Abstract
JNK is a member of the MAPK family and has essential roles in inflammation and cell differentiation and apoptosis. In recent years, there have been accumulating data indicating a novel role for JNK in cell growth and migration. In this report, we demonstrate that JNK activity is necessary for serotonin (5-HT)-induced proliferation and migration of bovine pulmonary artery smooth muscle cells (PASMCs). Stimulation with 5-HT was found to lead to activation of JNK with a maximal activation at 10 min. Inhibition of JNK with its specific inhibitor, SP-600125, or its dominant-negative form, DN-JNK, significantly reduced 5-HT-stimulated [3H]thymidine incorporation and cyclin D1 expression. A similar inhibitory effect on SMC migration produced by 5-HT, as detected by a wound healing assay, was observed with inhibition of JNK. Furthermore, inhibition of 5-HT receptors 1B and 2A, but not inhibition of the 5-HT transporter, blocked 5-HT-induced JNK activation. Inhibition of phosphatidylinositol 3-kinase (PI3K) with LY-294002 and wortmannin had little or no effect on 5-HT-induced JNK phosphorylation, but JNK inhibitor SP-600125 and DN-JNK blocked 5-HT-stimulated phosphorylation of Akt and its downstream effectors, p70S6K1 and S6, indicating that Akt is a downstream effector of JNK. Activation of Akt by 5-HT was blocked only minimally, if at all, by inhibitors of ERK and p38 MAPK, indicating a uniqueness of JNK MAPK in this activation of Akt. Coimmunoprecipitation showed binding of Akt to JNK, further supporting the interaction of JNK and Akt. Thus JNK is a critical molecule in 5-HT-induced PASMC proliferation and migration and may act at an important point for cross talk of the MAPK and PI3K pathways. Its activation by 5-HT is initiated through 5-HT 1B and 2A receptors, and its stimulation of SMC proliferation and migration occurs through the Akt pathway.
Keywords: cell migration, serotonin signaling
serotonin (5-hydroxytryptamine; 5-HT) has long been recognized to be one of the most potent naturally occurring pulmonary vasoconstrictors (5, 24). 5-HT also has been found to be a smooth muscle cell (SMC) mitogen in recent years (8). It was first implicated in the pathogenesis of pulmonary arterial hypertension (PAH) after an outbreak of the disease in Switzerland in the 1960s among patients taking aminorex fumarate, an appetite suppressant that inhibits serotonin uptake by platelets (5, 9). Since then, there have been many studies exploring the roles of serotonin in PAH (5, 6, 8, 21). We have previously demonstrated that ERK1/ERK2 MAPK (15), Rho/Rho kinase (ROCK; Ref. 20), and phosphatidylinositol 3-kinase (PI3K)-Akt pathways (18, 19) play important parallel roles in 5-HT-induced SMC proliferation and migration.
The MAPK family is evolutionarily conserved, and all eukaryotic cells possess multiple MAPK pathways. The MAPK family can be subdivided into three major groups: ERK, p38, and JNK (4, 12). JNK is activated by multiple growth factors and cytokines, such as EGF, PDGF, TGF-β, and TNF, in addition to cellular stress (4, 12). There is increasing evidence that MAPKs are in a central position for cell proliferation and migration.
JNK has frequently been suggested to have essential roles in inflammation, differentiation, and apoptosis (3, 11). Moreover, there are accumulating data indicating a role for JNK in cell migration and proliferation (1, 4, 10, 12, 13, 16, 28). However, the role of JNK in 5-HT-induced SMC proliferation and migration and the molecular mechanisms by which JNK mediates these functions have not been described previously.
In the present study, we have identified for the first time that JNK plays an important role in pulmonary artery proliferative and migratory responses to 5-HT. Specifically, JNK is instrumental in SMCs proliferation and migration and in the activation of Akt, a function not shared with ERK and p38 MAPKs. The activation of JNK in this case appears to occur via combined actions by 5-HT on 5-HT 1B/1D and 2A/2B receptors, but not the 5-HT transporter (5-HTT), and is unrelated to the action of 5-HT on PI3K.
MATERIALS AND METHODS
Reagents.
RPMI 1640 medium was purchased from GIBCO Laboratories (Grand Island, NY). FBS, 5-HT, paroxetine, imipramine, SB-215505, and ketanserin were purchased from Sigma Chemical (St. Louis, MO). GR-55562, GR-127935, and mianserin were purchased from Tocris (Ellisville, MO). U-0126, SP-600125, SB-203580, LY-294002, and wortmannin were purchased from Calbiochem (EMD Chemicals, Gibbstown, NJ). Antibodies to Akt, cyclin D1, and tubulin were from Santa Cruz Biotechnology (San Diego, CA). Rabbit polyclonal antibodies to phosphorylated (phospho-) JNK (Thr183/Tyr185), JNK, phospho-p44/42 MAPK (ERK1/ERK2; Thr202/Tyr204), phospho-p38 MAPK, phospho-Akt (Ser473), phospho-S6, phospho-p70S6, and p70S6 were from Cell Signaling Technology (Danvers, MA). Pierce nuclear and cytoplasmic extraction kits were purchased from Thermo Fisher Scientific (Pittsburgh, PA). Adenoviral constructs Ad-DN-JNK (dominant-negative type) and Ad vector (control) were from Dr. Hideaki Kaneto (Osaka University, Osaka, Japan).
Cell culture.
Bovine pulmonary artery SMCs were isolated from calf pulmonary arteries using a modification of the method of Ross as previously described (22, 23) and were cultured in RPMI 1640 medium supplemented with 10% FBS. Cells from passages 3 to 12 were used for the experiments.
[3H]thymidine incorporation assay.
SMCs seeded in 96-well plates were growth-arrested for 72 h in serum-free medium. Cells were incubated with and without 1 μmol/l 5-HT in the same medium for 20 h and then labeled with [methyl-3H]thymidine (20 μCi/ml) for 4 h. In some experiments, inhibitors were added 60 min before the 5-HT. DMSO (0.1%) was added to the vehicle control group. After labeling, experiments were terminated by aspiration of medium, and the cells were harvested onto 96-well microplate filter paper using a Tomtec harvester (Tomtec, Hamden, CT). Radioactivity was countered in a TriLux liquid scintillation and luminescence counter (PerkinElmer Life Sciences, Boston, MA).
Wound healing assay.
Cell migration was assessed in a wound healing assay. Wounds were made by scraping through the cell monolayer with a sterile 1-ml pipette tip. Three wounds were made in a plate. SMCs were starved with serum-free RPMI 1640 medium for 24 h and then preincubated with 5 μmol/l SP-600125 for 60 min before treatment with 10 μmol/l 5-HT. At 20 h after wounding, phase-contrast images at 3 wound sites along the wounding scratch were examined and photographed by Nikon phase-contrast microscopy at 100-fold magnification. The width of the wounding gap was measured on the photographs. Triplicate results were obtained in 3 separate experiments.
Immunoprecipitation.
Cell lysates were precleared with protein A-agarose and/or protein G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA), incubated with specific antibodies at 4°C for 2 h or overnight and immunoprecipitated with protein A-agarose and/or protein G-agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) at 4°C for another 2 h with constant rotation.
Adenoviral infection of SMCs.
Subconfluent bovine pulmonary artery SMCs were infected with adenoviruses at a multiplicity of infection of 10 in complete medium 24 h before starvation, when the virus-containing medium was replaced by serum-free medium and incubated overnight. Under optimal conditions, ∼80% of the cells were infected as determined by green fluorescent protein autofluorescence.
Preparation of cell nuclear extract.
Growth-arrested SMCs were treated with 5-HT for indicated times, and nuclear extracts from cells were prepared with NE-PER nuclear and cytoplasmic extraction kits according to the manufacturer's protocol (Pierce, Rockford, IL). Cells were collected by scraping them from a p60 plate into a 1.5-ml tube, which was centrifuged at 500 g for 5 min. The supernatant was removed, and 100 μl of ice-cold cytoplasmic extraction reagent (CER) I with protease and phosphatase inhibitors was added to the cell pellet. The mixture was vortexed vigorously for 15 s and incubated on ice for 10 min. Ice-cold CER II (5.5 μl) was added, and the mixture was again vortexed for 5 s before centrifuging at 4°C at full speed (∼16,000 g) for 5 min. The supernatant (cytoplasmic extract) fraction was transferred to a clean tube, and the pellet was washed with ice-cold PBS once. Ice-cold NER with protease and phosphatase inhibitors (50 μl) was added to the pellet, and the mixture was vortexed vigorously for 40 min in the cold-room. The tube was then centrifuged at 4°C at full speed (∼16,000 g) for 10 min, and the supernatant (nuclear extract) was transferred to a clean tube. All extracts were stored at −80°C.
Western blotting.
Cells were lysed in a modified RIPA buffer [50 mM Tris·HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40, 0.5% (wt/vol) sodium deoxycholate, 0.1% SDS, 0.2 mM phenylmethylsulfonyl fluoride, leupeptin (5 μg/ml), aprotinin (5 μg/ml), and 1 mM Na3VO4]. Cell nuclei were removed from lysates by centrifugation for 10 min, and the cell lysates were normalized for protein concentrations by using Bradford reagent (Bio-Rad, Hercules, CA). The lysate (30 μg) was resolved by SDS-PAGE (10%) and transferred onto a PVDF membrane. The membrane was blocked with 5% fat-free milk in PBST with 0.1% (vol/vol) Tween 20 for 1 h at room temperature and then incubated with a primary antibody in 1:1,000 dilution at 4°C overnight. The membrane was then washed three times in PBST and incubated for 1 h at room temperature with horseradish peroxidase-conjugated secondary antibody in 1:3,000 or 1:5,000 dilution (Bio-Rad), followed by ECL detection (Thermo Fisher Scientific). Densitometry measurements were performed with the software UN-SCAN-IT from Silk Scientific (Orem, UT). Fold changes were expressed in relation to the control.
Immunocytochemistry.
The growth-arrested SMCs were treated with 5-HT for indicated times. Cells were washed with PBS and fixed with 4% formaldehyde at room temperature for 10 min and then permeabilized with PBS-Triton X-100 (0.4%) for 10 min. Cells were washed with PBS, blocked with 5% goat serum in 1% BSA/PBS for 20 min, and then incubated with primary antibody in the dilution of 1:100 at 4°C overnight. Cells were then washed with PBS and incubated with FITC-conjugated secondary antibody in the dilution of 1:100 for 2 h at room temperature. Cells were washed with PBS, and coverslips were mounted in Citifluor and sealed with nail polish.
Statistical analysis.
Means ± SD were calculated, and statistically significant differences between groups were determined by one-way ANOVA followed by Tukey post hoc comparisons. An effect was considered significant at P < 0.05.
RESULTS
JNK is activated by 5-HT in pulmonary artery SMCs.
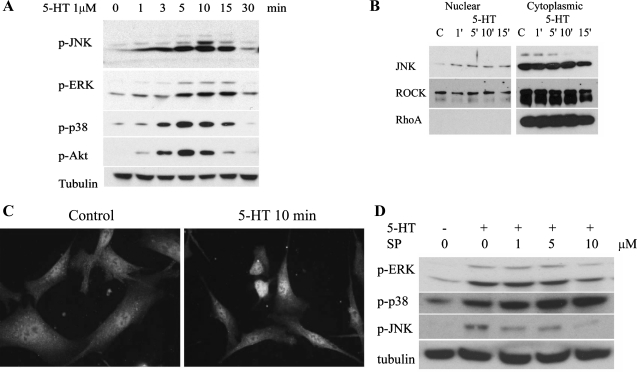
Starved bovine pulmonary artery SMCs were treated with 1 μM 5-HT for various periods of time ranging from 1 to 30 min. As shown in Fig. 1A, JNK was activated as early as 1 min after 5-HT stimulation as evidenced by the phosphorylation of JNK at Thr183 and Tyr185 sites. The stimulation peaked at 10 min and returned to basal level at 30 min. As shown in Fig. 1A, 5-HT also activated ERK, p38 MAPK, and Akt. Activation of JNK by 5-HT resulted in its import into the cellular nucleus (Fig. 1B). The absence of the protein RhoA served to control the purity of the nuclear fraction, and ROCK was used as a loading control. Immunocytochemistry was also performed to show the nuclear translocation of phospho-JNK by 5-HT stimulation (Fig. 1C). JNK activity was inhibited by SP-600125 at 1 or 5 μM, a dose showing no effect on ERK and p38 MAPKs (Fig. 1D).
Fig. 1.
A: JNK is activated by 5-HT. Bovine pulmonary artery smooth muscle cells (SMCs) were starved for 24 h and then treated with 1 μM 5-HT for various periods of time ranging from 1 to 30 min. Total cell lysates in RIPA buffer were resolved using PAGE and transferred to PVDF membrane and probed with antibodies to phosphorylated (p-) JNK, p-ERK, p38 MAPK, p-Akt, and tubulin as a loading protein. B and C: 5-HT stimulates JNK nuclear translocation. Growth-arrested SMCs were stimulated with 1 μM 5-HT for the indicated periods of time. Nuclear and cytoplasmic proteins were extracted from the cells, resolved with PAGE, transferred to PVDF membrane, and probed with antibodies to JNK, Rho/Rho kinase (ROCK), and RhoA (B). Immunocytochemistry was also performed to show the nuclear translocation of p-JNK by 5-HT stimulation (C). Various concentrations of the JNK inhibitor SP-600125 were tested against activations of MAPKs by 5-HT (D). C, control.
JNK activation by 5-HT leads to SMC proliferation and migration.
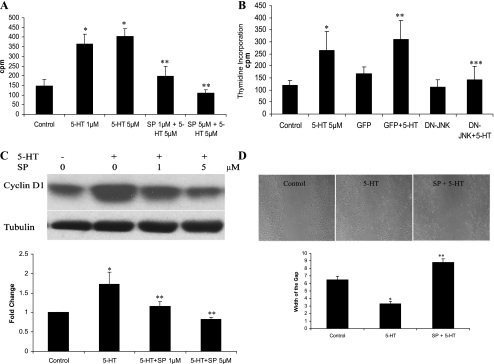
To determine whether the 5-HT-induced activation of JNK is involved in 5-HT-stimulated SMC growth, SMCs were pretreated with the specific JNK inhibitor SP-600125 (a competitive ATP inhibitor selective for JNK) for 60 min before stimulation with 5-HT. As shown in Fig. 2A, 5-HT-stimulated proliferation of SMCs was significantly suppressed with SP-600125. To exclude possible nonspecific inhibition by the chemical inhibitor, SMCs were also infected with a DN-JNK and then stimulated with 5-HT. As shown in Fig. 2B, DN-JNK also significantly blocked 5-HT-induced proliferation of SMCs, further confirming the function of JNK in regulating 5-HT-stimulated SMCs growth. Cyclin D1 is an important regulator of cell proliferation, and our (20) previous studies have shown that 5-HT upregulates cyclin D1 in SMCs. As shown in Fig. 2C, the JNK inhibitor SP-600125 blocked 5-HT-induced upregulation of cyclin D1 in the SMCs. A similar inhibitory effect on 5-HT-induced SMC migration as detected by a wound healing assay was observed with inhibition of JNK (Fig. 2D). Taken together, these data strongly support the concept that activation of JNK is an intermediate step in the mediation of proliferation and migration of pulmonary artery SMCs by 5-HT.
Fig. 2.
JNK participates in 5-HT-induced SMC proliferation and migration. A: JNK inhibitor SP-600125 (SP) reduced 5-HT-mediated SMCs proliferation. Quiescent SMCs were pretreated with JNK inhibitor SP-600125 at 1 or 5 μM for 60 min and then stimulated with 5 μM 5-HT for 24 h. DNA synthesis was determined by monitoring [3H]thymidine incorporation. Data presented are means ± SD for n = 3. *P < 0.05 compared with the control; **P < 0.05 compared with 5 μM 5-HT-stimulated cells. B: dominant-negative form of JNK (DN-JNK) blocked 5-HT-induced SMCs growth. SMCs were infected with adenoviruses encoding green fluorescent protein (GFP)-DN-JNK or GFP (vector) at multiplicity of infection (MOI) of 10 for 48 h before the experiment. Quiescent SMCs were incubated with 5 μM 5-HT for 24 h, and DNA synthesis was determined by monitoring [3H]thymidine incorporation. Data presented are means ± SD for n = 3. *P < 0.001 compared with the control; **P < 0.05 compared with GFP (vector) or control; ***P < 0.05 compared with 5-HT or GFP/5-HT. C: JNK regulates cyclin D1 expression by 5-HT. Growth-arrested bovine pulmonary artery SMCs were incubated with SP-600125 (1–5 μM) for 60 min and then stimulated with 5-HT (1 μM) for 6 h. Total cell lysates were immunoblotted with antibodies to cyclin D1 and tubulin. Data presented are means ± SD for n = 3. *P < 0.05 compared with control cells; **P < 0.05 compared with 5-HT-stimulated cells. D: JNK regulates SMC migration. In a wound healing assay, growth-arrested pulmonary artery SMCs were gently scraped with a pipette tip to produce a wound. Three wounds were made in a plate. Cells were stimulated with 10 μM 5-HT for 20 h. Pretreatment was with either 5 μM SP-600125 or its solvent DMSO for 60 min. Three wound sites along the wounding scratch were examined and photographed at 100-fold magnification. The width of the wounding gap was measured on the photographs. Triplicate results were obtained in 3 separate experiments. *P < 0.05 compared with the control; **P < 0.05 compared with the 5-HT-treated cells. cpm, Counts per minute.
Activation of JNK by 5-HT is PI3K-independent.
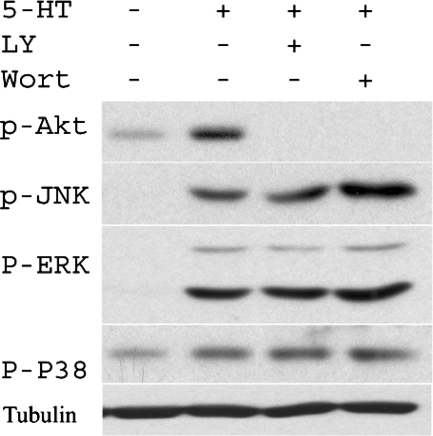
It has been reported that JNK is an important cell signaling component downstream of PI3K in PDGF-induced cell migration (1). To explore the mechanisms that mediate JNK activation by 5-HT, PI3K inhibitors, LY-294002 and wortmannin, were tested in the pulmonary artery SMCs. As shown in Fig. 3, both LY-294002 and wortmannin had little or no effect on 5-HT-induced JNK phosphorylation at Thr183 and Tyr185 but blocked 5-HT-induced Akt activation, confirming its effectiveness in inhibiting the PI3K pathway. As we (18) have noted previously, inhibition of PI3K by LY-294002 or wortmannin inhibits Akt phosphorylation by 5-HT.
Fig. 3.
Activation of JNK MAPK by 5-HT is independent of phosphatidylinositol 3-kinase (PI3K). Pulmonary artery SMCs were starved for 24 h and then pretreated with PI3K inhibitors LY-294002 (LY; 10 μM) and wortmannin (Wort; 1 μM) for 60 min before 1 μM 5-HT stimulation for 10 min. Total cell lysates in RIPA buffer were resolved using PAGE and transferred to PVDF membrane and probed with antibodies to p-JNK, p-ERK, p-p38 MAPK, p-Akt, and tubulin as a loading protein.
5-HT 1B/1D and 2A/2B receptors participate in activation of JNK by 5-HT.
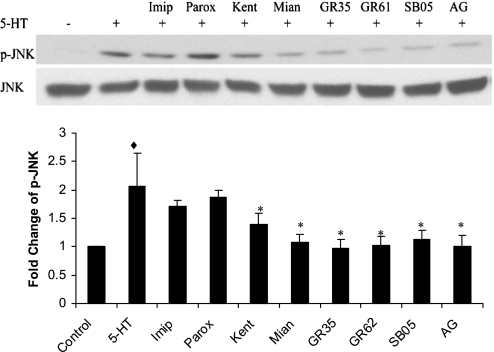
To determine whether specific 5-HT receptors or 5-HTT is involved in activation of JNK by 5-HT, starved bovine pulmonary artery SMCs were pretreated with the known inhibitors of 5-HT receptors and transporter for 60 min before 5-HT stimulation. As shown in Fig. 4, 5-HTT inhibitors, imipramine and paroxetine, failed to block 5-HT-induced activation of JNK, but 5-HT receptor 1B/1D inhibitors (GR-127935 and GR-55562), 2A inhibitors (ketanserin and mianserin), and a 2B inhibitor (SB-215505) significantly blocked 5-HT-induced phosphorylation of JNK, indicating that 5-HT receptors 1B/1D and receptors 2A/2B may be the upstream activators of JNK on 5-HT stimulation in bovine pulmonary artery SMCs. Interestingly, the PDGF receptor (PDGFR) inhibitor AG-1296 also blocked 5-HT-induced activation of JNK. We (19) previously reported that 5-HT transactivates PDGFR via 5-HTT. However, in the present study, 5-HTT does not appear to participate in the 5-HT-induced JNK activation, suggesting that there may be other mechanism(s) linking 5-HT to PDGFR and JNK.
Fig. 4.
5-HT receptors regulate JNK activation by 5-HT. Starved bovine pulmonary artery SMCs were pretreated with inhibitors of 5-HT transporter [10 μM imipramine (Imip) and 10 μM paroxetine (Parox)], 5-HT receptors 2A [1 μM ketanserin (Kent) and 10 μM mianserin (Mian)], 2B [10 μM SB-215505 (SB05)], 1B [10 μM GR-127935 (GR35) and 10 μM GR-55562 (GR62)], and PDGF receptor-β [PDGFRβ; 10 μM AG-1296 (AG)] for 60 min before 1 μM 5-HT. Data presented are means ± SD for n = 3. ♦P < 0.05 compared with control cells; *P < 0.05 compared with 5-HT-stimulated cells.
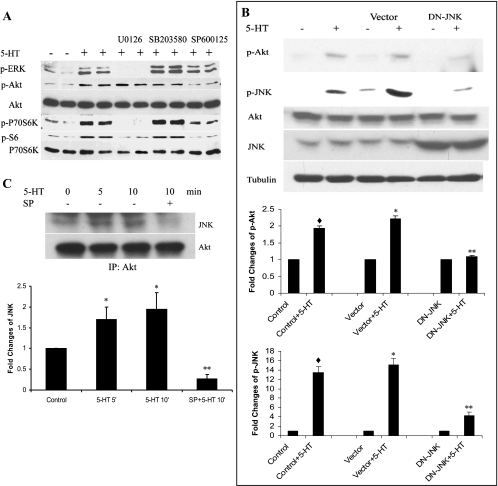
Regulation of the Akt pathway by JNK in 5-HT-stimulated bovine pulmonary artery SMCs.
Since we (15, 18, 20) have previously identified the importance of the ERK and Akt pathways in 5-HT-induced pulmonary artery SMC proliferation, we next undertook a series of studies to determine whether activation of the Akt pathway by 5-HT might be under the regulation of a MAPK. We knew from our (18) previous studies that the Akt pathway is not under regulation by ERK, and this was further confirmed in Fig. 5A. To our surprise, we found that JNK but not ERK or p38 MAPK was instrumental in the activation of Akt by 5-HT. Inhibition of JNK by its specific inhibitor SP-600125 resulted in decreased phosphorylation of Akt and its downstream effectors p70S6K1 and S6 (Fig. 5A). Infection of bovine pulmonary artery SMCs with adenovirus carrying DN-JNK further confirmed the findings with the pharmacological inhibitor (Fig. 5B).
Fig. 5.
JNK regulates Akt pathway. A: JNK, but not ERK or p38 MAPK, regulates 5-HT-induced Akt phosphorylation. Starved SMCs were pretreated with MAPK inhibitors 10 μM U-0126 (ERK), 20 μM SB-203580 (p38), and 5 μM SP-600125 (JNK) for 60 min before 1 μM 5-HT stimulation. Total cell lysates were immunoblotted with antibodies to p-Akt, Akt, p-ERK, p-S6 kinase, p-p70S6 kinase, and p70S6 kinase. B: DN-JNK blocks 5-HT-induced Akt activation. Pulmonary artery SMCs were infected with adenoviruses carrying DN-JNK or the adenovirus alone at MOI of 10 for 24 h and then starved for another 24 h before stimulation with 1 μM 5-HT for 10 min. Total cell lysates in RIPA buffer were resolved with PAGE, transferred to PVDF membrane, and probed with antibodies to p-JNK, JNK, p-Akt, Akt, and tubulin. Data presented are means ± SD for n = 3. ♦ P < 0.05 compared with nontransfected control; *P < 0.05 compared with vector-transfected control; **P < 0.05 compared with 5-HT-stimulated vector-transfected cells. C: interaction of JNK and Akt on 5-HT stimulation. SMCs were starved for 24 h and then treated with 1 μM 5-HT for 5 and 10 min. Pretreatment was with SP-600125 at 5 μM for 60 min. Coimmunoprecipitation was performed with the total cell lysates using antibody against Akt. The precipitates were resolved with PAGE, transferred to PVDF membrane, and probed with antibodies to JNK and Akt. Data presented are means ± SD for n = 3, *p < 0.05 compared with control; **P < 0.05 compared with 10 μM of 5-HT-stimulated cells. IP, immunoprecipitation.
We next explored how JNK regulates Akt on 5-HT stimulation. Coimmunoprecipitation experiments revealed that JNK could directly bind with Akt, and this binding was increased by 5-HT stimulation and blocked by the JNK inhibitor SP-600125 (Fig. 5C), further supporting the interaction of JNK and Akt in 5-HT-stimulated pulmonary artery SMCs. These data suggest that JNK may regulate pulmonary artery SMCs proliferation and migration via Akt and provide evidence that the MAPK pathway cross talks with the PI3K-Akt pathway at JNK-Akt.
DISCUSSION
We have demonstrated in this study that 5-HT stimulates the activation of JNK MAPK as well as other MAPKs in pulmonary artery SMCs and that the activation of JNK contributes to 5-HT-induced SMC proliferation and migration. Although JNK is usually suggested to have essential roles in inflammation, differentiation, and apoptosis, recent studies have shown that JNK is also involved in regulating cell proliferation and migration (7, 14, 27), which is consistent with our present observations. In our study, we found that inhibition of JNK could block the 5-HT-induced upregulation of cyclin D1, suggesting that JNK may regulate cell proliferation by controlling cell cycle. We have found multiple signaling pathways, including ERK MAPK (15), ROCK (20), PI3K-Akt pathways (18, 19), and now the JNK pathway, to be activated by 5-HT and involved in 5-HT-induced SMC proliferation and migration. Although these pathways function in parallel, they also integrate or cross talk with each other as demonstrated by the JNK-Akt interaction noted in our present study.
Our studies show that activation of JNK by 5-HT leads to activation of Akt, a previously established pathway in SMC proliferation (18). To the best of our knowledge, this is the first report to associate JNK with Akt activation in 5-HT stimulation. There is one previous report that shows activation of Akt via JNK in cardiomyocyte survival after hypoxic injury (25). Therefore, the results of this study reveal a new mechanism by which 5-HT may regulate SMC proliferation and migration by linking a MAP kinase pathway and the PI3K pathway. Furthermore, JNK plays a unique role in activation of Akt, as inhibitors of ERK and p38 MAPKs had no or little effect on this activation of Akt by 5-HT. The activation of JNK MAPK does not occur through PI3K, unlike the activation of the Akt pathway discussed in our (18) previous paper. Hence, there is more than one pathway for the activation of Akt in these cells. The PI3K-independent JNK pathway is a novel pathway that regulates Akt activity in pulmonary artery SMCs on 5-HT stimulation. We also observed the direct interaction of JNK and Akt, and this interaction was blocked by inhibition of JNK activity (Fig. 5C). This provides direct evidence that JNK regulates Akt. We (17) also have previously found that activation of PLD by 5-HT occurs through the 5-HT2A receptor and, like JNK, requires a mechanism that is PI3K-independent. We do not presently know whether the JNK pathway might participate in the activation of PLD by 5-HT.
The activation of JNK appears to occur via 5-HT 2A/2B and 1B/1D receptors and is independent of 5-HTT. The only other previous report, to our knowledge, of the activation of JNK by 5-HT is that of Turner et al. (26) where the authors showed that activation of JNK in Chinese hamster ovary fibroblasts induced apoptosis. In contrast to this finding, our studies show that activation of JNK by 5-HT results in enhanced proliferation and migration of pulmonary artery SMCs. Banes et al. (2) have previously reported an inability of 5-HT to activate either JNK or p38 MAPKs in rat aortic vascular SMCs. This result may be due to species or source differences in the cells.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-085260 (B. L. Fanburg).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Amagasaki K, Kaneto H, Heldin CH, Lennartsson J. c-Jun N-terminal kinase is necessary for platelet-derived growth factor-mediated chemotaxis in primary fibroblasts. J Biol Chem 281: 22173–22179, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Banes AK, Loberg RD, Brosius FC, 3rd, Watts SW. Inability of serotonin to activate the c-Jun N-terminal kinase and p38 kinase pathways in rat aortic vascular smooth muscle cells. BMC Pharmacol 1: 8–14, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr RK, Bogoyevitch MA. The c-Jun N-terminal protein kinase family of mitogen-activated protein kinases (JNK MAPKs). Int J Biochem Cell Biol 33: 1047–1063, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Bennett BL. c-Jun N-terminal kinase-dependent mechanisms in respiratory disease. Eur Respir J 28: 651–661, 2006 [DOI] [PubMed] [Google Scholar]
- 5.de Caestecker M. Serotonin signaling in pulmonary hypertension. Circ Res 98: 1229–1231, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Eddahibi S, Morrell N, d'Ortho MP, Naeije R, Adnot S. Pathobiology of pulmonary arterial hypertension. Eur Respir J 20: 1559–1572, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Eynott PR, Nath P, Leung SY, Adcock IM, Bennett BL, Chung KF. Allergen-induced inflammation and airway epithelial and smooth muscle cell proliferation: role of Jun N-terminal kinase. Br J Pharmacol 140: 1373–1380, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanburg BL, Lee SL. A new role for an old molecule: serotonin as a mitogen. Am J Physiol Lung Cell Mol Physiol 272: L795–L806, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Gurtner HP. Aminorex and pulmonary hypertension. A review. Cor Vasa 27: 160–171, 1985 [PubMed] [Google Scholar]
- 10.Gururajan M, Chui R, Karuppannan AK, Ke J, Jennings CD, Bondada S. c-Jun N-terminal kinase (JNK) is required for survival and proliferation of B-lymphoma cells. Blood 106: 1382–1391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)–from inflammation to development. Curr Opin Cell Biol 10: 205–219, 1998 [DOI] [PubMed] [Google Scholar]
- 12.Johnson GL, Nakamura K. The c-jun kinase/stress-activated pathway: regulation, function and role in human disease. Biochim Biophys Acta 1773: 1341–1348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang YJ, Jeon ES, Song HY, Woo JS, Jung JS, Kim YK, Kim JH. Role of c-Jun N-terminal kinase in the PDGF-induced proliferation and migration of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem 95: 1135–1145, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Kavurma MM, Khachigian LM. ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J Cell Biochem 89: 289–300, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Lee SL, Wang WW, Finlay GA, Fanburg BL. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am J Physiol Lung Cell Mol Physiol 277: L282–L291, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Leppa S, Bohmann D. Diverse functions of JNK signaling and c-Jun in stress response and apoptosis. Oncogene 18: 6158–6162, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Fanburg BL. Phospholipase D signaling in serotonin-induced mitogenesis of pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 295: L471–L478, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Fanburg BL. Serotonin-induced growth of pulmonary artery smooth muscle requires activation of phosphatidylinositol 3-kinase/serine-threonine protein kinase B/mammalian target of rapamycin/p70 ribosomal S6 kinase 1. Am J Respir Cell Mol Biol 34: 182–191, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Li M, Warburton RR, Hill NS, Fanburg BL. The 5-HT transporter transactivates the PDGFbeta receptor in pulmonary artery smooth muscle cells. FASEB J 21: 2725–2734, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase-induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res 95: 579–586, 2004 [DOI] [PubMed] [Google Scholar]
- 21.MacLean MR. Pulmonary hypertension and the serotonin hypothesis: where are we now? Int J Clin Pract Suppl: 27–31, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Ross R. The smooth muscle cell. II. Growth of smooth muscle in culture and formation of elastic fibers. J Cell Biol 50: 172–186, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ross R, Klebanoff SJ. The smooth muscle cell. I. In vivo synthesis of connective tissue proteins. J Cell Biol 50: 159–171, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudolph AM, Paul MH. Pulmonary and systemic vascular response to continuous infusion of 5-hydroxytryptamine (serotonin) in the dog. Am J Physiol 189: 263–268, 1957 [DOI] [PubMed] [Google Scholar]
- 25.Shao Z, Bhattacharya K, Hsich E, Park L, Walters B, Germann U, Wang YM, Kyriakis J, Mohanlal R, Kuida K, Namchuk M, Salituro F, Yao YM, Hou WM, Chen X, Aronovitz M, Tsichlis PN, Bhattacharya S, Force T, Kilter H. c-Jun N-terminal kinases mediate reactivation of Akt and cardiomyocyte survival after hypoxic injury in vitro and in vivo. Circ Res 98: 111–118, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Turner JH, Garnovskaya MN, Raymond JR. Serotonin 5-HT1A receptor stimulates c-Jun N-terminal kinase and induces apoptosis in Chinese hamster ovary fibroblasts. Biochim Biophys Acta 1773: 391–399, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Yang YM, Bost F, Charbono W, Dean N, McKay R, Rhim JS, Depatie C, Mercola D. C-Jun NH(2)-terminal kinase mediates proliferation and tumor growth of human prostate carcinoma. Clin Cancer Res 9: 391–401, 2003 [PubMed] [Google Scholar]
- 28.Zhan Y, Kim S, Izumi Y, Izumiya Y, Nakao T, Miyazaki H, Iwao H. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler Thromb Vasc Biol 23: 795–801, 2003 [DOI] [PubMed] [Google Scholar]