collect the bead-bound antibody, the samples were centrifuged at 14,000 rpm for 5 s, the supernatant was removed, and the beads were washed with 500 μl of IP buffer. The wash step was repeated two additional times, and 20 μl of 2× Laemmli sample buffer was added to the samples and boiled for 5 min. The samples were then divided equally and loaded onto duplicate 4–20% gradient gels (Gradipore, Frenchs Forest, Australia) and run to completion according to the manufacturer's instructions. The proteins were transferred to Immun-Blot polyvinylidene difluoride membrane (Bio-Rad Laboratories), and the membrane was blocked with 5% skim milk in TBST from 1 h to overnight. The membranes were probed with antibodies to either eNOS (to normalize for the immunoprecipitation efficiency) or 3-NT (EMD/Calbiochem), and reactive bands were visualized with the SuperSignal West Femto Maximum Sensitivity Substrate Kit (Pierce) and Kodak 440CF image station (New Haven, CT) as described above. Tissue lysate run in an adjacent lane without prior IP was used as a positive control. Relative nitrated eNOS was determined as a ratio of the 3-NT-eNOS/total eNOS signals.
Statistical analysis.
Means ± SD were calculated for the protein of interest. The means ± SD were calculated for the hemodynamic variables, NOS activities, and EPR amplitudes. Differences over time were determined by ANOVA for repeated measures, and Student-Newman-Keuls post hoc testing was performed. Values between groups were compared by ANOVA for repeated measures. A P value of less than 0.05 was considered statistically significant.
RESULTS
There were no differences in gestational age, weight, sex distribution, or baseline hemodynamic variables between vehicle-treated, PEG-SOD-treated, and non-constricted fetal lambs (data not shown).
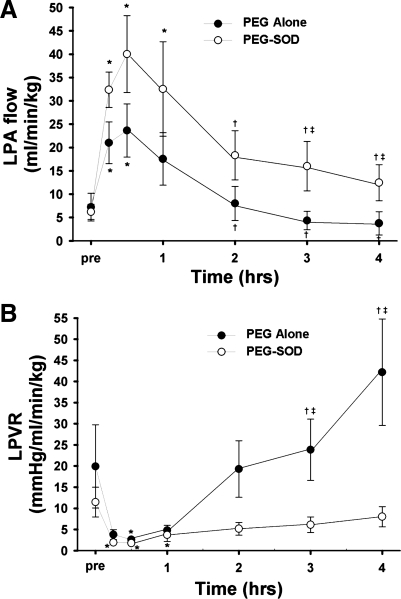
In vehicle (PEG-alone)-treated lambs, acute ductal constriction rapidly increased mean pulmonary arterial pressure and left pulmonary blood flow (P < 0.05). Left pulmonary vascular resistance decreased (P < 0.05). Mean systemic arterial pressure, left atrial pressure, and systemic arterial blood gases and pH were all unchanged (Table 1). During the 4-h study period, pulmonary arterial pressure remained increased (as per protocol), but left pulmonary blood flow and pulmonary vascular resistance returned to preconstriction values (Fig. 1). In fact, compared with 30-min postconstriction, pulmonary blood flow was decreased after 4 h, and pulmonary vascular resistance was increased (P < 0.05, Fig. 1).
Table 1.
Hemodynamic changes associated with acute ductal constriction in vehicle-treated fetal lambs
| Ductal Constriction |
|||||||
|---|---|---|---|---|---|---|---|
| Preconstriction | 15 Min | 30 Min | 1 H | 2 H | 3 H | 4 H | |
| PAP, mmHg | 54.5 + 3.7 | 66.6 + 8.4* | 65.2 + 7.4* | 68.8 + 7.9* | 68.2 + 8.5* | 64.7 + 10.1* | 65.5 + 8.5* |
| Left pulmonary vascular resistance, mmHg ml−1 min−1kg−1 | 20.3 + 19.6 | 3.3 + 2.5 | 2.3 + 1.2* | 4.3 + 2.7 | 16.3 + 15.2 | 20.7 + 16.6† | 35.5 + 29.9† |
| Left pulmonary blood flow, ml kg−1 min−1 | 7.3 + 6.6 | 21.1 + 9.9* | 25.9 + 10.9* | 17.7 + 12.4 | 8.1 + 8.0† | 4.4 + 4.4† | 3.8 + 5.4† |
| SAP, mmHg | 53.0 + 2.6 | 49.5 + 6.1 | 50.9 + 4.3 | 49.8 + 4.9 | 49.1 + 7.5 | 46.1 + 10.3 | 48.0 + 6.2 |
| SAP-PAP, mmHg | 1.4 + 1.6 | 17.1 + 3.7* | 16.1 + 4.5* | 19.0 + 6.1* | 19.2 + 5.1* | 18.5 + 8.3* | 17.5 + 4.6* |
| PH, units | 7.31 + 0.16 | 7.42 + 0.07 | 7.40 + 0.07 | 7.41 + 0.08 | 7.37 + 0.05 | 7.37 + 0.08 | 7.38 + 0.09 |
| Paco2, Torr | 47.4 + 9.4 | 43.0 + 5.9 | 42.7 + 8.2 | 42.7 + 8.2 | 44.4 + 6.8 | 45.4 + 8.8 | 47.6 + 9.9 |
| Pao2, Torr | 16.7 + 3.1 | 16.0 + 2.0 | 16.3 + 2.5 | 17 + 1.7 | 15.7 + 3.1 | 16 + 4.5 | 15.5 + 2.6 |
Values are means and SD; n = 5 lambs. PAP, pulmonary arterial pressure; SAP, systemic arterial pressure.
P < 0.05 vs. preconstriction values.
P < 0.05 vs. previous column.
Fig. 1.
Changes in left pulmonary blood flow (LPA; A) and left pulmonary vascular resistance (LPVR; B) before and after acute ductal constriction. Acute ducal constriction induces an initial increase in pulmonary blood flow and decrease in pulmonary vascular resistance. This is followed by active pulmonary vasoconstriction, resulting in a decrease in flow and increase in resistance. Pretreatment with PEG-SOD attenuated this response. N = 5 vehicle-treated lambs, n = 7 PEG-SOD-treated lambs. Values are means ± SD. *P < 0.05 vs. pre, †P < 0.05 vs. 30 min, ‡P < 0.05 between groups.
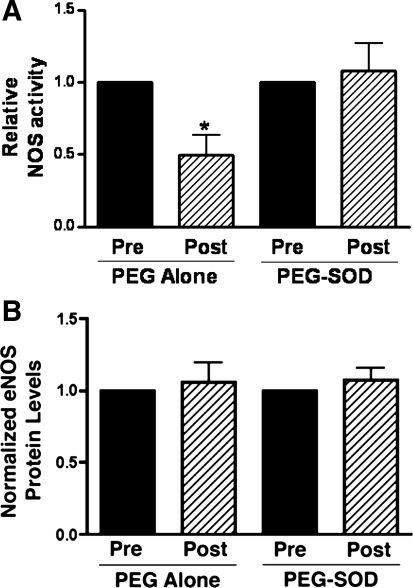
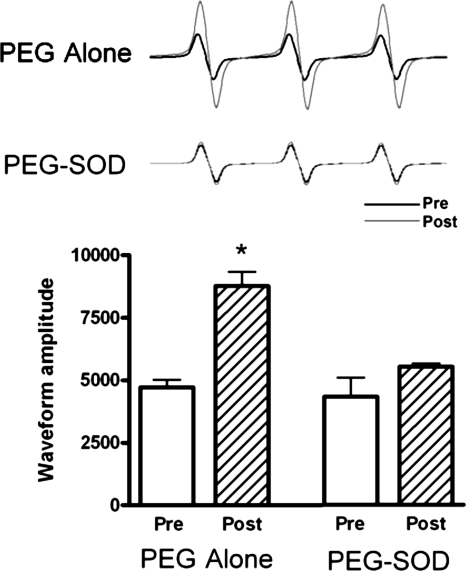
In vehicle-treated lambs, NOS activity decreased by ∼50% (P < 0.05, Fig. 2) 4 h following acute ductal constriction. These changes were independent of changes in eNOS protein levels and are consistent with our previous findings (17). Superoxide levels, as determined by EPR on peripheral lung, increased following acute ductal constriction in vehicle-treated fetal lambs (P < 0.05, Fig. 3). Specificity of the EPR assay for superoxide was confirmed by a significant reduction in the waveform amplitude with the addition of PEG-SOD to the samples.
Fig. 2.
Changes in lung tissue nitric oxide synthase (NOS) activity (A) and eNOS protein levels (B) before and 4 h after acute ductal constriction. Top, left: acute ductal constriction decreases total NOS activity in vehicle-treated lambs (n = 5). Values are means ± SE, normalized to preconstriction values, *P < 0.05. Bottom, left: densitometric values for eNOS protein from vehicle-treated lambs (normalized to β-actin; n = 5). Acute ductal constriction does not change eNOS protein levels. Values are means ± SD, normalized to preconstriction values. Top, right: however, total NOS activity does not decrease following acute ductal constriction in PEG-SOD-treated fetal lambs, whereas eNOS protein levels remain unchanged (bottom, right). N = 7 PEG-SOD. Values are means ± SD, normalized to preconstriction values.
Fig. 3.
Superoxide anion levels in lung tissue before and 4 h after acute ductal constriction, estimated by electron paramagnetic resonance assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine·HCl. Left: superoxide levels increased after acute ductal constriction in vehicle-treated lambs (n = 5) but were unchanged in PEG-SOD-treated lambs (right; n = 7). Values are means ± SD. *P < 0.05 vs. pre.
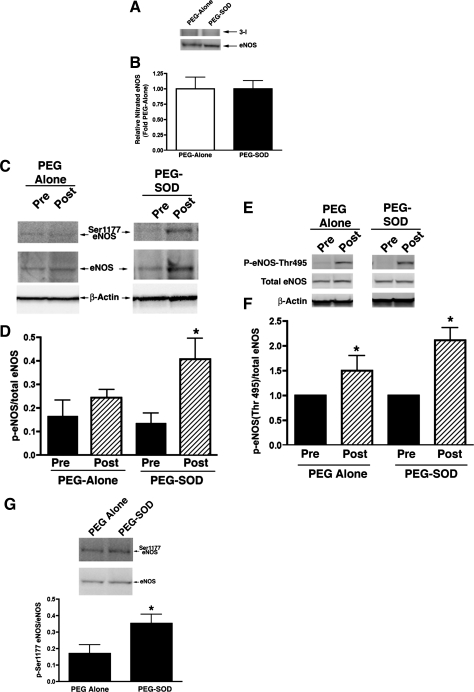
Posttranslational alterations in NOS can result in changes in NOS activity. Since ROS can alter both the nitration and phosphorylation status of NOS, we evaluated potential alterations in nitrated eNOS, Ser1177 eNOS, and eNOS Thr495 following acute constriction of the ductus arteriosus. As seen in Fig. 4, total amounts of nitrated eNOS and Ser1177 eNOS were not changed following acute ductal constriction. However, the amount of eNOS Thr495, which is associated with decreased NOS activity, was increased following acute ductal constriction (P < 0.05).
Fig. 4.
3-NT eNOS protein levels (A and B), Ser1177 eNOS protein levels (C and D), and eNOS Thr495 protein levels (E and F) in lung tissue before and 4 h after acute ductal constriction. Acute ductal constriction did not change 3-NT eNOS and Ser1177 eNOS levels in vehicle-treated lambs. However, eNOS Thr495 protein levels, a phosphorylated form of eNOS associated with decreased activity, were increased. Following PEG-SOD treatment, acute ductal constriction did not change 3-NT eNOS levels, but Ser1177 eNOS levels were increased. The increase in eNOS Thr495 levels were not affected by PEG-SOD treatment. In fact, when compared side by side, phospho-Ser1177 eNOS protein levels were greater in the PEG-SOD-treated lambs following ductal constriction than the PEG-alone-treated lambs (G). N = 5 vehicle-treated lambs, n = 7 PEG-SOD-treated lambs. Values are means ± SD. *P < 0.05 vs. Pre.
To determine potential NO-ROS interactions following ductal constriction, an additional group of fetal lambs was pretreated with PEG-SOD. In PEG-SOD-treated lambs, acute ductal constriction rapidly increased mean pulmonary arterial pressure and left pulmonary blood flow (P < 0.05). Left pulmonary vascular resistance decreased (P < 0.05). Mean systemic arterial pressure, left atrial pressure, and systemic arterial blood gases and pH were all unchanged (Table 2). During the 4-h study period, pulmonary arterial pressure remained increased (as per protocol), but left pulmonary vascular resistance remained unchanged (Table 2, Fig. 1). Left pulmonary blood flow decreased at hour 3 and 4 but remained higher than preconstriction values. In fact, after 3 and 4 h of ductal constriction, pulmonary blood flow was significantly increased, and pulmonary vascular resistance was significantly decreased in PEG-SOD-treated lambs compared with vehicle-treated lambs (P < 0.05, Fig. 1).
Table 2.
Hemodynamic changes associated with acute ductal constriction in PEG-SOD-treated fetal lambs
| Ductal Constriction |
|||||||
|---|---|---|---|---|---|---|---|
| Preconstriction | 15 Min | 30 Min | 1 H | 2 H | 3 H | 4 H | |
| PAP, mmHg | 49.2 + 7.2 | 60.9 + 8.1* | 60.2 + 10.0* | 61.9 + 5.2* | 61.9 + 7.4* | 57.1 + 6.1 | 55.3 + 10.4 |
| Left pulmonary vascular resistance, mmHg ml−1 min−1kg−1 | 11.6 + 9.5 | 1.9 + 0.5* | 1.7 + 0.8* | 3.7 + 4.0* | 5.2 + 3.9 | 6.2 + 4.8 | 8.1 + 6.3 |
| Left pulmonary blood flow, ml kg−1 min−1 | 6.2 + 4.2 | 32.3 + 9.8* | 39.9 + 21.79* | 32.5 + 26.8* | 18.3 + 13.9† | 16.0 + 14.0† | 12.4 + 10.2† |
| SAP, mmHg | 48.0 + 8.2 | 43.5 + 8.3 | 43.5 + 7.4 | 42.9 + 6.2 | 42.4 + 5.9 | 40.3 + 4.5 | 39.5 + 8.6 |
| PAP-SAP, mmHg | 1.2 + 1.7 | 17.5 + 4.9* | 16.7 + 5.7* | 18.9 + 3.9* | 19.5 + 3.4* | 16.7 + 3.9* | 15.8 + 5.2* |
| PH, units | 7.31 + 0.05 | 7.32 + 0.02 | 7.31 + 0.02 | 7.30 + 0.05 | 7.26 + 0.06 | 7.28 + 0.07 | 7.26 + 0.09 |
| Paco2, Torr | 50.1 + 5.0 | 48.0 + 5.0 | 48.6 + 11.1 | 52.6 + 8.4 | 53.4 + 8.6 | 56.4 + 11.6 | 57.3 + 14.9 |
| Pao2, Torr | 12.2 + 3.4 | 12.8 + 3.6 | 13.1 + 3.6 | 12.8 + 3.9 | 13.0 + 2.73.1 | 16 + 4.5 | 15.5 + 2.6 |
Values are means and SD; n = 7 lambs.
P < 0.05 vs. preconstriction values;
P < 0.05 vs. previous column.
In PEG-SOD-treated lambs, lung tissue NOS activity did not decrease following acute ductal constriction, although eNOS protein levels were unchanged (Fig. 2). Superoxide levels and 3-NT eNOS levels were also unchanged (Figs. 3 and 4). Interestingly, although phospho-Thr495 eNOS levels were elevated as in vehicle-treated lambs, levels of phospho-Ser1177 eNOS, a phosphorylated form of eNOS associated with increased activity, were increased following acute ductal constriction in PEG-SOD-treated lambs (P <0.05, Fig. 4). In fact, following ductal constriction, the levels of phospho-Ser1177 eNOS were higher in the PEG-SOD-treated lambs than the PEG-alone-treated lambs (P < 0.05).
No changes in hemodynamic variables, superoxide levels, NOS activity, and protein levels were noted in non-constricted (sham) fetal lambs (data not shown).
DISCUSSION
The regulation of the high basal pulmonary vascular tone, and its dramatic transition to a low-resistance circulation after birth, involves a complex, incompletely understood, interaction between anatomic alterations, mechanical forces, and a balance between varieties of vasoactive mediators with competing effects on basal tone. Although the maintenance of low fetal pulmonary blood flow and high pulmonary vascular resistance is considered adaptive given the placental circulation and the resulting non-dependence of pulmonary blood flow for gas exchange, aberrations in these mechanisms may result in incomplete transition to the low postnatal pulmonary resistance and persistent pulmonary hypertension of the newborn (PPHN). Recent studies have demonstrated a role for increased myogenic tone in the maintenance of the high fetal pulmonary vascular resistance, but its mechanisms are incompletely understood. Previously, we have demonstrated a role for decreased eNOS activity in the intense pulmonary vasoconstriction following an increase in pulmonary blood flow secondary to acute ductal constriction in the fetal lamb (17). In the current study, we found that the decrease in eNOS activity is associated with an increase in lung superoxide levels and changes in the phosphorylation status of eNOS. Furthermore, we found that pretreatment with SOD attenuates the vasoconstriction and preserves NOS activity, suggesting a role for ROS-eNOS interactions in the pulmonary myogenic response following acute ductal constriction in the fetus.
A novel finding of this study is the demonstration of an 82% increase in lung superoxide levels 4 h following acute constriction of the fetal ductus arteriosus (Fig. 3). Several potential mechanisms may be involved in this finding, including alterations in mechanical forces, ET-1, and the phosphorylation status of eNOS. Ductal constriction results in a sustained increase in pulmonary arterial pressure and a transient increase in flow. Interestingly, in vitro studies demonstrate that the mechanical forces associated with increased pressure (cyclic mechanical strain) increase superoxide production via an upregulation of NADPH oxidase (11, 14, 28), suggesting a possible mechanism for the increase in superoxide demonstrated in this study. In addition, we have previously shown that ET-1 levels are increased following acute ductal constriction, and in vitro data demonstrate that ET-1 can increase superoxide production in isolated fetal pulmonary artery smooth muscle cells (29). Last, the current study demonstrates changes in the phosphorylation status of eNOS, and data suggest that such changes may uncouple eNOS resulting in NOS-derived superoxide production (9). The exact mechanisms involved in the increase in superoxide production in this study warrant further investigation.
Associated with the increase in superoxide reduction was a 50% decrease in NOS activity (Fig. 2). This finding is consistent with our previous investigation (17). However, the mechanism is unclear. Changes in NOS activity can result from changes at the transcriptional, posttranscriptional, and posttranslational level (10). Similar to our previous study, the change in NOS activity was not associated with changes in gene expression, suggesting a posttranslational modification. Several posttranslational factors, including the nitrational and phosphorylation status of the enzyme, may dynamically regulate eNOS activity (10, 18, 20, 21), and, interestingly, several lines of evidence suggest that ROS can modulate many of these posttranslational alterations. For example, superoxide reacts rapidly with NO to form peroxynitrite, a strong oxidizing agent, which reacts readily with biological molecules and is capable of nitrating free or protein-associated tyrosines, including eNOS, rendering it inactive. In the current study, we found no differences in the amounts of nitrated eNOS following 4 h of acute ductal constriction between the PEG-alone- and PEG-SOD-treated lungs, suggesting that eNOS nitration was not responsible for the differences in eNOS activity between the two groups (Fig. 4). However, because of limitations in the amount of preconstriction tissue available, comparisons between pre- and postconstriction in the PEG-alone tissues could not be assessed to completely rule out the responsibility of changes in eNOS nitration in this preparation. Phosphorylation and dephosphorylation of eNOS is another important posttranslational modification that regulates activity. Although there are numerous phosphorylation sites, the best studied are the phosphorylation of the serine residue Ser1177 and the threonine residue Thr495. Phosphorylation of Ser1177 induces eNOS activation, whereas phosphorylation of Thr495 is inhibitory (3). We found that acute compression of the fetal ductus arterious resulted in a significant increase in eNOS-Thr495, whereas eNOS-Ser1177 was unchanged. The net result of these changes could explain, at least in part, the associated decrease in NOS activity. Other posttranslational modifications, such as intracellular location, protein-protein interactions, and substrate and cofactor availability, could participate in the changes in NOS activity during ductal constriction, and further investigations are planned to elucidate these complex processes.
Pretreatment with PEG-SOD prevented the reduction in NOS activity and attenuated the intense pulmonary vasoconstriction following ductal constriction, suggesting a role for ROS in this myogenic response. This lack of a decrease in NOS activity induced by PEG-SOD treatment was not associated with a decrease in eNOS-Thr495 levels, a change in nitrated eNOS, or a change in eNOS protein expression. Interestingly, PEG-SOD treatment was associated with an increase in the lung protein levels of the stimulatory eNOS-Ser1177, suggesting that the increase in eNOS-Ser1177, at least in part, is responsible for the restoration in NOS activity. However, ROS can be involved in several other potential NOS modifications that require further investigation.
Several important limitations of the present study are noteworthy. First, only one ROS, superoxide, was studied. Other ROS, such as hydrogen peroxide, may be altered by mechanical forces and may alter NOS activity, and thus warrant further study (34). Second, the model utilized in this study employs peripheral lung biopsies. Therefore, the biochemical analysis is performed on peripheral lung homogenate, excluding the possibility of examining specific arterial sites of the myogenic response within the lung vasculature, and isolating changes to specific cell types. Strengths of this study include the in vivo performance of these investigations in chronically instrumented late-gestation fetal sheep, and the preconstriction lung biopsy allows the determination of changes within the same animal over time.
We conclude that alterations in ROS participate in the hemodynamic and biochemical alterations associated with the myogenic response following fetal constriction of the ductus arteriosus. We speculate that aberrations in these adaptive mechanisms may participate in the pathophysiology of PPHN and the alterations in fetal blood flow patterns associated with certain congenital heart defects, and thus warrant further investigation.
GRANTS
This research was supported in part by National Institutes of Health Grants HL-60190, HL-67841, HL-72123, HL-70061, HL-084739-03, and HD-057406 (to S. M. Black); HD-047349 (to P. Oishi) and HL-61284 (to J. R. Fineman); and a Transatlantic Network Development Grant from the LeDuq Foundation (to S. M. Black and J. R. Fineman).
DISCLOSURES
No conflicts of interest (financial or otherwise) are declared by the author(s).
REFERENCES
- 1.Abman SH, Accurso FJ. Acute effects of partial compression of ductus arteriosus on fetal pulmonary circulation. Am J Physiol Heart Circ Physiol 257: H626–H634, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Abman SH, Shanley PF, Accurso FJ. Failure of postnatal adaptation of the pulmonary circulation after chronic intrauterine pulmonary hypertension in fetal lambs. J Clin Invest 83: 1849–1858, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, Sessa WC. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric-oxide synthase. J Biol Chem 278: 14841–14849, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Black SM, Bekker JM, Johengen MJ, Parry AJ, Soifer SJ, Fineman JR. Altered regulation of the ET-1 cascade in lambs with increased pulmonary blood flow and pulmonary hypertension. Pediatr Res 47: 97–106, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Black SM, Fineman JR. Oxidative and nitrosative stress in pediatric pulmonary hypertension: roles of endothelin-1 and nitric oxide. Vascul Pharmacol 45: 308–316, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Black SM, Johengen MJ, Soifer SJ. Coordinated regulation of genes of the nitric oxide and endothelin pathways during the development of pulmonary hypertension in fetal lambs. Pediatr Res 44: 821–830, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Bush PA, Gonzalez NE, Ignarro LJ. Biosynthesis of nitric oxide and citrulline from l-arginine by constitutive nitric oxide synthase present in rabbit corpus cavernosum. Biochem Biophys Res Commun 186: 308–314, 1992 [DOI] [PubMed] [Google Scholar]
- 8.Carpenter D, Larkin H, Chang A, Morris E, O'Neill J, Curtis J. Superoxide dismutase and catalase do not affect the pulmonary hypertensive response to group B streptococcus in the lamb. Pediatr Res 49: 181–188, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chen CA, Druhan LJ, Varadharaj S, Chen YR, Zweier JL. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem 283: 27038–27047, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol 280: F193–F206, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Hitomi H, Fukui T, Moriwaki K, Matsubara K, Sun GP, Rahman M, Nishiyama A, Kiyomoto H, Kimura S, Ohmori K, Abe Y, Kohno M. Synergistic effect of mechanical stretch and angiotensin II on superoxide production via NADPH oxidase in vascular smooth muscle cells. J Hypertens 24: 1089–1095, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ivy DD, Kinsella JP, Abman SH. Endothelin blockade augments pulmonary vasodilation in the ovine fetus. J Appl Physiol 81: 2481–2487, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Ivy DD, Ziegler JW, Dubus MF, Fox JJ, Kinsella JP, Abman SH. Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res 39: 435–442, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Matsushita H, Lee KH, Tsao PS. Cyclic strain induces reactive oxygen species production via an endothelial NAD(P)H oxidase. J Cell Biochem Suppl Suppl 36: 99–106, 2001 [DOI] [PubMed] [Google Scholar]
- 15.McMullan DM, Bekker JM, Johengen MJ, Hendricks-Munoz K, Gerrets R, Black SM, Fineman JR. Inhaled nitric oxide-induced rebound pulmonary hypertension: role for endothelin-1. Am J Physiol Heart Circ Physiol 280: H777–H785, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Morin FC., 3rd Ligating the ductus arteriosus before birth causes persistent pulmonary hypertension in the newborn lamb. Pediatr Res 25: 245–250, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Ovadia B, Bekker JM, Fitzgerald RK, Kon A, Thelitz S, Johengen MJ, Hendricks-Munoz K, Gerrets R, Black SM, Fineman JR. Nitric oxide-endothelin-1 interactions after acute ductal constriction in fetal lambs. Am J Physiol Heart Circ Physiol 282: H862–H871, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Papapetropoulos A, Rudic RD, Sessa WC. Molecular control of nitric oxide synthases in the cardiovascular system. Cardiovasc Res 43: 509–520, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Pollock DM, Pollock JS. Endothelin and oxidative stress in the vascular system. Curr Vasc Pharmacol 3: 365–367, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, Baker JE, Sessa WC. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem 276: 17621–17624, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol 64: 749–774, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 272: L1005–L1012, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. Acute intrauterine pulmonary hypertension impairs endothelium-dependent vasodilation in the ovine fetus. Pediatr Res 45: 575–581, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Storme L, Rairigh RL, Parker TA, Kinsella JP, Abman SH. In vivo evidence for a myogenic response in the fetal pulmonary circulation. Pediatr Res 45: 425–431, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Tamura Y, Chi LG, Driscoll EM, Jr, Hoff PT, Freeman BA, Gallagher KP, Lucchesi BR. Superoxide dismutase conjugated to polyethylene glycol provides sustained protection against myocardial ischemia/reperfusion injury in canine heart. Circ Res 63: 944–959, 1988 [DOI] [PubMed] [Google Scholar]
- 26.Turner GR, Levin DL. Prostaglandin synthesis inhibition in persistent pulmonary hypertension of the newborn. Clin Perinatol 11: 581–589, 1984 [PubMed] [Google Scholar]
- 27.Villamor E, Le Cras TD, Horan MP, Halbower AC, Tuder RM, Abman SH. Chronic intrauterine pulmonary hypertension impairs endothelial nitric oxide synthase in the ovine fetus. Am J Physiol Lung Cell Mol Physiol 272: L1013–L1020, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Wang DS, Proffit D, Tsao PS. Mechanotransduction of endothelial oxidative stress induced by cyclic strain. Endothelium 8: 283–291, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Wedgwood S, Dettman R, Black S. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol 281: L1058–L1067, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Wedgwood S, McMullan DM, Bekker JM, Fineman JR, Black SM. Role for endothelin-1-induced superoxide and peroxynitrite production in rebound pulmonary hypertension associated with inhaled nitric oxide therapy. Circ Res 89: 357–364, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Wild LM, Nickerson PA, Morin FC., 3rd Ligating the ductus arteriosus before birth remodels the pulmonary vasculature of the lamb. Pediatr Res 25: 251–257, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Wiseman DA, Wells SM, Hubbard M, Welker JE, Black SM. Alterations in zinc homeostasis underlie endothelial cell death induced by oxidative stress from acute exposure to hydrogen peroxide. Am J Physiol Lung Cell Mol Physiol 292: L165–L177, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Wiseman DA, Wells SM, Wilham J, Hubbard M, Welker JE, Black SM. Endothelial response to stress from exogenous Zn2+ resembles that of NO-mediated nitrosative stress, and is protected by MT-1 overexpression. Am J Physiol Cell Physiol 291: C555–C568, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Zhen J, Lu H, Wang XQ, Vaziri ND, Zhou XJ. Upregulation of endothelial and inducible nitric oxide synthase expression by reactive oxygen species. Am J Hypertens 21: 28–34, 2008 [DOI] [PubMed] [Google Scholar]