Abstract
Pathological lung overdistention associated with mechanical ventilation at high tidal volumes (ventilator-induced lung injury; VILI) compromises endothelial cell (EC) barrier leading to development of pulmonary edema and increased morbidity and mortality. We have previously shown involvement of microtubule (MT)-associated Rho-specific guanine nucleotide exchange factor GEF-H1 in the agonist-induced regulation of EC permeability. Using an in vitro model of human pulmonary EC exposed to VILI-relevant magnitude of cyclic stretch (18% CS) we tested a hypothesis that CS-induced alterations in MT dynamics contribute to the activation of Rho-dependent signaling via GEF-H1 and mediate early EC response to pathological mechanical stretch. Acute CS (30 min) induced disassembly of MT network, cell reorientation, and activation of Rho pathway, which was prevented by MT stabilizer taxol. siRNA-based GEF-H1 knockdown suppressed CS-induced disassembly of MT network, abolished Rho signaling, and attenuated CS-induced stress fiber formation and EC realignment compared with nonspecific RNA controls. Depletion of GEF-H1 in the murine two-hit model of VILI attenuated vascular leak induced by lung ventilation at high tidal volume and thrombin-derived peptide TRAP6. These data show for the first time the critical involvement of microtubules and microtubule-associated GEF-H1 in lung vascular endothelial barrier dysfunction induced by pathological mechanical strain.
Keywords: cyclic stretch, actin, microtubules, endothelium, Rho GEF, mechanical forces
reorganization of the endothelial cell (EC) cytoskeleton, which is composed of actin filaments, microtubules (MT), and intermediate filaments, leads to alteration in cell shape and provides a structural basis for an increase in vascular permeability, implicated in the pathogenesis of many diseases including asthma, sepsis, and acute lung injury (ALI) (24, 37, 41). MT depolymerization by MT inhibitors nocodazole or vinblastin in pulmonary EC results in increased myosin light chain (MLC) phosphorylation, stress fiber formation, contraction, and EC barrier dysfunction (9, 10, 19, 60, 66). These effects are linked to the activation of small GTPase Rho and can be attenuated by cell pretreatment with paclitaxel (taxol), which promotes MT stabilization (9, 19, 25).
Rho and its downstream target Rho-associated kinase (Rho-kinase) may directly catalyze MLC phosphorylation or act indirectly via inactivation of MLC phosphatase (MYPT1) (65, 67) by phosphorylating Thr695, Ser894, and Thr850 (28), and cause actomyosin-driven cell contraction and EC barrier dysfunction. Guanine nucleotide exchange factor H1 (GEF-H1) has been recently characterized as a Rho-specific GEF, which localizes on microtubules and exhibits Rho-specific activity (54). In MT-bound state, the guanine-exchange activity of GEF-H1 is suppressed, whereas GEF-H1 release caused by MT disassembly stimulates Rho-specific GEF activity (36). We and others have previously shown that barrier-disruptive effects of thrombin, TGF-β1, and TNFα are associated with partial disassembly of MT (7–10, 25, 50, 73), which activated Rho signaling, and, thus further enhanced Rho-dependent mechanisms of agonist-induced EC barrier dysfunction (10, 19, 25, 73). We have recently reported the protective effect of MT stabilization against thrombin-induced Rho activation and barrier compromise and demonstrated the essential role of MT-associated Rho-specific nucleotide exchange factor GEF-H1 in MT-mediated regulation of Rho activity, cytoskeletal remodeling, and EC permeability (8).
Clinical observations as well as animal and cell culture studies strongly suggest that mechanical forces play an important role in pathophysiological regulation of the lung barrier, as high magnitude pathological cyclic stretch increases vascular permeability (6), activates inflammatory cytokine production (32, 69) and apoptosis (31, 51, 56), which mirrors in vivo alveolar flooding, leukocyte infiltration, and hypoxemia leading to morbidity and mortality (39, 64). Previous studies by our group indicate the important role of Rho pathway in the lung EC barrier disruption induced by pathological cyclic stretch and inflammatory agents (11, 17). Our studies strongly suggest that attenuation of Rho activity and stimulation of Rac-dependent mechanisms reduces lung vascular leak and promotes barrier recovery in the in vitro and in vivo models of ALI/ventilator-induced lung injury (VILI) (11, 17, 47).
In the current study using in vitro and in vivo models of VILI, we evaluated the involvement of microtubule-associated Rho-specific GEF-H1 in the development of lung vascular dysfunction induced by mechanical stimulation.
MATERIALS AND METHODS
Cell culture and reagents.
Human pulmonary artery endothelial cells (HPAEC) and cell culture basal medium (EBM-2) with growth supplements were obtained from Lonza (Allendale, NJ), cultured according to the manufacturer's protocol, and used at passages 5–9. Di-phospho-MLC and GEF-H1 antibodies were obtained from Cell Signaling (Beverly, MA); phospho-MYPT antibodies were purchased from Upstate Biotechnology (Lake Placid, NY); VE-cadherin antibodies were obtained from BD Transduction Laboratories (San Diego, CA); β-tubulin antibodies were from Covance (Berkeley, CA). All reagents for immunofluorescence staining were purchased from Molecular Probes (Eugene, OR). Unless specified, biochemical reagents were obtained from Sigma (St. Louis, MO).
Cell culture under cyclic stretch.
Cyclic stretch (CS) experiments were performed according to previously described protocol (6, 58) using FX-4000T Flexecell Tension Plus system (Flexcell International, McKeesport, PA) equipped with 25-mm BioFlex Loading Station designed to provide uniform radial and circumferential strain across a membrane surface along all radii. Each BioFlex membrane is stretched over the post when under vacuum pressure, creating a single-plane uniformly stretched circle. The radial and circumferential strain was experimentally determined by vendor (Flexcell International). Experimental measurements demonstrated that the part of the membrane stretching over the post (25-mm diameter) receives uniform strain in the radial direction that was proportional to vacuum level. The nature of such strain results in preferential radial vs. tangential vector of strain at the more proximal areas (visible cell orientation differences at the distance of two-thirds of radius from the center of the membrane). These areas were used for immunofluorescence experiments to better illustrate effects of microtubule stabilization or GEF-H1 depletion on CS-induced cytoskeletal remodeling. In brief, after 72 h of culture, cells were exposed to high-magnitude (18% linear elongation, sinusoidal wave, 25 cycles/min) CS to recapitulate the mechanical stresses experienced by the alveolar endothelium at high tidal volume mechanical ventilation (6, 63). Control BioFlex plates with static EC culture were placed in the same cell culture incubator and processed similarly to CS-preconditioned cells. At the end of the experiment, cell lysates were collected for Western blot analysis, or CS-exposed endothelial monolayers were fixed and used for immunofluorescence staining.
Immunofluorescence staining and image analysis.
CS-exposed endothelial monolayers were fixed in 1.5% glutaraldehyde solution in PBS for 10 min at room temperature, washed three times with PBS, permeabilized with 0.2% Triton X-100 in PBS for 60 min at room temperature, and blocked with 1% sodium borohydrite in PBS three times for 10 min. Incubation with antibodies of interest were performed in blocking solution (2% BSA in PBS) for 1 h at room temperature, followed by staining with Alexa 488-conjugated secondary antibodies (Molecular Probes). Actin filaments were stained with Texas red-conjugated phalloidin for 1 h at room temperature. After immunostaining, the glass slides were prepared using mounting medium (Kirkegaard and Perry Laboratories, Gaithersburg, MD) and analyzed using Nikon video-imaging system (Nikon Instech, Tokyo, Japan) as previously described (5, 11, 12). Quantitative analysis of assembled MT was performed as previously described (10, 18, 19).
Knockdown of GEF-H1 in pulmonary EC.
To reduce the content of endogenous GEF-H1, cells were treated with gene-specific siRNA duplexes. Predesigned standard purity siRNA sets (Homo sapiens) were ordered from Dharmacon (Lafayette, CO), and transfection of EC with siRNA was performed as previously described (5, 16). After 48 h of transfection, cells were used for experiments or harvested for Western blot verification of specific protein depletion. For in vivo experiments, predesigned standard purity Stealth GEF-H1-specific mouse siRNA sets were purchased from Invitrogen (Carlsbad, CA). Polymer-based administration of nonspecific or specific siRNA conjugated with polycation polyethilenimine (PEI-22) shown to promote lung-specific DNA and siRNA delivery was used (59, 62). The optimal concentration of siRNA was determined in the series of preliminary experiments. Liposome-siRNA polyplexes were formed at ratio 1:10 (1 μg siRNA per 10 μg lipid). SiRNA at 40 μg/kg showed the most significant target gene inhibition after 72 h of transfection determined by Western blot analysis. Treated mice showed no signs of nonspecific siRNA-induced inflammation. Nonspecific, nontargeting siRNA (Dharmacon) was used as a control treatment for both in vitro and in vivo experiments.
qRT-PCR.
Reverse transcription (RT) was performed with 1 μg of total RNA isolated from lung tissue of control and Si-GEF-H1 treated mouse to obtain cDNA with SuperScript II and random primers (Invitrogen) as the primer in a 20-μl reaction volume. Each cDNA sample was diluted to 2.6 ng/μl in sterile ddH2O, and 5 μl of this dilution was used as template for qPCR. Mouse GEF-H1 primers: forward, 5′-GACTCTAGCCAGAGGG-ATCG-3′; reverse, 5′-CTGCCTGTAGGCCATGTAGA-3′, and mouse housekeeping gene GAPD: forward, GAGTCAACGGATTTGGTCGT-3′; reverse, 5′-TTGATTTTGGAGGGATCTCG-3′ for the qPCR reactions, designed for QRT with the length of the amplicons 106 bp and 238 bp, correspondingly. PCR reactions were performed in an optical 384-well plate with an ABI PRISM 7900 HT sequence detection system (Applied Biosystems, Foster City, CA), using QuantiTech PCR SYBR Green I Kit (Qiagen, Valencia, CA) to monitor dsDNA synthesis. Data were analyzed using the SDS 2.2.1 software (Applied Biosystems).
Immunoblotting.
After stimulation, cells were lysed, and protein extracts were separated by SDS-PAGE, transferred to nitrocellulose membrane, and probed with specific antibodies as previously described (5).
Mechanical ventilation protocol.
All experimental protocols involving the use of animals were approved by the University of Chicago Institutional Animal Care and Use Committee for the humane treatment of experimental animals. C57BL/6J mice (8–10 wk old, male) with average weight 20–25 g (Jackson Laboratories, Bar Harbor, ME) were anesthetized with an intraperitoneal injection of ketamine (75 mg/kg) and acepromazine (1.5 mg/kg). Tracheotomy was performed, and the trachea was cannulated with a 20-gauge, one-inch catheter (Penn-Century, Philadelphia, PA) that was tied into place to prevent air leak. The animals were placed on mechanical ventilator (Harvard Apparatus, Boston, MA). Mice were given a single dose of intratracheal TRAP6 (1.5 × 10−5 mol/kg) followed by 4 h of mechanical ventilation with high tidal volume (HTV; 30 ml/kg) ventilation. In experiments with taxol, mice were injected with taxol (3.75 × 10−7 mol/kg iv) before TRAP6 instillation and mechanical ventilation. After the experiment, animals were killed by exsanguination under anesthesia. Bronchoalveolar lavage (BAL) was performed using 1 ml of sterile Hanks’ balanced salt buffer, and measurements of cell count and protein concentration were conducted as previously described (13, 27). Measurement of Evans blue accumulation in the lung tissue was performed according to the protocol described previously with few modifications (44, 47). In brief, Evans blue dye (EBD; 30 ml/kg) was injected into the external jugular vein 2 h before termination of ventilation. At the end of the experiment, thoracotomy was performed, and the lungs were perfused free of blood with PBS containing 5 mM EDTA. Both left lung and right lung were excised and imaged by Kodak digital camera. After imaging, lungs were blotted dry, weighed, and homogenized in PBS (1 ml/100 μg tissue). Homogenized tissue was incubated with 2 vol formamide (18 h, 60°C), centrifuged at 12,000 g for 20 min. Optical density of the supernatant was determined by spectrophotometry at 620 and 740 nm. EBD accumulation (micrograms of Evans blue dye per g lung) in lung homogenates was calculated against a standard curve. For histological assessment of lung injury, the lungs were harvested without lavage collection and stained with hematoxylin and eosin as previously described (27, 47). Tissue sections were evaluated at ×40 magnification.
Statistical analysis.
Results are expressed as means ± SD of three to six independent experiments. Experimental samples were compared with controls by unpaired Student's t-test. For multiple-group comparisons, a one-way ANOVA and post hoc multiple comparisons tests were used. P < 0.05 was considered statistically significant.
RESULTS
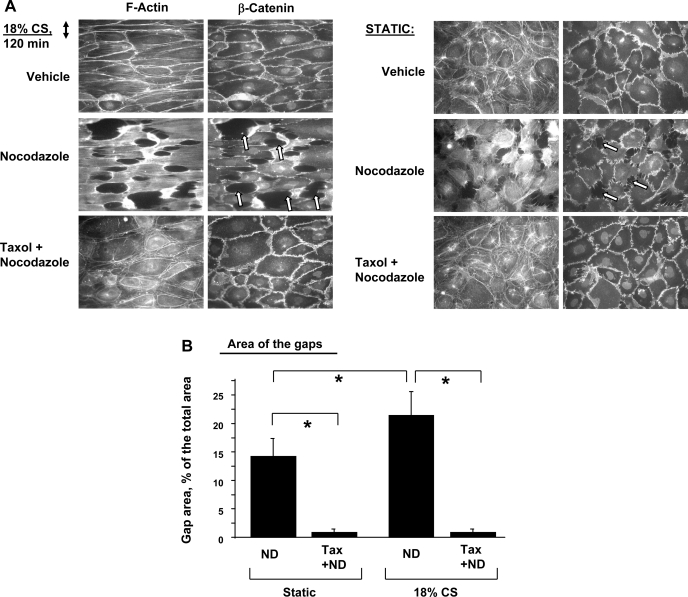
MT stabilization inhibits pulmonary EC gap formation induced by a combination of pathological CS and nocodazole. Previous reports have shown disruptive effects of MT inhibitor nocodazole in static endothelial cell cultures (8, 19, 66). This study examined effects of pathological CS on EC barrier failure-induced MT disassembly. Human pulmonary EC grown to confluence on Flexcell plates were exposed to 18% CS for 2 h and treated with vehicle or nocodazole for 30 min with continuing CS. Cytoskeletal and adherens junction remodeling was monitored by double immunofluorescence staining for F-actin and β-catenin. In consistence with previous reports (15, 19), nocodazole induced monolayer disruption in static EC manifested by stress fiber formation, paracellular gap formation, and disruption of adherens junctions (Fig. 1A). Quantitative image analysis of control and stimulated EC monolayers after immunofluorescence staining shows that EC exposure to 18% CS further enhanced nocodazole-induced paracellular gap formation (Fig. 1B). Importantly, EC pretreatment with the MT stabilizer taxol completely inhibited nocodazole-induced barrier disruption in both static and stretched EC. These data suggest a role of MT in stretch-activated signaling.
Fig. 1.
Involvement of microtubule (MT) in cyclic stretch (CS)-mediated endothelial cell (EC) remodeling. Pulmonary EC grown to confluence on Flexcell plates were exposed to 18% CS or left under static conditions. At 2 h, cells were stimulated with nocodazole (0.5 μM, 30 min) with or without taxol pretreatment (2 μM, 30 min). A: double immunofluorescence staining was performed with Texas red phalloidin to detect actin filaments and with β-catenin antibodies to visualize adherens junctions. Paracellular gaps are marked by arrows. B: quantitative analysis of gap formation on static and stretched human pulmonary artery endothelial cells (HPAEC). Data are expressed as means ± SD of 5 independent experiments; *P < 0.05.
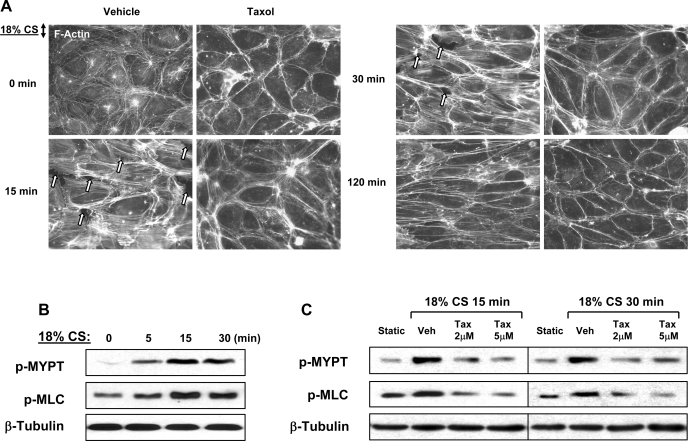
MT stabilization attenuates CS-induced cell alignment. Actin cytoskeletal remodeling in EC exposed to CS occurs in two phases (6, 71). Early phase (0–30 min) is characterized by increased stress fiber formation, F-actin reorientation, and transient appearance of paracellular gaps associated with cell reorientation and reestablishment of monolayer integrity. At later time points (2 h), EC monolayer reorientation is complete, and centrally localized stress fibers partially disappear while peripheral F-actin accumulation is enhanced. The following experiments tested the involvement of MT in cytoskeletal remodeling and cell orientation in response to CS. EC were pretreated with vehicle or taxol for 30 min followed by 18% CS exposure for various periods of time. CS induced rapid cell reorientation at 15 min accompanied by formation of stress fibers and appearance of paracellular gaps (Fig. 2A). At 30 min, cells became more oriented in the direction perpendicular to the main distension vector, while stress fibers and paracellular gaps remained evident. At later time points of CS exposure, EC revealed more pronounced orientation accompanied by gradual decrease in number of stress fibers and resealing of paracellular gaps (data not shown). In agreement with a previous report (6), CS-induced cell alignment was complete by 120 min and characterized by formation of circumferential F-actin rim, disappearance of central stress fibers, and complete resealing of paracellular gaps. Remarkably, stabilization of MT by taxol before CS stimulation significantly decreased stress fiber formation, prevented appearance of paracellular gaps during acute phase of CS (15–30 min), and attenuated CS-induced cell orientation at later time points (120 min) (Fig. 2A).
Fig. 2.
Effect of MT stabilization on CS-induced cytoskeletal remodeling. Human pulmonary EC were pretreated with vehicle or taxol (2 μM, 30 min) followed by exposure to 18% CS for various periods of time. A: actin cytoskeletal remodeling was examined by immunofluorescence staining with Texas red-conjugated phalloidin. Paracellular gaps are marked by arrows. B: levels of myosin light chain (MLC) phosphorylation in the total lysates were determined by Western blot analysis. C: MYPT phosphorylation was analyzed by Western blot in static and CS-challenged EC with or without the presence of taxol.
Previous findings suggest involvement of Rho pathway in acute CS-induced stress fiber formation and cytoskeletal remodeling (6, 58). We next examined effects of 18% CS on site-specific phosphorylation of myosin-binding subunit of MYPT1 at Thr850, which is mediated by Rho-associated kinase. EC exposure to 18% CS rapidly increased phospho-MYPT1 levels, as well as MLC phosphorylation, the two parameters of EC contraction activated by Rho pathway (Fig. 2B). In turn, MT stabilization by taxol pretreatment suppressed CS-induced MYPT1 and MLC phosphorylation during acute phase of CS (15–30 min) (Fig. 2C). These results demonstrate the essential role of MT in CS-mediated cytoskeletal rearrangement and intracellular signaling.
Pathological CS alters MT organization.
Effects of CS on MT organization were examined in more detail. Analysis of MT structure was performed at time points corresponding to acute phase (30 min) of EC response to CS and at the time point when cell reorientation is complete (120 min). The results were compared with nonstretched EC. Analysis of MT structure shows that under static conditions, MT organized into faint uniformly distributed lattice network (Fig. 3A). Acute CS induced alignment of MT perpendicular to the main distension vector, and this rearrangement was accompanied by MT depolymerization and significant reduction in a number of assembled MT, which was detected by quantitative image analysis of MT staining (Fig. 3, A and B). After 120 min of CS, pulmonary EC exhibited strong MT alignment perpendicular to the main distension vector, which was accompanied by complete restoration of MT network (Fig. 3, A and B).
Fig. 3.
Effects of pathological acute CS on MT dynamics. HPAEC grown on Flexcell plates were subjected to 18% CS for various periods of time. A: MT structure was analyzed by immunofluorescence staining for β-tubulin. Arrows indicate main direction of CS vector. B: quantitative analysis of assembled MT. Data are expressed as means ± SD of 5 independent experiments; *P < 0.05. C: pool of stable MT was determined in stretched EC by Western blot analysis with antibodies against acetylated tubulin. D: effect of taxol (2 μM, 30 min) treatment on CS-induced alteration of stable MT was evaluated by Western blot with antibodies against acetylated tubulin.
The pool of stable MT undergoes posttranslational modifications such as acetylation and detyrosination (46), which may reflect stability of MT network under particular conditions (48, 49, 52). In the following experiments, we analyzed a pool of acetylated MT in CS-stimulated HPAEC. Exposure of EC to 18% CS significantly decreased the amount of acetylated MT during acute phase of CS, as detected by Western blot analysis (Fig. 3C). Stabilization of MT with taxol completely restored levels of acetylated tubulin in CS-subjected EC compared with static cell culture (Fig. 3D). These results strongly suggest a role of CS in the modulation of MT organization. These data are also consistent with completion of actin cytoskeletal remodeling, cell alignment, and restoration of EC monolayer integrity at 2 h of CS (Fig. 2A). Importantly, protective effects of taxol against CS-induced partial MT disassembly correlate well with the taxol-induced suppression of CS-induced Rho signaling (Fig. 2B) and suggest a link between CS-induced partial MT disassembly and Rho activation. The next experiments examined a role of MT-associated signaling in CS-induced Rho activation.
GEF-H1 knockdown attenuates CS-induced cytoskeletal rearrangement.
We have previously described a key role for MT-associated Rho-specific GEF-H1 in the regulation of Rho activity by changes in MT assembly/disassembly status (8). We next tested the involvement of GEF-H1 in intracellular signaling and cytoskeletal remodeling in pulmonary EC exposed to pathological CS using GEF-H1 knockdown by specific siRNA. HPAEC were transiently transfected with GEF-H1-specific or nonspecific siRNA for 72 h. GEF-H1 knockdown was confirmed by Western blot (Fig. 4A). Actin cytoskeletal remodeling in control and siRNA-treated EC monolayers exposed to 18% CS was analyzed by immunofluorescence staining of F-actin. In contrast to nontransfected EC (Fig. 2A) or EC transfected with nonspecific RNA, GEF-H1 knockdown decreased CS-induced stress fiber formation and paracellular gap formation, prevented cell reorientation after 30 min of CS (Fig. 4A), and impaired EC alignment after 120 min of CS compared with nonspecific siRNA controls (Fig. 4A) or nontransfected cells (Fig. 2A). Biochemical analysis of Rho pathway activation showed that GEF-H1 knockdown decreased phosphorylation of Rho downstream targets MYPT1 (Fig. 4B) and MLC (Fig. 4C) in response to acute 18% CS (5–45 min) compared with cells transfected with nonspecific siRNA. In static EC culture, silencing of GEF-H1 did not significantly affect actin arrangement and levels of MLC and MYPT phosphorylation (data not shown). These data delineate involvement of GEF-H1 in the mechanisms of mechanotransduction.
Fig. 4.
Role of GEF-H1 in CS-induced cytoskeletal rearrangement. Human pulmonary EC were transfected with GEF-H1-specific or nonspecific siRNA. After 72 h of transfection, cells were subjected to 18% CS for various periods of time. A: cytoskeletal remodeling in control and stretched EC monolayers was analyzed by immunofluorescence staining for F-actin. Inset: Western blot analysis of GEF-H1 depletion in HPAEC. B and C: phosphorylation status of MYPT (B) or MLC (C) was determined by Western blot analysis in static and CS-exposed EC transfected with nonspecific or GEF-H1-specific siRNA.
GEF-H1 controls stretch-dependent MT dynamics in pulmonary EC.
We have previously reported a positive feedback mechanism of agonist-induced Rho activation and increased EC permeability via transient disassembly of MT network and release of MT-associated Rho-specific guanine nucleotide exchange factor GEF-H1 (10). To test the role of GEF-H1 in the regulation of MT stability by mechanical forces, endogenous GEF-H1 was depleted using specific siRNA, and MT structure in control and stretched pulmonary EC was analyzed by immunofluorescence staining with tubulin antibody. Similar to nontransfected cells (Fig. 3A), acute 18% CS (30 min) caused MT reorientation accompanied by MT disassembly in EC treated with nonspecific siRNA. In contrast, GEF-H1 depletion inhibited MT reorientation and prevented MT disassembly in EC exposed to 30-min CS (Fig. 5A). These data were further confirmed by quantitative image analysis of assembled MT (Fig. 5B). Prolonged CS treatment (2 h) showed restoration and alignment of MT network in both GEF-H1-depeleted and control cells (Fig. 5A). Analysis of stable MT pool by Western blot with acetylated tubulin antibody showed that GEF-H1 knockdown significantly attenuated decline in the levels of acetylated tubulin induced by 30-min CS (Fig. 5C). These findings support a critical role of GEF-H1 in CS-mediated regulation of MT organization.
Fig. 5.
Involvement of GEF-H1 in CS-mediated alterations of MT network. Pulmonary EC grown on Flexcell plates were treated with GEF-H1-specific or nonspecific siRNA for 72 h, followed by 18% CS exposure for 0, 30, or 120 min. A: MT structure was analyzed by immunofluorescence staining for β-tubulin. Arrows indicate main direction of CS vector. B: morphometric analysis of assembled MT. Data are expressed as means ± SD of 3 independent experiments; *P < 0.05. C: pools of stable MT were detected by Western blot for acetylated tubulin in samples from static and stretched EC transfected with nonspecific or GEF-H1-specific siRNA.
Microtubule stabilization attenuates VILI in vivo.
To link in vitro results with Rho-dependent signal transduction in vivo, we used a clinically relevant two-hit model of lung injury induced by HTV mechanical ventilation and TRAP6, the thrombin-derived nonthrombogenic peptide that serves as a PAR1 receptor ligand. Development of VILI in vivo is a complex process mediated by activation of several cell types (i.e., endothelium, neutrophils, macrophages, alveolar epithelium) by pathological mechanical stretch as well as by elevations of circulating proinflammatory cytokines and mediators (IL-8, IL-1β, coagulation proteins, etc.), and a two-hit (or even multiple-hit) model of VILI is a currently accepted model (39). Increased production of proinflammatory cytokines in animal models becomes evident after two and more hours of mechanical ventilation (30, 38) and remains elevated for several hours thereafter. Therefore, increased lung vascular barrier permeability is observed even after 4 h of HTV mechanical ventilation. Reversibility of CS- and thrombin-induced EC barrier dysfunction observed in cell cultures may be explained by inactivation of thrombin or receptor desensitization in vitro, whereas downregulation of pathological mediator signaling and synergistic effects of mechanical stretch in the inflamed lung requires a more prolonged period of time. Our recent report (14) demonstrates that TRAP6 alone at concentrations used in this study (1.5 × 10−5 mol/kg) has no effect on lung permeability. Higher TRAP6 doses (3 × 10−5 mol/kg) increased cell counts and protein concentration in the BAL fluid in spontaneously ventilated mice, but at the same time increased lethality in mice exposed to HTV ventilation. Thus, potentiation of ventilator-induced lung permeability by subthreshold concentrations of TRAP6 may adequately represent a two-hit model of VILI.
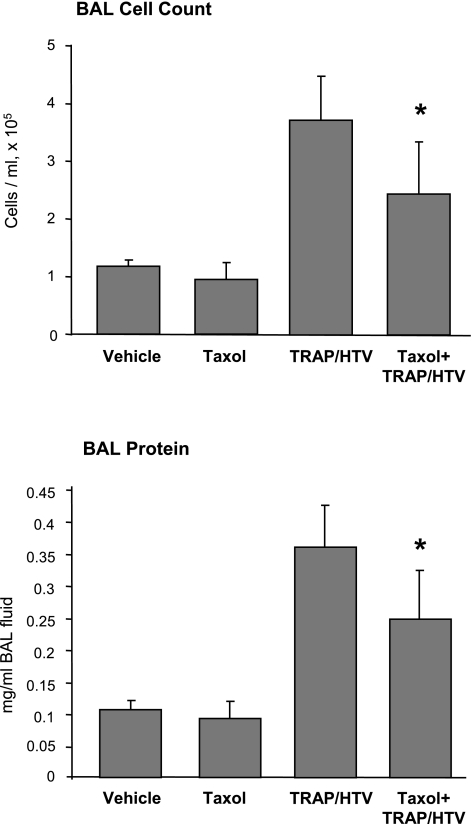
In the following experiments, we investigated effects of MT stabilization by taxol on parameters of lung injury in the TRAP6/HTV model. Mice were intravenously injected with vehicle or taxol before mechanical ventilation at HTV (30 ml/kg, 4 h) in the presence of TRAP6. At the end of experiments, BAL and tissue harvesting were performed as described in materials and methods. Taxol administration significantly reduced TRAP6/HTV-induced increases in BAL cell count and protein concentration (2.48 ± 0.87 × 105 cells/ml vs. 3.69 ± 0.73 × 105 cells/ml in TRAP6/HTV, P < 0.01; and 0.25 ± 0.08 mg/ml vs. 0.36 ± 0.07 mg/ml in TRAP6/HTV, P < 0.03, respectively) (Fig. 6). These data suggest a role for MT and MT-mediated signaling in the development of VILI.
Fig. 6.
Role of taxol in the development of ventilator-induced lung injury (VILI). Mice were treated with vehicle or taxol (3.75 × 10−7 mol/kg iv) before TRAP6 instillation (1.5 × 10−5 mol/kg it) and mechanical ventilation at HTV (30 ml/kg, 4 h). BAL cell count and protein concentration were determined as described in materials and methods. Data are expressed as means ± SD of 4 independent experiments; *P < 0.05.
GEF-H1 depletion attenuates VILI in vivo.
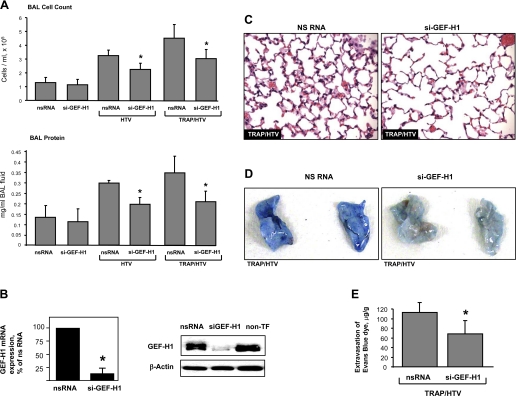
In the following experiments, we utilized an in vivo TRAP6/HTV model to address a role of MT-associated GEF-H1-mediated signaling in lung injury induced by mechanical ventilation. Using siRNA approach, we performed in vivo knockdown of GEF-H1. Mice were transfected with nonspecific or GEF-H1-specific siRNA for 72 h followed by mechanical ventilation at HTV with or without TRAP6 administration. In mice transfected with nonspecific RNA, HTV caused a prominent increase in BAL cell count and protein concentration (3.25 ± 0.39 × 105 cells/ml vs. 1.31 ± 0.41 × 105 cells/ml in control, P < 0.001; and 0.30 ± 0.01 mg/ml vs. 0.14 ± 0.06 mg/ml in control, P < 0.001, respectively) (Fig. 7A). In agreement with our previous data (14), TRAP6 instillation further promoted HTV-induced lung injury, detected by measurements of BAL cell count and protein concentration (4.49 ± 0.96 × 105 cells/ml in TRAP6/HTV vs. 3.25 ± 0.39 × 105 cells/ml in HTV alone, P < 0.01; and 0.35 ± 0.08 mg/ml in TRAP6/HTV vs. 0.30 ± 0.01 mg/ml in HTV alone, P < 0.04, respectively). Importantly, GEF-H1 depletion significantly reduced lung injury in both models, as detected by measurements of cell count and protein content levels in BAL fluid from HTV- and TRAP6/HTV-subjected animals (2.39 ± 0.35 × 105 cells/ml vs. 3.25 ± 0.39 × 105 cells/ml in HTV, P < 0.01; 3.01 ± 0.57 × 105 cells/ml vs. 4.49 ± 0.96 × 105 cells/ml in TRAP6/HTV, P < 0.01, for cell counts; and 0.20 ± 0.03 mg/ml vs. 0.30 ± 0.01 mg/ml in HTV, P < 0.01; 0.21 ± 0.05 mg/ml vs. 0.35 ± 0.08 mg/ml in TRAP6/HTV, P < 0.01, for protein concentration) (Fig. 7A). Knockdown of GEF-H1 was confirmed by qRT-PCR and by Western blot analysis of lung tissue (Fig. 7B).
Fig. 7.
Role of GEF-H1 in the development of VILI. Mice were transfected with nonspecific or GEF-H1-specific siRNA for 72 h followed by mechanical ventilation at HTV (30 ml/kg, 4 h) with or without TRAP6 instillation (1.5 × 10−5 mol/mouse, it). A: BAL cell count and protein concentration were determined as described in materials and methods. Data are expressed as means ± SD of 4 independent experiments; *P < 0.01. B: downregulation of GEF-H1 expression was confirmed by qRT-PCR and by Western blot analysis of lung tissue samples. C: whole lungs were fixed, embedded in paraffin, and used for histological evaluation by hematoxylin and eosin staining (n = 3–5 per group). D and E: effect of GEF-H1 knockdown on HTV-induced vascular leak was analyzed by Evans blue-labeled albumin extravasation into the lung tissue (D). The quantitative analysis of Evans blue-labeled albumin extravasation was performed by spectrophotometric analysis of Evans blue extracted from the lung tissue samples (E) (n = 4 per group; *P < 0.05).
Histological analysis of lung sections stained with hematoxylin and eosin revealed that in animals transfected with nonspecific RNA, TRAP6/HTV caused inflammatory cell infiltration in the lung parenchyma and alveolar hemorrhage, which indicate vascular barrier compromise. These effects were attenuated by GEF-H1 downregulation with siRNA (Fig. 7C).
The role of GEF-H1 in the development of VILI was further assessed by measurement of Evans blue leakage into the lung tissue. Consistent with our previous data (47), TRAP6/HTV induced Evans blue leakage from the vascular space into the lung parenchyma in mice transfected with nonspecific RNA. Importantly, TRAP6/HTV-induced Evans blue accumulation was significantly suppressed in the lungs from animals with depleted GEF-H1 (Fig. 7D). There were no significant histological changes and Evans blue tissue accumulation in the lungs from control mice treated with GEF-H1-specific siRNA compared with animals treated with nonspecific RNA (data not shown). Collectively, these data show the essential role of GEF-H1 signaling in the development of lung injury in the two-hit model of VILI.
DISCUSSION
ALI is a devastating clinical syndrome characterized by acute lung inflammation and vascular barrier disruption that affects >200,000 patients per year in the U.S. and leads to 30–50% mortality (1). Mechanical ventilation, particularly with HTV, can worsen or even cause de novo lung injury. Direct contribution of Rho signaling in the lung vascular barrier dysfunction has been shown in the animal models of ALI/VILI and in pulmonary endothelial cell monolayers exposed to pathological CS and edemagenic agonists in vitro (13, 26, 47, 61). In turn, attenuation of Rho signaling by barrier-protective oxidized phospholipids, sphingosine 1-phosphate, or prostacyclin analogs reduced lung endothelial hyperpermeability and improved indices of lung injury in the animal models of ALI/VILI (20, 40, 47, 61).
Similar to Rho activation by edemagenic and inflammatory mediators, applied mechanical strain transmitted from extracellular matrix to focal adhesions of vascular lung endothelial cells may also activate Rho (42), which via Rho kinase may coordinate the entire process of focal adhesion maturation and induce myosin II-driven tension (3) leading to increased lung vascular leak. Studies of cells exposed to mechanical forces in vitro show that mechanical strain or centripetal pulling of the cell by micropipette causes redistribution of focal adhesions, their elongation, and increases in size (55, 58). This process activates Rho GTPase leading to activation of Rho kinase-dependent actomyosin contraction (55) and signaling in the focal adhesions (45, 70). Although several intriguing hypotheses have been proposed to explain mechanisms of mechanosensing and stretch-induced Rho activation by focal adhesions (3, 57), they await further experimental validation.
The results of this study show that MT stabilization prevents stretch-induced transient gap formation (Fig. 2A, 15- and 30-min time points) and Rho kinase-dependent phosphorylation of MYPT1 in pulmonary EC, whereas combination of mechanical stretch and MT disassembly caused by nocodazole dramatically enhances EC barrier disruption via stimulation of Rho pathway of EC permeability. These data indicate the involvement of MT-associated factor(s) in CS-induced control of Rho signaling. Our previous studies show that partial MT destabilization by edemagenic agonists leads to release and activation of MT-associated Rho-specific guanine nucleotide exchange factor GEF-H1 (8). Because pathological CS also induced partial MT disassembly, we hypothesized that GEF-H1 may serve as a mechanotransducer involved in CS-induced Rho activation in endothelial cells. To test this hypothesis, we depleted GEF-H1 using siRNA approach. GEF-H1 knockdown prevented CS-induced activation of Rho pathway judged by reduced phospho-MYPT1 and phospho-MLC levels and markedly attenuated parameters of lung barrier dysfunction in the murine model of TRAP6/VILI.
Activation of Rho pathway leads to increased permeability, stress fiber formation, actomyosin contraction, and paracellular gap formation in LPS-stimulated endothelial cells and mediates lung barrier dysfunction in the animal models of LPS and VILI (13, 26, 61). A recent report by Mirzapoiazova et al. (43) showed that microtubule stabilization with taxol attenuated lung inflammation and vascular leak induced by intratracheal LPS instillation. Taxol pretreatment decreased LPS-induced infiltration of proteins and inflammatory cells into BAL fluid, reduced lung myeloperoxidase activity, and decreased extravasation of Evans blue-labeled albumin into lung tissue. Therefore, observed lung protection by taxol may be explained by the mechanism described in this study, i.e., MT stabilization and downregulation of GEF-H1 activity leading to reduction of Rho signaling.
This study shows that molecular inhibition of GEF-H1 attenuated TRAP6-independent lung barrier dysfunction caused by HTV alone. However, TRAP6 in the two-hit model of VILI may engage additional mechanisms leading to activation of Rho signaling and development of lung edema. A study by Jenkins et al. (33) showed that intratracheal instillation of the PAR1-specific peptide TFLLRN may increase lung edema during HTV ventilation and proposed additional mechanism of PAR1-mediated activation of αvβ6-integrins leading to activation of latent TGF-β. TGF-β activation is known to trigger Rho signaling and also causes partial destabilization of microtubules in pulmonary endothelial cells associated with increased permeability (14). Thus, GEF-H1-dependent microtubule destabilization caused by pathological mechanical stretch may overlap with MT perturbations associated with activation of TGF-β signaling, and attenuation of lung vascular permeability by microtubule stabilization or GEF-H1 depletion described in this study may target both mechanisms.
The exact mechanism of CS-induced MT disassembly remains unknown. Interestingly, step-wise tensile mechanical stress increases MT assembly in vascular smooth muscle cells, whereas compressive mechanical strain decreases MT assembly and induces Rho membrane localization indicative of Rho activation (53). CS stimulation of alveolar epithelial cell line A549 decreased the MT network but increased the acetylation state of remaining polymerized tubulin (29). Such increased acetylation of polymerized tubulin has been hypothesized to serve as protection of remaining MT network from further disassembly by continuing mechanical strain. In addition, initial disruption of EC monolayer integrity induced by CS may trigger mechanisms aimed at restoration of endothelial barrier. For example, disruption of EC junctions activates small GTPase Rap1, which then triggers Rac1 signaling leading to activation of cortical cytoskeleton and reannealing of adherens junctions (35). Interestingly, such Rac-mediated reparation mechanism may lead to inhibition of Rho activity by activating adherens junction-associated Rho inhibitor p190RhoGAP (68). In addition, Rac activation also contributes to stabilization of microtubule network (21), and, as a consequence, association of GEF-H1 with microtubules and GEF-H1 inactivation.
Our experiments show that microtubule stabilization by taxol decreases parameters of VILI. These data suggest a direct role of MT destabilization leading to GEF-H1 release and activation, in the HTV-induced lung vascular leak. However, we do not exclude that GEF-H1 activation by mechanical signals may be combined with GEF-H1 activation by other mechanisms. Prolonged mechanical ventilation at HTV stimulates the release of proinflammatory mediators stimulating lung inflammation, a condition defined as biotrauma (23). TNF-α is one inflammatory cytokine released as a result of lung overinflation (2) and has been recently identified as an activator of GEF-H1-mediated Rho signaling via Erk MAP kinase-dependent mechanism (34). Together, this study and published data demonstrate complexity of cellular mechanisms triggered by lung overinflation that are dictated by cross-talk between mechanotransduction pathways and signaling induced by spectrum of inflammatory mediators.
The results of this study and published data (4, 8, 36) show that pathological GEF-H1 signaling occurs under circumstances that either lead to MT depolymerization or interfere with ability of GEF-H1 to associate with microtubules. In this connection, GEF-H1 has been recently suggested as a potential drug target for diseases that involve aberrant Rho signaling and altered dynamics of microtubule cytoskeleton (4). Consideration of GEF-H1 drug targeting may be advantageous compared with direct pharmacological inhibitors of Rho or Rho-kinase activity, because pharmacological targeting of GEF-H1 will inhibit the aberrant or excessive Rho signaling resulting from pathological conditions associated with MT disruption, whereas basal Rho functions regulated by other mechanisms will remain unchanged. In addition to MT-dependent mechanism, GEF-H1 activity may be negatively regulated by Rac mechanism via PAK1- or PAK-4-mediated phosphorylation at Ser810 (22, 72). This mechanism may explain inhibition of Rho signaling by Rac-activating agents such as oxidized phospholipids, hepatocyte growth factor, or sphingosine 1-phosphate, which ultimately lead to barrier-protective effects in the models of ALI.
In summary, this study describes a novel mechanism of stretch-induced activation of barrier-disruptive Rho signaling by MT-associated Rho-specific guanine nucleotide exchange factor GEF-H1. Our results suggest beneficial effects of stabilization of MT network and GEF-H1 targeting to MT that may be considered as future therapies for the treatment of VILI and ARDS.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-087823 and HL-58064 (for Birukov) and Grant HL-089257, the American Heart Association Midwest Affiliate Grant-in-Aid, and the American Lung Association Biomedical Research Grant (for A. A. Birukova).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Belperio JA, Keane MP, Lynch JP, 3rd, Strieter RM. The role of cytokines during the pathogenesis of ventilator-associated and ventilator-induced lung injury. Semin Respir Crit Care Med 27: 350–364, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol 19: 677–695, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Birkenfeld J, Nalbant P, Yoon SH, Bokoch GM. Cellular functions of GEF-H1, a microtubule-regulated Rho-GEF: is altered GEF-H1 activity a crucial determinant of disease pathogenesis? Trends Cell Biol 18: 210–219, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Birukov KG, Bochkov VN, Birukova AA, Kawkitinarong K, Rios A, Leitner A, Verin AD, Bokoch GM, Leitinger N, Garcia JG. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ Res 95: 892–901, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Birukov KG, Jacobson JR, Flores AA, Ye SQ, Birukova AA, Verin AD, Garcia JG. Magnitude-dependent regulation of pulmonary endothelial cell barrier function by cyclic stretch. Am J Physiol Lung Cell Mol Physiol 285: L785–L797, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Birukova AA, Adyshev D, Gorshkov B, Birukov KG, Verin AD. ALK5 and Smad4 are involved in TGF-beta1-induced pulmonary endothelial permeability. FEBS Lett 579: 4031–4037, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Birukova AA, Adyshev D, Gorshkov B, Bokoch GM, Birukov KG, Verin AA. GEF-H1 is involved in agonist-induced human pulmonary endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol 290: L540–L548, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Birukova AA, Birukov KG, Adyshev D, Usatyuk P, Natarajan V, Garcia JG, Verin AD. Involvement of microtubules and Rho pathway in TGF-beta1-induced lung vascular barrier dysfunction. J Cell Physiol 204: 934–947, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Birukova AA, Birukov KG, Smurova K, Adyshev DM, Kaibuchi K, Alieva I, Garcia JG, Verin AD. Novel role of microtubules in thrombin-induced endothelial barrier dysfunction. FASEB J 18: 1879–1890, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Birukova AA, Chatchavalvanich S, Rios A, Kawkitinarong K, Garcia JG, Birukov KG. Differential regulation of pulmonary endothelial monolayer integrity by varying degrees of cyclic stretch. Am J Pathol 168: 1749–1761, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birukova AA, Cokic I, Moldobaeva N, Birukov KG. Paxillin is involved in the differential regulation of endothelial barrier by HGF and VEGF. Am J Respir Cell Mol Biol 40: 99–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birukova AA, Fu P, Chatchavalvanich S, Burdette D, Oskolkova O, Bochkov VN, Birukov KG. Polar head groups are important for barrier-protective effects of oxidized phospholipids on pulmonary endothelium. Am J Physiol Lung Cell Mol Physiol 292: L924–L935, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Birukova AA, Fu P, Xing J, Cokic I, Birukov KG. Lung endothelial barrier protection by iloprost in the 2-hit models of ventilator-induced lung injury (VILI) involves inhibition of Rho signaling. Transl Res 155: 44–54, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birukova AA, Liu F, Garcia JG, Verin AD. Protein kinase A attenuates endothelial cell barrier dysfunction induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 287: L86–L93, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Birukova AA, Malyukova I, Mikaelyan A, Fu P, Birukov KG. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J Cell Physiol 211: 608–617, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol 295: L612–L623, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birukova AA, Smurova K, Birukov KG, Kaibuchi K, Garcia JGN, Verin AD. Role of Rho GTPases in thrombin-induced lung vascular endothelial cells barrier dysfunction. Microvasc Res 67: 64–77, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Birukova AA, Smurova K, Birukov KG, Usatyuk P, Liu F, Kaibuchi K, Ricks-Cord A, Natarajan V, Alieva I, Garcia JG, Verin AD. Microtubule disassembly induces cytoskeletal remodeling and lung vascular barrier dysfunction: role of Rho-dependent mechanisms. J Cell Physiol 201: 55–70, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Birukova AA, Zagranichnaya T, Alekseeva E, Fu P, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE2 and PGI2 promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res 313: 2504–2520, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem 72: 743–781, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 118: 1861–1872, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Dos Santos CC, Slutsky AS. Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89: 1645–1655, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct 21: 317–326, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Essler M, Staddon JM, Weber PC, Aepfelbacher M. Cyclic AMP blocks bacterial lipopolysaccharide-induced myosin light chain phosphorylation in endothelial cells through inhibition of Rho/Rho kinase signaling. J Immunol 164: 6543–6549, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Fu P, Birukova AA, Xing J, Sammani S, Murley JS, Garcia JG, Grdina DJ, Birukov KG. Amifostine reduces lung vascular permeability via suppression of inflammatory signalling. Eur Respir J 33: 612–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 22: 32–39, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Geiger RC, Kaufman CD, Lam AP, Budinger GR, Dean DA. Tubulin acetylation and histone deacetylase 6 activity in the lung under cyclic load. Am J Respir Cell Mol Biol 40: 76–82, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman RB, Pugin J, Lee JS, Matthay MA. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev 14: 523–535, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Hammerschmidt S, Kuhn H, Grasenack T, Gessner C, Wirtz H. Apoptosis and necrosis induced by cyclic mechanical stretching in alveolar type II cells. Am J Respir Cell Mol Biol 30: 396–402, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Jafari B, Ouyang B, Li LF, Hales CA, Quinn DA. Intracellular glutathione in stretch-induced cytokine release from alveolar type-2 like cells. Respirology 9: 43–53, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Jenkins RG, Su X, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis GE, Chambers RC, Matthay MA, Sheppard D. Ligation of protease-activated receptor 1 enhances alpha(v)beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest 116: 1606–1614, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem 284: 11454–11466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci 120: 17–22, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol 4: 294–301, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Lum H, Malik AB. Mechanisms of increased endothelial permeability. Can J Physiol Pharmacol 74: 787–800, 1996 [DOI] [PubMed] [Google Scholar]
- 38.Matthay MA, Bhattacharya S, Gaver D, Ware LB, Lim LH, Syrkina O, Eyal F, Hubmayr R. Ventilator-induced lung injury: in vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol 283: L678–L682, 2002 [DOI] [PubMed] [Google Scholar]
- 39.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167: 1027–1035, 2003 [DOI] [PubMed] [Google Scholar]
- 40.McVerry BJ, Garcia JG. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell Signal 17: 131–139, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279–367, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, Puzon-McLaughlin W, Tarui T, Shyy JY, Takada Y, Usami S, Chien S. Differential regulation of Rho GTPases by beta1 and beta3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci 115: 2199–2206, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Mirzapoiazova T, Kolosova IA, Moreno L, Sammani S, Garcia JG, Verin AD. Suppression of endotoxin-induced inflammation by taxol. Eur Respir J 30: 429–435, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Moitra J, Sammani S, Garcia JG. Re-evaluation of Evans Blue dye as a marker of albumin clearance in murine models of acute lung injury. Transl Res 150: 253–265, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. Pp125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene 17: 455–463, 1998 [DOI] [PubMed] [Google Scholar]
- 46.Nogales E. Structural insights into microtubule function. Annu Rev Biochem 69: 277–302, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Nonas S, Birukova AA, Fu P, Xing J, Chatchavalvanich S, Bochkov VN, Leitinger N, Garcia JG, Birukov KG. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit Care 12: R27, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palazzo AF, Cook TA, Alberts AS, Gundersen GG. mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat Cell Biol 3: 723–729, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Palazzo AF, Eng CH, Schlaepfer DD, Marcantonio EE, Gundersen GG. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science 303: 836–839, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Petrache I, Birukova A, Ramirez SI, Garcia JG, Verin AD. The role of the microtubules in tumor necrosis factor-alpha-induced endothelial cell permeability. Am J Respir Cell Mol Biol 28: 574–581, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Pimentel DR, Amin JK, Xiao L, Miller T, Viereck J, Oliver-Krasinski J, Baliga R, Wang J, Siwik DA, Singh K, Pagano P, Colucci WS, Sawyer DB. Reactive oxygen species mediate amplitude-dependent hypertrophic and apoptotic responses to mechanical stretch in cardiac myocytes. Circ Res 89: 453–460, 2001 [DOI] [PubMed] [Google Scholar]
- 52.Piperno G, LeDizet M, Chang XJ. Microtubules containing acetylated alpha-tubulin in mammalian cells in culture. J Cell Biol 104: 289–302, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Putnam AJ, Cunningham JJ, Pillemer BB, Mooney DJ. External mechanical strain regulates membrane targeting of Rho GTPases by controlling the state of microtubule polymerization. Am J Physiol Cell Physiol 284: C627–C639, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Ren Y, Li R, Zheng Y, Busch H. Cloning and characterization of GEF-H1, a microtubule-associated guanine nucleotide exchange factor for Rac and Rho GTPases. J Biol Chem 273: 34954–34960, 1998 [DOI] [PubMed] [Google Scholar]
- 55.Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol 153: 1175–1186, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchez-Esteban J, Wang Y, Cicchiello LA, Rubin LP. Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 282: L448–L456, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Schwartz MA, Desimone DW. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol 20: 551–556, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shikata Y, Rios A, Kawkitinarong K, DePaola N, Garcia JG, Birukov KG. Differential effects of shear stress and cyclic stretch on focal adhesion remodeling, site-specific FAK phosphorylation, and small GTPases in human lung endothelial cells. Exp Cell Res 304: 40–49, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Singleton PA, Chatchavalvanich S, Fu P, Xing J, Birukova AA, Fortune JA, Klibanov AM, Garcia JG, Birukov KG. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ Res 104: 978–986, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tar K, Birukova AA, Csortos C, Bako E, Garcia JG, Verin AD. Phosphatase 2A is involved in endothelial cell microtubule remodeling and barrier regulation. J Cell Biochem 92: 534–546, 2004 [DOI] [PubMed] [Google Scholar]
- 61.Tasaka S, Koh H, Yamada W, Shimizu M, Ogawa Y, Hasegawa N, Yamaguchi K, Ishii Y, Richer SE, Doerschuk CM, Ishizaka A. Attenuation of endotoxin-induced acute lung injury by the Rho-associated kinase inhibitor, Y-27632. Am J Respir Cell Mol Biol 32: 504–510, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Thomas M, Ge Q, Lu JJ, Chen J, Klibanov AM. Cross-linked small polyethylenimines: while still nontoxic, deliver DNA efficiently to mammalian cells in vitro and in vivo. Pharm Res 22: 373–380, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tschumperlin DJ, Margulies SS. Alveolar epithelial surface area-volume relationship in isolated rat lungs. J Appl Physiol 86: 2026–2033, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol 282: L892–L896, 2002 [DOI] [PubMed] [Google Scholar]
- 65.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000 [DOI] [PubMed] [Google Scholar]
- 66.Verin AD, Birukova A, Wang P, Liu F, Becker P, Birukov K, Garcia JG. Microtubule disassembly increases endothelial cell barrier dysfunction: role of MLC phosphorylation. Am J Physiol Lung Cell Mol Physiol 281: L565–L574, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Vouret-Craviari V, Boquet P, Pouyssegur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell 9: 2639–2653, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127: 1027–1039, 2006 [DOI] [PubMed] [Google Scholar]
- 69.Yang G, Im HJ, Wang JH. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363: 166–172, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yano Y, Saito Y, Narumiya S, Sumpio BE. Involvement of rho p21 in cyclic strain-induced tyrosine phosphorylation of focal adhesion kinase (pp125FAK), morphological changes and migration of endothelial cells. Biochem Biophys Res Commun 224: 508–515, 1996 [DOI] [PubMed] [Google Scholar]
- 71.Yoshigi M, Clark EB, Yost HJ. Quantification of stretch-induced cytoskeletal remodeling in vascular endothelial cells by image processing. Cytometry A 55: 109–118, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Zenke FT, Krendel M, DerMardirossian C, King CC, Bohl BP, Bokoch GM. p21-activated kinase 1 phosphorylates and regulates 14–3-3 binding to GEF-H1, a microtubule-localized Rho exchange factor. J Biol Chem 279: 18392–18400, 2004 [DOI] [PubMed] [Google Scholar]
- 73.Zhang D, Wang Z, Jin N, Li L, Rhoades RA, Yancey KW, Swartz DR. Microtubule disruption modulates the Rho-kinase pathway in vascular smooth muscle. J Muscle Res Cell Motil 22: 193–200, 2001. [DOI] [PubMed] [Google Scholar]