Abstract
Exercise training enhances agonist-mediated relaxation in both control and collateral-dependent coronary arteries of hearts subjected to chronic occlusion, an enhancement that is mediated in part by nitric oxide. The purpose of the present study was to elucidate exercise training-induced adaptations in specific cellular mechanisms involved in the regulation of endothelial nitric oxide synthase (eNOS) in coronary arteries of ischemic hearts. Ameroid constrictors were surgically placed around the proximal left circumflex coronary artery (LCX) of adult female Yucatan miniature swine. Eight weeks postoperatively, animals were randomized into sedentary (pen-confined) or exercise training (treadmill run; 5 days/wk; 14 wk) protocols. Coronary artery segments (∼1.0 mm luminal diameter) were isolated from collateral-dependent (LCX) and control (nonoccluded left anterior descending) arteries 22 wk after ameroid placement. Endothelial cells were enzymatically dissociated, and intracellular Ca2+ responses (fura 2) to bradykinin stimulation were studied. Immunofluorescence and laser scanning confocal microscopy were used to quantify endothelial cell eNOS and caveolin-1 cellular distribution under basal and bradykinin-stimulated conditions. Immunoblot analysis was used to determine eNOS, phosphorylated (p)-eNOS, protein kinase B (Akt), pAkt, and caveolin-1 protein levels. Bradykinin-stimulated nitrite plus nitrate (NOx; nitric oxide metabolites) levels were assessed via HPLC. Exercise training resulted in significantly enhanced bradykinin-mediated increases in endothelial Ca2+ levels, NOx levels, and the distribution of eNOS-to-caveolin-1 ratio at the plasma membrane in endothelial cells of control and collateral-dependent arteries. Exercise training also significantly increased total eNOS and phosphorylated levels of eNOS (pSer1179) in collateral-dependent arteries. Total eNOS protein levels were also significantly increased in collateral-dependent arteries of sedentary animals. These data provide new insights into exercise training-induced adaptations in cellular mechanisms of nitric oxide regulation in collateral-dependent coronary arteries of chronically occluded hearts that contribute to enhanced nitric oxide production.
Keywords: coronary artery disease, coronary circulation, nitrate, nitrite, porcine
coronary artery disease is associated with progressive impairment of endothelial function, including changes in endothelium-dependent vasomotor reactivity (11–14). Previous reports demonstrate that exercise training reverses impaired vasomotor responsiveness of the coronary vasculature observed under pathological conditions, primarily through improvements in endothelial function in both experimental models of coronary artery disease and patients with symptomatic coronary artery disease (11, 13, 14, 18). Specifically, exercise training attenuated paradoxical vasoconstriction and increased coronary blood flow in response to acetylcholine in coronary artery disease patients (13, 14). Furthermore, in a porcine model of chronic coronary occlusion, endothelium-dependent relaxation to bradykinin and ADP was enhanced following exercise training (10). These improvements in endothelial function have been associated with improvements in nitric oxide bioavailability following exercise training (10, 11, 13, 40). However, cellular and molecular mechanisms responsible for improvements in nitric oxide bioavailability in coronary artery disease subsequent to exercise training have not been fully elucidated.
Intracellular Ca2+ is one of the primary mediators of agonist-mediated activation of endothelial nitric oxide synthase (eNOS). Binding of Ca2+-dependent calmodulin is required for the transfer of electrons from the reductase domain to the oxygenase domain for the enzyme to achieve maximal activity (32). Furthermore, in plasmalemmal caveolae, eNOS association with caveolin-1 suppresses eNOS activity under basal conditions (21). Agonist-stimulated increases in intracellular Ca2+ also destabilize the eNOS/caveolin-1 complex through the binding of Ca2+-calmodulin, enhancing eNOS activity (26) and nitric oxide production. Previous findings that endothelium-dependent relaxation is improved after exercise training in the underlying setting of coronary artery disease suggested to us that alterations in agonist-mediated endothelial Ca2+ levels may contribute substantially to adaptations associated with exercise training. Furthermore, cellular redistribution of caveolin-1 and eNOS after agonist-mediated dissociation of the complex is also thought to contribute to the regulation of nitric oxide. Although the regulation of eNOS activity by its association with caveolin-1 has been established and well studied, it remains unclear how the association of these proteins and the regulatory actions of caveolin-1 on eNOS activity are influenced by coronary artery disease and exercise training.
Phosphorylation of eNOS is also recognized as a regulatory mechanism of eNOS activity. Although numerous phosphorylation sites on eNOS have been identified, the functional consequences of porcine Ser1179 and Thr497 (equivalent to Ser1177 and Thr495 in humans) appear to be the best understood. Previous reports indicate that increased phosphorylation at Ser1179 results in enhanced electron flux and increased Ca2+ sensitivity of eNOS (5, 25). On the other hand, phosphorylation at Thr497 appears to interfere with the binding of Ca2+-calmodulin to the Ca2+-calmodulin binding domain and thereby inhibits eNOS activity (7). Dephosphorylation of this residue is generally associated with agonist-stimulated increases in intracellular Ca2+ levels, removing the inhibitory actions of this phosphorylated residue (7). Exercise training has been reported to increase phosphorylation levels of Ser1179 in the left internal mammary artery of coronary artery disease patients; however, the effects of exercise training on the phosphorylation levels of Thr497 have not been assessed previously in diseased patients or clinically relevant animal models.
Based on the paucity of information related to the effects of exercise training on these regulatory pathways of eNOS in the underlying setting of coronary artery disease, the purpose of the present study was to elucidate exercise training-induced adaptations in specific cellular mechanisms involved in the regulation of eNOS in coronary arteries of ischemic hearts.
MATERIALS AND METHODS
Experimental animals and surgical procedures.
All animal protocols were in accordance with “Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training” and approved by the Institutional Animal Care and Use Committee at Texas A&M University in accordance with the Association for the Accreditation of Laboratory Animal Care procedures. Furthermore, all methods conformed to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services Publication NIH 85-23, Office of Science and Health Reports, Bethesda, MD). Adult female Yucatan miniature swine (Sinclair Research Center, Auxvasse, MO) were surgically instrumented with ameroid constrictors around the proximal left circumflex coronary (LCX) artery as described in detail previously (10, 17). Animals were preanesthetized with glycopyrrolate (0.004 mg/kg im) and midazolam (0.5 mg/kg im). Anesthesia was induced with ketamine (20 mg/kg im) and maintained with 3% isoflurane and 97% O2 throughout aseptic surgery. Sham-operated pigs were subjected to the entire surgical procedure with the exception of placement of the ameroid constrictor. Animals recovered from the surgery for 8 wk before the experimental protocols were initiated. Overall mortality in this study was 11.7% of instrumented pigs, which was attributable to sudden cardiac death occurring at the approximate time that the LCX artery typically becomes completely occluded.
Exercise training.
Ameroid-occluded animals were randomly assigned to either a sedentary (n = 58) or exercise training (n = 60) group. Sham-operated pigs (n = 6) were assigned to the sedentary protocol. Exercise-trained pigs underwent a progressive treadmill exercise training program (5 days/wk for 14 wk) as described previously (10, 17). Pigs were run at ∼75% maximal O2 consumption, based on O2 consumption data obtained from Yucatan miniature swine by McAllister and colleagues (24). Sedentary animals were confined to their pens for the duration of the experimental protocol. Effectiveness of the exercise training program was determined by comparing heart-to-body weight ratio and skeletal muscle citrate synthase activity as previously described (17).
Coronary angiography.
Angiography was performed 22 wk after surgical placement of the ameroid constrictor with the animal under general anesthesia. Hemodynamics were monitored throughout the procedure. Arterial access was obtained by surgical cutdown of the carotid artery. The left main coronary artery was catheterized with a 6-Fr guiding catheter (Vista Brite Tip; Cordis) introduced over a 0.035-in. guidewire. Selective coronary angiography was performed with nonionic contrast (Oxilan 350; Guerbet).
Preparation of coronary arteries.
After ameroid placement (22 wk), animals were anesthetized with ketamine (30 mg/kg) and pentobarbital sodium (30 mg/kg). The hearts were removed and placed in ice-cold Krebs bicarbonate buffer (0–4°C) for isolation of the collateral-dependent LCX and the nonoccluded, control left anterior descending (LAD) coronary arteries. Visual inspection at the ameroid occluder during dissection of the LCX artery indicated 100% occlusion in all animals used in this study. With the aid of a dissection microscope, segments of the LCX (distal to the site of occlusion) and LAD arteries were trimmed of fat and connective tissue and cut into rings.
Endothelial cell dissociation.
Segments of collateral-dependent LCX and nonoccluded LAD coronary arteries (∼1.0 mm luminal diameter) were cut longitudinally and pinned lumen side up in low-Ca2+ (0.1 mM) physiological buffer containing 294 U/ml collagenase, 5 U/ml elastase, 2 mg/ml BSA, 1 mg/ml soybean trypsin inhibitor, and 0.4 mg/ml DNase I. For fura 2 studies, cells were enzymatically dissociated by incubation in a 37°C water bath for 20 min. The enzyme solution was then replaced with enzyme-free low-Ca2+ solution, and isolated cells were obtained by repeatedly directing a stream of low-Ca2+ solution over the artery via fire-polished Pasteur pipette. For immunofluorescence studies, enzymatically dissociated cells were collected and transferred to a 15-ml conical tube, and 10 ml of enzyme-free low-Ca2+ solution were added to the tube. The cells were centrifuged (Sorvall RT7 Plus; swinging bucket rotor RTH-750; 900 rpm) for 5 min, supernatant was removed, and the pellet was resuspended in 4 ml of DMEM complete medium.
Endothelial cell free Ca2+ measurement.
Freshly dissociated endothelial cells were incubated with the fluorescent Ca2+ indicator fura 2-AM (2.5 μM) at 37°C for 20 min. Cells were washed at 37°C for 20 min in sterile modified Eagle's minimal essential storage media to cleave extracellular fura 2-AM. Isolated cells were maintained in low-Ca2+ solution at 4°C until use (0–4 h). Fura 2-loaded cells were placed in a superfusion chamber and observed using an epifluorescence microscopy system, which permitted simultaneous evaluation of fura 2 fluorescence from multiple user-selected endothelial cells throughout an experimental protocol. Cells were excited with a 175-W xenon arc lamp with dual scanning galvanometers with 340- and 380-nm interference filters used for excitation wavelength selection (Sutter Instruments; Lambda DG-5). Fluorescence emission (510 nm) from selected endothelial cells was synchronized with the appropriate excitation wavelength and reflected to an interline-transfer, progressive-scan, cooled charge-coupled device video camera (CoolSNAP HQ; Photometrics) with a dichroic mirror. The microscope was equipped with a ×40 oil immersion objective, numerical aperature of 1.3. Fluorescent images were acquired using Metafluor dual wavelength Ca2+ imaging software (Universal Imaging). Final data for estimates of intracellular free Ca2+ are expressed as fluorescence ratio (F340/F380 nm) because of uncertainties in extrapolating in vitro calibrations to in situ measures as described previously (42). Experiments were conducted at room temperature (22–25°C), and fluorescence data were sampled every 2 s. Cells were continuously superfused under gravity flow with physiological saline solution (PSS). Agonist additions at appropriate concentrations were made to PSS for determination of agonist-stimulated intracellular Ca2+ responses.
Immunolabeling of freshly isolated endothelial cells.
Resuspended cells were seeded into eight wells of chambered cover glass, precoated with Cell-Tak to ensure rapid cell attachment. Cells were then incubated at 37°C for 1 h with ambient CO2 of 5%. The culture medium was removed, and fresh DMEM (0.2 ml) with or without bradykinin (10−6 M) was added, followed by incubation at 37°C with 5% CO2 for 1 or 5 min. Cells were fixed in cold methanol-acetone (1:1) immediately after bradykinin treatment as previously described (36). Primary rabbit anti-eNOS IgG, mouse anti-caveolin-1 IgM, and mouse anti-CD146 IgG were diluted at 1:100 in PBS, added to the appropriate wells, and incubated at room temperature for 1 h. After five washes with PBS, pH 7.4, and 0.05% Tween 20, secondary antibodies (1:100; goat anti-rabbit IgG-FITC, goat anti-mouse IgM-Rhodamine Red X, or anti-mouse IgG-Cy5; Jackson ImmunoResearch Laboratories) were added to the wells and incubated for 1 h at room temperature followed by five washes with PBS (pH 7.4). Optimal dilutions for antibodies were based on experiments where staining was performed with a range of dilutions. Control experiments were also performed with normal rabbit and mouse sera, omitting the primary antibodies and using secondary antibodies. These procedures assured optimization of the concentration for both antibodies and hence minimized nonspecific antibody staining.
Laser scanning confocal microscopy.
An MRC-1024 point scanning laser confocal microscopy system (Bio-Rad) equipped with a Zeiss Axiovert 135 inverted microscope, fitted with ×63, 1.4 numerical aperture oil immersion lens was used. FITC, Rhodamine Red X, or Cy5 was excited with a 15-mW Kr-Ar laser using the 488-, 568-, or 647-nm bands, respectively. FITC, Rhodamine Red X, or Cy5 was detected through a DF522/35 filter, HQ598/40 band-pass filter, or DF522/35 filter, respectively. Confocal images were acquired with Laser Sharp Software (Bio-Rad) and analyzed with Laser Sharp, Metamorph (Molecular Devices), and Adobe Photoshop as described earlier (36). The total pixel intensity of endothelial cell eNOS and caveolin-1 was determined in double-immunolabeled cells using Metamorph software.
Plasma membrane distribution of eNOS and caveolin-1.
Distribution of both eNOS and caveolin-1 at the plasma membrane and intracellular sites was calculated as a percent distribution, as described for markers of other organelles (19). Briefly, individual cells were selected, and user-defined regions were outlined to discern total and cytosolic pixel intensity. Intracellular intensity distribution of eNOS or caveolin-1 was estimated as total anti-eNOS or total anti-caveolin-1 pixels (I1), respectively, excluding pixels near the plasma membrane (I2). The percentage of eNOS and caveolin-1 associated with the plasma membrane were individually estimated according to the expression, % = (I1 − I2)/I1 × 100.
Immunoblots.
Arterial rings (∼5 mm length; ∼1.0 mm diameter) were isolated from both the collateral-dependent LCX and nonoccluded LAD, and either incubated with bradykinin (30 nM) or PSS (basal) for specified durations at 37°C, then quick-frozen and stored at −80°C for later immunoblot analysis, as described in detail previously (8). Arterial lysate (30 μg total protein) was subjected to SDS-PAGE (12% gel), transferred to a polyvinylidene difluoride membrane, and probed overnight with primary antibody. Primary antibody sources and dilutions were as follows: eNOS (no. 610297, 1:750); phosphorylated (p)-eNOS (pSer1179, no. 612393, 1:250); p-eNOS (pThr497, no. 612707, 1:200); caveolin-1 (no. 610407, 1:2,000); protein kinase B (Akt) (no. 610861, 1:1,000) all from BD Biosciences; pAkt (pSer473, no. 4051, 1:250; Cell Signaling Technology); and β-actin (no. 600–501, 1:10,000; Novus Biologicals).
Measurement of nitrite plus nitrate levels.
First-order arterial branches (∼1–1.5 cm length) were isolated from both the collateral-dependent LCX and nonoccluded LAD and incubated with bradykinin (1 μM) for 10 min duration at 37°C. Rings were removed, and both supernatant and arteries were snap-frozen and stored at −80°C until processed. Nitrite plus nitrate (NOx), the stable oxidation products of nitric oxide, were measured in supernatant as indicators of nitric oxide synthesis using a fluorometric HPLC method modified from that described previously (20). Briefly, nitrate was converted to nitrite by nitrate reductase, followed by the derivatization of nitrite with 2,3-diaminonaphthalene to form the highly fluorescent 2,3-naphthotriazole (NAT). NAT was separated on an XBridge 3.5-μm reversed-phase C18 column (150 × 4.6 mm ID; Waters) guarded by a 5-μm reversed-phase C18 column (20 × 4.6 mm ID) and eluted with a 55% methanol-water mobile phase (flow rate, 0.5 ml/min). Fluorescence was monitored with excitation at 375 nm and emission at 415 nm. Mean retention time for NAT was 4.5 min. The fluorescence intensity of NAT was linear within the 1–5 μM standard curve employed.
Isometric tension studies.
Coronary rings were measured with a calibrated Filar micrometer eyepiece (Hitschfel Instruments) in a relaxed, unstretched state and then mounted on two stainless steel wires passed through the artery lumen. One wire was fixed to a force transducer (Grass FT03; Grass Instruments) and the other to a micrometer microdrive (Stoelting/Prior Microdrive) to allow precise changes in circumferential length of the vessel. The mounted arterial ring was lowered into a 20-ml tissue bath containing Krebs bicarbonate buffer at 37 ± 0.5°C and aerated with 95% O2-5% CO2. Coronary rings were progressively stretched to the maximum of the length-developed tension relationship at which pharmacological responsiveness was evaluated, as described previously (16).
Solutions.
Krebs buffer for isometric tension studies contained (in mM): 131.5 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 13.5 NaHCO3 and 0.025 EDTA. PSS used for fura 2 and NOx experiments contained (in mM): 2 CaCl2, 143 NaCl, 1 MgCl2, 5 KCl, 10 HEPES, and 10 glucose, pH 7.4. Cells used for fura 2 experiments were rinsed in sterile modified Eagle's minimal essential storage media that contained (in mM): 135 NaCl, 5 KCl, 0.34 NaH2PO4, 1 MgCl2, 2 CaCl2, 10 glucose, 2.6 NaHCO3, 0.44 KH2PO4, and 20 HEPES and (vol/vol) 0.02 amino acids, 0.01 vitamins, 0.002 phenol red, 0.01 penicillin/streptomycin, and 2% horse serum, pH 7.2 with NaOH at 23°C and passed through a sterile filter to a final pH of 7.4. Fura 2-AM loading solution contained 2.5 μM fura 2-AM, 0.5% cremophor, and 5% BSA. The loading solution was vortexed for 1 min and then sonicated for an additional 1 min to increase solubilization of fura 2-AM. Drugs were obtained from Sigma Chemical (St. Louis, MO) unless otherwise noted. Endothelial cell dispersion chemicals were obtained from Worthington Chemicals. Nitrate reductase and NADPH were obtained from Roche.
Statistical analysis.
Body weight, heart-to-body weight ratio, and citrate synthase values were compared using Student's t-tests. Isometric tension studies were analyzed using one-way ANOVA with planned orthogonal contrasts for post hoc analysis (4). Other data sets were compared using one-way ANOVA followed by Bonferroni or Games-Howell post hoc analysis, as appropriate based on homogeneity of variances, when a main effect was identified. For all analyses, a P value ≤0.05 was considered significant. Data are presented as means ± SE, and n values in parentheses reflect the number of animals studied or the number of animals and cells studied for fura 2 experiments. For isometric studies, when more than one vascular ring from a single coronary artery was used in identical protocols, the responses from these rings were averaged before data analyses were conducted.
RESULTS
Efficacy of the exercise training program.
Effectiveness of the 14-wk exercise training program was demonstrated by significant increases in skeletal muscle oxidative enzyme capacity and an increased heart-to-body weight ratio in exercise-trained compared with sedentary animals. Citrate synthase activity increased significantly (P < 0.001 for all comparisons) in the deltoid muscle (30.0 ± 1.4 vs. 23.5 ± 1.2 μmol·min−1·g−1) and the lateral (27.4 ± 1.5 vs. 20.5 ± 1.2 μmol·min−1·g−1), medial (34.7 ± 1.8 vs. 25.0 ± 1.4 μmol·min−1·g−1), and long (23.9 ± 1.1 vs. 19.2 ± 0.8 μmol·min−1·g−1) heads of the triceps brachii muscle in exercise-trained (n = 60) compared with sedentary (n = 58) pigs. Although body weight did not differ between sedentary and exercise-trained animals at the time of death (38.5 ± 0.8 vs. 38.2 ± 0.7 kg, respectively), heart-to-body weight ratio was significantly greater from exercise trained compared with sedentary pigs (5.8 ± 0.1 vs. 4.9 ± 0.1 g/kg, respectively; P < 0.001).
Angiography of chronic coronary artery occlusion and collateral perfusion.
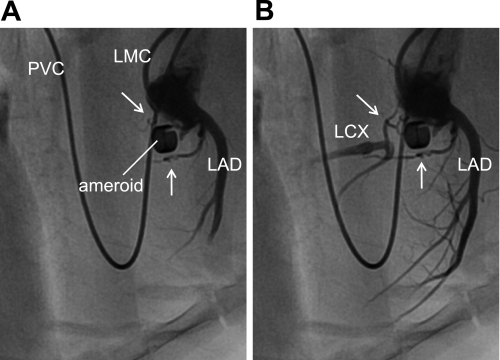
Figure 1 displays serial coronary angiographic images that confirm that blood flow through the LCX at the site of occlusion was completely abolished by the ameroid constrictor. Relatively large, tortuous collateral vessels supplying the LCX artery distal to occlusion are clearly visible in both panels of the angiogram. These collaterals appear to originate from the proximal LAD and LCX (proximal to occluder) and “bypass” the occlusion. Smaller intramyocardial collaterals are likely also present (45) but are difficult to visualize by angiography. Importantly, because complete ameroid-induced LCX occlusion is evident, the myocardial region formerly perfused by the native LCX artery is, by definition, now fully dependent upon perfusion via collateral vessels such as those observed in Fig. 1.
Fig. 1.
Angiography of porcine model of chronic coronary artery occlusion and collateral perfusion. The left main coronary artery was catheterized for performance of selective coronary angiography. A and B: regionally enhanced serial images emphasizing the cardiac silhouette to provide detail of coronary vasculature. White arrows in A and B identify collateral vessels supplying the left circumflex artery (LCX) distal to occlusion. LAD, left anterior descending artery; LMC, left main coronary artery catheter; PVC, pulmonary vein catheter for procedure not related to the data associated with this study.
Basal and bradykinin-stimulated intracellular Ca2+ levels in conduit endothelial cells.
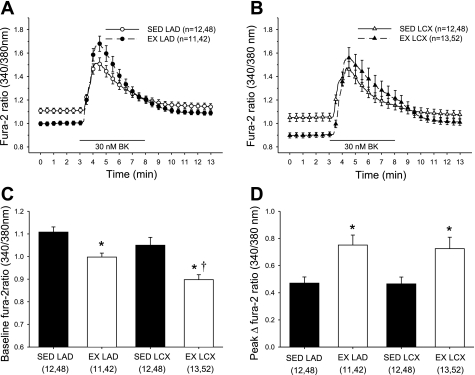
We evaluated cytosolic free Ca2+ levels (fura 2) under both basal and bradykinin-stimulated conditions in isolated endothelial cells from both the collateral-dependent and nonoccluded arteries. Figure 2, A and B, shows our experimental protocol and demonstrates mean Ca2+ responses in endothelial cells from both nonoccluded (Fig. 2A) and collateral-dependent (Fig. 2B) arteries of sedentary and exercise-trained animals. As shown in Fig. 2C, basal cytosolic Ca2+ levels were significantly lower in endothelial cells from arteries of exercise-trained compared with respective arteries from sedentary animals. Furthermore, basal Ca2+ levels were significantly lower in endothelial cells from collateral-dependent compared with control arteries of exercise-trained pigs (Fig. 2C). The peak cytosolic Ca2+ response to bradykinin stimulation was calculated by subtraction of basal Ca2+ levels from the peak Ca2+ response to bradykinin to generate the peak Δfura 2 ratio (Fig. 2D). Importantly, exercise training significantly enhanced the peak Ca2+ response to bradykinin in coronary endothelial cells isolated from both control and collateral-dependent arteries compared with cells from sedentary pigs (Fig. 2D). Peak cytosolic Ca2+ response to bradykinin stimulation in sham-operated animals did not differ between endothelial cells isolated from the LAD (0.37 ± 0.04 ratio units; n = 6) and LCX (0.42 ± 0.08 ratio units; n = 6) arteries and was not significantly different from the control LAD of chronically occluded animals (Fig. 2D). Based on our observation of a delayed recovery of the bradykinin-stimulated Ca2+ response in cells from the collateral-dependent artery from exercise-trained pigs (Fig. 2B), we also assessed the area under the Ca2+ response curve to bradykinin in the same cells. Area under the curve was calculated for the duration of bradykinin exposure (minutes 3–8) using basal Ca2+ levels as the baseline for the area under the curve. Area under the curve was significantly increased in endothelial cells from both the control and collateral-dependent arteries of exercise-trained pigs compared with cells from the sedentary counterparts (data not shown). Despite an apparent delayed recovery in endothelial cells from collateral-dependent compared with control arteries of exercise-trained pigs (Fig. 2B), the area under the curve was only slightly increased in cells from the collateral-dependent arteries. Occlusion alone did not significantly alter basal or bradykinin-stimulated Ca2+ levels in cells from sedentary animals.
Fig. 2.
Intracellular Ca2+ response to bradykinin stimulation in isolated endothelial cells. Time course responses showing average bradykinin-stimulated intracellular free Ca2+ (fura 2) responses in cells isolated from control (LAD; A) and collateral-dependent (LCX; B) arteries of sedentary (SED) and exercise-trained (EX) pigs. Endothelial cells were superfused with physiological saline solution (PSS) for 3 min, followed by 5 min exposure to bradykinin (30 nM) and 5 min recovery in PSS. C: baseline Ca2+ levels (average of initial 3 min PSS exposure) were significantly decreased in endothelial cells from arteries of exercise-trained compared with sedentary pigs in both control and collateral-dependent arteries. D: bradykinin-stimulated peak Ca2+ response was significantly increased in endothelial cells from exercise-trained compared with sedentary animals in both control and collateral-dependent arteries. Nos. in parentheses are animals, cells. Values are means ± SE of the no. of animals in parentheses. *Significantly different from respective SED. †Significantly different from EX LAD.
Immunofluorescent confirmation of endothelial cell isolation from coronary arteries.
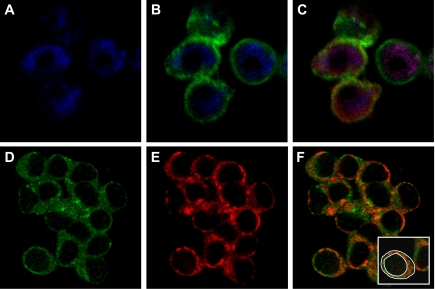
In immunofluoresence studies, endothelial cells were morphologically distinguishable from other cell types as flat, round-shaped single cells or cell clusters. Generally, >75% of cells from multiple viewing fields were endothelial cells distinguished by their distinct shape, staining pattern, and uniformity. Nearly all endothelial cells visualized were studied. Because of the flat morphology of endothelial cells and the limits of light microscopy optical resolution, it was possible to only obtain a few confocal slices through each cell. Consequently, the plasma membrane region in medial confocal slices through the cell appeared much broader than that obtained from very thick, nonflattened cells such as fibroblasts (19). Cells isolated from coronary arteries stained intensely for CD146, a marker specific for endothelial cells (Fig. 3A, blue pixels). Double immunolabeling with anti-CD146 (blue) and anti-eNOS (green) showed that the CD146-containing cells also stained brightly for eNOS (green), also found primarily in endothelial cells (Fig. 3B). CD146 was localized in the cytoplasm, whereas eNOS was primarily localized at the plasma membrane with some distribution in the cytosol. Triple immunolabeling with anti-CD146, anti-eNOS, and anti-caveolin-1 (red) detected the presence of all three proteins (Fig. 3C). These data validate that the cells utilized for these studies were freshly isolated coronary artery endothelial cells.
Fig. 3.
Confirmation of endothelial cell isolation from porcine coronary arteries and localization of endothelial nitric oxide synthase (eNOS) and caveolin-1. Endothelial cells were isolated from control arteries of sedentary pigs. Cells were fixed and triple immunolabeled with anti-CD146, anti-eNOS, and anti-caveolin-1, followed by fluorescent-labeled secondary antisera: anti-mouse-IgG-Cy5, anti-rabbit-IgG-FITC, and anti-mouse-IgM-Rhodamine Red X for detection of anti-CD146, -eNOS, and -caveolin-1, respectively. FITC, Rhodamine Red X, or Cy5 was excited with a 15-mW Kr-Ar laser using the 488-, 568-, and 647-nm bands, respectively. FITC, Rhodamine Red X, or Cy5 was detected through a DF522/35 filter, HQ598/40 band-pass filter, or DF522/35 filter, respectively. A: cells stained for CD146 (blue pixels), a marker specific for endothelial cells. B: superimposition of simultaneously acquired signals from anti-CD146 (blue pixels) and anti-eNOS (green pixels) further confirm the isolation of endothelial cells. C: superposition of simultaneously acquired signals from anti-CD146 (blue pixels), anti-eNOS (green pixels), and anti-caveolin-1 (red pixels). D: cellular images indicate that eNOS (green pixels) is primarily localized to the plasma membrane with some cytosolic distribution in freshly isolated endothelial cells. E: similarly, caveolin-1 (red pixels) was primarily distributed at the plasma membrane with some cytosolic distribution. F: superimposition of simultaneously acquired images of eNOS and caveolin-1 indicate prominent colocalization of these proteins at the plasma membrane. As illustrated (inset), the intensity of total (outer white circle) and plasma membrane-associated (inner white circle) green or red pixels for each cell was separately measured as described in materials and methods.
Colocalization of eNOS and caveolin-1 at the plasma membrane of endothelial cells.
Endothelial cells and cell clusters stained intensely with both anti-eNOS and anti-caveolin-1. Similar to our findings for eNOS distribution (Fig. 3D, green pixels), caveolin-1 was also primarily localized to the endothelial cell plasma membrane, with less intense staining in the cytoplasm (Fig. 3E, red pixels). To determine if eNOS colocalized with caveolin-1, cells were double immunolabeled with anti-eNOS and anti-caveolin-1. Superimposition of the simultaneously acquired images revealed colocalization of eNOS and caveolin-1 primarily at the plasma membrane (Fig. 3F, orange-red pixels).
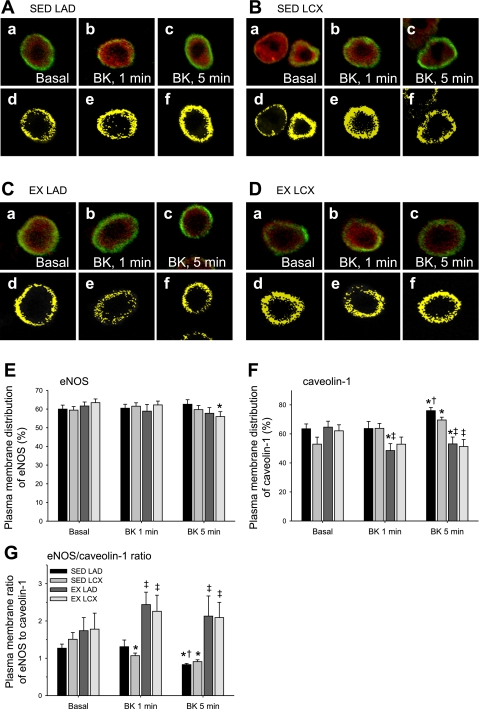
Effect of chronic occlusion and exercise training on the cellular distribution of eNOS and caveolin-1.
The effect of chronic coronary artery occlusion and exercise training on the cellular distribution of eNOS and caveolin-1 was quantitatively assessed in double-immunolabeled endothelial cells. In Fig. 4, A–D, a–c show the cellular distribution of both eNOS (green pixels) and caveolin-1 (red pixels) under basal conditions (a) and after 1-min (b) and 5-min (c) incubation periods with bradykinin. In Fig. 4, A–D, d–f show the distribution of colocalized eNOS and caveolin-1 (yellow pixels) simultaneously acquired under the same conditions. Figure 4, E and F, displays the quantitative data of images taken from 40–70 cells for each treatment group. Under basal conditions, neither chronic occlusion nor exercise training altered the percentage of cellular eNOS distributed at the plasma membrane (Fig. 4E). Similarly, the basal distribution of caveolin-1 at the plasma membrane was not significantly altered by occlusion or exercise training (Fig. 4F). Importantly, the plasma membrane cellular distribution of eNOS and caveolin-1 in sham-operated animals did not differ between endothelial cells isolated from the LAD (60 ± 8%) and LCX (63 ± 6%) arteries and was not significantly different from chronically occluded animals. Taken together, these data indicate that chronic coronary occlusion and exercise training did not alter the basal cellular distribution of eNOS or caveolin-1.
Fig. 4.
Effect of chronic occlusion and exercise training on basal and bradykinin (BK)-stimulated cellular distribution of eNOS and caveolin-1. Endothelial cells were isolated from control and collateral-dependent arteries of sedentary and exercise-trained pigs. Cells were fixed and probed for eNOS (green pixels) and caveolin-1 (red pixels) as described in Fig. 3. A–D, a–c: superimposed distributions of eNOS and caveolin-1 simultaneously acquired from endothelial cells of control and collateral-dependent arteries of sedentary and exercise-trained pigs. Cells were either untreated (a, basal) or treated with bradykinin for 1 min (b) or 5 min (c). A–D, d–f: to more clearly show the distribution of colocalized eNOS and caveolin-1, only the colocalized pixels (yellow pixels) obtained from the images corresponding to a, b, and c are displayed. E and F: quantitative distributions (%) of eNOS and caveolin-1 at the plasma membrane under basal conditions and following treatment with bradykinin for 1 and 5 min were calculated as described in materials and methods. G: the ratio of eNOS/caveolin-1 pixel intensity for each endothelial cell was determined from the percentage of eNOS and caveolin-1 distributed to the plasma membrane calculated as described in materials and methods. Graph legend in G pertains to E–G. Values represent means ± SE of 40–70 cells for each treatment group. *Significantly different from respective basal condition. †Significantly different from respective 1 min bradykinin. ‡Significantly different from respective sedentary condition.
The cellular distribution of eNOS and caveolin-1 was also determined after a 1-min (Fig. 4, A–D, b and e) or 5-min (Fig. 4, A–D, c and f) incubation with bradykinin. Results from quantitative analysis (Fig. 4E) indicated that bradykinin treatment for 1 min did not significantly alter eNOS distribution at the plasma membrane of endothelial cells isolated from the control or collateral-dependent arteries of sedentary or exercise-trained pigs. In contrast, 5 min bradykinin exposure produced a small but significant decrease in eNOS distribution at the plasma membrane in the collateral-dependent arteries of exercise-trained pigs compared with basal conditions.
Caveolin-1 distribution at the plasma membrane was not altered following 1 min bradykinin exposure in the control arteries of sedentary animals or collateral-dependent arteries of sedentary or exercise-trained animals compared with basal conditions (Fig. 4F). In contrast, the percentage of caveolin-1 associated with the plasma membrane after 1 min bradykinin exposure was significantly decreased in cells of the control artery of exercise-trained pigs. Interestingly, the response of endothelial cells from arteries of sedentary and exercise-trained animals to 5 min incubation with bradykinin was highly diverse. The distribution of caveolin-1 at the plasma membrane was significantly increased in endothelial cells of both the control and collateral-dependent arteries from sedentary animals following 5 min bradykinin exposure. In contrast, 5 min bradykinin treatment significantly decreased the distribution of caveolin-1 at the plasma membrane of endothelial cells from control and collateral-dependent arteries of exercise-trained pigs.
Effect of chronic occlusion and exercise training on basal and bradykinin-stimulated eNOS-to-caveolin-1 ratio in endothelial cells.
Although exercise training tended to increase the basal eNOS-to-caveolin-1 ratio at the plasma membrane of endothelial cells from both control and collateral-dependent arteries compared with that of sedentary pigs, this effect did not achieve statistical significance (Fig. 4G). Bradykinin exposure significantly decreased the eNOS/caveolin-1 ratio at the plasma membrane in both the control and collateral-dependent arteries of sedentary animals. In contrast, bradykinin exposure significantly enhanced the eNOS/caveolin-1 ratio at the plasma membrane in endothelial cells from both arteries of exercise-trained pigs. The distinct response of endothelial cells from arteries of sedentary and exercise-trained pigs to bradykinin treatment resulted in eNOS/caveolin-1 ratios that were 2.6- and 2.3-fold higher in endothelial cells of the control and collateral-dependent arteries of exercise-trained compared with sedentary pigs. This disparate response in the eNOS/caveolin-1 ratio was primarily attributable to the diversity in the bradykinin-mediated redistribution of caveolin-1 at the plasma membrane.
Basal levels of phosphorylated and total eNOS and Akt and caveolin-1 proteins.
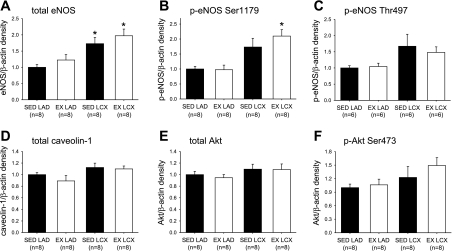
Determination of protein levels by immunoblot revealed that exercise training significantly increased eNOS (Fig. 5A), as well as phosphorylated (pSer1179) eNOS, protein content in collateral-dependent arteries (Fig. 5B). The protein content of total eNOS was also significantly increased in collateral-dependent arteries of sedentary pigs, and p-eNOS (pSer1179) also tended to be increased by occlusion alone (P = 0.09) (Fig. 5B). On the other hand, the protein content of p-eNOS (pThr497) was not altered (P = 0.10) by occlusion or exercise training (Fig. 5C). Similarly, caveolin-1, Akt, and pAkt (pSer473) were not altered significantly by occlusion or exercise training (Fig. 5, D–F).
Fig. 5.
Effect of chronic occlusion and exercise training on eNOS, phosphorylated (p)-eNOS, caveolin-1, protein kinase B (Akt), and pAkt protein levels as determined by immunoblot. Protein levels for total eNOS (A), p-eNOS (pSer1179) (B), p-eNOS (pThr497) (C), caveolin-1 (D), total Akt (E), and pAkt (pSer473) (F). Proteins of interest were quantified by densitometry analysis, normalized to β-actin, and expressed relative to density of control arteries of sedentary pigs (SED LAD). LAD, nonoccluded control artery; LCX, collateral-dependent artery. Values are means ± SE. No. of animals studied is provided in parentheses. *Significantly different from respective LAD artery.
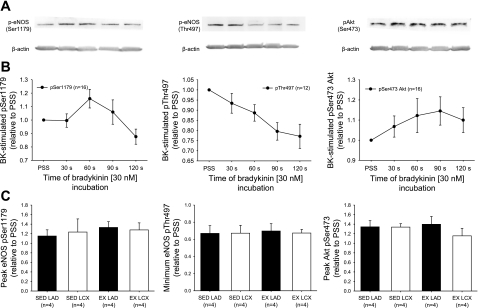
Time course of bradykinin-induced changes in phosphorylation status of eNOS and Akt.
We examined the time course effect of bradykinin treatment on phosphorylated levels of eNOS both at pSer1179 and pThr497, as well as phosphorylation of Akt at pSer473. Figure 6A provides representative immunoblots for the phosphorylated proteins of interest and β-actin, as loading control, of arterial rings that were untreated (PSS) or treated with bradykinin for specified durations as indicated. The average time course response of bradykinin-stimulated phosphorylation for pSer1179-eNOS, pThr497-eNOS, and pSer473-Akt from all arterial rings studied is shown in Fig. 6B. These studies demonstrate that bradykinin treatment elicited a transient increase in pSer1179-eNOS protein levels, with the average peak at the 60-s incubation and recovering to below basal levels at the 120-s incubation. The peak response, relative to PSS-treated rings and independent of specific time of bradykinin treatment, between control and collateral-dependent arteries from sedentary and exercise-trained pigs was not significantly different, suggesting that neither occlusion nor exercise training altered the bradykinin-stimulated phosphorylation of eNOS at Ser1179 (Fig. 6C). As also shown in Fig. 6C, bradykinin stimulation of endothelial cells resulted in dephosphorylation of Thr497-eNOS throughout the bradykinin time course. Although a nadir was not obtained in the time course presented, our preliminary data indicated that phosphorylation levels of Thr497-eNOS started to return toward basal levels after 3 min of bradykinin treatment. It is also evident that the reduction in phosphorylation levels begins to taper off at the 120-s time point (Fig. 6B). The greatest level of dephosphorylation obtained between control and collateral-dependent arteries from sedentary and exercise-trained pigs was not significantly different, suggesting that neither occlusion nor exercise training altered the bradykinin-stimulated dephosphorylation of eNOS at Thr497 (Fig. 6C). Examination of bradykinin-stimulated phosphorylation of Akt at Ser473 revealed that the peak response occurred at 90 s and began to recover toward basal levels at the 120-s incubation.
Fig. 6.
Time course of bradykinin treatment on phosphorylated protein levels of eNOS (pSer1179 and pThr497) and Akt (pSer473). A: representative immunoblots demonstrate the effect of various durations of treatment with bradykinin (30 nM) on phosphorylation levels of proteins as well as the loading control, β-actin. B: control and collateral-dependent rings of sedentary and exercise-trained pigs responded similarly to bradykinin treatment; thus, rings from all treatment groups are averaged to show effect of bradykinin on phosphorylated protein levels at times specified. Values are normalized to β-actin and expressed relative to density of PSS-treated rings. C: the greatest degree of phosphorylation or dephosphorylation, relative to PSS-treated rings and independent of specific time of bradykinin treatment, is averaged for control (LAD) and collateral-dependent (LCX) arteries of sedentary (SED) and exercise trained (EX) pigs. Values are means ± SE. No. of animals studied is provided in parentheses.
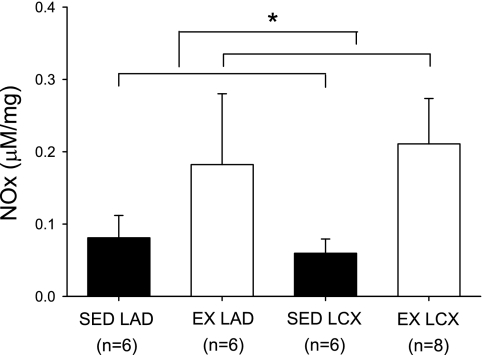
Bradykinin-stimulated NOx levels.
NOx levels in response to bradykinin stimulation were significantly greater in arterial rings isolated from exercise-trained compared with sedentary pigs (Fig. 7). Occlusion did not significantly alter bradykinin-stimulated NOx levels in sedentary or exercise-trained animals.
Fig. 7.
Effect of chronic occlusion and exercise training on bradykinin-stimulated nitrate plus nitrite (NOx) levels in coronary arteries. NOx levels were determined in arterial segments isolated from control and collateral-dependent rings from sedentary and exercise-trained pigs and normalized to tissue mass. Values are means ± SE of the no. of animals in parentheses. *Significantly greater in arterial rings isolated from exercise-trained compared with sedentary pigs.
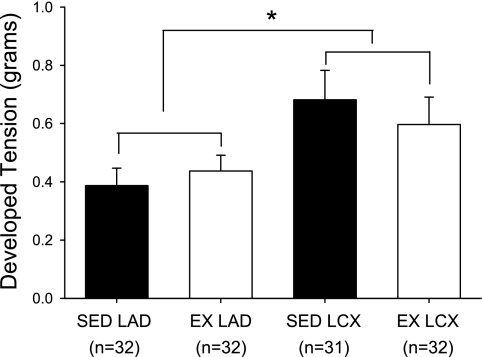
Contribution of nitric oxide to resting tension.
For isometric tension studies, analyses of dimensional characteristics revealed that both the outer and luminal diameters of collateral-dependent LCX arterial rings from sedentary animals were slightly, but significantly, smaller than control LAD rings of both sedentary and exercise-trained pigs (Table 1). Incubation of coronary arterial rings with the nitric oxide synthase inhibitor NG-nitro-l-arginine methyl ester (l-NAME, 100 μM; Fig. 8) increased resting tension in all treatment groups; however, collateral-dependent arteries displayed a significantly greater increase in tension than control rings. The basal effect of l-NAME in sham-operated animals did not differ between the LAD (0.41 ± 0.09 g) and LCX (0.38 ± 0.14 g) arteries and was not significantly different from the control artery of chronically occluded sedentary animals (0.39 ± 0.06 g).
Table 1.
Dimensional characteristics of coronary artery rings for isometric tension studies
| n | Outer Diameter, mm | Lumen Diameter, mm | Wall Thickness, mm | Axial Length, mm | |
|---|---|---|---|---|---|
| SED LAD | 32 | 1.56 ± 0.05 | 1.06 ± 0.05 | 0.12 ± 0.01 | 3.52 ± 0.09 |
| SED LCX | 32 | 1.35 ± 0.05* | 0.82 ± 0.03* | 0.11 ± 0.01 | 3.29 ± 0.11 |
| EX LAD | 31 | 1.63 ± 0.05 | 1.09 ± 0.04 | 0.13 ± 0.01 | 3.51 ± 0.08 |
| EX LCX | 32 | 1.47 ± 0.08 | 0.98 ± 0.06 | 0.12 ± 0.01 | 3.49 ± 0.09 |
Values are means ± SE; n, no. of animals studied. LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; SED, sedentary; EX, exercise trained.
Significantly different from SED LAD and EX LAD.
Fig. 8.
Effect of chronic occlusion and exercise training on nitric oxide contribution to basal active tone in coronary arteries. Increase in resting tension of arterial rings in response to nitric oxide synthase inhibition (NG-nitro-l-arginine methyl ester; 100 μM). Values are means ± SE of the no. of animals in parentheses. *Significantly greater tension development than control (LAD) arteries.
DISCUSSION
In this study, we document several novel findings regarding the effect of exercise training on the cellular and molecular mechanisms involved in the regulation of eNOS in the underlying setting of chronic coronary artery occlusion. Our data reveal that exercise training augments the bradykinin-stimulated increase in cytosolic free Ca2+ levels of endothelial cells isolated from both control and collateral-dependent coronary arteries compared with Ca2+ levels from sedentary animals. Exercise training also produced increases in bradykinin-stimulated NOx levels that corresponded closely with the peak Ca2+ responses. Despite no significant effect of exercise training on the basal eNOS/caveolin-1 ratio at the plasma membrane, bradykinin exposure significantly enhanced the eNOS/caveolin-1 ratio at the plasma membrane of endothelial cells from both the control and collateral-dependent arteries of exercise-trained pigs. In contrast, bradykinin exposure significantly decreased the eNOS/caveolin-1 ratio at the plasma membrane in endothelial cells from both the control and collateral-dependent arteries of sedentary pigs. These distinct responses between sedentary and exercise-trained animals in the eNOS/caveolin-1 ratio were primarily attributable to the diversity in the bradykinin-mediated redistribution of caveolin-1 at the plasma membrane. We also provide the first demonstration that protein levels for total eNOS and phosphorylated eNOS at Ser1179 were increased in collateral-dependent arteries of exercise-trained pigs.
The enhanced bradykinin-stimulated NOx levels and Ca2+ responses in endothelial cells of both control and collateral-dependent arteries after exercise training provide compelling support regarding previous observations in this model that control and collateral-dependent coronary arteries of exercise-trained swine display enhanced bradykinin- and ADP-mediated relaxation compared with sedentary pigs (10). These previous studies also reported that the enhanced agonist-mediated relaxation responses were partly dependent on the nitric oxide signaling pathway. Indeed, cytosolic Ca2+ levels are a crucial determinant of eNOS activity since eNOS achieves maximal activation only when cytosolic Ca2+ reaches an adequate level to stimulate calmodulin binding to eNOS and facilitates the electron flux necessary for catalytic activity of the enzyme and nitric oxide production (32). Taken together, these data suggest that the enhanced bradykinin-mediated increase in endothelial cytosolic Ca2+ likely contributes to greater eNOS activity, nitric oxide production, and enhanced bradykinin-induced relaxation in both control and collateral-dependent coronary arteries from exercise-trained pigs (10). In addition to the nitric oxide signaling pathway, increases in endothelial cytosolic Ca2+ levels also have been shown to activate the cyclooxygenase and endothelium-derived hyperpolarization pathways. Thus the small increases in bradykinin-stimulated relaxation in arteries from exercise-trained pigs that persist in the presence of nitric oxide synthase inhibition (10) may be attributable to other Ca2+-dependent endothelium-mediated vasodilatory pathways.
Our data also demonstrate that exercise training produced significantly decreased basal Ca2+ levels in endothelial cells from both the control and collateral-dependent arteries that were most pronounced in cells from the collateral-dependent artery. This interesting adaptation may compensate for potential upregulation of other pathways contributing to vasodilatation in the basal state. For example, in the collateral-dependent artery of exercise-trained pigs, our data reveal increased eNOS protein levels under basal conditions. Reduced basal endothelial cytosolic Ca2+ levels in concert with enhanced eNOS protein would limit vasodilatation under resting conditions, yet allow for rapid Ca2+ mobilization, eNOS activation, and enhanced nitric oxide production and coronary vasodilatation under conditions of increased cardiac metabolic demand (i.e., exercise). However, the exercise training-mediated reduction in basal Ca2+ levels in cells from the control artery may also be an adaptive response to increased activity of another mechanism of vasodilatation not revealed in this study. Unfortunately, direct measures of basal NOx levels were not included in this study.
Ca2+-dependent activation of calmodulin is also important in the displacement of caveolin-1 from eNOS. Caveolin-1, an integral membrane protein in caveolae, serves as a structural scaffold for numerous signaling molecules, including eNOS. The interaction of eNOS and caveolin-1 suppresses eNOS catalytic activity, but this tonic inhibition by caveolin-1 can be displaced by calmodulin (21) in a Ca2+-dependent manner (26). Bradykinin stimulation appeared to have a greater effect on the cellular distribution of caveolin-1 rather than eNOS in both sedentary and exercise-trained pigs. Exposure to bradykinin for 5 min resulted in a net increase in caveolin-1 associated with the plasma membrane of endothelial cells from both control and collateral-dependent arteries of sedentary animals. Because a similar response was not observed after 1 min incubation with bradykinin, we speculate that this may be an adaptive response to relatively long-term exposure to bradykinin. Indeed, evaluation of bradykinin-mediated relaxation in arterial rings reveals that responses are transient and peak within 1–2 min after bradykinin addition. These results suggest that the increased distribution of caveolin-1 at the plasma membrane may function as a compensatory mechanism to limit continued stimulation of plasma membrane-associated eNOS. On the contrary, 1 and 5 min bradykinin treatment decreased caveolin-1 distribution to the plasma membrane in cells of both the control and collateral-dependent arteries of exercise-trained pigs. These seemingly contradictory adaptations are difficult to reconcile but may be attributable to differences in additional adaptations in cellular signaling pathways not examined. The resultant eNOS/caveolin-1 ratio at the plasma membrane was significantly enhanced in exercise-trained compared with sedentary animals in cells from both the control and collateral-dependent arteries. These data suggest that the bradykinin-stimulated distribution of caveolin-1 away from the plasma membrane may contribute to increased eNOS activity following exercise training.
Previous studies have also documented that the interaction of eNOS with the bradykinin receptor inhibits eNOS activity, whereas bradykinin treatment disrupts this interaction and enhances eNOS activity by redistributing the enzyme away from the plasma membrane (6, 21, 26, 27). Thus any change in eNOS distribution in response to bradykinin may contribute to alterations in eNOS activation status. The plasma membrane distribution of eNOS in endothelial cells isolated from collateral-dependent arteries of exercise-trained pigs was significantly reduced after bradykinin treatment, suggesting net migration of eNOS from the plasma membrane to the cytosolic compartment. This bradykinin-induced redistribution of eNOS may contribute to the restored bradykinin-mediated relaxation observed in coronary vasculature distal to chronic occlusion in exercise-trained animals (10). Whether a change in the association of eNOS from caveolar to noncaveolar lipid rafts of the plasma membrane is altered by coronary occlusion and/or exercise training is unknown.
In our study, the majority of eNOS and caveolin-1 was localized at the plasma membrane of arterial endothelial cells under basal conditions. This distribution is consistent with the basal distribution reported previously for cultured bovine aortic endothelial cells (31). The lack of redistribution of eNOS away from the plasma membrane after bradykinin stimulation is consistent with some studies (23) but opposes others (31, 41). Indeed, the fate of eNOS localization after agonist-induced dissociation from caveolin-1 is somewhat controversial, primarily limited to study of cultured cell lines (23, 31, 41), and appears to display a heterogeneity of responses across vascular beds (2).
In addition to activation of eNOS by Ca2+-dependent mechanisms, the dynamic regulation of eNOS also involves phosphorylation at numerous sites, including Ser1179 and Thr497. Phosphorylation of Ser1179 increases eNOS catalytic activity by increasing electron flux at the reductase domain and increasing the Ca2+ sensitivity of the enzyme (5, 25). Similar to previous observations in porcine aortic endothelial cells (7), stimulation with bradykinin elicited a transient increase in phosphorylation at Ser1179 that returned to basal levels with more prolonged agonist stimulation. It is interesting to note that basal levels of Ser1179 phosphorylation appeared more robust in cells of our intact arterial rings than levels observed in cultured endothelial cells (7), suggesting that the recent exposure of our arterial rings to mechanical forces associated with blood flow likely contributed to enhanced basal levels of Ser1179 phosphorylation. Similar to our findings, these previous studies also reported a transient dephosphorylation of Thr497 with bradykinin stimulation (7) that recovered to basal levels with more prolonged exposure. The phosphorylation of the protein kinase Akt, a primary determinant of eNOS phosphorylation at Ser1179, was not altered significantly by bradykinin exposure, as reported previously (7). Previously, our group has reported that exercise training increases both total eNOS and phosphorylated eNOS (pSer1179) protein levels in small coronary arteries (∼200 μm luminal diameter) from the collateral-dependent compared with the control, nonoccluded region (15). Interestingly, previous studies in control pigs have reported no significant effect of exercise training on eNOS protein levels in conduit coronary arteries (22).
Coronary angiograms from our pigs demonstrate that the myocardial region formerly perfused by the native LCX artery is completely dependent upon collateral vessels for blood flow after ameroid-induced occlusion. Based on previous studies by Roth and colleagues (33), collateral vessel development in this porcine model is sufficient to maintain normal blood flow into the collateral-dependent region under resting conditions, although perfusion is significantly attenuated during acute exercise. Furthermore, histological analyses (33, 34) demonstrate that the porcine model of chronic occlusion and exercise training displays infarct size of ∼5–7% of the LCX-supplied myocardial region or ∼1% of the left ventricle. These investigators also report that, at rest, myocardial function in the collateral-dependent LCX region was similar to that observed in the control LAD region, whereas, during severe exercise, contractile function of the collateral-dependent region was reduced markedly (33). Taken together, these data indicate that the porcine model of progressive, chronic occlusion is a model of stress-induced ischemic heart disease that closely mimics human chronic coronary disease.
Limitations
Measures of endothelial cell free Ca2+ using fura 2 were completed at room temperature based on previous studies that revealed that bradykinin-stimulated Ca2+ transients in porcine aortic endothelial cells were most pronounced at 20–25°C, whereas transients at 35–40°C were approximately half of that observed at the lower temperatures (29). Therefore, to ensure that we obtained a sufficient number of cells with quantifiable fura 2 transients in our experiments, we completed our studies at room temperature (22–25°C). Furthermore, performing fura 2 studies in isolated cells at room temperature is considered an accepted approach, as indicated by numerous publications from multiple laboratories in this field (28, 43, 44, 46). Irrespective, our studies utilized endothelial cells isolated from sedentary and exercise-trained animals studied under identical conditions and provided the first evidence for training-induced changes in coronary endothelial cell Ca2+ mobilization in a model of chronic coronary occlusion and collateral development.
Clinical Implications and Conclusions
Distal vascular resistance within collateral-dependent myocardium has been shown to contribute to the regulation of coronary tone and myocardial perfusion to ischemic myocardium in human patients (3, 14, 30, 35, 37) and experimental animal models of coronary artery disease (1, 8–11, 38, 39), including effects on vasodilator reserve capacity (35, 37). Furthermore, impaired endothelial function of collateral-dependent vasculature (8–11, 14, 38) may have profound effects on blood flow to the myocardium-at-risk, as well as implicate potential beneficial mechanisms of exercise in coronary artery disease. Indeed, as suggested by Hambrecht et al. (14), the most potent vascular effects of exercise training may be upon improved endothelium-dependent vasodilatation and coronary blood flow reserve in diseased hearts (10, 11, 13, 14). Our results provide new insights into endothelial mechanisms that may underlie these effects in coronary artery disease.
Our isometric tension data (Fig. 8) suggested that nitric oxide contributed to basal tone to a greater extent in collateral-dependent than control arteries of both sedentary and exercise-trained pigs. It is interesting to note that arteries with the greatest contribution of nitric oxide to basal tone also displayed the highest protein levels for total and phosphorylated eNOS, suggesting that basal nitric oxide levels may be most attributable to eNOS phosphorylation levels. An increased contribution of nitric oxide to basal tone in the collateral-dependent arteries may maintain these arteries in a more dilated state under resting conditions to enhance resting blood flow to the ischemic region. In contrast, bradykinin-stimulated NOx levels closely paralleled the peak Ca2+ response in the four artery treatment groups, suggesting that Ca2+-calmodulin-mediated stimulation of eNOS appears to be the primary mediator of eNOS activity in agonist-stimulated conditions. Relevant to these findings, Hambrecht and colleagues (13) subjected patients to a 4-wk exercise training program before coronary artery bypass surgery. Subsequent ex vivo evaluation of a sample of the left internal mammary artery taken at surgery revealed improved endothelium-dependent relaxation and increases in eNOS protein and mRNA expression and enhanced phosphorylation of eNOS at Ser1179 and Akt at Ser473 in patients that were exercise trained compared with sedentary counterparts (13).
The present studies suggest that exercise training elicits adaptations in cellular mechanisms involved in the regulation of eNOS that likely contribute to the enhanced nitric oxide observed in coronary arteries of ischemic hearts. Taken together with previous findings that agonist-mediated, nitric oxide-dependent relaxation is enhanced in both control and collateral-dependent arteries of exercise-trained animals (9, 10), the current studies support the concept that exercise training enhances the regulatory role for nitric oxide in coronary function in the underlying setting of coronary artery disease. We speculate that chronic, intermittent increases in coronary blood flow that are associated with exercise training generate mechanical forces, including shear stress and distention, at the vascular wall that may contribute to the adaptations observed in these studies. In addition to the nitric oxide signaling pathway, increases in endothelial cytosolic Ca2+ levels also have been shown to activate the cyclooxygenase and endothelium-derived hyperpolarization pathways. Thus exercise training-induced adaptations in agonist-stimulated endothelial cell Ca2+ mobilization could potentially have profound effects on coronary vasodilatation and thus contribute to improved perfusion and enhanced vasodilator reserve that would be especially effective in the collateral-dependent myocardium.
GRANTS
These studies were supported by research funds from the National Institutes of Health, R01-HL-064931 (C. L. Heaps and J. L. Parker) and R01-GM-31651 (F. Schroeder).
ACKNOWLEDGMENTS
We appreciate the technical and surgical expertise of Mildred Mattox and technical contributions of Erin Ashmore and Jeff Bray.
REFERENCES
- 1.Altman JD, Klassen CL, Bache RJ. Cyclooxygenase blockade limits blood flow to collateral-dependent myocardium during exercise. Cardiov Res 30: 697–704, 1995 [PubMed] [Google Scholar]
- 2.Andries LJ, Brutsaert DL, Sys SU. Nonuniformity of endothelial constitutive nitric oxide synthase distribution in cardiac endothelium. Circ Res 82: 195–203, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Billinger M, Fleisch M, Eberli FR, Meier B, Seiler C. Collateral and collateral-adjacent hyperemic vascular resistance changes and the ipsilateral coronary flow reserve. Cardiov Res 49: 600–608, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Birnbaum D. Take two orthogonals and call me in the morning. Infect Control Hosp Epidemiol 24: 544–547, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999 [DOI] [PubMed] [Google Scholar]
- 6.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem 273: 3125–3128, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: e68–e75, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Fogarty JA, Delp MD, Muller-Delp JM, Laine GA, Parker JL, Heaps CL. Neuropilin-1 is essential for enhanced VEGF165-mediated vasodilatation in collateral-dependent coronary arterioles of exercise trained pigs. J Vasc Res 46: 152–161, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fogarty JA, Muller-Delp JM, Delp MD, Mattox ML, Laughlin MH, Parker JL. Exercise training enhances vasodilation responses to vascular endothelial growth factor in porcine coronary arterioles exposed to chronic coronary occlusion. Circulation 109: 664–670, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Griffin KL, Laughlin MH, Parker JL. Exercise training improves endothelium-mediated vasorelaxation after chronic coronary occlusion. J Appl Physiol 87: 1948–1956, 1999 [DOI] [PubMed] [Google Scholar]
- 11.Griffin KL, Woodman CR, Price EM, Laughlin MH, Parker JL. Endothelium-mediated relaxation of porcine collateral-dependent arterioles is improved by exercise training. Circulation 104: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Halcox JPJ, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KRA, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation 106: 653–658, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med 342: 454–460, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Heaps CL, Mattox ML, Kelly KA, Meininger CJ, Parker JL. Exercise training increases basal tone in arterioles distal to chronic coronary occlusion. Am J Physiol Heart Circ Physiol 290: H1128–H1135, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heaps CL, Parker JL, Sturek M, Bowles DK. Altered calcium sensitivity contributes to enhanced contractility of collateral-dependent coronary arteries. J Appl Physiol 97: 310–316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heaps CL, Sturek M, Rapps JA, Laughlin MH, Parker JL. Exercise training restores adenosine-induced relaxation in coronary arteries distal to chronic occlusion. Am J Physiol Heart Circ Physiol 278: H1984–H1992, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation 100: 1194–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Starodub O, McIntosh A, Kier AB, Schroeder F. Liver fatty acid-binding protein targets fatty acids to the nucleus. Real time confocal and multiphoton fluorescence imaging in living cells. J Biol Chem 277: 29139–29151, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Jobgen WS, Jobgen SC, Li H, Meininger CJ, Wu G. Analysis of nitrite and nitrate in biological samples using high-performance liquid chromatography. J Chrom B 851: 71–82, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric-oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol 90: 501–510, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Garcia-Cardena G, Sessa WC. Biosynthesis and palmitoylation of endothelial nitric oxide synthase: mutagenesis of palmitoylation sites, cysteines-15 and/or -26, argues against depalmitoylation-induced translocation of the enzyme. Biochemistry 34: 12333–12340, 1995 [DOI] [PubMed] [Google Scholar]
- 24.McAllister RM, Newcomer SC, Pope ER, Turk JR, Laughlin MH. Effects of chronic nitric oxide synthase inhibition on responses to acute exercise in swine. J Appl Physiol 104: 186–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCabe TJ, Fulton D, Roman LJ, Sessa WC. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem 275: 6123–6128, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin counterbalancing allosteric modulators of endothelial nitric oxide synthase. J Biol Chem 272: 25907–25912, 1997 [DOI] [PubMed] [Google Scholar]
- 28.Nakano T, Watanabe H, Ozeki M, Asai M, Katoh H, Satoh H, Hayashi H. Endoplasmic reticulum Ca2+ depletion induces endothelial cell apoptosis independently of caspase-12. Cardiov Res 69: 908–915, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Paltauf-Doburzynska J, Graier WF. Temperature dependence of agonist-stimulated Ca2+ signaling in cultured endothelial cells. Cell Calcium 21: 43–51, 1997 [DOI] [PubMed] [Google Scholar]
- 30.Piek JJ, van Liebergen RA, Koch KT, de Winter RJ, Peters RJ, David GK. Pharmacological modulation of the human collateral vascular resistance in acute and chronic coronary occlusion assessed by intracoronary blood flow velocity analysis in an angioplasty model. Circulation 96: 106–115, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem 273: 27383–27388, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Presta A, Liu J, Sessa WC, Stuehr DJ. Substrate binding and calmodulin binding to endothelial nitric oxide synthase coregulate its enzymatic activity. Nitric Oxide 1: 74–87, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Roth DM, Maruoka Y, Rogers J, White FC, Longhurst JC, Bloor CM. Development of coronary collateral circulation in left circumflex ameroid-occluded swine myocardium. Am J Physiol 25: 1279–1288, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Roth DM, White FC, Nichols ML, Dobbs SL, Longhurst JC, Bloor CM. Effect of long-term exercise on regional myocardial function and coronary collateral development after gradual coronary artery occlusion in pigs. Circulation 82: 1778–1789, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Sambuceti G, Parodi O, Giorgetti A, Salvadori P, Marzilli M, Dabizzi P, Marzullo P, Neglia D, L'Abbate A. Microvascular dysfunction in collateral-dependent myocardium. J Am Coll Cardiol 26: 615–623, 1995 [DOI] [PubMed] [Google Scholar]
- 36.Schroeder F, Zhou M, Swaggerty CL, Atshaves BP, Petrescu AD, Storey SM, Martin GG, Huang H, Helmkamp GM, Ball JM. Sterol carrier protein-2 functions in phosphatidylinositol transfer and signaling. Biochemistry 42: 3189–3202, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Seiler C, Fleisch M, Billinger M, Meier B. Simultaneous intracoronary velocity- and pressure-derived assessment of adenosine-induced collateral hemodynamics in patients with one- to two-vessel coronary artery disease. J Am Coll Cardiol 34: 1985–1994, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Sellke FW, Kagaya Y, Johnson RG, Sharique R, Schoen FJ, Grossman W, Weintraub RM. Endothelial modulation of porcine coronary microcirculation perfused via immature collaterals. Am J Physiol Heart Circ Physiol 262: H1669–H1675, 1992 [DOI] [PubMed] [Google Scholar]
- 39.Sorop O, Merkus D, de Beer VJ, Houweling B, Pistea A, McFalls EO, Boomsma F, van Beusekom HM, van der Giessen WJ, VanBavel E, Duncker DJ. Functional and structural adaptations of coronary microvessels distal to a chronic coronary artery stenosis. Circ Res 102: 795–803, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JWE, Price EM, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem Biophys Res Comm 226: 703–710, 1996 [DOI] [PubMed] [Google Scholar]
- 42.Wagner-Mann C, Bowman L, Sturek M. Primary action of endothelin on Ca release in bovine coronary artery smooth muscle cells. Am J Physiol Cell Physiol 260: C763–C770, 1991 [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Lau F, Li L, Yoshikawa A, van BC. Acetylcholine-sensitive intracellular Ca2+ store in fresh endothelial cells and evidence for ryanodine receptors. Circ Res 77: 37–42, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Reznick S, Li P, Liang W, van BC. Ca(2+) removal mechanisms in freshly isolated rabbit aortic endothelial cells. Cell Calcium 31: 265–277, 2002 [DOI] [PubMed] [Google Scholar]
- 45.White FC, Carroll SM, Magnet A, Bloor CM. Coronary collateral development in swine after coronary artery occlusion. Circ Res 71: 1490–1500, 1992 [DOI] [PubMed] [Google Scholar]
- 46.Zoratti C, Kipmen-Korgun D, Osibow K, Malli R, Graier WF. Anandamide initiates Ca(2+) signaling via CB2 receptor linked to phospholipase C in calf pulmonary endothelial cells. Br J Pharmacol 140: 1351–1362, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]