Abstract
Heme oxygenase (HO)-1 is a cytoprotective enzyme with anti-inflammatory properties. HO-1 is induced during a systemic inflammatory response, and expression of HO-1 is beneficial during sepsis of a Gram-positive source. Systemic infection from Gram-positive organisms has emerged as an important cause of sepsis, with Staphylococcus aureus as a common etiology. An important mediator of Gram-positive infections is peptidoglycan (PGN), a cell wall component of these organisms. Here, we demonstrate that HO-1 played an important, protective role in vivo, as mice deficient in HO-1 were very sensitive to the lethal effects of PGN derived from S. aureus. PGN induced HO-1 protein and mRNA levels, and this regulation occurred at the level of gene transcription. The PGN-responsive region of the HO-1 promoter (from −117 to −66 bp) contains a functional EBS, and Ets proteins are known to be involved in the regulation of inflammatory responses. We showed previously that Ets factors (activators Ets-2 and Ets-1 and repressor Elk-3) regulate HO-1 expression by Gram-negative endotoxin. However, during exposure to a Gram-positive stimulus in the present study, Elk-1 was a potent activator of HO-1 in conjunction with PGN. The ability of Elk-1 to induce HO-1 promoter activity was independent of direct DNA binding, but rather occurred by interacting with the CCAAT/enhancer-binding protein-α (C/EBPα), which binds to DNA. Moreover, silencing of C/EBPα in macrophages prevented induction of HO-1 promoter activity by either Elk-1 or PGN. These data provide further insight into the regulation and function of HO-1 by a mediator of Gram-positive bacteria.
Keywords: inflammatory response, Gram-positive, macrophage, gene regulation, transcription
heme oxygenase (HO) is the rate-limiting enzyme in heme degradation that produces equimolar concentrations of carbon monoxide (CO), biliverdin, and ferrous iron (38, 39). Biliverdin is subsequently converted into bilirubin by biliverdin reductase (33, 35). Three different isoforms of HO have been described, and HO-1 is a very inducible isoform in response to many pathophysiological stimuli, including proinflammatory mediators (2). HO-1 and its products, CO and bilirubin, have anti-inflammatory and antioxidant properties, respectively (1, 24, 31, 32). These properties are critical during the inflammatory response of LPS exposure from Gram-negative bacteria, as underscored by our (44) previous work showing increased mortality, end-organ damage, and oxidative stress in HO-1 null (HO-1−/−) mice compared with wild-type (HO-1+/+) mice during endotoxemia. We (13) have also demonstrated that vascular overexpression of HO-1 decreases mortality due to a Gram-positive Enterococcus faecalis infection during abdominal sepsis. The increased HO-1 expression and subsequent production of CO increased bacterial phagocytosis and the endogenous antimicrobial response to this Gram-positive organism (13). The improved phagocytic response by HO-1 was dependent on nucleotide-binding oligomerization domain (NOD)-2 expression.
Peptidoglycan (PGN), a conserved component of the cell wall of Gram-positive bacteria, is a polymer constituted of glycan strands of N-acetylglucosamine and N-acetylmuramic acid that are cross-linked by peptide bridges (16, 22). PGN is released into the circulation on lysis of the bacterial cell wall, and PGN from Staphylococcal species has been shown to stimulate the production of proinflammatory cytokines (26) and chemokines via pathogen recognition receptors in macrophages (16, 17, 19, 34). Furthermore, Staphylococcus aureus PGN is capable of inducing a systemic proinflammatory response and organ dysfunction in rodents (42). PGN in conjunction with lipoteichoic acid (LTA; another cell wall component of Gram-positive bacteria) has also been used to produce a sepsis-like syndrome (14, 40, 41). However, when administered alone, a higher concentration of PGN is required to elicit a response similar to LPS, as a small fraction of naïve PGN is degraded into complex branched stem peptides, which contain the inflammatory properties (25). Because of the beneficial effects of HO-1 in Gram-positive infection in our (13) previous study, and the fact that little is known about the production of anti-inflammatory mediators after PGN stimulation, we sought to investigate the regulation and function of HO-1 induced by PGN from a Gram-positive bacterial source, S. aureus.
The Ets proteins are a family of transcription factors that are known to be involved with inflammatory responses or to interact with transcription factors activated by inflammatory responses (3, 45). The majority of Ets family members bind to GGAA/T-containing DNA elements as monomeric proteins; however, some Ets factors regulate the transcriptional activity of adjacent cis-acting elem ents, for example the interaction of Ets-1 and activator protein-1 (AP-1) proteins on enhancer elements containing adjacent Ets and AP-1 binding sites (6). Recently, we (11) demonstrated that positive transactivators such as Ets-2 and, to a lesser extent, Ets-1 contribute to the LPS induction of HO-1 in macrophages via an Ets binding site, EBS2, located in the proximal promoter of mouse HO-1. Moreover, LPS-induced downregulation of the repressor Elk-3, which belongs to the ternary complex factors (TCF) subfamily of Ets factors, facilitates HO-1 induction in macrophages via a different Ets binding site, EBS1, just upstream of EBS2 (12). Because of the importance of these two EBS in the proximal HO-1 promoter during LPS stimulation, we also wanted to elucidate the potential role of Ets family members and their associated binding sites in driving HO-1 transcription by PGN of a Gram-positive bacterial source.
MATERIALS AND METHODS
Cell culture and reagents.
Murine macrophages (RAW 264.7) (15) and fibroblasts (NIH/3T3) were grown according to the recommendations of American Type Culture Collection. PGN from S. aureus was purchased from Sigma-Aldrich. PGN is dissolved in 50% DMSO and sonicated (Model 500 Digital Sonic Dismembrator; Fisher Scientific) before use.
Mouse model of a systemic Gram-positive stimulus.
HO-1−/− mice were generated as described previously (47). Female mice were used for these studies, and all mice were on a pure BALB/c genetic background. HO-1−/− and HO-1+/+ mice were injected intraperitoneally with PGN (20 mg/kg). Survival was assessed every 8 h for 7 days. Spleens, kidneys, and lungs were harvested at baseline and after 6, 12, and 24 h of PGN stimulation. All PGN experiments in mice were performed in accordance with National Institutes of Health (NIH) guidelines and were approved by Harvard Medical Area standing committee on animals.
RNA isolation and Northern blot and real-time PCR analyses.
Extraction of total RNA from cultured cells and mouse tissues was performed using the RNeasy Mini RNA isolation kit (Qiagen). Northern blot analysis was performed as described previously (11), using a random-primed, [α-32P]dCTP-labeled HO-1 cDNA probe. To correct for the differences in RNA loading, blots were subsequently hybridized to a 32P-labeled oligonucleotide probe complementary to 18S rRNA. Radioactivity was quantitated on a Phosphorimager using ImageQuant software (Molecular Dynamics). Real-time PCR was performed as described previously (5, 13). PCR primers for mouse HO-1 (NM_010442) were designed from 681 to 700 bp (5′-tgctcgaatgaacactctgg-3′) and 803 to 784 bp (5′-tcctctgtcagcatcacctg-3′).
Luciferase reporter constructs and expression plasmids.
The luciferase reporter-promoter plasmids of HO-1 were generated by subcloning these fragments into the pGL2-Basic Vector (Promega) as previously described (11, 12, 28). Expression plasmids pCI-Ets-1, pCI-Ets-2, pCI-Elk-3, and pCI-NERF2 were a generous gift from Dr. Peter Oettgen (Beth Israel Deaconess Medical Center, Boston, MA), and pcDNA3-Elk-1 was a gift from Dr. F. M. Stanley (New York University School of Medicine, New York, NY). mEBS1 and mEBS2 (mutants of EBS at −125 and −93 bp, respectively) in HO-1 (−295/+74) plasmid were generated as previously described (11, 12). pCI-CCAAT/enhancer-binding protein-α (pCI-C/EBPα), pCI-C/EBPβ, and pCI-C/EBPδ were a gift from Dr. Mark Feinberg (Brigham and Women's Hospital, Boston, MA).
Site-directed mutagenesis.
Mutant C/EBP binding site (mC/EBP) at −90 bp was generated by site-directed mutagenesis of the HO-1 (−295/+74) plasmid using Pfu polymerase (Stratagene). PCR primers encoding mC/EBP binding site, −82 to −90, were generated with TGTTcCcAC substituted for TGTTGCAAC in mC/EBP (5′-CTCCGGGCTGGATGTTCCCACAGCAGCGAGAAC-3′ and 5′-GTTCTCGCTGCTGTGGGAACATCCAGCCCGGAG-3′). Mutated sequences are underlined. The PCR products were digested with DpnI, and the undigested plasmids were transformed into XL2-Blue bacteria (Stratagene). Individual plasmids were sequenced to verify incorporation of the C/EBP site mutation. Deletion of Elk-1 DNA binding domain (Elk-1 ΔDBD), from the 2nd to the 93rd amino acid, was performed by site-directed mutagenesis of the Elk-1 plasmid, the same method as described above. In brief, PCR primers encoding upstream and downstream sequences of the deletion region were generated (5′-CTCAAACAGACACCATGTGCTCCACTGAGGACTG-3′ and 5′-cagtcctcagtggagcacatggtgtctgtttgag-3′). Single underline indicates 1st amino acid sequence, and double underline indicates 94th amino acid sequence.
Transient transfection and reporter activity assays.
Transient transfection assays were performed using FuGENE 6 transfection reagent (Roche Applied Science) as described previously (11, 12). The HO-1 promoter-reporter plasmid (200 ng/well) and the indicated amounts of Ets factors, C/EBP factors, and empty vector were cotransfected into murine macrophages. For assays in RAW 264.7 cells, 3 × 105 cells/well were plated in triplicate on 6-well plates and incubated for 24 h. Twenty-four hours later, vehicle or PGN (1 μg/ml) was administered. The cells were harvested for luciferase activity using the Luciferase Assay System (Promega) 24 h after treatment. Luciferase activity was measured in a Wallace Victor3 1420 multilabel counter (PerkinElmer).
Silencing of C/EBPα in mouse macrophages.
Short hairpin RNA (shRNA) plasmids for C/EBPα (Clone NM_007678.1-898s1c1; Sigma-Aldrich) and vector control (pLKO.1-puro) were transfected into RAW 264.7 cells, and stable clones were selected using puromycin (5 μg/ml) resistance. Silencing of C/EBPα was confirmed by Western blot analysis. Clones silencing C/EBPα and control vector were subsequently transfected with a HO-1 promoter-reporter construct and an expression plasmid for Elk-1 or stimulated with PGN as described for the reporter activity assays.
EMSA.
EMSA were performed as described previously (8), using nuclear extracts from RAW 264.7 cells stimulated with vehicle or PGN (1 μg/ml) for 1 h. Double-stranded oligonucleotide probes encoding region −98 to −74 of the HO-1 5′-flanking sequence, C/EBP probe (5′-GGGCTGGATGTTGCAACAGCAGCGA-3′; the C/EBP binding site is underlined) and mC/EBP probe (5′-GGGCTGGATGTTcCcACAGCAGCGA-3′), were used. The probes were end-labeled with [γ-32P]ATP using T4 polynucleotide kinase (New England Biolabs). Labeled DNA (∼50,000 counts/min) was used in each binding reaction. In cold competition assays, a 100-fold molar excess of unlabeled double-stranded oligonucleotides as indicated were added to the binding reactions. For mobility supershift experiments, 1 μg of C/EBPα rabbit polyclonal antibody (Santa Cruz Biotechnology) was added to the binding reaction and incubated at room temperature for 2 h before the addition of radioactive probe.
Total protein isolation and Western blot analysis.
Protein isolation and Western blotting were performed as described previously (11). Rabbit polyclonal anti-HO-1 (Stressgen Biotechnologies) antibody was diluted to 1:2,000 before use. Rabbit polyclonal anti-Elk-1 and anti-C/EBPα antibodies (Santa Cruz Biotechnology) were diluted to 1:1,000 before use. The blots were exposed to X-ray film and evaluated with NIH ImageJ software.
Coimmunoprecipitation assays.
Protein G Plus/Protein A Agarose (Calbiochem) beads were washed by PBS and resuspended with dilution buffer (PBS + 1 mg/ml BSA). Elk-1 antibody or C/EBPα antibody, 5 μg in dilution buffer, was added at 1:1 ratio to the beads. Dimethyl pimelimidate (1 mg/ml) was added to prevent IgG binding to the beads. The beads were agitated for 30 min at room temperature and then washed three times with buffer (0.2 M triethanolamine in PBS) before quenching the cross-linking with 50 mM ethanolamine in PBS. Finally, unlinked antibody was washed out with 1 M glycine, pH 3, and the cross-linked beads were reconstituted in PBS.
RAW 264.7 cells were treated with vehicle or PGN (1 μg/ml). Cells were harvested 1 h after treatment, and the nuclear protein extract was isolated with lysis buffer [20 mM Tris·HCl, pH 7.5, 25% sucrose, 420 mM NaCl, 5 mM dithiothreitol, 2 mM MgCl2, 0.2 mM EDTA, 1× protease inhibitor mixture (Roche Applied Science)]. Lysate proteins (400 μg) were incubated with the antibody-cross-linked beads at 4°C overnight. The beads were then washed four times with a cold buffer (20 mM Tris·HCl, pH 7.2, 1 mM EDTA, 0.1% Triton X-100, 150 mM NaCl, 1 mg/ml BSA, and 1× protease inhibitor mixture), and bounded proteins were separated via gradient SDS-PAGE gel (4–20%) and transferred to nitrocellulose membranes for Western blot analysis (48).
Statistics.
Comparisons between groups where indicated were made by factorial ANOVA followed by Fisher protected least significant differences test. Comparisons of mortality were made by analyzing Kaplan-Meier survival curves and then log-rank test to assess for differences in survival. Statistical significance is accepted at P < 0.05.
RESULTS
Endogenous HO-1 is induced by PGN, and absence of HO-1 increases mortality during PGN-induced sepsis-like syndrome.
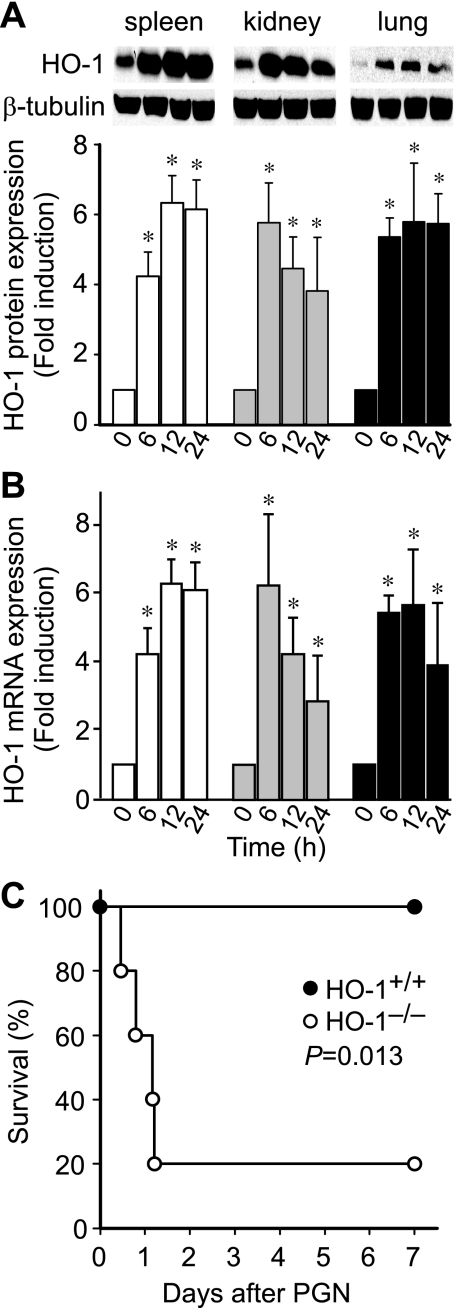
To determine whether the expression of HO-1 is regulated by PGN in vivo, we administered PGN (20 mg/kg ip) to wild-type mice and assessed HO-1 protein expression in spleen, kidney, and lung tissue after 0, 6, 12, and 24 h. Western blot analysis revealed increased HO-1 protein expression at 6 h, and protein levels of HO-1 remained elevated throughout 24 h in all three organs (Fig. 1A). HO-1 mRNA levels had a similar pattern (Fig. 1B). Next, to assess the functional significance of this HO-1 expression, we assessed survival of HO-1+/+ and HO-1−/− mice after administration of PGN (20 mg/kg ip). HO-1−/− mice had a very high mortality rate when exposed to PGN, reaching 80% by day 2. In contrast, this dose of PGN was not lethal in HO-1+/+ mice (Fig. 1C). These data suggest that expression of endogenous HO-1 plays a vital role in protecting mice from the lethal consequences of systemic PGN administration.
Fig. 1.
Absence of heme oxygenase (HO)-1 during peptidoglycan (PGN)-induced sepsis-like syndrome leads to increased mortality in mice. HO-1+/+ mice (n = 12) were injected intraperitoneally with PGN (20 mg/kg). The mice were killed 0, 6, 12, and 24 h after PGN administration, and protein (A) and RNA (B) were extracted from the spleen (open bars), kidney (gray filled bars), and lung (black filled bars) tissues. Western blot analyses were performed using anti-HO-1 and anti-β-tubulin antibodies, and Northern blot analyses were performed using 32P-labeled HO-1 and 18S rRNA probes, as described in materials and methods. Experiments in A and B were performed 3 independent times. *P < 0.005 vs. matched 0 h of each organ. C: HO-1+/+ (n = 6; filled circles) and HO-1−/− (n = 5; open circles) mice were injected intraperitoneally with PGN (20 mg/kg). Mortality was recorded every 8 h for 7 days. P = 0.013.
Induction of HO-1 by PGN in macrophages in vitro.
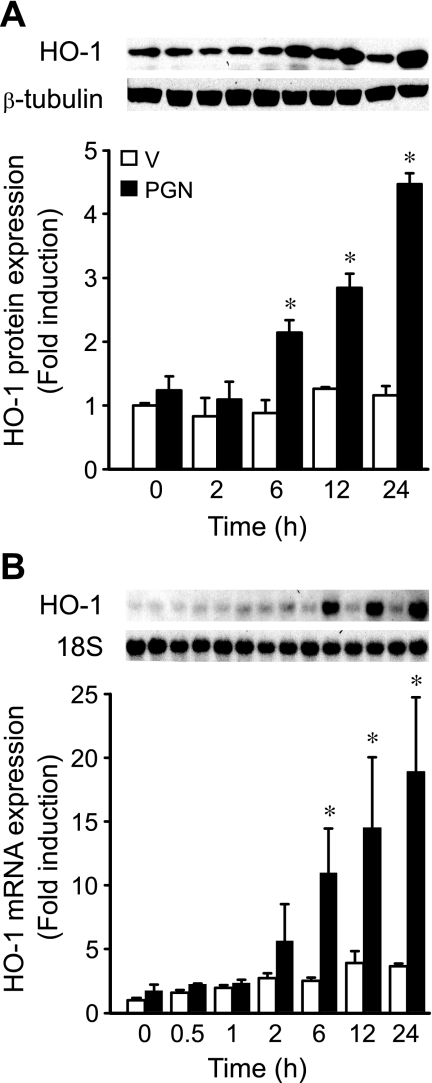
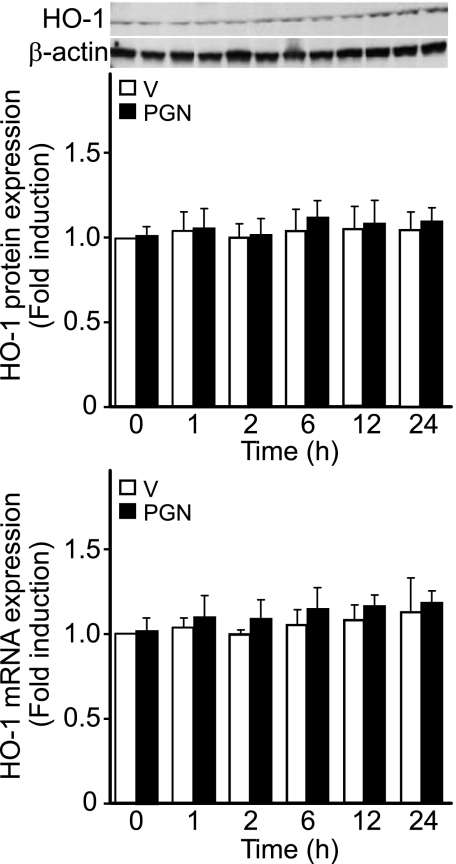
Macrophages are important cellular mediators during sepsis and endotoxemia (21), and these cells have also been widely used in the study of PGN response (45). Thus we wanted to determine whether PGN would induce HO-1 expression in macrophages. We found that PGN increased HO-1 protein and mRNA levels in a dose-dependent manner (with a dose as low as 0.5 μg/ml and up to 5 μg/ml, data not shown) in mouse macrophages (RAW 264.7 cells). A dose of 1 μg/ml PGN was chosen for further experiments. To determine the temporal expression pattern of HO-1 during PGN exposure, we administered 1 μg/ml PGN to RAW 264.7 cells and harvested protein and total RNA at various time points, including 0, 0.5, 1, 2, 6, 12, and 24 h. Similar to the in vivo analysis of HO-1 in tissues, Western blot analysis revealed that protein levels of HO-1 increased by 6 h and remained elevated throughout 24 h. HO-1 induction was 2.1-, 2.8-, and 4.5-fold at 6, 12, and 24 h, respectively, compared with 0 h (P < 0.05; Fig. 2A). By Northern blot analysis, HO-1 mRNA began to increase slightly by 2 h, but the levels of mRNA were significantly increased by 6, 12, and 24 h, with 11.0-, 14.5-, and 18.9-fold increases, respectively, compared with 0 h (P < 0.05; Fig. 2B). We also stimulated another cell type, fibroblasts (NIH/3T3 cells), with a comparable dose of PGN (1 μg/ml) and found no significant induction of HO-1 protein or mRNA (Fig. 3) in contrast to macrophages. Other investigators have shown in fibroblasts (from the synovium of rheumatoid arthritis patients, thus already in an inflammatory environment) that much higher doses of PGN (a minimum of 10 μg/ml and up to 30–100 μg/ml) are required to further induce the immune mediator IL-6 (9). These data suggest that HO-1 induction in macrophages is more sensitive to PGN stimulation, as demonstrated by the HO-1 response in macrophages compared with fibroblasts.
Fig. 2.
Induction of HO-1 protein and mRNA by PGN in macrophages. A: mouse macrophages (RAW 264.7) were treated with vehicle (V; open bars) or PGN (1 μg/ml; filled bars). Protein was extracted 0, 2, 6, 12, and 24 h after vehicle or PGN administration, and Western blot analyses were performed as described in materials and methods, using anti-HO-1 and anti-β-tubulin antibodies. B: mouse macrophages were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars). Total RNA was extracted 0, 0.5, 1, 2, 6, 12, and 24 h after vehicle or PGN administration, and Northern blot analyses were performed as described in materials and methods, using 32P-labeled HO-1 and 18S rRNA probes. Experiments in A and B were performed 3 independent times. *P < 0.05 vs. time-matched vehicle.
Fig. 3.
Response of HO-1 protein and mRNA to PGN stimulation in fibroblasts. Top: mouse fibroblasts (NIH/3T3 cells) were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars). Protein was extracted 0, 1, 2, 6, 12, and 24 h after vehicle or PGN administration, and Western blot analyses were performed as described in materials and methods, using anti-HO-1 and anti-β-actin antibodies. Bottom: mouse fibroblasts were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars). Total RNA was extracted 0, 1, 2, 6, 12, and 24 h after vehicle or PGN administration, and real-time PCR analyses were performed as described in materials and methods. Experiments in top and bottom were performed 2 independent times.
PGN response element(s) are between −117 and −66 bp of the HO-1 promoter.
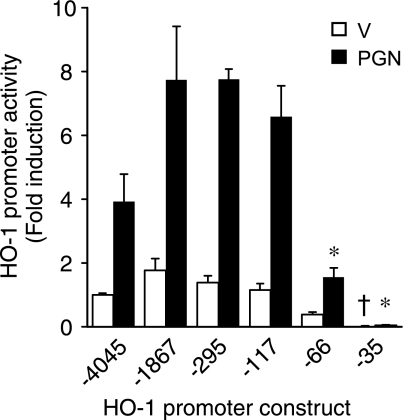
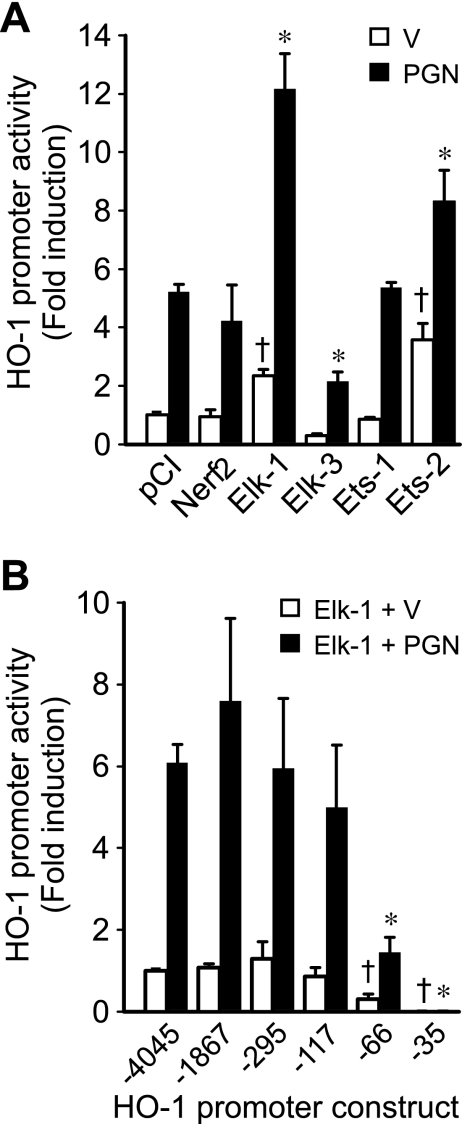
HO-1 is well-known to be regulated at the level of gene transcription (2). Thus, to determine the location of the PGN response element(s) in the HO-1 promoter, we made deletion mutants of construct HO-1 (−4,045/+74) and transiently transfected these constructs into RAW 264.7 cells exposed to vehicle or PGN (1 μg/ml). In the absence of PGN (Fig. 3, open bars), promoter activity began to decrease in construct HO-1 (−66/+74), but a significant and marked drop in activity did not occur until HO-1 (−35/+74) compared with all other constructs (P < 0.05; Fig. 4). As expected, HO-1 promoter activity (−4,045/+74) was significantly induced by PGN (Fig. 4, filled bars). However, different from the vehicle experiments, the most dramatic decrease in PGN-induced promoter activity (P < 0.05; Fig. 4) occurred with deletion to construct HO-1 (−66/+74). These results suggest that critical cis-acting element(s) responsible for HO-1 induction by PGN in macrophages are located between −117 and −66 bp of the HO-1 promoter.
Fig. 4.
HO-1 promoter has PGN-responsive element(s) downstream of −117 bp. Cells were transiently transfected with deletion mutants of the HO-1 promoter (200 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. HO-1 promoter activity was calculated as fold induction compared with the activity of construct HO-1 (−4,045/+74) exposed to vehicle. Four independent experiments were performed in triplicate. *P < 0.05 vs. activity of HO-1 (−4,045/+74), (−1,867/+74), (−295/+74), and (−117/+74) promoter constructs stimulated with PGN. †P < 0.05 vs. all other constructs exposed to vehicle.
Elk-1 is an activator of the HO-1 promoter.
We (11) have previously shown that the EBS2 site (−93 bp) is important for induction of HO-1 by LPS stimulation. Thus we hypothesized that Ets transcription factors may also be important for regulation of HO-1 by PGN. To determine which Ets factors may contribute to HO-1 induction by PGN, we examined the ability of a panel of Ets factors to transactivate the HO-1 promoter. RAW 264.7 cells were transiently cotransfected with HO-1 (−4,045/+74) and expression plasmids for NERF2, Elk-1, Elk-3, Ets-1, and Ets-2 in the presence of vehicle or PGN (1 μg/ml). In the absence of PGN (Fig. 5, open bars), Elk-1 and Ets-2 were the most potent inducers of the HO-1 promoter (P < 0.05; Fig. 5A). To further evaluate the induction of HO-1 promoter activity by Elk-1 alone, we cotransfected HO-1 (−4,045/+74) with increasing amounts of the Elk-1 expression plasmid and demonstrated an increase in HO-1 promoter activity in a dose-dependent manner (up to 4.8-fold with 400 ng/well, data not shown). In the presence of PGN (Fig. 5, filled bars), Elk-1 and Ets-2 further increased HO-1 promoter activity by 12.2- and 7.5-fold, respectively, compared with baseline pCI control (P < 0.05; Fig. 5A). To localize the Elk-1-responsive element(s) in the HO-1 promoter, we transiently cotransfected deletion mutants of the HO-1 promoter and an Elk-1 expression plasmid into RAW 264.7 cells in the presence or absence of PGN (1 μg/ml). We found that, analogous to PGN (Fig. 4), induction of HO-1 promoter activity by Elk-1 alone or Elk-1 + PGN significantly decreased at deletion mutant HO-1 (−66/+74) compared with constructs containing additional upstream regions of the promoter (−117 through −4,045 bp; Fig. 5B). These data verify that Elk-1 is a transactivator of the HO-1 promoter and that the region of the HO-1 promoter responsible for Elk-1 transactivation lies in the same location as the element(s) responsible for induction of the HO-1 promoter by PGN (between −117 and −66 bp).
Fig. 5.
Ets factors transactivate the HO-1 promoter, and Elk-1-responsive element(s) are located in the region analogous to PGN-responsive element(s). A: cells were transiently cotransfected with HO-1 (−4,045/+74) promoter construct (200 ng/well) and Ets expression plasmids (pCI-NERF2, pCI-Elk-1, pCI-Elk-3, pCI-Ets-1, and pCI-Ets-2) or empty pCI vector (400 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.05 vs. pCI control with PGN. †P < 0.05 vs. pCI control with vehicle. B: cells were transiently cotransfected with deletion mutants of the HO-1 promoter (200 ng/well) and pCI-Elk-1 expression plasmid (400 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.05 vs. activity of HO-1 (−4,045/+74), (−1,867/+74), (−295/+74), and (−117/+74) promoter constructs stimulated with PGN. †P < 0.05 vs. HO-1 (−4,045/+74) promoter construct with vehicle.
EBS are not responsible for HO-1 induction by Elk-1 and PGN.
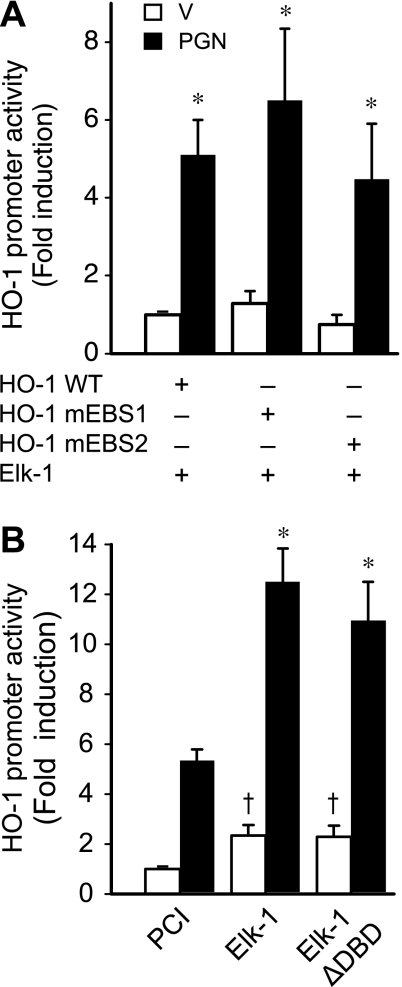
To identify the cis-acting element(s) responsible for Elk-1 and PGN transactivation of the HO-1 promoter, we mutated two EBS in promoter construct HO-1 (−295/+74), termed mEBS1 and mEBS2 (11). The wild-type, mEBS1, or mEBS2 HO-1 (−295/+74) promoter constructs were cotransfected into RAW 264.7 cells with Elk-1 expression plasmid and exposed to either vehicle or PGN (1 μg/ml). The promoter activity in the presence of Elk-1 or Elk-1 + PGN was not different between wild-type, mEBS1, and mEBS2 HO-1 promoter constructs (Fig. 6A). These data suggest that Elk-1 may activate the HO-1 promoter independent of binding to EBS1 or EBS2. To test this hypothesis, we generated an Elk-1 mutant expression plasmid with Elk-1 ΔDBD. The empty pCI vector, wild-type Elk-1, or Elk-1 ΔDBD expression plasmids were cotransfected with HO-1 (−295/+74) promoter into RAW 264.7 cells and exposed to vehicle or PGN (1 μg/ml). Both wild-type Elk-1 and Elk-1 ΔDBD were capable of increasing HO-1 promoter activity compared with empty pCI vector. Furthermore, wild-type Elk-1 and Elk-1 ΔDBD were able to enhance transactivation of the HO-1 promoter in the presence of PGN (Fig. 6B). These data suggest that DNA binding is not required for Elk-1 to transactivate the HO-1 promoter and support our data showing no difference in activity of wild-type and mEBS promoter constructs exposed to Elk-1 and PGN.
Fig. 6.
Ets binding sites (EBS) are not essential for induction of HO-1 promoter activity by PGN or Elk-1. A: cells were transiently cotransfected with HO-1 (−295/+74) promoter constructs containing wild-type (HO-1 WT) or mutated EBS at −125 (HO-1 mEBS1) or −93 (HO-1 mEBS2; 200 ng/well) and pCI-Elk-1 expression plasmid (400 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.05 vs. constructs receiving vehicle. B: cells were transiently cotransfected with HO-1 (−295/+74) promoter construct (200 ng/well) and expression plasmids for empty pCI vector, WT Elk-1, or Elk-1 containing deletion of its DNA binding domain (Elk-1 ΔDBD; 400 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.05 vs. pCI control with PGN. †P < 0.05 vs. pCI control with vehicle.
C/EBPα together with Elk-1 induce HO-1 promoter activity.
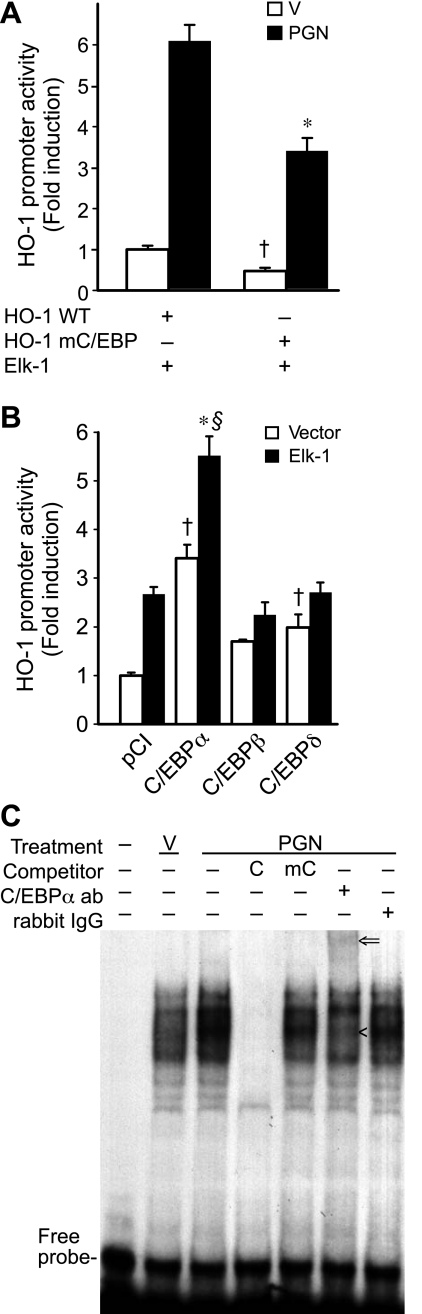
A cis-acting element just downstream of EBS2 in the HO-1 promoter, C/EBP binding site, was considered a potential mediator of this PGN response because C/EBP proteins are transcription factors known to be activated by oxidative stress (10). We thus mutated the C/EBP binding site starting at −90 bp (designated mC/EBP) in the HO-1 (−295/+74) construct. The wild-type and mC/EBP HO-1 (−295/+74) promoter constructs were cotransfected into RAW 264.7 cells with Elk-1 expression plasmid in the presence or absence of PGN (1 μg/ml). The mC/EBP HO-1 promoter activity in the presence of Elk-1 alone or Elk-1 + PGN was significantly decreased compared with the wild-type HO-1 promoter (Fig. 7A). These data suggest that the C/EBP binding site is important for activation of the HO-1 promoter by Elk-1 and PGN and support the hypothesis that Elk-1 may activate the HO-1 promoter through the C/EBP binding site.
Fig. 7.
CCAAT/enhancer-binding protein-α (C/EBPα) induces HO-1 promoter activity together with Elk-1 and binds to a C/EBP binding site. A: cells were transiently cotransfected with HO-1 (−295/+74) promoter construct containing WT (HO-1 WT) or mutated C/EBP binding site at −90 (HO-1 mC/EBP; 200 ng/well) and pCI-Elk-1 expression plasmid (400 ng/well). After the transfection, cells were treated with vehicle (open bars) or PGN (1 μg/ml; filled bars) for 24 h and then harvested for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.05 compared with HO-1 WT construct stimulated with PGN. †P < 0.05 vs. HO-1 WT with vehicle. B: cells were transiently cotransfected with HO-1 (−295/+74) promoter construct (200 ng/well) and either empty pCI vector (open bars; 400 ng/well) or pCI-Elk-1 expression plasmid (filled bars; 400 ng/well). Both groups were also cotransfected with empty pCI vector, pCI-C/EBPα, pCI-C/EBPβ, or pCI-C/EBPδ expression plasmids (400 ng/well). After the transfections, cells were harvested at 48 h for luciferase assay. Four independent experiments were performed in triplicate. *P < 0.001 vs. pCI control with Elk-1. †P < 0.05 vs. pCI control without Elk-1. §P < 0.05 vs. pCI-C/EBPα without Elk-1. C: EMSA was performed using nuclear proteins harvested from cells 1 h following treatment with vehicle or PGN (1 μg/ml). Nuclear proteins were first incubated with a reaction mixture containing cold C/EBP site probe (C), cold mutated C/EBP site probe (mC), anti-C/EBPα antibody (ab), or control rabbit IgG as indicated. A radiolabeled C/EBP site probe (−98/−74) was then added into the reaction mixture and subjected to electrophoresis. Details are described in materials and methods. <, Disrupted complex; arrow, supershifted band. This experiment was performed 3 independent times.
To determine whether C/EBP transcriptional factors could transactivate the HO-1 promoter, empty pCI vector or expression plasmids for C/EBPα, C/EBPβ, or C/EBPδ were cotransfected with HO-1 (−295/+74) promoter into RAW 264.7 cells in the presence or absence of Elk-1. The most potent inducer of the HO-1 promoter was C/EBPα (3.4-fold; P < 0.05), followed to a lesser extent by C/EBPδ (2.0-fold) and no significant induction by C/EBPβ (Fig. 7B). In addition, the combination of C/EBPα and Elk-1 further increased HO-1 promoter activity (5.5-fold), which was significantly greater than C/EBPα or Elk-1 alone and not produced by other C/EBP factors (P < 0.05).
PGN induces C/EBPα binding to the HO-1 promoter.
To further understand the role of C/EBPα in the induction of HO-1 by PGN, we performed an EMSA with a radiolabeled probe encoding the C/EBP binding site at −89 to −82 bp of the HO-1 promoter. The probe was incubated with nuclear extracts from RAW 264.7 cells in the presence or absence of PGN (1 μg/ml). Four inducible protein complexes bounded at the C/EBP binding site, and the intensity of these complexes increased after PGN treatment (Fig. 7C, lane 3 compared with lane 2). To evaluate the specificity of binding, a 100-fold molar excess of unlabeled identical (Fig. 7C, lane 4) or mutated (Fig. 7C, lane 5) oligonucleotide competitors was incubated with the reaction mixture. The identical competitor completely disrupted the DNA-protein complexes, and this disruption did not occur with the mC/EBP competitor, suggesting specific binding of these complexes to the C/EBP probe. To determine whether C/EBPα was present within the binding complex, C/EBPα antibody (Fig. 7C, lane 6) or rabbit IgG (control; Fig. 7C, lane 7) was incubated with the reaction mixture. This revealed that the C/EBPα antibody disrupted the 3rd DNA-protein complex (Fig. 7C, “<”) and supershifted the complex (Fig. 7C, arrow), whereas rabbit IgG had no effect. These results indicated that the binding of C/EBPα to the C/EBP binding site on the HO-1 promoter is specific and increased by PGN.
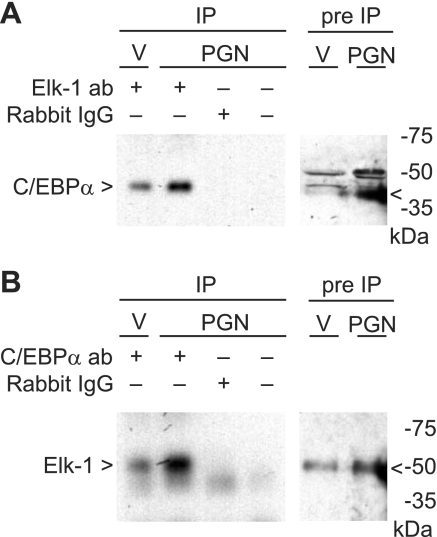
PGN induces C/EBPα and Elk-1 interaction.
To demonstrate whether C/EBPα and Elk-1 proteins interact after PGN stimulation, we performed coimmunoprecipitation (co-IP) assays using antibodies to C/EBPα and Elk-1. Elk-1 antibody-cross-linked protein A/G agarose beads were incubated with nuclear protein extracts from RAW 264.7 cells in the presence or absence of PGN (1 μg/ml). The precipitated proteins were separated by SDS-PAGE for Western blot analysis using C/EBPα antibody. C/EBPα expression in the cell extracts, before IP, was increased after exposure to PGN (Fig. 8A, lanes 5 and 6). C/EBPα expression in Elk-1 antibody-immunoprecipitated proteins was also increased after PGN (Fig. 8A, lanes 1 and 2), but no expression was evident in the rabbit IgG and negative control immunoprecipitates (Fig. 8A, lanes 3 and 4). These results revealed that C/EBPα interacts with Elk-1, and this interaction is increased after PGN stimulation. We repeated the experiment using C/EBPα antibody-cross-linked protein A/G agarose beads that were incubated with nuclear protein extracts from RAW 264.7 cells in the presence or absence of PGN (1 μg/ml). The precipitated proteins were separated by SDS-PAGE for Western blot analysis using Elk-1 antibody. Elk-1 protein was slightly increased by PGN in cell extracts before IP (Fig. 8B, lanes 5 and 6). Analogous to the prior experiment, treatment with PGN caused an increase in Elk-1 expression in C/EBPα antibody-immunoprecipitated proteins (Fig. 8B, lanes 1 and 2), but no expression was present in the rabbit IgG and negative control immunoprecipitates (Fig. 8B, lanes 3 and 4). Both results revealed that the protein-protein interaction between C/EBPα and Elk-1 increased after PGN stimulation.
Fig. 8.
PGN induces C/EBPα and Elk-1 interaction. A: coimmunoprecipitation (co-IP) was performed using nuclear protein extract harvested from cells 1 h following treatment with vehicle or PGN (1 μg/ml). Cellular proteins were incubated with Elk-1 antibody-cross-linked protein A/G beads, rabbit IgG-cross-linked protein A/G beads, or protein A/G beads alone. The bounded proteins were separated with SDS-PAGE. Western blot analysis of IP-bounded proteins (lanes 1-4) and pre-IP cellular proteins (lanes 5 and 6) were performed using C/EBPα antibody. B: co-IP was performed as described above. Cellular proteins were incubated with C/EBPα antibody-cross-linked protein A/G beads, rabbit IgG-cross-linked protein A/G beads, or protein A/G beads alone. The bounded proteins were separated with SDS-PAGE. Western blot analysis of IP-bounded proteins (lanes 1-4) and pre-IP cellular proteins (lanes 5 and 6) were performed using Elk-1 antibody. Details are described in materials and methods. <, Specific band identified by antibody. Experiments in A and B were performed 2 independent times.
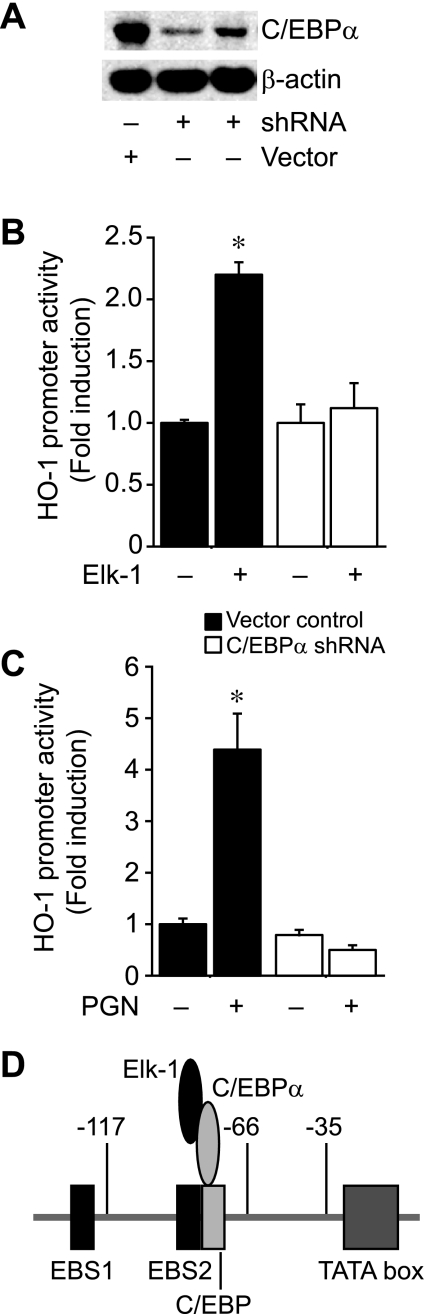
To further confirm that C/EBPα, in conjunction with Elk-1, is important for HO-1 transactivation by PGN, we silenced the expression of C/EBPα in RAW 264.7 cells and assessed HO-1 promoter activity compared with wild-type (vector control) cells. Western blot analysis revealed that shRNA decreased C/EBPα expression by 78.3 ± 2.1% in mouse macrophages compared with vector control cells (Fig. 9A). Next, we transfected C/EBPα shRNA cells and control cells with construct HO-1 (−295/+74) in the presence or absence of an expression plasmid for Elk-1. Compared with vector control cells, which showed an induction of the HO-1 promoter to a similar degree as wild-type cells (Fig. 5A), Elk-1 did not increase HO-1 promoter activity in C/EBPα-silenced cells (Fig. 9B). In addition, C/EBPα shRNA cells showed no increase in HO-1 promoter activity when exposed to PGN (1 μg/ml), in contrast with the induction seen in vector control cells (Fig. 9C). Taken together, these data demonstrate the importance of C/EBPα for HO-1 transactivation by Elk-1 and PGN. Figure 9D provides a schema of how the interaction of Elk-1 and C/EBPα, through the C/EBP binding site, leads to HO-1 promoter transactivation independent of DNA binding by Elk-1.
Fig. 9.
Silencing of C/EBPα prevents Elk-1 and PGN induction of HO-1. A: mouse macrophages (RAW 264.7) were stably transfected with short hairpin RNA (shRNA) plasmids for C/EBPα (2 separate transfections) or vector control. Protein was extracted, and Western blot analysis was performed as described in materials and methods, using anti-HO-1 and anti-β-actin antibodies. B: cells were transiently transfected with HO-1 (−295/+74) promoter construct (200 ng/well), in the presence (+) or absence (−) of an Elk-1 expression plasmid (400 ng/well), after stable C/EBPα silencing (open bars) or no silencing (vector control; filled bars). C: cells were transiently transfected with HO-1 (−295/+74) promoter construct (200 ng/well), in the presence (+) or absence (−) of PGN (1 μg/ml), after stable C/EBPα silencing (open bars) or no silencing (vector control; filled bars). In B and C, 3–5 independent experiments were performed in triplicate. *P < 0.05 vs. all other groups. D: the diagram depicts the interaction of Elk-1 and C/EBPα and binding to the C/EBP site to drive HO-1 promoter activity independent of Elk-1 binding to DNA.
DISCUSSION
To sort out the complex pathways of sepsis over the years, both in vivo and in vitro, investigators have traditionally focused on the use of LPS from Gram-negative bacteria (7). This was, in part, due to the fact that sepsis was considered by many to be near-synonymous with Gram-negative endotoxemia. However, recently it has become more obvious that the concept of sepsis can arise from microbial infections of different sources (4). For example, Gram-positive bacteria now account for up to 50% of all cases of sepsis (4, 27). Thus we decided to investigate a Gram-positive mediator and its role in a systemic inflammatory response. The major component of the cell wall of Gram-positive bacterial is PGN (27) along with LTA (36). Toll-like receptor (TLR)-2 is a known receptor of LTA, and recently it has been shown that LTA induces HO-1 through the TLR2/MyD88/TRAF6 pathway, causing a Nrf2-dependent transactivation of the HO-1 promoter in tracheal smooth muscle cells (23). TLR2 was also initially identified as the receptor of PGN (19, 34), however, this concept has been challenged using more highly purified preparations of PGN (27, 41). Although the role of TLR2 in PGN signaling may be in question, specific PGN moieties are detected by the cytoplasmic proteins of the NOD family, including NOD2. Since HO-1 plays a protective role in the host defense response to Gram-positive bacteria (13), and this response occurs, in part, through the expression of NOD2, we focused our study on the regulation of the HO-1 gene by PGN from S. aureus in inflammatory cells.
The biological significance of HO-1 expression during S. aureus PGN exposure was demonstrated by the fact that only 20% of HO-1−/− mice survived this Gram-positive inflammatory stimulus, whereas HO-1+/+ mice showed no lethality (100% survival) to PGN administration (20 mg/kg ip; Fig. 1C). Systemic administration of PGN is a pure inflammatory stimulus, suggesting that the anti-inflammatory and antioxidant properties of HO-1, and its products of heme catabolism, play a cytoprotective role (1, 24, 31, 32). However, in the setting of a Gram-positive microbial infection, we (13) showed previously that HO-1-derived CO is also able to modulate the function of inflammatory cells, promoting increased phagocytosis of bacteria. Thus we believe the beneficial effects of HO-1 during a systemic inflammatory response are multifactorial. Moreover, although mortality is increased in the absence of HO-1, it is clear that increased endogenous expression of HO-1 is not necessarily sufficient to completely protect against death due to endotoxemia or sepsis. To confirm this concept, we showed that overexpression of HO-1, or administration of CO, rescued mice deficient in HO-1 or improved outcome during endotoxemia (37) and sepsis (13) in wild-type mice.
Since expression of HO-1 is critical for survival during systemic administration of PGN, we further explored the mechanism responsible for HO-1 gene regulation by PGN. HO-1 was inducible at both the protein and mRNA level in vivo in tissue (Fig. 1) and in vitro in macrophages (Fig. 2), and this regulation occurred at the level of gene transcription as HO-1 promoter constructs were induced by PGN (Fig. 4). The region of the HO-1 promoter responsible for PGN induction was between −117 and −66 bp (Fig. 4), a region not known to be regulated by Nrf2 (a transcription factor involved in HO-1 regulation by LTA) (23). In fact, this region of the HO-1 promoter is more similar to the LPS-responsive region (11, 12), containing one of the downstream EBS (located at −93 bp, EBS2). However, different from LPS stimulation, expression of Elk-1 in conjunction with PGN (more than Ets-2 or Ets-1) was the most prominent inducer of HO-1 (Fig. 5A). Elk-1 is a member of the TCF subfamily of Ets transcription factors (20, 43, 46), and previously it has been shown that Elk-1 contributes to the generation of proinflammatory cytokines (such as tumor necrosis factor-α) by PGN in macrophages (45).
Interestingly, the induction of HO-1 did not depend on the direct interaction of Elk-1 with DNA (Fig. 6). A prior study reported the ability of C/EBPβ and Elk-1 to interact and drive gene transcription of the immediate early gene family member c-fos (18). Because of this fact, and the presence of a C/EBP binding site at −90 bp (between −117 and −66 bp and in close proximity to EBS2), we further explored the potential role of C/EBP factors in conjunction with Elk-1 to drive PGN-induced HO-1 promoter activity. Different from disruption of EBS2 and EBS1, mutation of the C/EBP binding site significantly reduced HO-1 promoter activity by Elk-1 in the presence or absence of PGN (Fig. 7A). Analysis of C/EBP factors demonstrated that the most potent transactivator of HO-1 was C/EBPα, and this response was further increased by coexpression with Elk-1 (Fig. 7B). In addition, stimulation of macrophages with PGN increased the co-IP of C/EBPα and Elk-1 (Fig. 8), establishing the interaction of endogenous Elk-1 and C/EBPα in the cell. The activity and/or expression of C/EBP factors are known to be regulated by inflammatory stimuli, including LPS, with induction of C/EBPβ and C/EBPδ, and reduction of C/EBPα, in a number of cell types (29, 30). However, in our study, C/EBPα and its interaction with Elk-1 was increased by PGN, and silencing C/EBPα in macrophages completely abrogated induction of HO-1 promoter by either Elk-1 or PGN (Fig. 9, B and C). These data provide another means by which a C/EBP factor may modulate an inflammatory response, by the regulation of a gene with known anti-inflammatory properties.
Taking together the HO-1 promoter evaluation, the protein-DNA binding studies, and the protein-protein interaction studies, we believe Elk-1 interacts with C/EBPα, and this complex binds to the C/EBP site of the HO-1 promoter, contributing to its transactivation by PGN (Fig. 9D, illustration). In reviewing the literature regarding the transcriptional regulation of HO-1, Alam and Cook (2) hypothesized a similar scenario regarding the regulation of HO-1 by NF-κB. Studies using inhibitors of NF-κB have implicated a role for this transcription factor in the regulation of HO-1, even with the absence of a clearly identified functional binding site in the promoter. Thus the authors (2) hypothesized that a transcription factor may have its effects independent of direct DNA binding, but rather through its association with other DNA-binding proteins within a complex on the HO-1 promoter. In the present study, we demonstrate this concept, showing that Elk-1 drives HO-1 transcription without evidence of direct binding to DNA, but rather by interacting with another DNA-binding protein, C/EBPα. Our study provides further insight into the regulation of HO-1 by an inflammatory mediator of a Gram-positive bacterial source.
GRANTS
This work was supported by NIH Grants HL-060788, GM-053249, and AI-061246 to M. A. Perrella and grants from Ulsan University (2008-0014) and the Korea Research Foundation (KRF-2008-331-C00216) to S. W. Chung.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic Biol Med 39: 1–25, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Alam J, Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol 36: 166–174, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Anderson KL, Perkin H, Surh CD, Venturini S, Maki RA, Torbett BE. Transcription factor PU.1 is necessary for development of thymic and myeloid progenitor-derived dendritic cells. J Immunol 164: 1855–1861, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet 35: 63–78, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Arikan MC, Shapiro SD, Mariani TJ. Induction of macrophage elastase (MMP-12) gene expression by statins. J Cell Physiol 204: 139–145, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bassuk AG, Leiden JM. A direct physical association between ETS and AP-1 transcription factors in normal human T cells. Immunity 3: 223–237, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Beutler B, Rietschel ET. Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3: 169–176, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Chen YH, Layne MD, Watanabe M, Yet SF, Perrella MA. Upstream stimulatory factors regulate aortic preferentially expressed gene-1 expression in vascular smooth muscle cells. J Biol Chem 276: 47658–47663, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Chiu YC, Lin CY, Chen CP, Huang KC, Tong KM, Tzeng CY, Lee TS, Hsu HC, Tang CH. Peptidoglycan enhances IL-6 production in human synovial fibroblasts via TLR2 receptor, focal adhesion kinase, Akt, and AP-1-dependent pathway. J Immunol 183: 2785–2792, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Choi AM, Knobil K, Otterbein SL, Eastman DA, Jacoby DB. Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am J Physiol Lung Cell Mol Physiol 271: L383–L391, 1996 [DOI] [PubMed] [Google Scholar]
- 11.Chung SW, Chen YH, Perrella MA. Role of Ets-2 in the regulation of heme oxygenase-1 by endotoxin. J Biol Chem 280: 4578–4584, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Chung SW, Chen YH, Yet SF, Layne MD, Perrella MA. Endotoxin-induced down-regulation of Elk-3 facilitates heme oxygenase-1 induction in macrophages. J Immunol 176: 2414–2420, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Chung SW, Liu X, Macias AA, Baron RM, Perrella MA. Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J Clin Invest 118: 239–247, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Kimpe SJ, Kengatharan M, Thiemermann C, Vane JR. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA 92: 10359–10363, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ejima K, Layne MD, Carvajal IM, Nanri H, Ith B, Yet SF, Perrella MA. Modulation of the thioredoxin system during inflammatory responses and its effect on heme oxygenase-1 expression. Antioxid Redox Signal 4: 569–576, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 18: 521–540, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 278: 8869–8872, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Hanlon M, Bundy LM, Sealy L. C/EBP beta and Elk-1 synergistically transactivate the c-fos serum response element. BMC Cell Biol 1: 2, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwaki D, Mitsuzawa H, Murakami S, Sano H, Konishi M, Akino T, Kuroki Y. The extracellular toll-like receptor 2 domain directly binds peptidoglycan derived from Staphylococcus aureus. J Biol Chem 277: 24315–24320, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Kasza A, O'Donnell A, Gascoigne K, Zeef LAH, Hayes A, Sharrocks AD. The ETS domain transcription factor Elk-1 regulates the expression of its partner protein, SRF. J Biol Chem 280: 1149–1155, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Koay MA, Gao X, Washington MK, Parman KS, Sadikot RT, Blackwell TS, Christman JW. Macrophages are necessary for maximal nuclear factor-kappa B activation in response to endotoxin. Am J Respir Cell Mol Biol 26: 572–578, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Labischinski H, Maidhof H. Bacterial Peptidoglycan: Overview and Evolving Concepts. Amsterdam: Elsevier, 1994 [Google Scholar]
- 23.Lee IT, Wang SW, Lee CW, Chang CC, Lin CC, Luo SF, Yang CM. Lipoteichoic acid induces HO-1 expression via the TLR2/MyD88/c-Src/NADPH oxidase pathway and Nrf2 in human tracheal smooth muscle cells. J Immunol 181: 5098–5110, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Maines MD, Gibbs PE. 30 Years of heme oxygenase: from a “molecular wrecking ball” to a “mesmerizing” trigger of cellular events. Biochem Biophys Res Commun 338: 568–577, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Majcherczyk PA, Langen H, Heumann D, Fountoulakis M, Glauser MP, Moreillon P. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J Biol Chem 274: 12537–12543, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Mattsson E, Verhage L, Rollof J, Fleer A, Verhoef J, van Dijk H. Peptidoglycan and teichoic acid from Staphylococcus epidermidis stimulate human monocytes to release tumour necrosis factor-alpha, interleukin-1 beta and interleukin-6. FEMS Immunol Med Microbiol 7: 281–287, 1993 [DOI] [PubMed] [Google Scholar]
- 27.Myhre AE, Aasen AO, Thiemermann C, Wang JE. Peptidoglycan–an endotoxin in its own right? Shock 25: 227–235, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Pellacani A, Wiesel P, Sharma A, Foster LC, Huggins GS, Yet SF, Perrella MA. Induction of heme oxygenase-1 during endotoxemia is downregulated by transforming growth factor-β1. Circ Res 83: 396–403, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Poli V. The role of C/EBP isoforms in the control of inflammation and native immunity functions. J Biol Chem 273: 29279–29282, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J 365: 561–575, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic implications. Physiol Rev 86: 583–650, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ryter SW, Morse D, Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol 36: 175–182, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salim M, Brown-Kipphut BA, Maines MD. Human biliverdin reductase is autophosphorylated, and phosphorylation is required for bilirubin formation. J Biol Chem 276: 10929–10934, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning CJ. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem 274: 17406–17409, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science 235: 1042–1046, 1987 [DOI] [PubMed] [Google Scholar]
- 36.Sutcliffe IC, Shaw N. Atypical lipoteichoic acids of Gram-positive bacteria. J Bacteriol 173: 7065–7069, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takamiya R, Hung CC, Hall SR, Fukunaga K, Nagaishi T, Maeno T, Owen C, Macias AA, Fredenburgh LE, Ishizaka A, Blumberg RS, Baron RM, Perrella MA. High-mobility group box 1 contributes to lethality of endotoxemia in heme oxygenase-1-deficient mice. Am J Respir Cell Mol Biol 41: 129–135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenhunen R, Marver H, Schmid R. Microsomal heme oxygenase, characterization of the enzyme. J Biol Chem 244: 6388–6394, 1969 [PubMed] [Google Scholar]
- 39.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA 61: 748–755, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thiemermann C. Interactions between lipoteichoic acid and peptidoglycan from Staphylococcus aureus: a structural and functional analysis. Microbes Infect 4: 927–935, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Travassos LH, Girardin SE, Philpott DJ, Blanot D, Nahori MA, Werts C, Boneca IG. Toll-like receptor 2-dependent bacterial sensing does not occur via peptidoglycan recognition. EMBO Rep 5: 1000–1006, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang JE, Dahle MK, Yndestad A, Bauer I, McDonald MC, Aukrust P, Foster SJ, Bauer M, Aasen AO, Thiemermann C. Peptidoglycan of Staphylococcus aureus causes inflammation and organ injury in the rat. Crit Care Med 32: 546–552, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci 23: 213–216, 1998 [DOI] [PubMed] [Google Scholar]
- 44.Wiesel P, Patel AP, DiFonzo N, Marria PB, Sim CU, Pellacani A, Maemura K, LeBlanc BW, Marino K, Doerschuk CM, Yet SF, Lee ME, Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1 deficient mice. Circulation 102: 3015–3022, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Xu Z, Dziarski R, Wang Q, Swartz K, Sakamoto KM, Gupta D. Bacterial peptidoglycan-induced TNF-alpha transcription is mediated through the transcription factors Egr-1, Elk-1, and NF-kappaB. J Immunol 167: 6975–6982, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Yang SH, Bumpass DC, Perkins ND, Sharrocks AD. The Ets domain transcription factor Elk-1 contains a novel class of repression domain. Mol Cell Biol 22: 5036–5046, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yet SF, Perrella MA, Layne MD, Hsieh CM, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest 103: R23–R29, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Azhar G, Zhong Y, Wei JY. Identification of a novel serum response factor cofactor in cardiac gene regulation. J Biol Chem 279: 55626–55632, 2004. [DOI] [PubMed] [Google Scholar]