Abstract
Pulmonary fibroblasts regulate extracellular matrix production and degradation and are critical in maintenance of lung structure, function, and repair, but they also play a central role in lung fibrosis. cAMP-elevating agents inhibit cytokine- and growth factor-stimulated myofibroblast differentiation and collagen synthesis in pulmonary fibroblasts. In the present study, we overexpressed adenylyl cyclase 6 (AC6) in pulmonary fibroblasts and measured cAMP production and collagen synthesis. AC6 overexpression enhanced cAMP production and the inhibition of collagen synthesis mediated by isoproterenol and beraprost, but not the responses to butaprost or PGE2. To examine if increased AC6 expression would impact the development of fibrosis in an animal model, we generated transgenic mice that overexpress AC6 under a fibroblast-specific promoter, FTS1. Lung fibrosis was induced in FTS1-AC6+/− mice and littermate controls by intratracheal instillation of saline or bleomycin. Wild-type mice treated with bleomycin showed extensive peribronchial and interstitial fibrosis and collagen deposition. By contrast, FTS1-AC6+/− mice displayed decreased fibrotic development, lymphocyte infiltration (as determined by pathological scoring), and lung collagen content. Thus, AC6 overexpression inhibits fibrogenesis in the lung by reducing pulmonary fibroblast-mediated collagen synthesis and myofibroblast differentiation. Because AC6 overexpression does not lead to enhanced basal or PGE2-stimulated levels of cAMP, we conclude that endogenous catecholamines or prostacyclin is produced during bleomycin-induced lung fibrosis and that these signals have antifibrotic potential.
Keywords: cAMP, lipid rafts
current therapeutic strategies for interstitial lung diseases have a limited ability to reverse underlying pathophysiological abnormalities, especially in settings such as pulmonary fibrosis (5, 16). Pulmonary fibroblasts play critical roles in the formation and maintenance of lung structure and function. Through their ability to synthesize collagen and other extracellular matrix (ECM) proteins, fibroblasts establish the scaffolding that sustains pulmonary architecture, including alveolar orientation and structure. Dysregulated production of ECM and fibroblast proliferation/differentiation can contribute to pathology, particularly in pulmonary fibrosis, by altering alveolar structure and function (41, 44).
While the pathogenesis of pulmonary fibrosis is not well understood, it is clear that alveolar epithelial cells and fibroblasts (likely derived from several sources) are central to disease progression. Injury of alveolar epithelial cells leads to activation of these cells to produce a number of fibrogenic mediators, including transforming growth factor (TGF)-β, TNF-α, platelet-derived growth factor, and others (45). These mediators serve to initiate wound repair processes, including immune cell invasion and activation of resident fibroblasts. Fibroblasts differentiate into activated myofibroblasts upon exposure to cytokines such as TGF-β (46). Myofibroblasts, so named because of their phenotypic expression of α-smooth muscle actin, take on a pseudocontractile phenotype and have high capacity for synthesizing various profibrotic mediators (TGF-β, fibroblast growth factor, angiotensin, endothelin, granulocyte-macrophage colony-stimulating factor, IL-6, and IL-8) and producing ECM proteins (collagens, laminin, and fibronectin) (4, 46, 51). This increase in cytokines and inflammatory mediators recruits immune cells to the site of injury and activates fibroblast proliferation, migration, and differentiation. The wound accumulates fibroblast-like cells that likely derive from several sources, including interstitial fibroblasts, circulating fibrocytes, infiltrating macrophages, and even epithelial cells that undergo epithelial-mesenchymal transition (48). While fibroblasts and immune cells appear to synergize each other's existence at a site of wound repair, fibroblasts can sustain their activated state through their autocrine functions.
Adenylyl cyclases (ACs) are enzymes regulated by G protein-coupled receptors (GPCRs) via heterotrimeric G proteins that stimulate (Gs) or inhibit (Gi) AC activity and the formation of cAMP. cAMP, via activation of PKA and exchange protein activated by cAMP (Epac), initiates rapid actions, such as regulation of ion channels and intermediary metabolism, as well as more delayed effects, such as changes in gene expression, cell growth, and proliferation. Agents that increase cAMP levels inhibit various measures of pulmonary fibroblast function, including collagen synthesis, cell proliferation and migration, and collagen gel contraction (12, 20, 31). Administration of a β-adrenergic receptor (β-AR) antagonist causes an elevation in pulmonary collagen, while treatment with PGE2, prostacyclin, or aminophylline [which increases cAMP via inhibition of phosphodiesterases (PDEs)] attenuates bleomycin-stimulated pulmonary fibrosis (10, 23, 24, 29, 33). Thus, agents working through the cAMP signaling cascade appear to have antifibrotic potential, but no therapies using these mechanisms have proven effective in humans. This may relate to an inherent lack of efficacy in the cAMP signaling system or an inability to sufficiently target drug action to immune and fibroblast cells.
There are nine different G protein-regulated AC isoforms, each with different tissue distribution and regulation (17). Such differences in regulation include stimulation or inhibition by βγ-subunits of activated G proteins, Ca2+, and various protein kinases. Among the isoforms, AC2, AC6, AC8, and AC9 predominate in the lung (7). We have detected AC5, AC6, AC8, and AC9 in a pulmonary fibroblast cell line, WI-38 (25). AC6 is inhibited by PKA, low concentrations (0.1–1 μM) of free Ca2+, nitric oxide, and directly by Gαi and Gβγ (2, 18, 32). Because of its susceptibility to inhibition, AC6 may be particularly useful in production of tightly regulated, but significantly enhanced, agonist-stimulated cAMP formation.
We tested the hypothesis that overexpression of AC6 will enhance cAMP formation in pulmonary fibroblasts and inhibit fibrogenic responses. Our results indicate that AC6 overexpression in isolated pulmonary fibroblasts increases cAMP-generating capacity stimulated by β-AR or prostacyclin receptor (IPR) agonists, but not by other Gs-coupled receptors. Furthermore, these same agents’ ability to inhibit collagen production or myofibroblast differentiation was also enhanced by gene transfer of AC6. Finally, we show that transgenic overexpression of AC6 under a fibroblast-specific promoter reduced bleomycin-induced lung fibrosis. We conclude that circulating hormones or locally generated mediators exist in sufficient quantity to suppress lung fibrosis in mice when cAMP signaling is enhanced via overexpression of AC.
MATERIALS AND METHODS
Materials.
Primary antibody for Flag (monoclonal, F1804) was obtained from Sigma-Aldrich. Primary antibodies for caveolin-1 (monoclonal, 610057) and caveolin-2 (monoclonal, 610684) were obtained from BD Biosciences. Primary antibodies for AC5/6 (polyclonal, sc-25500), AC2 (polyclonal, sc-587), AC7 (polyclonal, sc-68138), β2-AR (polyclonal, sc-569), PGE2 receptor (EP2R; polyclonal, sc-20675), α-smooth muscle actin (monoclonal, sc-130617), collagen I (monoclonal, sc-59772), and collagen III (monoclonal, sc-59816) and secondary antibodies were obtained from Santa Cruz Biotechnology. 5′-N-ethylcarboxamidoadenosine (NECA) was obtained from Tocris Bioscience. Beraprost and butaprost were obtained from Cayman Chemical. All other chemicals and reagents were obtained from Sigma-Aldrich.
Cell isolation and culture.
Pulmonary fibroblasts were isolated from Sprague-Dawley rats or FVB/N mice. Lungs were rapidly excised and then minced, pooled, and placed in a collagenase-pancreatin digestion solution. After five sequential digestions, the fibroblasts were pelleted and resuspended in DMEM supplemented with penicillin, streptomycin, amphotericin B (Fungizone), and 10% FBS (Atlanta Biologicals). After a 30-min period of attachment to uncoated culture plates, cells that were weakly attached or unattached were rinsed free and discarded. Cells were then cultured for 2–4 days. The purity of lung fibroblast cultures was >95% as determined by positive staining for vimentin and negative staining for smooth muscle actin and von Willebrand factor, as previously described (39). All animals were treated according to the National Institutes of Health principles of laboratory animal care (NIH Publication No. 85-23, revised 1985) and applicable US law. All procedures were submitted to the University of Tennessee Health Science Center Institutional Animal Care and Use Committee, which approved the studies prior to their initiation.
Measurement of cAMP accumulation.
Rat or mouse pulmonary fibroblasts were grown to 80% confluency on 24-well plates. Cells were washed three times with serum- and NaHCO3-free DMEM supplemented with 20 mM HEPES, pH 7.4. After equilibration at 37°C for 30 min, the cells were incubated with the indicated drugs plus 0.2 mM isobutylmethylxanthine, a broadly specific PDE inhibitor, for 10 min. Assay medium was aspirated, and 200 μl of lysis buffer (GE Healthcare) were added to each well to terminate each reaction. cAMP content of the lysis buffer extract was quantified using the Biotrak ELISA kit (GE Healthcare) according to the manufacturer's acetylation protocol. Data were normalized to the amount of protein in each sample, as determined using a dye-binding protein assay (Bio-Rad).
Nondetergent isolation of caveolar and noncaveolar membranes.
Rat lung fibroblasts were fractionated using a detergent-free method. Plates (10 cm) at 70–80% confluency were washed twice in ice-cold PBS and scraped into a total of 1.5 ml of 500 mM sodium carbonate, pH 11. Cells were homogenized with 20 strokes in a tissue grinder followed by three 20-s bursts with an ultrasonic cell disruptor. A full 1-min rest period was included between ultrasonic bursts. One milliliter of homogenate was brought to 45% sucrose by addition of an equal volume of 90% sucrose in MBS (25 mM MES and 150 mM NaCl, pH 6.5) and loaded in an ultracentrifuge tube. A discontinuous sucrose gradient was layered on top of the sample as follows: 2 ml of 35% sucrose prepared in MBS with 250 mM sodium carbonate and then 1 ml of 5% sucrose (also in MBS-Na2CO3). The gradient was centrifuged at 46,000 rpm on a SW55Ti rotor (Beckman Coulter, Fullerton, CA) for 16–18 h at 4°C. The faint light-scattering band was collected from the 5–35% sucrose interface (lipid raft fractions), while the bottom of the gradient (45% sucrose) was collected as nonraft material. Raft and nonraft fractions, along with whole cell lysate, were then analyzed by SDS-PAGE (with loading of equal proportions of each fraction) and immunoblot.
Immunoblot analysis.
Whole cell lysates were obtained as follows: cells were scraped in modified RIPA lysis buffer [0 mM Tris·HCl, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, plus mammalian protease inhibitor cocktail (catalog no. P-8340, Sigma Aldrich)] and homogenized by sonication. Equal protein amounts of each lysate [as determined using a dye-binding protein assay (Bio-Rad)] were separated on 10% SDS-polyacrylamide gels by electrophoresis before being transferred to polyvinylidene difluoride membranes (Millipore) by electroblotting. Membranes were blocked in 20 mM PBS with 3% nonfat dry milk and incubated with primary antibody for 2–12 h at 4°C with constant rocking. Bound primary antibodies were visualized using appropriate secondary antibody with conjugated horseradish peroxidase (Santa Cruz Biotechnology) and enhanced chemiluminescence (ECL) reagent (Pierce). Some primary antibodies recognized multiple nonspecific protein species. In these cases, appropriately sized immunoreactive bands were identified on the basis of expected molecular weight of the protein of interest, and only these bands are shown.
Assay of collagen synthesis.
Collagenase-sensitive [3H]proline incorporation was measured according to a method described previously (27, 39). Rat pulmonary fibroblasts were cultured in 12-well plates and then serum-starved for 24 h in 0.25% FBS. [3H]proline (1 μCi/well; PerkinElmer Life and Analytical Sciences, Boston, MA) was added to each well, along with vehicle, various drugs of interest, and 2.5% FBS for 24 h (as indicated). After media were aspirated and cells were washed, cells were removed by trypsinization, and the cellular protein was precipitated in trichloroacetic acid (20%). Precipitated protein was pelleted by centrifugation, washed in Tris-CaCl2-N-ethymaleimide buffer, and then digested with collagenase (2 mg/ml; Worthington Biochemicals, Freehold, NJ). Nondigested proteins were precipitated with 10% trichloroacetic acid and centrifugation. The collagenase-sensitive [3H]proline content of the supernatant was determined by liquid scintillation counting.
Production and screening of FTS1-AC6 mice.
We cloned the full-length promoter of the fibroblast-specific protein-1 gene, termed FTS1, from a mouse genomic library. This promoter has been shown to drive expression specifically in fibroblasts of many different tissues (35). After deletion of the cytomegalovirus promoter of pEGFP-N1 by restriction enzyme digestion, FTS1 was ligated into pEGFP-N (Clontech) for validation of the promoter. We then removed the enhanced green fluorescence protein (EGFP) gene from the resulting vector by restriction digest and ligated the human AC6 gene (NM_015270) with an NH2 terminally fused Flag epitope by PCR using PfuUltra High Fidelity DNA polymerase (Stratagene). The FTS1-AC6 construct was transiently transfected into human embryonic kidney (HEK-293) and COS-7 cells for validation. The linearized FTS1-AC6 construct was then purified and microinjected into fertilized FVB/N eggs. Two founder lines were individually backcrossed with wild-type mice to generate FTS1-AC6+/− mice. Genotyping was performed by automated real-time quantitative PCR (Transnetyx).
Bleomycin-induced pulmonary fibrosis.
Animals were intratracheally instilled with 2 U/kg bleomycin hydrochloride in sterile saline (Nippon Kayaku, Tokyo, Japan) in a total volume of ≤50 μl saline on day 0. Control mice received an intratracheal injection of saline on day 0. Intratracheal instillation was performed on anesthetized mice via a 27-gauge needle through the exposed trachea. The 2-wk survival rate was >80%. On day 14, the mice were killed and the lungs were dissected for analysis. After the superior vena cava was severed, 5 ml of saline were perfused through the left ventricle, and then 3 ml of 10% neutralized buffered formalin were infused. The lung was dissected, and the right lobes were immediately frozen and the left lobes were placed in 10% formalin solution. Left lung tissues were processed for histology by the University of Tennessee Health Science Center Pathology Laboratory. Sections (5-μm-thick) from paraffin-embedded tissues were mounted on glass slides, and serial sections were stained with hematoxylin and eosin or Masson's trichrome. Entire slides were then digitally scanned on a digital slide scanner (ScanScope XT, Aperio Technologies). The scoring system of Ashcroft et al. (1) was used by an investigator blinded to the treatment conditions to evaluate the extent of inflammation or fibrosis. All fields of each section were evaluated and scored, and the mean value for each animal is reported.
Assay of tissue collagen, 6-keto-PGF1α, and norepinephrine and epinephrine content.
Acid-soluble collagen was measured in homogenates from frozen lung tissues using the Sircol assay according to the manufacturer's protocol (Biocolor). Tissue was diced and then homogenized in 0.5 M acetic acid and protease inhibitor cocktail and stirred overnight at 3°C. Then 200 μl of the test sample were mixed with 1 ml of dye reagent for 30 min at room temperature. The collagen-dye complex was precipitated by centrifugation at 14,000 g for 5 min, washed twice with ethanol, and dissolved in 1 ml 0.5 M NaOH. The absorbance of each sample was measured at 540 nm using a plate-reading spectrophotometer (Biotek). Collagen standards (provided by the manufacturer) were processed similarly and used to calculate the amount of collagen in each sample. The assay was performed in triplicate for each lung sample, and the mean was used as the sample value. To assay for 6-keto-PGF1α levels in lung, frozen tissues were homogenized in cold methanol and then cooled in a −80°C freezer for 30 min. Samples were then spun at 2,000 g for 30 min, the supernatant was dried under a stream of nitrogen, and the samples were reconstituted in enzyme immunoassay buffer and assayed for 6-keto-PGF1α levels by ELISA (Cayman Chemical) following the manufacturer's protocols. Lung catecholamine concentrations were measured from frozen lung tissues by homogenization in modified RIPA lysis buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, and 1% Igepal CA-630) with 10 mM ascorbic acid and 0.1 mM EDTA to prevent oxidation. Lysates were then centrifuged for 5 min at 1,000 g, and supernatants were assayed for epinephrine or norepinephrine levels by ELISA (Rocky Mountain Diagnostics) following the manufacturer's protocols. Data were normalized to the wet weight of the total lung tissue for each sample.
Data analysis and statistics.
Data are presented as means ± SE and, in some cases, as representative images of at least three separate experiments. For in vivo studies of bleomycin-induced fibrosis, we estimated from published studies a survival rate of 70% and that 10–15 animals would be required for statistical confidence. We treated animals in groups of five wild-type plus five FTS1-AC6+/− and monitored outcomes to minimize the number of animals used in the study. GraphPad Prism 5.0 (GraphPad Software) was used for statistical comparisons (t-tests and 1-way analysis of variance) and graphics.
RESULTS
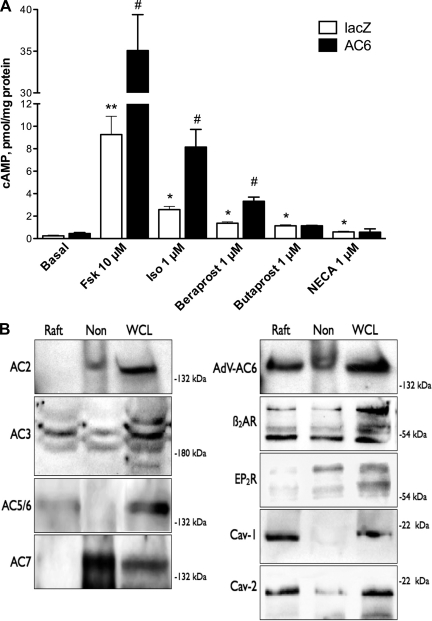
Rat pulmonary fibroblasts respond to several hormones and agonists with increases in cAMP formation. To test if these cognate GPCRs selectively link to a particular AC isoform, we treated cells with recombinant adenoviruses expressing lacZ (as a control for adenoviral infection) or AC6 and then measured cAMP generation. We found that forskolin (a direct activator of AC, 10 μM), isoproterenol (a β-AR agonist, 1 μM), beraprost (an IPR agonist, 1 μM), butaprost (an EP2R-selective agonist), and NECA (an adenosine receptor agonist) led to increases in cAMP production in control cells (Fig. 1). AC6 overexpression did not alter basal cAMP production but induced a sizable increase in production stimulated by forskolin, isoproterenol, and beraprost compared with responses to these agonists in control cells (Fig. 1). By contrast, butaprost- and NECA-stimulated cAMP production was unchanged in cells that overexpress AC6.
Fig. 1.
Adenylyl cyclase (AC) 6 overexpression selectively enhances cAMP production stimulated by various receptor agonists due to colocalization in lipid rafts. A: rat pulmonary fibroblasts were cultured on 24-well plates and incubated with an adenovirus expressing lacZ (control) or human AC6 for 24 h. Cells were equilibrated in serum-free medium for 1 h and then treated for 10 min with the indicated drug in the presence of a phosphodiesterase inhibitor. cAMP levels in cell lysates were measured by ELISA. Fsk, forskolin; Iso, isoproterenol; NECA, 5′-N-ethylcarboxamidoadenosine. Values are means ± SE (n = 5). *P < 0.05; **P < 0.01 vs. basal (no drug; by paired t-test). #P < 0.05 vs. lacZ (by paired t-test). B: untreated rat pulmonary fibroblasts (control) and cells incubated for 24 h with an adenovirus expressing human AC6 (Adv-AC6) were fractionated using a nondetergent method to isolate lipid rafts and caveolae. After sucrose density centrifugation, fractions 2–4 were combined as the raft fraction and fractions 7–10 were combined as the nonraft fraction. Each pooled fraction and a whole cell lysate (WCL) control were separated by SDS-PAGE and analyzed by immunoblot. β2-AR, β2-adrenergic receptor; EP2R, PGE2 receptor; Cav, caveolin. Images are representative of 3 experiments.
Previous studies reported compartmentation of AC6 in lipid rafts, microdomains of the plasma membrane enriched in sphingolipid and cholesterol, and selective coupling of β-AR and IPRs to AC6 because of their colocalization in lipid rafts (28, 37). We used sucrose density centrifugation to fractionate rat lung fibroblasts and isolate lipid rafts and used immunoblot analysis to assay for AC isoforms and GPCR localization. Buoyant lipid raft fractions contained the vast majority of caveolin-1 and caveolin-2 immunoreactivity (Fig. 1B). We detected expression of AC2, AC3, AC5/6, and AC7, with AC3 and AC7 the most readily detected isoforms in rat lung fibroblasts. Only AC3 and AC5/6 immunoreactivity was observed in lipid raft fractions. When we fractionated cells overexpressing AC6 (AdV-AC6), AC5/6 immunoreactivity was observed primarily in lipid raft fractions. We also detected β2-AR and EP2R immunoreactivity in rat pulmonary fibroblasts. β2-ARs were detected in lipid raft and nonraft fractions, while the bulk of EP2R immunoreactivity was observed in heavy nonraft fractions. These data imply that AC6 overexpression in rat lung fibroblasts localizes primarily in lipid rafts and that these microdomains include β2-ARs but exclude EP2Rs.
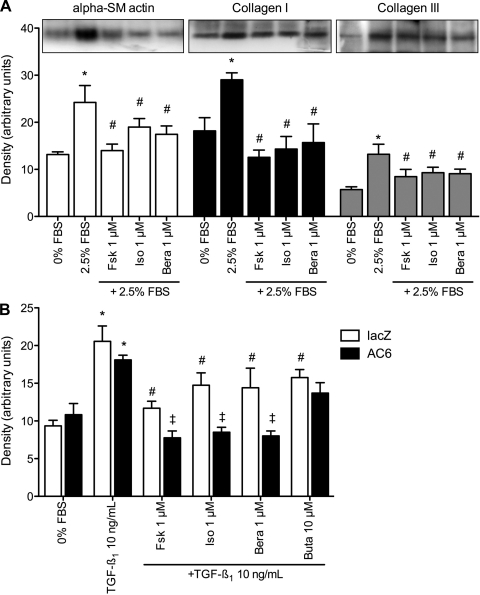
To examine if agents that elevate cAMP production might lead to alterations in fibroblast function at the cellular level, we used similar conditions to measure collagen synthesis and α-smooth muscle actin expression. Treatment of serum-starved rat pulmonary fibroblasts for 18 h with 2.5% FBS significantly increased immunoreactivity for collagen I, collagen III, and α-smooth muscle actin (Fig. 2A). Cotreatment with 1 μM forskolin, isoproterenol, or beraprost reduced immunoreactivity of each of these proteins. PGE2 (10 μM) or butaprost (1 μM) also decreased immunoreactivity of collagen I, collagen III, and α-smooth muscle actin (data not shown). Thus, cAMP-elevating agents have a clear and consistent effect: they reduce production of two primary forms of lung collagen and inhibit differentiation into myofibroblasts.
Fig. 2.
cAMP-elevating agents inhibit α-smooth muscle (SM) actin and collagen expression. A: immunoblot analysis of α-smooth muscle actin, collagen I, and collagen III in lysates from serum-starved rat pulmonary fibroblasts treated with serum (FBS) and the indicated drugs for 24 h. Top: representative immunoblot image; bottom: densitometric analysis of immunoreactive bands. Bera, beraprost. B: immunoblot analysis of α-smooth muscle actin in lacZ (control) and AC6-overexpressing rat pulmonary fibroblasts treated with transforming growth factor (TGF)-β1 and various cAMP-elevating agents. Densitometric analysis of immunoreactive bands is shown. Values are means ± SE (n = 3–4). *P < 0.05 vs. no-serum (by paired t-test). #P < 0.05 vs. 2.5% FBS or TGF-β1 alone (by paired t-test). ‡P < 0.05 vs. lacZ.
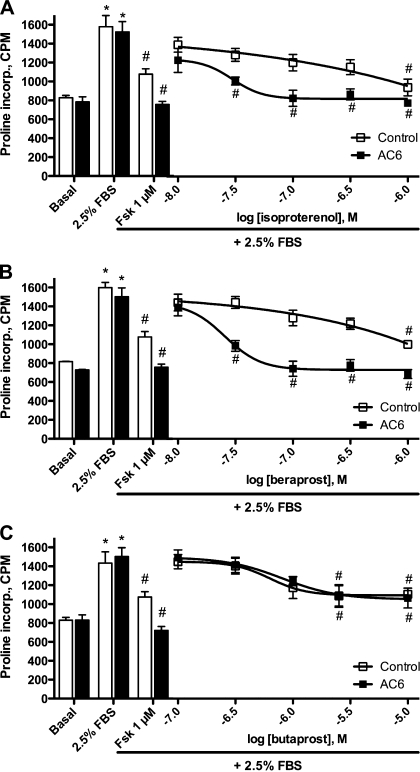
We next examined if increased expression of AC6 would lead to enhancement of these receptor-mediated cellular responses. AC6 overexpression enhanced the inhibition of TGF-β1-stimulated α-smooth muscle actin expression by forskolin, isoproterenol, and beraprost but did not alter the response to butaprost (Fig. 2B). These results are consistent with the effect of AC6 overexpression on cAMP production stimulated by these same agonists. We also assayed for collagenase-sensitive proline incorporation in cells incubated with lacZ (control) or AC6 recombinant adenoviruses to examine the concentration response of these cAMP-elevating agents. Rat pulmonary fibroblasts were stimulated with 2.5% FBS with and without forskolin or various concentrations of isoproterenol, beraprost, or butaprost. Forskolin-mediated inhibition of proline incorporation was increased by overexpression of AC6 (Fig. 3). In control cells, <1 μM isoproterenol did not produce a statistically significant decrease in proline incorporation. However, in cells where AC6 was overexpressed, significant inhibition of proline incorporation was observed at isoproterenol concentrations as low as 30 nM (Fig. 3A). Similar effects of AC6 overexpression were observed with increasing concentrations of beraprost (Fig. 3B), but no effect was observed at any concentration of butaprost (Fig. 3C). Taken together, these data are consistent with the idea that increased AC6 expression enhances cAMP production stimulated by β-ARs and IPRs, but not EP2Rs, causing an increased inhibitory effect on collagen synthesis and myofibroblast differentiation.
Fig. 3.
Effect of AC6 overexpression on isoproterenol-, beraprost-, or butaprost-mediated inhibition of collagen synthesis. Rat pulmonary fibroblasts were cultured on 12-well plates and incubated with recombinant adenovirus expressing lacZ (control, open bars and symbols) or human AC6 (AC6, solid bars and symbols) for 24 h. Cells were cultured in serum-free medium for 24 h and then treated for 24 h with 2.5% FBS, the indicated drug, and [3H]proline. Collagenase-sensitive proline incorporation [counts/min (cpm)] was assayed as a measure of collagen synthesis. Bars show basal and stimulated levels of proline incorporation along with forskolin inhibition of the response (positive control). Line graphs show effect of indicated agonist on FBS-stimulated proline incorporation. Values are means ± SE (n = 6). *P < 0.05 vs. basal (no FBS; by paired t-test). #P < 0.05 vs. 2.5% FBS alone (by paired t-test).
To directly test the idea that increased cAMP generation by fibroblasts can be antifibrotic, we generated a transgenic mouse overexpressing AC6 under a fibroblast-specific promoter. We cloned the FTS-1 promoter upstream of fibroblast-specific protein-1 (22, 35) and ligated it with the human AC6 gene. The FTS-1 promoter appears to be activated in fibroblast cells of various origins, including those arising from epithelial-mesenchymal transitions and from infiltrating macrophages, and its activity is increased during lung fibrogenesis (22, 36). We generated transgenic mice expressing FTS1-AC6 in an FVB/N background strain. These mice show no outward phenotype from birth to 20 mo of age, reproduce normally, and have a normal life span. Routine histology of major organs of 4- and 20-mo-old FTS1-AC6+/− mice revealed no alterations or abnormalities. Homozygous FTS1-AC6+/+ mice also display no outward phenotype.
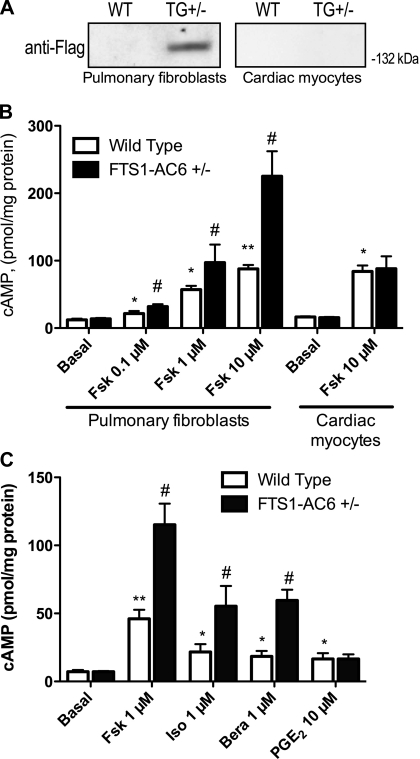
We examined the specificity of the FTS-1 promoter by isolating cardiac myocytes and pulmonary fibroblasts from FTS1-AC6+/− mice and wild-type littermate controls from two different founder lines. Immunoblot analysis for the epitope-tagged AC6 showed that pulmonary fibroblasts express the epitope-tagged AC6 protein encoded by the transgene but that wild-type littermate control mice do not (Fig. 4A). By contrast, cardiac myocytes isolated from FTS1-AC6+/− and wild-type mice were negative for Flag-AC6 immunoreactivity. We also measured cAMP production in isolated cells in response to multiple concentrations of forskolin. Maximal cAMP production (10 μM forskolin) was increased ∼2.5-fold in pulmonary fibroblasts from FTS1-AC6+/− mice but was not different in cardiac myocytes isolated from wild-type littermates (Fig. 4B). Forskolin-stimulated cAMP was similarly enhanced in isolated cardiac fibroblasts (data not shown). Thus the FTS1 promoter drives expression of AC6 in fibroblasts, but not in cardiac myocytes.
Fig. 4.
FTS1-AC6 transgenic mice display enhanced cAMP production in fibroblasts. A: immunoblot analysis for the Flag epitope on AC6 in whole cell lysates from pulmonary fibroblasts or cardiac myocytes isolated from wild-type (WT) or FTS1-AC6+/− (TG+/−) mice. B: cAMP production in pulmonary fibroblasts or cardiac myocytes isolated from FTS1-AC6+/− mice or littermate controls (wild-type). C: cAMP production stimulated by various receptor agonists or forskolin in pulmonary fibroblasts isolated from FTS1-AC6+/− mice or littermate controls (wild-type). Cells cultured on 24-well plates were equilibrated in serum-free medium for 1 h and then treated for 10 min with the indicated drug in the presence of a phosphodiesterase inhibitor. cAMP levels in cell lysates were measured by ELISA. Values are means ± SE (n = 3–4). *P < 0.05; **P > 0.01 vs. basal (no drug; by paired t-test). #P < 0.05 vs. wild-type (by paired t-test).
We then measured cAMP production stimulated by various receptor agonists in isolated mouse lung fibroblasts from FTS1-AC6+/− mice and littermate controls. We found a pattern of receptor coupling to AC6 that mirrored the pattern observed in rat lung fibroblasts with AC6 overexpressed via adenovirus (Fig. 1). cAMP production in response to isoproterenol and butaprost was significantly higher in cells isolated from FTS1-AC6+/− mice than in cells from wild-type animals (Fig. 4C). The levels of cAMP stimulated by addition of PGE2 were not different between FTS1-AC6+/− and wild-type mice, indicating that transgenic overexpression of AC6 also selectively couples to β-ARs and IPRs over EP2Rs.
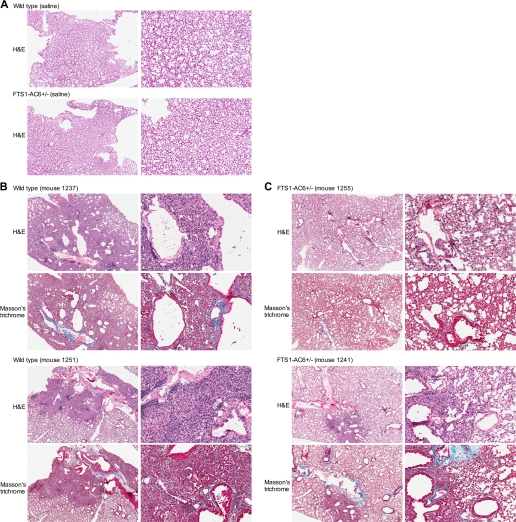
To examine in vivo fibrogenesis, heterozygous FTS1-AC6+/− mice (n = 10, 5 males and 5 females) or littermate controls (n = 9, 5 males and 4 females) were intratracheally injected with 2 U/kg bleomycin or vehicle control (<50 μl total volume). Mice were killed 14 days after treatment and perfused systemically with formalin, and the lungs were removed for histological examination. The survival rate was 60% for bleomycin-treated wild-type mice and 83% for FTS1-AC6+/− mice (Table 1). Left lobes of the lung from surviving mice were embedded in paraffin and sectioned in a left-right medial plane. Serial sections of the left lobe were stained with Masson's trichrome and hematoxylin-and-eosin, and each slide was examined in a blinded fashion. FTS1-AC6+/− and wild-type mice intratracheally instilled with saline showed normal lung morphology (Fig. 5A). The bleomycin-treated wild-type mice showed extensive peribronchial and interstitial fibrosis, inflammation, and collagen deposition (Fig. 5A). By contrast, FTS1-AC6+/− mice displayed more limited fibrotic development, less inflammation, and less peribronchial and perivascular collagen deposition (Fig. 5B). A statistically significant decrease in body weight was observed bleomycin-treated wild-type mice, but not in FTS1-AC6+/− mice (Table 1).
Table 1.
Survival rate and body weight 14 days after bleomycin administration
| Survival, % | Body Weight, g | |
|---|---|---|
| Wild-type | ||
| Vehicle | 100 | 31.7 ± 0.8 |
| Bleomycin | 60 | 26.8 ± 2.1* |
| FTS1-AC6+/− | ||
| Vehicle | 100 | 31.1 ± 0.5 |
| Bleomycin | 83 | 28.0 ± 1.7 |
Values for body weight are means ± SE. Deceased animals are not included in survival or body weight measurements.
P < 0.05 vs. vehicle.
Fig. 5.
Histological examination of lungs from FTS1-AC6+/− or wild-type control mice following intratracheal administration of bleomycin. Bleomycin (2 U/kg, <50 μl total volume) or saline was instilled directly into the trachea of FTS1-AC6+/− mice or wild-type littermate controls. At 14 days following treatment, lungs were dissected, sectioned, and stained with hematoxylin and eosin (H & E) or Masson's trichrome. Images of similar fields for each stain were captured at ×2 and ×10 magnification. Saline-treated animals displayed normal lung architecture (A). Images from 2 bleomycin-treated animals from each group (wild-type mice in B and FTS1-AC6+/− mice in C) are representative of 9 wild-type and 10 FTS1-AC6+/− mice.
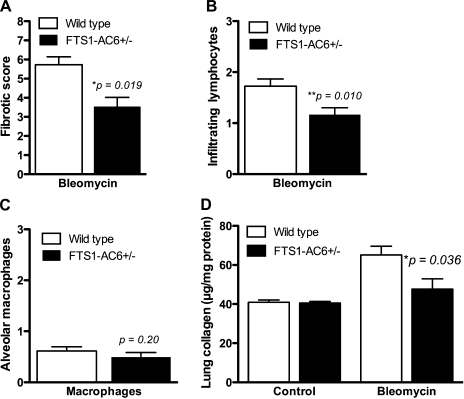
Histological scoring for fibrosis, inflammation, and intra-alveolar macrophages was performed by a blinded investigator on all fields from multiple sections of each lung. The method of Ashcroft et al. (1) was used for fibrotic scoring, while the degree of inflammation was assessed on a scale of 1–3. Scores were assigned to each microscopic field under a ×10 objective and then averaged to yield a single score for each individual animal. The data indicate a statistically significant effect of the FTS1-AC6 transgene on fibrotic score and degree of inflammation (Fig. 6, A and B). By contrast, the amount of foamy macrophages evident in alveolar spaces (an index of the degree of injury induced by intratracheal administration of bleomycin, also scored on a scale of 1–3) was not different between the transgenic and nontransgenic littermates (Fig. 6C). The Sircol dye binding assay was used to measure total acid-soluble collagen content in the right lobe of each lung. Collagen content of lungs from FTS1-AC6+/− mice did not differ in the vehicle control group but was significantly lower in the bleomycin-treated group (Fig. 6D). Statistical analysis of variance by sex showed no difference in any of these measures between males and females. Taken together, these data lead us to the conclusion that FTS1-AC6+/− mice are resistant to bleomycin-induced pulmonary fibrosis because of reduced inflammation and collagen synthesis.
Fig. 6.
Pathological scoring of fibrosis, inflammation, and collagen content of the lung following bleomycin instillation. Mice were treated with intratracheal bleomycin, and lungs were dissected for histological analysis 14 days later. An investigator blinded to the transgene status of the animals examined the stained sections and assessed the fibrotic score on a scale of 1–8 using the method of Ashcroft et al. (1) (A) and the number of infiltrating lymphocytes (B) and alveolar macrophages (C) on a scale of 1–3. Lung collagen content was measured by the Sircol assay and expressed as total acid-soluble collagen/mg protein (D). Values are means ± SE (n = 9–10). Data were analyzed by 1-way ANOVA with Tukey's post hoc tests, and P values are shown.
Basal levels of cAMP production are unchanged in isolated lung fibroblasts and transgenic mice overexpressing AC6 (Figs. 1 and 4). Because our studies of lung fibrosis did not employ infused pharmacological agents, we hypothesized that endogenous hormones exist during fibrogenesis that are sufficient to activate GPCRs coupled to increases in cAMP production in lung fibroblasts. We used ELISAs to measure the endogenous levels of hormones capable of activating β2-ARs or IPRs from mouse lungs. Concentrations of 6-keto-PGF1α, the stable metabolite of prostacyclin, were elevated in bleomycin-treated compared with saline-treated animals (Table 2). There was no statistical difference in 6-keto-PGF1α concentration between wild-type and FTS1-AC6+/− mice. Tissue concentrations of norepinephrine and epinephrine were below detectable levels (<5 ng/g) in lungs of vehicle-treated animals but were readily detectable in wild-type and FTS1-AC6+/− mice treated with bleomycin (Table 2). Both catecholamines were detected at significantly lower concentrations in bleomycin-treated FTS1-AC6+/− mice than in similarly treated wild-type mice. The lower levels of tissue catecholamines in FTS1-AC6+/− mice may be a reflection of the reduced stress in these animals as a result of decreased disease severity or other factors. We conclude that levels of prostacyclin and catecholamines are elevated during lung fibrogenesis and could be responsible for driving the antifibrotic phenotype observed in FTS1-AC6+/− mice.
Table 2.
6-Keto-PGF1α and catecholamine levels in lungs from saline- and bleomycin-treated mice
| 6-Keto-PGF1α, ng/g | Norepinephrine, ng/g | Epinephrine, ng/g | |
|---|---|---|---|
| Wild-type | |||
| Vehicle | 38.8 ± 6.5 | BD | BD |
| Bleomycin | 59.3 ± 2.7* | 67.0 ± 14.4 | 43.9 ± 11.5 |
| FTS1-AC6+/− | |||
| Vehicle | 36.5 ± 2.3 | BD | BD |
| Bleomycin | 62.7 ± 10.2* | 27.2 ± 6.3† | 11.87 ± 5.6† |
Values are means ± SE. BD, below limits of detection (∼5 ng/g).
P < 0.05 vs. vehicle.
P < 0.05 vs. wild-type.
DISCUSSION
Several reports have demonstrated that AC6 overexpression only enhances signaling by certain GPCRs because of compartmentation of receptors and AC6 in lipid rafts (37). These and other data support the idea that signaling components are spatially organized within plasma membrane microdomains and perhaps prearranged in signaling complexes (8, 47). We previously reported that AC6 overexpression selectively enhances signaling by β-ARs and IPRs in cardiac fibroblasts and that this led to enhanced inhibition of collagen synthesis and myofibroblast differentiation by these receptors (28). The present data extend these findings to pulmonary fibroblasts but, further, demonstrate that these actions on cell function have physiological relevance to an in vivo model of pulmonary fibrosis.
We and others have examined the potential antifibrotic role of many agents coupled to increased intracellular cAMP production in pulmonary fibroblasts (21, 25, 26). We have also shown that pulmonary fibroblasts derived from fibrotic lung possess a defect in the cAMP-PKA-cAMP response element-binding protein signaling pathway that may explain their increased fibrotic activity (27). cAMP appears to exert its antifibrotic action via multiple intracellular signaling pathways. One is the inhibition of Smad-mediated transcription via competition between cAMP response element-binding protein and Smad for key transcriptional coactivators (26). Another is cAMP-mediated inhibition of certain non-Smad signaling pathways activated by TGF-β, including ERK1/2 and JNK MAP kinases (26). These, and likely other, antifibrotic actions of cAMP appear to occur through activation of PKA and Epac-1 (19, 52). Because of the widespread deleterious effects of prolonged infusion of PKA inhibitors and the lack of Epac inhibitors, we are unable to confirm that the antifibrotic phenotype of FTS1-AC6+/− mice is due to their increased cAMP signaling. However, AC's sole enzymatic products are cAMP and pyrophosphate, and our data from studies of isolated cells using specific receptor agonists corroborate these in vivo findings. Also, FTS1-AC6+/− mice display no outward phenotype until they are subjected to a disease model, likely because of the highly regulated activity of AC6. Taken together, our findings support the notion that sustained increases in intracellular levels of cAMP negatively regulate fibroblast function.
Other studies demonstrate the potential of prostanoid mediators, such as PGE2 and prostacyclin, to inhibit fibrogenesis in the lung. Early studies indicated that a macrophage-derived product liberated PGE2 in the lung during bleomycin-induced fibrosis and caused an inhibition of collagen synthesis (3). It was later reported that PGE2 acts via elevation of cAMP production in fibroblasts to inhibit collagen synthesis and cell proliferation (11, 49). Patients with idiopathic pulmonary fibrosis or rats treated with bleomycin have lung fibroblasts with diminished PGE2-generating capacity (34, 50). Prostacyclin or its analogs can inhibit the development of bleomycin-induced pulmonary fibrosis (29, 33). The action of endogenously produced prostacyclin appears sufficient for this antifibrotic action, while endogenous PGE2 is incapable of such action (29). Thus, prostacyclin appears to be generated by the lung during fibrogenesis and, by virtue of its ability to increase cAMP production in fibroblasts, appears to have antifibrotic action.
The present data support the role of prostacyclin and catecholamines as endogenous antifibrotic mediators. We show in isolated lung fibroblasts that overexpression of AC6 increases cAMP production in response to β-AR and IPR, but not EP2R, agonists. Pulmonary fibroblasts isolated from FTS1-AC6 mice display this same selectivity of action and are resistant to the development of bleomycin-induced fibrosis. In our studies of lung fibrosis, the mice received no adjunct therapies. We detected increased levels of endogenous catecholamines and prostacyclin in lungs from bleomycin-treated mice, with FTS1-AC6+/− mice displaying similar levels of the prostacyclin metabolite but lower levels of catecholamines than their wild-type counterparts. These data lead us to conclude that these endogenous hormones lack sufficient efficacy to limit lung fibrogenesis in wild-type animals but, when AC6 is overexpressed, possess sufficient efficacy to limit the development of fibrosis. This shortfall in inherent efficacy of these signaling systems may mean that traditional pharmacotherapies targeting these receptors (β-AR or IPR agonists) would be ineffective at slowing fibrogenesis.
Hormones acting through GPCRs often initiate multiple signaling events in addition to the canonical cAMP pathway. This diversity in receptor signaling, via βγ-subunits of the G protein or other modalities, leads to diverse effects on cell function. AC activity, by contrast, leads to generation of a single signaling molecule, and expression levels of AC appear to limit the ability of GPCRs to stimulate cAMP accumulation (13, 40). Twenty- to 100-fold overexpression of β-ARs or Gsα in cells or transgenic mice yields only small increases in maximal cAMP production or functional correlates (9, 14, 30). Moreover, β2-AR overexpression in the heart leads to temporary increases in cardiac function that eventually give way to cardiomyopathies (6, 15). By contrast, increased expression of cardiac myocyte AC6 increases cardiac responsiveness to catecholamines, improves cardiac physiology in experimental heart failure, and extends life span (42, 43). Thus, GPCRs induce pleiotropic signaling and diverse cellular responses, some of which may be deleterious in pathological settings. On the other hand, increased AC expression amplifies only the cAMP signaling component by GPCRs to yield more specific alterations in response. These phenomena may also contribute to the observed effect of AC6 overexpression on lung fibrogenesis.
It is unclear if AC isoforms other than AC6 can play similar antifibrotic roles. One would expect that all ACs, by virtue of their ability to catalyze the synthesis of cAMP, would have the potential to enhance antifibrotic signals. However, cAMP signaling can be compartmentized in cells, and different AC isoforms reside in distinct cellular microdomains (38, 47). Our data show that EP2Rs can elicit antifibrotic responses, meaning that the cAMP emanating from these activated receptors is capable of antifibrotic signaling. This fact makes it tempting to speculate that overexpression of an AC isoform that couples to EP2/4Rs would enhance antifibrotic responses to PGE2 or its analogs. AC6 appears to be a minor isoform in pulmonary fibroblasts of mice, with AC3 and AC7 the most readily detected isoforms in our studies. It would be of interest to examine the effects of overexpression of AC3 or AC7 in lung fibroblasts.
Traditional pharmacological approaches have used receptors as the preferred sites to achieve specificity of effects and to minimize toxicity. This strategy has been enormously successful, since different GPCRs have different patterns of cellular expression and regulation. However, classical therapeutic approaches have largely failed to slow pulmonary fibrosis in humans. The present data suggest that targeting specific intracellular signaling pathways may effectively enhance signaling by endogenous hormones, such that disease progression can be slowed. Alternatively, combinatorial approaches for enhancing cAMP generation and action (including traditional receptor agonists and PDE inhibitors) may prove effective therapies for lung fibrosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-079166.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
The authors thank Dr. Ioannis Dragatsis and the University of Tennessee Health Science Center Transgenic Core Facility for generation of FTS1-AC6 founder lines and Dr. Arnold Postlethwaite for assistance with bleomycin instillation in mice.
REFERENCES
- 1.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol 41: 467–470, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayewitch ML, Avidor-Reiss T, Levy R, Pfeuffer T, Nevo I, Simonds WF, Vogel Z. Inhibition of adenylyl cyclase isoforms V and VI by various Gβγ subunits. FASEB J 12: 1019–1025, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Clark JG, Kostal KM, Marino BA. Bleomycin-induced pulmonary fibrosis in hamsters. An alveolar macrophage product increases fibroblast prostaglandin E2 and cyclic adenosine monophosphate and suppresses fibroblast proliferation and collagen production. J Clin Invest 72: 2082–2091, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clozel M, Salloukh H. Role of endothelin in fibrosis and anti-fibrotic potential of bosentan. Ann Med 37: 2–12, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwartz DA, Schultz GS, Wagner CR, Musson RA. Future research directions in idiopathic pulmonary fibrosis: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 166: 236–246, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Dash R, Kadambi V, Schmidt AG, Tepe NM, Biniakiewicz D, Gerst MJ, Canning AM, Abraham WT, Hoit BD, Liggett SB, Lorenz JN, Dorn GW, 2nd, Kranias EG. Interactions between phospholamban and β-adrenergic drive may lead to cardiomyopathy and early mortality. Circulation 103: 889–896, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Defer N, Best-Belpomme M, Hanoune J. Tissue specificity and physiological relevance of various isoforms of adenylyl cyclase. Am J Physiol Renal Physiol 279: F400–F416, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Dessauer CW. Adenylyl cyclase-a-kinase anchoring protein complexes: the next dimension in cAMP signaling. Mol Pharmacol 76: 935–941, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drazner MH, Peppel KC, Dyer S, Grant AO, Koch WJ, Lefkowitz RJ. Potentiation of β-adrenergic signaling by adenoviral-mediated gene transfer in adult rabbit ventricular myocytes. J Clin Invest 99: 288–296, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Failla M, Genovese T, Mazzon E, Fruciano M, Fagone E, Gili E, Barera A, La Rosa C, Conte E, Crimi N, Cuzzocrea S, Vancheri C. 16,16-Dimethyl prostaglandin E2 efficacy on prevention and protection from bleomycin-induced lung injury and fibrosis. Am J Respir Cell Mol Biol 41: 50–58, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Fine A, Goldstein RH. The effect of PGE2 on the activation of quiescent lung fibroblasts. Prostaglandins 33: 903–913, 1987 [DOI] [PubMed] [Google Scholar]
- 12.Fine A, Poliks CF, Donahue LP, Smith BD, Goldstein RH. The differential effect of prostaglandin E2 on transforming growth factor-β and insulin-induced collagen formation in lung fibroblasts. J Biol Chem 264: 16988–16991, 1989 [PubMed] [Google Scholar]
- 13.Gao M, Ping P, Post S, Insel PA, Tang R, Hammond HK. Increased expression of adenylyl cyclase type VI proportionately increases β-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA 95: 1038–1043, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, Vatner DE, Homcy CJ. Overexpression of Gsα protein in the hearts of transgenic mice. J Clin Invest 95: 1676–1683, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE. Common genomic response in different mouse models of β-adrenergic-induced cardiomyopathy. Circulation 108: 2926–2933, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 345: 517–525, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu Rev Pharmacol Toxicol 41: 145–174, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hill J, Howlett A, Klein C. Nitric oxide selectively inhibits adenylyl cyclase isoforms 5 and 6. Cell Signal 12: 233–237, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Huang SK, Wettlaufer SH, Chung J, Peters-Golden M. Prostaglandin E2 inhibits specific lung fibroblast functions via selective actions of PKA and Epac-1. Am J Respir Cell Mol Biol 39: 482–489, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kohyama T, Liu X, Wen FQ, Zhu YK, Wang H, Kim HJ, Takizawa H, Cieslinski LB, Barnette MS, Rennard SI. PDE4 inhibitors attenuate fibroblast chemotaxis and contraction of native collagen gels. Am J Respir Cell Mol Biol 26: 694–701, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Kolodsick JE, Peters-Golden M, Larios J, Toews GB, Thannickal VJ, Moore BB. PGE2 inhibits fibroblast to myofibroblast transition via EP2 signaling and cAMP elevation. Am J Respir Cell Mol Biol 29: 537–544, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Lawson WE, Polosukhin VV, Zoia O, Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG, Blackwell TS. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am J Respir Crit Care Med 171: 899–907, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Lindenschmidt RC, Witschi H. Attenuation of pulmonary fibrosis in mice by aminophylline. Biochem Pharmacol 34: 4269–4273, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Lindenschmidt RC, Witschi HP. Propranolol-induced elevation of pulmonary collagen. J Pharmacol Exp Ther 232: 346–350, 1985 [PubMed] [Google Scholar]
- 25.Liu X, Ostrom RS, Insel PA. cAMP-elevating agents and adenylyl cyclase overexpression promote an antifibrotic phenotype in pulmonary fibroblasts. Am J Physiol Cell Physiol 286: C1089–C1099, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Sun SQ, Hassid A, Ostrom RS. cAMP inhibits transforming growth factor-β-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and Smad signaling in cardiac fibroblasts. Mol Pharmacol 70: 1992–2003, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Sun SQ, Ostrom RS. Fibrotic lung fibroblasts show blunted inhibition by cAMP due to deficient cAMP response element-binding protein phosphorylation. J Pharmacol Exp Ther 315: 678–687, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Thangavel M, Sun SQ, Kaminsky J, Mahautmr P, Stitham J, Hwa J, Ostrom RS. Adenylyl cyclase type 6 overexpression selectively enhances β-adrenergic and prostacyclin receptor-mediated inhibition of cardiac fibroblast function because of colocalization in lipid rafts. Naunyn Schmiedebergs Arch Pharmacol 377: 359–369, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovgren AK, Jania LA, Hartney JM, Parsons KK, Audoly LP, Fitzgerald GA, Tilley SL, Koller BH. COX-2-derived prostacyclin protects against bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 291: L144–L156, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Milano CA, Allen LF, Rockman HA, Dolber PC, McMinn TR, Chien KR, Johnson TD, Bond RA, Lefkowitz RJ. Enhanced myocardial function in transgenic mice overexpressing the β2-adrenergic receptor. Science 264: 582–586, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Mio T, Adachi Y, Carnevali S, Romberger DJ, Spurzem JR, Rennard SI. β-Adrenergic agonists attenuate fibroblast-mediated contraction of released collagen gels. Am J Physiol Lung Cell Mol Physiol 270: L829–L835, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Mons N, Decorte L, Jaffard R, Cooper DM. Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci 62: 1647–1652, 1998 [DOI] [PubMed] [Google Scholar]
- 33.Murakami S, Nagaya N, Itoh T, Kataoka M, Iwase T, Horio T, Miyahara Y, Sakai Y, Kangawa K, Kimura H. Prostacyclin agonist with thromboxane synthase inhibitory activity (ONO-1301) attenuates bleomycin-induced pulmonary fibrosis in mice. Am J Physiol Lung Cell Mol Physiol 290: L59–L65, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Ogushi F, Endo T, Tani K, Asada K, Kawano T, Tada H, Maniwa K, Sone S. Decreased prostaglandin E2 synthesis by lung fibroblasts isolated from rats with bleomycin-induced lung fibrosis. Int J Exp Pathol 80: 41–49, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okada H, Danoff TM, Fischer A, Lopez-Guisa JM, Strutz F, Neilson EG. Identification of a novel cis-acting element for fibroblast-specific transcription of the FSP1 gene. Am J Physiol Renal Physiol 275: F306–F314, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol Renal Physiol 273: F563–F574, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Ostrom RS, Gregorian C, Drenan RM, Xiang Y, Regan JW, Insel PA. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J Biol Chem 276: 42063–42069, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Ostrom RS, Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol 143: 235–245, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, Insel PA, Brunton LL, Meszaros JG. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J Biol Chem 278: 24461–24468, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Ostrom RS, Post SR, Insel PA. Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving Gs. J Pharmacol Exp Ther 294: 407–412, 2000 [PubMed] [Google Scholar]
- 41.Ramos C, Montano M, Garcia-Alvarez J, Ruiz V, Uhal BD, Selman M, Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol 24: 591–598, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, Hammond HK. Adenylyl cyclase increases survival in cardiomyopathy. Circulation 105: 1989–1994, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation 99: 3099–3102, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Sansores RH, Ramirez-Venegas A, Perez-Padilla R, Montano M, Ramos C, Becerril C, Gaxiola M, Pare P, Selman M. Correlation between pulmonary fibrosis and the lung pressure-volume curve. Lung 174: 315–323, 1996 [DOI] [PubMed] [Google Scholar]
- 45.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med 134: 136–151, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res 250: 273–283, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Steinberg SF, Brunton LL. Compartmentation of G protein-coupled signaling pathways in cardiac myocytes. Annu Rev Pharmacol Toxicol 41: 751–773, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med 55: 395–417, 2004 [DOI] [PubMed] [Google Scholar]
- 49.Tobey RA, Valdez JG, Valdez YE, Lehnert BE. Proliferation of rat and human lung fibroblasts following exposure to prostaglandin E2. Exp Lung Res 16: 235–255, 1990 [DOI] [PubMed] [Google Scholar]
- 50.Wilborn J, Crofford LJ, Burdick MD, Kunkel SL, Strieter RM, Peters-Golden M. Cultured lung fibroblasts isolated from patients with idiopathic pulmonary fibrosis have a diminished capacity to synthesize prostaglandin E2 and to express cyclooxygenase-2. J Clin Invest 95: 1861–1868, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wynn T. Cellular and molecular mechanisms of fibrosis. J Pathol 214: 199–210, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yokoyama U, Patel HH, Lai NC, Aroonsakool N, Roth DM, Insel PA. The cyclic AMP effector Epac integrates pro- and anti-fibrotic signals. Proc Natl Acad Sci USA 105: 6386–6391, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]