Abstract
Inflammation is associated with various pulmonary diseases and contributes to the pathogenesis of acute lung injury. We previously identified a proinflammatory signaling pathway triggered by G protein-coupled receptors (GPCRs) in which stimulation of Gq-coupled GPCRs results in activation of the transcription factor NF-κB. Because damage to the lung causes the release of multiple mediators acting through Gq-coupled GPCRs, this signaling pathway is likely to contribute to inflammatory processes in the injured lung. In an effort to identify novel inhibitors of lung inflammation, the National Institutes of Health Clinical Collection, a library of 446 compounds, was screened for inhibitory activity toward production of IL-8 induced by stimulation of the Gq-coupled tachykinin 1 receptor with substance P in A549 cells. Twenty-eight compounds that significantly inhibited substance P-induced IL-8 production were identified. The most potent inhibitor was triptolide, a diterpenoid compound from Tripterygium wilfordii Hook F, a vine used in traditional Chinese medicine for the treatment of autoimmune diseases. Triptolide inhibited IL-8 production induced by substance P with an IC50 of 2.3 × 10−8 M and inhibited NF-κB activation in response to an agonist of the protease-activated receptor 2 with an IC50 of 1.4 × 10−8 M. Anti-inflammatory effects of triptolide were assessed in vivo using a chlorine gas lung injury model in mice. Triptolide inhibited neutrophilic inflammation and the production of KC (Cxcl1) in the lungs of chlorine-exposed mice. The results demonstrate that triptolide exhibits anti-inflammatory activity in cultured lung cells and in an in vivo model of acute lung injury.
Keywords: chlorine gas, acute lung injury, substance P, nuclear factor-κB, tachykinin 1 receptor
exposure of the lung to toxic substances damages pulmonary cells and can result in acute lung injury and the related clinical manifestation of acute respiratory distress syndrome. Damage to pulmonary epithelial and endothelial cells compromises the barriers between the alveolar space, the lung interstitium, and the bloodstream, resulting in pulmonary edema and impaired respiratory function. Acute lung injury is typically associated with an inflammatory response characterized by chemokine production and influx of neutrophils. Such a response can be beneficial in terms of fighting bacterial infection; however, excessive or uncontrolled inflammation may contribute to further tissue damage and be detrimental to the process of resolving lung injury. Although a variety of drugs with anti-inflammatory activity have been developed, none has been clinically approved for the treatment of acute respiratory distress syndrome. Therefore, the identification of novel anti-inflammatory compounds may be therapeutically beneficial for acute lung injury.
Chlorine is a widely used industrial compound that is considered a chemical threat agent. Human exposure to chlorine gas, leading to lung injury, has occurred through accidental release in industrial or transportation settings and through intentional release as a chemical weapon (2). Animal models of chlorine gas inhalation exposure have been developed as a means to characterize the pathology of this type of acute lung injury and to test potential treatment strategies (3, 10, 13). We previously developed and characterized a model of chlorine-induced lung injury in mice (18). Chlorine gas inhalation in inbred mice led to epithelial cell death, pulmonary edema, plasma protein leakage, chemokine production, and neutrophil influx. This model can be used to develop and test therapeutic countermeasures for chlorine-induced lung injury.
Pulmonary injury results in the release of inflammatory mediators, including chemokines, neuropeptides, nucleotides, kinins, chemotactic factors, and proteinases, which act through G protein-coupled receptors (GPCRs). We showed previously that substance P, a neuropeptide released from sensory nerves in response to injury, stimulates chemokine production and release through binding to its cognate GPCR [tachykinin 1 receptor (Tacr1), also known as neurokinin 1 receptor] (23). Signal transduction studies showed that Tacr1 stimulated activation of the proinflammatory transcription factor NF-κB through a pathway dependent on the Gq type of G protein, phospholipase C, and calcium release (1, 23). Similar processes were triggered by Gq-coupled GPCRs in lung epithelial cell lines, as well as in primary lung epithelial and endothelial cells. In the present study, we developed an in vitro system to screen for drugs that inhibited proinflammatory gene expression triggered by Tacr1 activation. Screening of a small compound library identified multiple chemicals that inhibited this process. The compound with the most potent inhibitory activity, triptolide, was investigated further for anti-inflammatory activity in vitro and in a mouse model of chlorine-induced lung injury.
MATERIALS AND METHODS
Materials.
The National Institutes of Health (NIH) Clinical Collection compound library was obtained from BioFocus DPI (San Diego, CA). Human Tacr1 cDNA was obtained from the University of Missouri-Rolla cDNA Resource Center (Rolla, MO). IL-8 ELISA reagents were purchased from Pierce (Rockford, IL), the XTT cell viability kit from Sigma-Aldrich (St. Louis, MO), substance P from Tocris Bioscience (Ellisville, MO), human TNF-α (TNF) from PeproTech (Rocky Hill, NJ), and protease-activated receptor 2 (PAR-2) agonist II (2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-NH2) from EMD Biosciences (San Diego, CA). Cell culture reagents were obtained from Invitrogen (Carlsbad, CA), except for hygromycin B, which was obtained from Roche (Indianapolis, IN).
Cell lines.
A549 human lung adenocarcinoma cells were obtained from the American Type Culture Collection (Manassas, VA). To generate Tacr1 stable transfectants, A549 cells were transfected with a linearized plasmid (obtained from the University of Missouri-Rolla cDNA Resource Center) containing the pcDNA3.1+ backbone and a human hemagglutinin-tagged Tacr1 cDNA. Transfectants were selected with 700 μg/ml G418, and cells from surviving colonies were cloned by limiting dilution in 96-well plates. Clones were screened for Tacr1 expression by RT-quantitative PCR using reagents from Applied Biosystems (Foster City, CA). A549/Tacr1 cells were maintained in DMEM plus Glutamax, 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 700 μg/ml G418. A459 cells stably transfected with NF-κB-luciferase were purchased from Panomics (Fremont, CA) and maintained in DMEM plus Glutamax, 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml hygromycin B.
Screening of the compound library.
A549/Tacr1 cells were seeded into 96-well dishes at a density of 5 × 104 cells/well. On the following day, standard medium was replaced with serum-free medium, and cells were pretreated with test compounds from the NIH Clinical Collection (14b) at a concentration of 10 μM. As most clinically effective inhibitors will be active in the nanomolar range, 10 μM was chosen as a dose likely to produce maximal inhibition for effective inhibitors. After 30 min, substance P was added to a final concentration of 100 nM, or TNF-α was added to 10 ng/ml. Cells were incubated with test compounds plus substance P or TNF for 4 h, and culture medium was collected and stored at −80°C for subsequent analysis of IL-8 levels.
IL-8, XTT, and luciferase assays.
IL-8 in cell culture medium was measured by ELISA using reagents from Pierce as directed by the supplier. Cell viability was measured by XTT assay. Cells were seeded into 96-well plates and cultured in medium without phenol red. On the following day, wells were rinsed with serum-free medium without phenol red and then replaced with the same medium containing 200 μg/ml XTT. After addition of test compounds, plates were incubated for 4 h, and then the absorbance of each well was determined at 450 nm. NF-κB-luciferase activity was measured using the Luciferase Assay System from Promega (Madison, WI).
Chlorine exposure and triptolide treatment.
Experiments involving animals were approved by the University of Louisville Institutional Animal Care and Use Committee and were conducted in accordance with the Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals (6). FVB/N mice were obtained from the Jackson Laboratory and were exposed to chlorine gas as described elsewhere (18). Briefly, mice were subjected to whole body chlorine exposure in a 54-liter polyester chamber. Gas from a 1% chlorine source was mixed with room air to achieve the desired concentration. Exposure levels were determined by sampling using a modified version of an American Society for Testing Materials method for airborne chlorine (16, 18), except chlorine levels (as produced iodine) were measured spectrophotometrically at 405 nm, rather than by specific-ion electrode. Animals were exposed for 1.1 h to a target dose of 260 ppm-h. Actual doses averaged 255 ± 3 (SD) ppm-h. Mice were treated intraperitoneally with a single dose of triptolide administered 1 h after the end of the chlorine exposure. Triptolide was dissolved in dimethyl sulfoxide at 12.5 mg/ml and diluted with Dulbecco's PBS (D-PBS) to a concentration of 1 mg/ml. Further dilutions were prepared using D-PBS. Vehicle-treated animals were injected with 0.8% dimethyl sulfoxide in D-PBS.
Analysis of chlorine-induced lung inflammation.
Separate groups of mice were used for collection of lung lavage and fixation of lung tissue. Lung lavage fluid was collected and differential cell counts were performed as described previously (18). The levels of KC (Cxcl1), a mouse CXC chemokine that is a chemoattractant for neutrophils, were measured using commercially available ELISA reagents (mouse KC DuoSet, R&D Systems, Minneapolis, MN). Lungs were fixed by intratracheal instillation of 10% neutral buffered formalin at a pressure of 25 cmH2O. Lungs were embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin. Immunohistochemistry for the neutrophil marker Ly-6G was performed as described elsewhere (18), except antigen retrieval was achieved by incubation of sections in sodium citrate, pH 6.0, containing 0.05% Tween 20 at 95°C for 30 min. Ly-6G cell counts were performed on one high-power field from each of four lobes per mouse. The counts from individual lobes were combined to yield a single number of Ly-6G-positive cells per unit area for each mouse.
Data analysis.
Group means were compared using ANOVA with Bonferroni's correction for multiple comparisons (StatView) or Tukey's multiple comparison test (GraphPad Prism). Correlation between inhibition of substance P- and TNF-induced IL-8 production was analyzed using Microsoft Excel. The criterion for statistical significance was set at P < 0.05.
RESULTS
Screening for inhibitors of substance P-induced NF-κB activation.
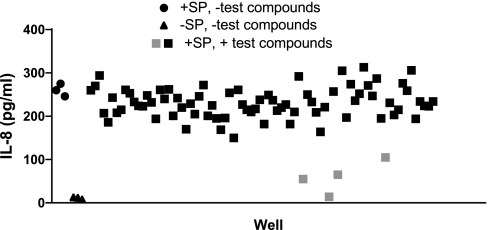
A library of compounds was screened to identify drugs that would inhibit activation of the proinflammatory transcription factor NF-κB by a Gq-coupled GPCR. For this purpose, we used A549 human lung epithelial cells that were stably transfected with Tacr1 (A549/Tacr1 cells). We previously showed that substance P treatment of A549 cells transiently transfected with Tacr1 results in the activation of NF-κB and upregulation of IL-8 through a Gq-dependent mechanism. To search for inhibitors of this pathway, chemicals from the NIH Clinical Collection, a library of 446 compounds, were screened for the ability to inhibit, at a concentration of 10 μM, the production of IL-8 in A549/Tacr1 cells treated with substance P in 96-well plates. Figure 1 shows representative results from one of the six plates used to conduct the screening. IL-8 levels were 11 ± 3 (SE) pg/ml in untreated wells and 260 ± 14 pg/ml in substance P-treated wells with no test compounds. IL-8 production in most wells clustered around the values in the wells not treated with test compounds, indicating no significant inhibitory effects. However, some wells had lower IL-8 levels (14–105 pg/ml for 4 samples on this plate), suggesting possible inhibition of substance P-induced IL-8 production by the compounds in these wells. On the basis of the initial screening results, a total of 28 compounds appeared to inhibit IL-8 production and were rescreened to confirm inhibitory activity. Five compounds appeared to stimulate IL-8 production but were not subjected to further analysis. The 28 potential inhibitory chemicals were tested in a second experiment using three wells per test compound. All 28 compounds exhibited significant inhibition of substance P-induced IL-8 production (P < 0.001 for each compound vs. substance P treatment alone; Table 1). The identification of multiple compounds with known anti-inflammatory effects (e.g., corticosteroids) corroborated the success of the screening method in identifying compounds with the desired activity. The chemical with the most potent inhibitory activity (99% inhibition) was triptolide, a diterpenoid compound isolated from Tripterygium wilfordii Hook F, a vine used in traditional Chinese medicine for the treatment of autoimmune diseases.
Fig. 1.
Inhibition of substance P (SP)-induced IL-8 production by test compounds. A549 cells stably transfected with tachykinin 1 receptor (Tacr1) were treated with test compounds for 30 min and then with 100 nM substance P for 4 h. IL-8 in cell culture supernatants was measured by ELISA. Baseline IL-8 levels were low in untreated cells (▴) and increased with substance P treatment (●). Most test compounds did not inhibit the substance P-induced increase in IL-8 production (black squares), but a minority appeared to cause significant inhibition (gray squares).
Table 1.
Inhibitors of substance P-induced IL-8 production
| %Inhibition |
||||||
|---|---|---|---|---|---|---|
| Chemical | Description | SP* | TNF | P Value (TNF) | %Viability | P Value (XTT) |
| Triptolide | Diterpenoid | 99.3 | 100.1 | <0.001 | 105.6 | NS |
| Beclomethasone | Corticosteroid | 93.5 | 77.0 | <0.001 | 97.5 | NS |
| Homoharringtonine | Protein synthesis inhibitor | 93.0 | 98.4 | <0.001 | 89.8 | <0.001 |
| Idarubicin | DNA intercalator | 91.6 | 94.2 | <0.001 | 97.6 | NS |
| Lamivudine | Reverse transcriptase inhibitor | 89.4 | 80.9 | <0.001 | 95.8 | NS |
| Loteprednol | Corticosteroid | 87.3 | 75.6 | <0.001 | 95.5 | NS |
| Desoximetsone | Corticosteroid | 87.0 | 80.8 | <0.001 | 101.2 | NS |
| Tropisetron | Serotonin receptor antagonist | 84.3 | 81.2 | <0.001 | 102.7 | NS |
| 3-[3,5-Dibromo-4-hydroxybenzoyl]-2-ethylbenzofuran | Stimulator of uric acid excretion | 84.2 | 58.9 | <0.001 | 104.1 | NS |
| δ1-Hydrocortisone | Corticosteroid | 82.0 | 74.9 | <0.001 | 105.9 | NS |
| Fluticasone | Corticosteroid | 82.0 | 81.3 | <0.001 | 103.3 | NS |
| Halometasone | Corticosteroid | 81.9 | 80.7 | <0.001 | 103.4 | NS |
| Linezolide | Protein synthesis inhibitor | 78.4 | 70.0 | <0.001 | 97.7 | NS |
| Secnidazole | Nitroimidazole | 77.7 | 63.5 | <0.001 | 102.5 | NS |
| Nitazoxanide | 5-Nitrothiazolyl | 72.0 | 38.9 | <0.001 | 106.5 | <0.05 |
| Cefixime | Cell wall synthesis inhibitor | 69.5 | 45.9 | <0.001 | 92.2 | <0.01 |
| 2H-indol-2-one,1,3-dihydro-1-phenyl-3,3-bis(4-pyridinylmethyl) | Stimulator of neurotransmitter release | 69.1 | 61.8 | <0.001 | 98.4 | NS |
| Doxorubicin | DNA intercalator | 67.6 | 80.4 | <0.001 | 93.4 | <0.05 |
| Corticosterone | Corticosteroid | 67.5 | 42.2 | <0.01 | 99.7 | NS |
| Reichstein's substance S | Corticosteroid | 67.4 | 41.2 | <0.001 | 96.7 | NS |
| Epirubicin | DNA intercalator | 59.1 | 83.1 | <0.001 | 97.5 | NS |
| Megestrol | Reproductive hormone | 55.1 | 52.3 | <0.001 | 100.6 | NS |
| Irbesartan | Angiotensin receptor antagonist | 51.1 | 31.9 | <0.01 | 104.6 | NS |
| Sertraline | Serotonin reuptake inhibitor | 48.5 | 27.7 | <0.01 | 88.9 | <0.001 |
| 10,11-Diol,5,6,6a,7,8,12b-hexahydrobenzo[a]phenanthridine | Dopamine receptor agonist | 44.2 | 53.4 | <0.001 | 100.2 | NS |
| Epigallocatechin gallate | Antioxidant | 37.2 | 16.5 | NS | 101.3 | NS |
| Medroxyprogesterone | Reproductive hormone | 36.3 | 34.7 | <0.001 | 101.3 | NS |
| Nelfinavir | Protease inhibitor | 26.8 | −6.6 | NS | 103.0 | NS |
P < 0.001 for all compounds vs. substance P (SP) alone. NS, not significant.
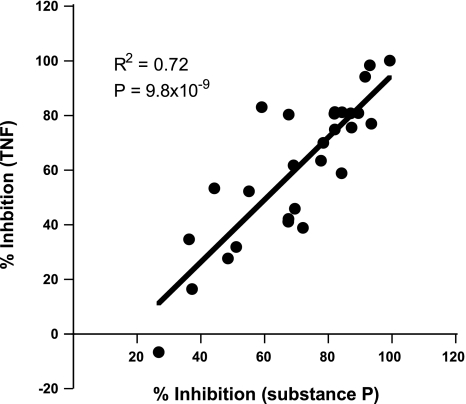
To examine whether the action of any of the inhibitory compounds was specific toward intracellular signaling pathways activated by substance P, an additional experiment was performed in which A549/Tacr1 cells were treated with test compounds and then stimulated with TNF. Twenty-six of the 28 compounds identified in the initial screen significantly inhibited IL-8 production following TNF treatment (Table 1). Examination of the relative inhibitory activities for IL-8 production induced by substance P and TNF revealed a highly significant correlation between the two (Fig. 2). This result suggested that, in general, the compounds affected signaling events occurring downstream of the point where signaling pathways activated by substance P and TNF converge, for example, at the level of NF-κB activation.
Fig. 2.
Correlation between inhibition of IL-8 production induced by substance P and TNF-α (TNF). Percent inhibition of substance P-induced vs. TNF-induced IL-8 production is shown as a scatter plot, with each point representing results from a compound in Table 1.
Cell viability was measured by XTT assay in A549/Tacr1 cells treated with the 28 chemicals that inhibited substance P-induced IL-8 production to determine whether any of the inhibitory effects were caused by nonspecific cellular toxicity. For 24 of the 28 compounds, a concentration of 10 μM did not result in significant reduction of cell viability (Table 1), suggesting that most of the chemicals had a specific effect on proinflammatory signaling pathways.
Anti-inflammatory effects of triptolide in vitro.
A dose-response analysis for the effect of triptolide on substance P-induced IL-8 production in A549/Tacr1 cells was performed (Fig. 3). Triptolide inhibited IL-8 production with an IC50 of 2.3 × 10−8 M (Fig. 3A). We showed previously that the upregulation of IL-8 in response to Tacr1 stimulation was dependent on NF-κB activation (23). To examine directly whether triptolide inhibits NF-κB-dependent gene transcription in lung epithelial cells, A549 cells stably transfected with an NF-κB-luciferase reporter gene were used. In these A549/NF-κB-luc cells, treatment with an agonist for the Gq-coupled PAR-2 results in NF-κB activation as determined by upregulation of the reporter gene (Fig. 3B). Triptolide induced a dose-dependent inhibition of NF-κB-luciferase activity in response to a PAR-2 agonist with an IC50 of 1.4 × 10−8 M (Fig. 3B). Triptolide had no effect on cell viability as determined by XTT assay at any of the concentrations tested up to 10−5 M (Fig. 3C). These data show that triptolide inhibits NF-κB-dependent gene transcription triggered through an endogenously expressed Gq-coupled GPCR.
Fig. 3.
Effect of triptolide on proinflammatory gene expression and cell viability. A: dose response for the effect of triptolide on IL-8 production in A549/Tacr1 cells. Cells were treated with the indicated concentrations of triptolide for 30 min and then with 100 nM substance P for 4 h. IL-8 in cell culture supernatants was measured by ELISA. Each point represents mean ± SE for 3 wells per group. B: dose response for the effect of triptolide on NF-κB-luciferase activity in A549/NF-κB-luc cells. Cells were treated with the indicated concentrations of triptolide for 30 min and then with 10 μM protease-activated receptor 2 (PAR-2) agonist (2-furoyl-Leu-Ile-Gly-Arg-Leu-Orn-NH2) for 4 h. Luciferase activity was measured in cellular extracts. Each point represents mean ± SE for 3 wells per group. C: effect of triptolide on cell viability. Cells were treated with XTT and the indicated concentration of triptolide for 4 h, and absorbance of the wells was measured at 450 nm (A450). Values are means ± SE for 8 wells/group.
Inhibition of chlorine-induced lung inflammation by triptolide.
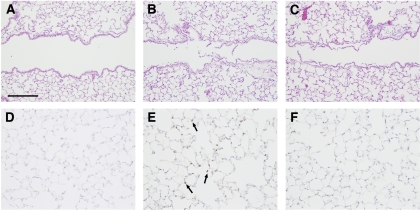
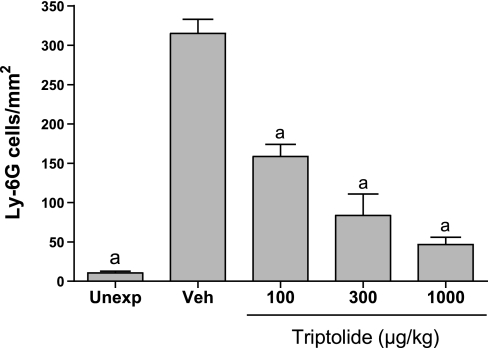
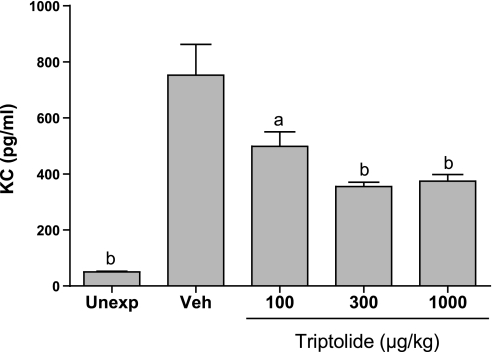
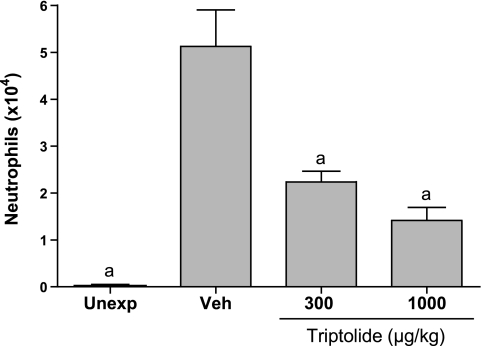
The effects of triptolide on inflammation associated with acute lung injury were examined in mice exposed to chlorine gas. This model is characterized by neutrophil influx into the lung parenchyma, with a peak 6 h after exposure, and increased neutrophils in lung lavage fluid that are highest at 48 h. This neutrophilic inflammation is correlated with an increased level in lavage fluid of the CXC chemokine KC (Cxcl1), which peaks 6 h after exposure. FVB/N mice were exposed to a target dose of 260 ppm-h chlorine and were treated with triptolide intraperitoneally 1 h after the end of the exposure. Lungs were fixed 6 h after exposure for histological analysis and immunostaining of neutrophils in lung tissue or were lavaged 6 and 48 h after exposure for analysis of lavage fluid KC and neutrophils, respectively. Chlorine inhalation resulted in pronounced epithelial damage, as evidenced by shedding of epithelial cells from airways (Fig. 4). Triptolide did not appear to affect the extent of chlorine-induced epithelial injury. Staining of lung sections for the neutrophil marker Ly-6G revealed that chlorine caused a dramatic increase in neutrophils in lung tissue that was inhibited by triptolide (Figs. 4 and 5). The decreased neutrophil influx was associated with a decrease in the neutrophil chemoattractant KC in mice treated with triptolide (Fig. 6). Neutrophils were decreased in lung lavage fluid collected 48 h after chlorine exposure (Fig. 7). Triptolide had no significant effect on other measures of lung injury, including lung weight (a measure of pulmonary edema) and lavage fluid protein content (an indicator of epithelial/endothelial barrier function). Collectively, the results demonstrate anti-inflammatory effects of triptolide in vivo following lung injury.
Fig. 4.
Lung histology in chlorine-exposed mice. Mice were exposed to chlorine, and lungs were fixed for histological analysis 6 h after exposure. Lung sections were stained with hematoxylin and eosin (A–C) or with antibodies to the neutrophil marker Ly-6G (D–F). A and D are from unexposed mice: A shows normal airway structure, and D shows lack of Ly-6G staining in typical high-power field (blue staining is hematoxylin counterstain). B and E are from chlorine-exposed, vehicle-treated mice: B shows airway epithelial damage, and E shows neutrophil influx (brown staining, arrows). C and F are from chlorine-exposed, triptolide-treated mice: triptolide did not affect airway epithelial injury (C) but effectively blocked neutrophil influx (F).
Fig. 5.
Inhibition of neutrophil influx in lungs of chlorine-exposed mice. Mice were exposed to inhaled chlorine and treated with triptolide 1 h after the end of the exposure. At 6 h after exposure, lungs were fixed for immunohistochemistry. Control groups were unexposed mice (Unexp) and chlorine-exposed mice treated with vehicle (Veh). Histological sections of lungs were stained with antibodies against the neutrophil marker Ly-6G, and stained cells in lung parenchyma were counted. Values are means ± SE for 5–7 mice per group. aP < 0.001 vs. Veh.
Fig. 6.
Inhibition of KC in lung lavage fluid from chlorine-exposed mice. Mice were exposed to inhaled chlorine and treated with triptolide 1 h after the end of the exposure. At 6 h after exposure, lung lavage was performed, and KC (Cxcl1) was measured in lavage fluid by ELISA. Control groups were unexposed mice and chlorine-exposed mice treated with vehicle. Values are means ± SE for 6–8 mice per group. aP < 0.05 vs. Veh; bP < 0.001 vs. Veh.
Fig. 7.
Inhibition of neutrophils in lung lavage fluid from chlorine-exposed mice. Mice were exposed to inhaled chlorine and treated with triptolide 1 h after the end of the exposure. At 48 h after exposure, lung lavage was performed, and differential cell counts were determined. Control groups were unexposed mice and chlorine-exposed mice treated with vehicle. Values are means ± SE for 5–7 mice per group. aP < 0.001 vs. Veh.
DISCUSSION
In the present study, a novel screening methodology was used to identify anti-inflammatory compounds from a chemical library. This type of approach is a common one in drug discovery efforts and requires a high-throughput screening assay, in addition to an appropriate collection of chemicals containing the desired activity. An advantage of this strategy is that it represents an unbiased approach that does not require assumptions about the mechanism of action or chemical structure of the compounds. However, the results will depend critically on the size and diversity of the compound library. In the present study, the screening was based on a signal transduction pathway previously characterized by us in which Gq-coupled GPCRs stimulate proinflammatory gene expression (1, 23). Gq, phospholipase C, calcium, protein kinase C, Ras, Raf, and Erk, and NF-κB were identified as necessary signaling components in this pathway. Thus, compounds that interfered with any of these intermediate steps would be identified as inhibitors of IL-8 production in the assay. The chemicals that were screened comprise the NIH Clinical Collection, a library of compounds that have been used in clinical trials (14b). An advantage of this collection is that the compounds have been used in humans and have known safety profiles, which increases the potential for identifying activities of chemicals that could be readily translated to clinical use. The validity of the assay was confirmed by the identification in the screen of multiple drugs with known anti-inflammatory or immunosuppressive activity, including corticosteroid compounds, triptolide, epigallocatechin gallate, idarubicin, doxorubicin, epirubicin, megestrol, and medroxyprogesterone. Anti-inflammatory effects of other identified compounds are not well documented and, thus, may represent hitherto unknown activities in this regard.
Compounds that were identified as having inhibitory activity toward Tacr1-induced IL-8 production were subsequently tested for inhibition in cells treated with TNF as a means of screening for Gq-specific inhibitors. All the test compounds that exhibited high inhibitory activity toward Tacr1-induced IL-8 expression also inhibited TNF-triggered IL-8 production. In addition, there was a striking correlation between the extent of inhibition in the two assays, suggesting that the test compounds were inhibiting similar intracellular processes in cells treated with substance P or TNF. Stimulation of Tacr1 or TNF receptors results in activation of NF-κB, but by distinct upstream signaling events. The likely convergence point for these signaling pathways is at the level of IκB phosphorylation and degradation, leading to NF-κB activation. Our data are consistent with most of the compounds identified in the screen acting as inhibitors of NF-κB activation or NF-κB-dependent transcription. Screening of larger compound libraries with more diverse structures and mechanisms of action would be necessary to identify compounds that specifically inhibit Gq signaling.
The present studies demonstrate that triptolide is a potent inhibitor of NF-κB-dependent gene expression and chemokine production induced by GPCRs. These measures were used as in vitro surrogates for anti-inflammatory activity. Effects of triptolide on proinflammatory gene expression were shown on an endogenous gene in cells overexpressing Tacr1 and on an NF-κB-luciferase reporter gene in response to an endogenous GPCR. Effects of triptolide in both systems were observed in the nanomolar range. These data provide evidence that triptolide inhibits GPCR-induced chemokine production through inhibition of NF-κB activity. Mechanisms by which triptolide produces such inhibitory effects have not been fully established. Because triptolide has been reported not to inhibit IκB degradation or NF-κB DNA binding, the available data suggest a possible effect on transcriptional activation (14, 15, 24). Triptolide inhibits gene expression induced by multiple transcription factors, including heat shock factor protein 1, nuclear factor of activated T cells, and activating protein 1, in addition to NF-κB (7, 14, 15, 22). Triptolide had no effect in cell-free in vitro transcription assays, indicating it is not a direct inhibitor of RNA polymerase II complex formation or transcript elongation (14, 19). In cells, triptolide did produce global decreases in transcription, although subsets of genes were preferentially susceptible to its effects (14, 19).
Potential anti-inflammatory activity of triptolide following lung injury in vivo was investigated using a chlorine gas inhalation exposure model. Treatment of mice with triptolide resulted in decreased production of the neutrophil chemoattractant KC and inhibition of neutrophil influx into the lung. Triptolide appears to interfere with key events involved in the initiation of inflammation, because a single administration soon after injury produced effects that could be observed as late as 48 h after exposure. These results showing that triptolide can inhibit inflammation when administered subsequent to chlorine exposure suggest the potential value of triptolide for postexposure treatment of acute lung injury. The results also suggest that the in vitro screen used to identify compounds via their effects on IL-8 production was an adequate surrogate for detecting anti-inflammatory activity in vivo.
Our results are consistent with previous studies showing effects of triptolide in chronic and acute inflammatory states. For example, triptolide inhibited cytokine production, inflammation, and clinical progression of disease in a murine rheumatoid arthritis model (11). In experimental autoimmune encephalitis, triptolide was shown to decrease clinical symptoms and inflammatory cell infiltrates (20). Triptolide suppressed colitis and inflammatory cell infiltrates in IL-10 knockout mice (21). Triptolide treatment has also been shown to be effective in lung inflammation and injury models. Triptolide reduced pulmonary eosinophilic inflammation in mice sensitized and challenged with ovalbumin (12). A water-soluble form of triptolide inhibited inflammatory cell infiltrates and obliterative bronchiolitis in a tracheal allograft model (9). Triptolide derivatives reduced lung inflammation and fibrosis in mice treated with bleomycin (8, 17). Triptolide is also being evaluated for human use in a clinical trial for autosomal dominant polycystic kidney disease (14a). The results of the present study, in conjunction with previous work (8, 9, 11, 17, 20, 21), demonstrate broad inhibition of proinflammatory gene expression and repression of inflammatory effects by triptolide in multiple in vivo disease models.
GRANTS
This work was supported by the National Institutes of Health CounterACT Program through the National Institute of Environmental Health Sciences and the Office of the Director, National Institutes of Health (Award U01 ES-015673).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Chang WY, Chen J, Schlueter CF, Hoyle GW. Common pathways for activation of proinflammatory gene expression by G protein-coupled receptors in primary lung epithelial and endothelial cells. Exp Lung Res 35: 324–343, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans RB. Chlorine: state of the art. Lung 183: 151–167, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Gunnarsson M, Walther SM, Seidal T, Bloom GD, Lennquist S. Exposure to chlorine gas: effects on pulmonary function and morphology in anaesthetised and mechanically ventilated pigs. J Appl Toxicol 18: 249–255, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Laboratory Animal Resources, Commission of Life Sciences, National Research Council Guide for the Care and Use of Laboratory Animals Washington, DC: National Academy Press, 1996 [Google Scholar]
- 7.Jiang XH, Wong BC, Lin MC, Zhu GH, Kung HF, Jiang SH, Yang D, Lam SK. Functional p53 is required for triptolide-induced apoptosis and AP-1 and nuclear factor-κB activation in gastric cancer cells. Oncogene 20: 8009–8018, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Krishna G, Liu K, Shigemitsu H, Gao M, Raffin TA, Rosen GD. PG490-88, a derivative of triptolide, blocks bleomycin-induced lung fibrosis. Am J Pathol 158: 997–1004, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonard CT, Soccal PM, Berry GJ, Doyle RL, Theodore J, Duncan SR, Rosen GD. PG490-88, a derivative of triptolide, attenuates obliterative airway disease in a mouse heterotopic tracheal allograft model. J Heart Lung Transplant 21: 1314–1318, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol 295: L733–L743, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin N, Liu C, Xiao C, Jia H, Imada K, Wu H, Ito A. Triptolide, a diterpenoid triepoxide, suppresses inflammation and cartilage destruction in collagen-induced arthritis mice. Biochem Pharmacol 73: 136–146, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Mao H, Chen XR, Yi Q, Li SY, Wang ZL, Li FY. Mycophenolate mofetil and triptolide alleviating airway inflammation in asthmatic model mice partly by inhibiting bone marrow eosinophilopoiesis. Int Immunopharmacol 8: 1039–1048, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Martin JG, Campbell HR, Iijima H, Gautrin D, Malo JL, Eidelman DH, Hamid Q, Maghni K. Chlorine-induced injury to the airways in mice. Am J Respir Crit Care Med 168: 568–574, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Matta R, Wang X, Ge H, Ray W, Nelin LD, Liu Y. Triptolide induces anti-inflammatory cellular responses. Am J Transl Res 1: 267–282, 2009. [PMC free article] [PubMed] [Google Scholar]
- 14a.Nanjing University School of Medicine Randomized Clinical Trial of Triptolide Woldifii for Autosomal Dominant Polycistic Kidney Disease (Online). http://clinicaltrials.gov/ct2/show/study/NCT00801268?term=triptolide&rank=1 [15December2009].
- 14b.National Institutes of Health NIH Clinical Collection (Online). http://www.nihclinicalcollection.com [5March2010].
- 15.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-κB transcriptional activation. J Biol Chem 274: 13443–13450, 1999 [DOI] [PubMed] [Google Scholar]
- 16.Rando RJ, Hammad YY. A diffusive sampler for gaseous chlorine utilizing an aqueous sulfamic acid collection medium and specific ion electrode analysis. Appl Occup Environ Hyg 5: 700–706, 1990 [Google Scholar]
- 17.Ren YX, Zhou R, Tang W, Wang WH, Li YC, Yang YF, Zuo JP. (5R)-5-hydroxytriptolide (LLDT-8) protects against bleomycin-induced lung fibrosis in mice. Acta Pharmacol Sin 28: 518–525, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Tian X, Tao H, Brisolara J, Chen J, Rando RJ, Hoyle GW. Acute lung injury induced by chlorine inhalation in C57BL/6 and FVB/N mice. Inhal Toxicol 20: 783–793, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet C, Guilbaud N, Barret JM, Bailly C. Triptolide is an inhibitor of RNA polymerase I and II-dependent transcription leading predominantly to down-regulation of short-lived mRNA. Mol Cancer Ther 8: 2780–2790, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Mei Y, Feng D, Xu L. Triptolide modulates T-cell inflammatory responses and ameliorates experimental autoimmune encephalomyelitis. J Neurosci Res 86: 2441–2449, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Wei X, Gong J, Zhu J, Wang P, Li N, Zhu W, Li J. The suppressive effect of triptolide on chronic colitis and TNF-α/TNFR2 signal pathway in interleukin-10 deficient mice. Clin Immunol 129: 211–218, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem 281: 9616–9622, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Williams R, Zou X, Hoyle GW. The tachykinin 1 receptor stimulates proinflammatory gene expression in lung epithelial cells through activation of NF-κB via a Gq-dependent pathway. Am J Physiol Lung Cell Mol Physiol 292: L430–L437, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Zhao G, Vaszar LT, Qiu D, Shi L, Kao PN. Anti-inflammatory effects of triptolide in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 279: L958–L966, 2000. [DOI] [PubMed] [Google Scholar]