Abstract
We have recently demonstrated that the inhibition of histone deacetylases (HDAC) protects the heart against ischemia-reperfusion (I/R) injury. The mechanism by which HDAC inhibition confers myocardial protection remains unknown. The purpose of this study is to investigate whether the disruption of NF-κB p50 would eliminate the protective effects of HDAC inhibition. Wild-type and NF-κB p50-deficient mice were treated with trichostatin A (TSA; 0.1 mg/kg ip), a potent inhibitor of HDACs. Twenty-four hours later, the hearts were perfused in Langendorff model and subjected to 30 min of ischemia and 30 min of reperfusion. Inhibition of HDACs by TSA in wild-type mice produced marked improvements in left ventricular end-diastolic pressure, left ventricular rate pressure product, and the reduction of infarct size compared with non-TSA-treated group. TSA-induced cardioprotection in wild-type animals was absent with genetic deletion of NF-κB p50 subunit. Notably, Western blot displayed a significant increase in nuclear NF-κB p50 and the immunoprecipitation demonstrated a remarkable acetylation of NF-κB p50 at lysine residues following HDAC inhibition. EMSA exhibited a subsequent increase in NF-κB DNA binding activity. Luciferase assay demonstrated an activation of NF-κB by HDAC inhibition. The pretreatment of H9c2 cardiomyoblasts with TSA (50 nmol/l) decreased cell necrosis and increased in cell viability in simulated ischemia. The resistance of H9c2 cardiomyoblasts to simulated ischemia by HDAC inhibition was eliminated by genetic knockdown of NF-κB p50 with transfection of NF-κB p50 short interfering RNA but not scrambled short interfering RNA. These results suggest that NF-κB p50 acetylation and activation play a pivotal role in HDAC inhibition-induced cardioprotection.
Keywords: ischemia, myocardium, myocardial infarction
histone acetyltransferases (HAT) and histone deacetylases (HDAC) have recently emerged as important mechanisms in the regulation of a variety of cellular responses. Histone acetylation is mediated by HAT. The resulting modification in the structure of chromatin leads to nucleosomal relaxation and altered transcriptional activation. The reverse reaction is mediated by HDAC, which induces deacetylation, chromatin condensation, and transcriptional repression (5, 14, 22, 29, 31). In addition to histones, growing evidence suggests that HAT and HDAC target nonhistone proteins, including transcriptional factor (4), which may represent general regulatory mechanisms in biological signaling.
Since the identification of HDAC 1 (named HD 1; Ref. 15), 18 HDACs have been described in mammals and are divided into three distinct classes on the basis of secondary structure (34). Class I HDACs consist of HDAC 1, 2, 3, and 8, which are predominantly nuclear proteins and ubiquitously expressed. Class II HDACs include HDAC 4, 5, 7, and 9. In contrast to class I, class II HDACs exhibit a tissue specific pattern of expression. HDAC 4 and HDAC 5 are found at high levels in the heart, brain, and skeletal muscle (8, 11, 37). All class IIa HDACs shuttle between the nucleus and cytoplasm. Class III HDACs were identified on the basis of sequence similarity with Sir, a yeast transcriptional repressor that requires the cofactor NDA+ for its deacetylase activity. HDAC inhibitors have shown efficacy as anticancer reagents in human, and animal models and are emerging as an exciting clinical treatment targeting solid and hematological malignancies (35).
Inhibition of class II HDACs silences the fetal gene activation, renders myocytes insensitive to hypertrophic agonists, blocks cardiac hypertrophy, and prevents cardiac remodeling (1, 17, 18). Specific mutation of HDAC has resulted in attenuation of cardiac hypertrophy and reduced ischemia-reperfusion (I/R) injury (12). Furthermore, HDAC inhibition has previously been shown to markedly decrease infarct size in focal cerebral ischemia model of rats (26). We and others (12, 19, 40) have recently demonstrated that inhibition of HDAC with trichostatin A (TSA) protects the heart against ischemic injury. Taken together, these data support the pivotal role of HDAC inhibition in cardioprotection. However, the molecular signaling event that underlies HDAC inhibition-induced cardioportection is still poorly understood.
NF-κB is sequestered in the cytoplasm by the inhibitory protein I-κB. Ultraviolet radiation, T-cell activation, bacterial LPS viral gene products, and inflammatory cytokines promote IκB degradation, thereby allowing NF-κB to enter the nucleus and induce gene transcription (3, 28). The increased NF-κB in postischemic myocardium has been reported (21). Although these studies (23, 24, 38, 42) have indicated that NF-κB plays an essential role in regulation of myocardial I/R , whether NF-κB is involved in HDAC inhibition-induced cardioprotection has never been elucidated. Interestingly, it has been recently reported that NF-κB p50 can be acetylated by acetyltransferase p300 (6). However, there is no evidence to identify the acetylation of NF-κB p50 in myocardium and its implication in mediating myocardial I/R. Given that HDAC inhibition is known to result in hyperacetylation, it is imperative to assess whether HDAC inhibition induced cardioprotection through acetylation of NF-κB p50. The objectives of our study were to determine as follows: 1) whether the cardioprotective effects generated with the HDAC inhibitor TSA could be diminished with the targeted deletion of NF-κB p50 in mice; 2) whether NF-κB p50 could be acetylated following HDAC inhibition and whether acetylation of NF-κB p50 was associated with an increase in NF-κB DNA binding activity; 3) whether HDAC inhibition directly resulted in an activation of NF-κB; 4) whether HDAC inhibition would increase the resistance of H9c2 cardiomyoblasts to a subsequent simulated ischemic stress in the in vitro cell culture model; and 5) whether genetic suppression of NF-κB p50 with small interfering (si)RNA would eliminate the protection of H9c2 cardiomyoblasts from the simulated ischemia following HDAC inhibition. Investigation of the cause and effect relationship of HDAC inhibition with NF-κB p50 in the heart could provide insight into our understanding of novel mechanisms of ischemic heart disease and develop therapeutic strategies for clinical implication.
MATERIALS AND METHODS
Animals.
Adult male B6.129 wild-type control and homozygous p105−/− mice on the B6;129 background were supplied by Jackson Laboratories (Bar Harbor, ME). All animal experiments were conducted in accordance with the guidelines on humane use and care of laboratory animals for biomedical research published by the National Institutes of Health. The experimental protocol was approved and carried out in accordance with the guidelines adhered to the Institutional Animal Care and Use Committee of Rhode Island Hospital.
Chemical supplies and antibodies.
TSA was obtained from Calbiochem (San Diego, CA). N-(2-mercaptopropionyl)-gel electrophoresis supplies were obtained from Bio-Rad Laboratories (Hercules, CA). The perfusion chemicals triphenyltetrazolium chloride (TTC), 2-deoxy-d-glucose, and potassium cyanide were purchased from Sigma (St. Louis, MO). Mouse monoclonal acetylated-lysine (Ac-K-103) antibody was purchased from Cell Signaling (New England, MA). NF-κB p50 polyclonal rabbit antibody, β-actin, siRNA NF-κB p50 RNA, and negative control scrambled siRNA were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-rabbit IgG(H+L) horseradish peroxidase-conjugated secondary antibody was purchased from Amersham (Hercules, CA). Goat anti-mouse IgG(H+L) horseradish peroxidase conjugate secondary antibody was purchased from Invitrogen (Carlsbad, CA).
Langendorff isolated heart perfusion.
The methodology of Langendorff's isolated perfused heart preparation has been described previously in detail (42–44). Briefly, mice were anesthetized with an intraperitoneal injection of pentobarbital sodium (120 mg/kg). The hearts were rapidly excised and arrested in ice-cold Krebs-Henseleit buffer. They were then cannulated via the ascending aorta for retrograde perfusion by the Langendorff method using Krebs-Henseleit buffer containing the following (in mM): 110 NaCl, 4.7 KCl, 1.2 MgSO4 7H2O, 2.5 CaCl2 2H2O, 11 glucose, 1.2 KH2PO4, 25 NaHCO3, and 0.5 EDTA. The buffer, aerated with 95% O2-5% CO2 to give a pH of 7.4 at 37°C, was perfused at a constant pressure of 55 mmHg. A water-filled latex balloon, attached to the tip of polyethylene tubing, was then inflated sufficiently to provide a left ventricular end-diastolic pressure (LVEDP) of 10 mmHg. Myocardial function was measured including left ventricular developed pressure (LVDP), LVEDP, rate pressure product (RPP), heart rate, and coronary flow. LVDP was calculated by subtracting LVEDP from the peak systolic pressure. RPP, an index of cardiac work, was calculated by multiplying LVDP with heart rate.
Experimental protocol.
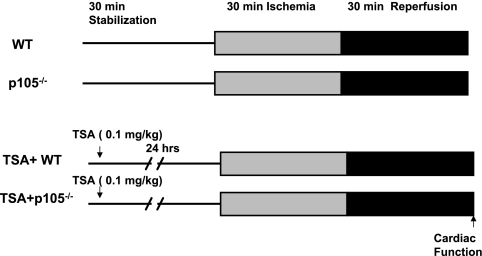
Mice were randomized into four experimental groups that underwent the following treatments, as shown in Fig. 1. Control wild-type B6.129 and NF-κB p105−/− mice were subjected to 30 min of stabilization and 30 min of ischemia followed by 30 min of reperfusion. To examine the effects of NF-κB p50 on cardioprotection elicited by HDAC inhibition, our established preconditioning protocol was used. Wild-type B6.129 and NF-κ B p105−/− mice were treated with TSA. Animals were divided into two groups: group 1: TSA + B6.129 mice (n = 5), B6.129 mice were injected with TSA (0.1 mg/kg ip); and group 2: TSA + NF-κB p105−/− mice (n = 7), p105−/− mice were injected with TSA (0.1 mg/kg ip). Twenty-four hours later, the hearts were subjected to 30 min of ischemia followed by 30 min of reperfusion.
Fig. 1.
Experimental protocol. See Materials and Methods for groups and treatments. WT, wild type; TSA, trichostatin A.
Another subset of wild-type animals without sustained I/R was treated with or without TSA solely for the purpose of measuring acetylated NF-κB p50. Animals were treated with TSA for 30 min, and heart tissues were collected. The hearts were frozen in liquid nitrogen, and cardiac lysates were extracted as previously described (42). Briefly, frozen hearts were ground and suspended in 1 ml of lysis buffer containing 50 mM Tris·HCl (pH 7.4), 0.1 mM sodium orthovanadate, 50 mM sodium fluoride, 150 mM sucrose, 1 mM PMSF, 5 mM EDTA, 5 mM EGTA, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 5 μg/ml pepstatin A. Mixtures were homogenized and microcentrifuged at 14,000 rpm for 20 min. The protein content of the supernatant was collected and determined using a protein assay (Bio-Rad).
Measurement of infarct size.
The infarct size was measured with a well-established method as previously described (40, 41). At the end of reperfusion, hearts were perfused with 10% TTC and then removed from the Langendorff perfusion apparatus. Hearts were then frozen and cut from apex to base into transverse slices. After being stained, 10% TTC buffer was replaced, and then the slices were fixed in formaldehyde for measurement of the infarcted areas using computer morphometry NIH image software (Image J 1.36). The infarct size was calculated and presented as the percentage of risk area, defined as the sum of the total ventricular area minus cavities (42–44).
Preparation of nuclear and cytosolic fractions.
Nuclear extracts were prepared using a modification of the method previously described (42, 43). Briefly, the tissue samples were pulverized in liquid nitrogen. The tissue was ground, suspended, and then lysed twice with lysis buffer containing 20 mM Tris pH 7.9, 140 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 0.5% NP-40, 0.5 mM sodium orthovanadate, and protease inhibitors (1 mM PMSF, 2 μg/ml aprotinin, and 2 μg/ml leupeptin). The nuclei were washed once with 1 ml of lysis buffer without NP-40 and resuspended in 150 μl of nuclear extraction buffer (50 mM Tris·HCI pH 7.9, 60 mM KCl, 1 mM EDTA, 1 mM EGTA, 2 mM DTT, 1 mM PMSF, and 0.5 mM sodium orthovanadate). After three cycles of freeze-thawing, the nuclear extracts were obtained by centrifugation at 10,000 g for 15 min and then used for Western blot analysis and EMSA. The cytosolic fraction of NF-κB was prepared according to the previously described method (43).
Western blot analysis.
The proteins were detected using the following antibodies: anti-acetylated lysine and anti-NF-κB p50 polyclonal antibodies. For immunoblotting, protein (50 μg/lane) was separated by SDS-PAGE using 10% SDS for NF-κB p50. Proteins were then transferred onto a nitrocellulose membrane for 2 h at 100 V. The membrane was blocked with 5% nonfat dry milk in 1× Tris-buffered saline containing 0.5% Tween 20 for 1 h. The blots were incubated with NF-κB p50 polyclonal antibody (1:1,000 dilution) for 2 h and visualized by incubation with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:5,000 dilution) for 1 h. The immunoblots were developed with the ECL Chemiluminescence Detection Reagent (Amersham Pharmacia Biotech). For the measurement of the acetylation of p50, cardiac tissue lysates (200 μg) were immunoprecipitated with an antibody (1 μg) specific for NF-κB p50 and gently agitated for 2 h at 4°C, followed by additional incubation with EZview red protein A affinity gel (Sigma). The beads were washed three times with the lysis buffer and one time with 50 mM Tris (pH 6.8). Then, 50 μl SDS of sample buffer were added, and the samples were heated for 5 min at 100°C. The supernatant was applied to SDS-PAGE gels. Immunoprecipitated protein was analyzed by Western blotting for immunoreaction with anti-acetylated lysine antibody (1:1,000 dilution), and antigen-antibody complexes were visualized as described above.
EMSA.
The EMSA was carried out by the LightShift Chemiluminescent EMSA kit (Pierce) that uses a nonisotopic method to detect DNA-protein interactions (44). Biotin end-labeled DNA double-stranded 22-mer oligonucleotide with the sequence 5′-AGT TGA GGG GAC TTT AGG C-3′ (Promega) containing a putative binding site for κB was incubated with the nuclear extracts (42). The binding reactions were performed in a final volume of 20 μl containing 10 μg of protein in buffer (10 mm Tris pH 7.5, 50 mM KCl, 5 mm MgCl2, 1 mm dithiothreitol, 0.05% Nonidet P-40, and 2.5% glycerol), 1 μg of poly(dI-dC), and 2 nm of biotin-labeled DNA. The reaction mixture was incubated for 30 min at room temperature. The competition reactions were performed by addition of 100-fold excess unlabeled double-stranded oligonucleotide to the reaction mixture. After the reaction, the DNA-protein complexes were subjected to a 5% native PAGE and transferred to a Biodyne nylon membrane (Pierce). After transfer, the membrane was immediately cross-linked for 10 min with ultraviolet 254-nm bulbs. A chemiluminescent detection method utilizing a luminol/enhancer solution was used as described by the manufacturer (Pierce), and membranes were exposed to X-ray film. The densitometric results were normalized to the control group and expressed as percentages of the control values.
Cell culture and siRNA transfection.
H9c2 cardiomyoblast cells, a clonal line derived from rat heart (ATCC, Rockville, MD), were grown in DMEM with 10% FCS, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The siRNA transfections were done according to the description in manufacturer's protocol. The negative control (scrambled) siRNA and NF-κB p50 siRNA were mixed with Lipofectamine 2000 (Invitrogen) at a final concentration at 500 nmol/l of siRNA in medium, respectively. Protein expression was determined by Western blot using a specific antibody to NF-κB p50 to confirm the significant reduction of NF-κB p50 after 24 h. At 24 h of posttransfection, H9c2 cardiomyoblasts were subjected to simulated ischemia. For TSA treatments, H9c2 cardiomyoblast cells were exposed to TSA (50 nmol/l) for 30 min before subsequent 30 min of simulated ischemia was initiated. The SI medium consists of the following (mM): 115 NaCl, 5 KCl, 1 KH2PO4, 1.2 MgSO4, 2 CaCl2 2H2O, and 25 HEPES containing 5 mM potassium cyanide and 20 mM 2-deoxy-d-glucose for 30 min in a 5% CO2 at 37°C. Cyanide treatment was terminated by being rinsed twice with PBS. The medium was then replaced by DMEM to mimic 30 min of the reperfusion.
Measurement of cell viability.
The assessment of H9c2 cardiomyoblast viability was based on the description and the principle of reduction of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT; Sigma) into blue formazan pigments in viable cells (7). At the end of the experiment, the medium was removed and the cells were washed with PBS. MTT (0.01 g/ml) was dissolved in PBS, and 500 μl were added to each well. Cells were subsequently incubated for 2 h at 37°C. Cells were then washed twice with PBS, and 1 ml of HCl-isopropanol-Triton (1% HCl in isopropanol:0.1% Triton X-100; 50:1) was added to each well for 5 min. The suspension was then centrifuged at 14,000 rpm for 2 min. The optical density was determined spectrophotometrically at a wavelength of 540 nm, and the values are expressed as percentages of control values.
Measurements of cell necrosis.
Loss of plasma membrane integrity (cell necrosis) was assessed by measurement of the activity of lactate dehydrogenase (LDH) in the supernatant. A 100-μl supernatant was transferred into a 96-well plate. The release of LDH in the culture medium from H9c2 cardiomyoblast cells at the end of reperfusion was determined as an indicator of cell necrosis using a commercially available kit (Roche), as per manufacturer's protocol with a modification (30). The optical density was determined spectrophotometrically at a wavelength of 490 nm, and the values are expressed as percentages of control values.
Luciferase activity assay.
NF-κB reporter gene assays were described previously with the appropriate modification (45) that the H9C2 cardiomyoblasts were transiently transfected with luciferase reporter construct 2×κB or empty vector by the Lipofectamine 2000 method (Invitrogen). Twenty-four hours after transfection, cells were treated with or without TSA (50 nM) in the presence or absence of simulated ischemia, as described above. Cell lysates were generated and luciferase activity was determined according to the manufacturer's instructions by using the Luciferase Assay System (BioVision). Luciferase activities were measured with TD-20/20 Luminometer (Turner Designs Instrument) three times, and results were normalized to the basal levels of the empty vector group.
Statistics.
All measurements are expressed as means ± SE. Differences among the groups were analyzed by one-way ANOVA, followed by post hoc Bonferroni correction or Student's unpaired t-test for two groups. Statistical differences were considered significant with a value of P < 0.05.
RESULTS
Baseline ventricular function.
Baseline functional parameters, including LVSP, LVEDP, LVDP, RPP, and heart rate, were recorded among the groups. As shown in Table 1, there were no significant differences among the groups before ischemia.
Table 1.
Baseline functional parameters
| Groups | LVSP, mmHg | LVEDP, mmHg | RPP, mmHg/min ×103 | CF, ml/min | Heart Rate, beats/min |
|---|---|---|---|---|---|
| WT (n = 6) | 89 ± 4 | 5 ± 2 | 31 ± 3 | 2.7 ± 0.5 | 385 ± 33 |
| p105−/− (n = 6) | 96 ± 7 | 5 ± 1 | 36 ± 2 | 3 ± 0.3 | 425 ± 27 |
| TSA + WT (n = 5) | 107 ± 11 | 1 ± 1 | 43 ± 7 | 4 ± 0.4 | 392 ± 29 |
| TSA + p105−/− (n = 7) | 90 ± 17 | 4 ± 1 | 39 ± 10 | 3.4 ± 0.7 | 343 ± 19 |
| P values | 0.71 | 0.18 | 0.68 | 0.41 | 0.19 |
Data are means ± SE; n = no. of animals. No significant differences were found between the experimental groups for any of the functional parameters. WT, wild type; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end-diastolic pressure; RPP, rate pressure product; CF, coronary effluent; TSA, trichostatin A.
Disruption of NF-κB p50 attenuates HDAC inhibition-induced ventricular functional improvement.
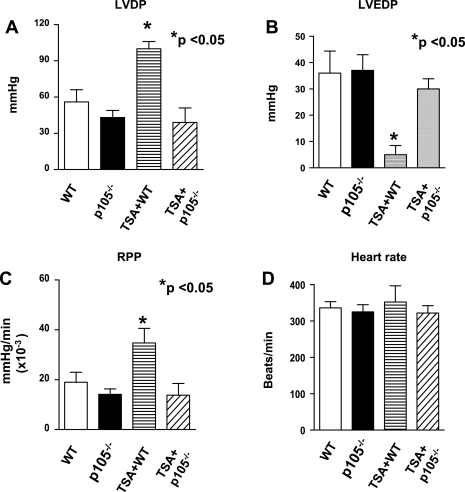
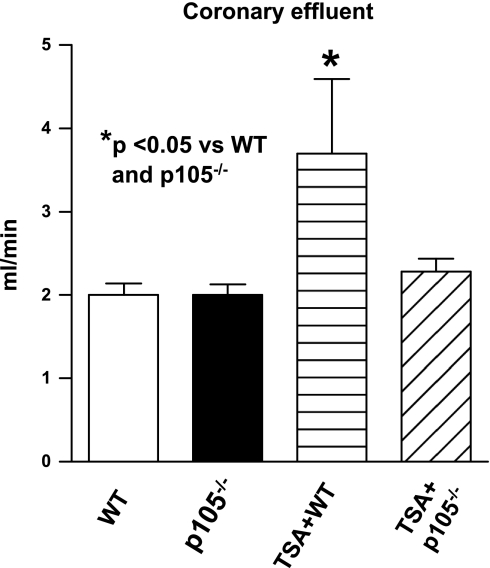
As shown in Fig. 2, A-D, left ventricular functional recovery declined dramatically compared with baseline, but there were no significant differences in ventricular functional recoveries between wild-type and NF-κB p50-deficient mice in the absence of HDAC inhibition. However, postischemic ventricular function was improved after TSA treatment in wild-type mice. TSA-induced postischemic improvement in LVEDP was abrogated in animals with disruption of the p50 subunit of NF-κB (30 ± 4 mmHg vs. 5 ± 4 mmHg in TSA + B6.129 WT mice; P < 0.05). In addition, the improvement of LVDP in TSA + B6.129 was also eliminated by deletion of p105 (P < 0.05). Similarly, disruption of NF-κB p105 mitigated the recovery of RPP (13.8 ± 4.7 × 103 mmHg/min vs. 34 ± 5.8 × 103 mmHg/min; P < 0.05). As shown in Fig. 3, there was no difference in coronary effluent between wild-type and NF-κB p105 deficient mice; however, TSA treatment resulted in a marked improvement in coronary effluents in postischemic myocardium (P < 0.05), which disappeared in TSA-treated NF-κB p105−/− mice.
Fig. 2.
Effect of TSA on postischemic ventricular function in WT and NF-κB p105−/− mice. A: left ventricular developed pressure (LVDP). B: left ventricular end-diastolic pressure (LVEDP). Rate pressure product (RPP; C) and heart rate (HR; D). Values are means ± SE (n = 5–7/group). *P < 0.05 vs. WT, p105−/−, and TSA + p105−/− groups.
Fig. 3.
Effect of TSA on coronary effluent in WT and NF-κB p105−/− mice mice. Values are means ± SE (n = 5–7). *P < 0.05 vs. WT and p105−/− groups.
Infarct-sparing effect of HDAC inhibition depends on NF-κB p50.
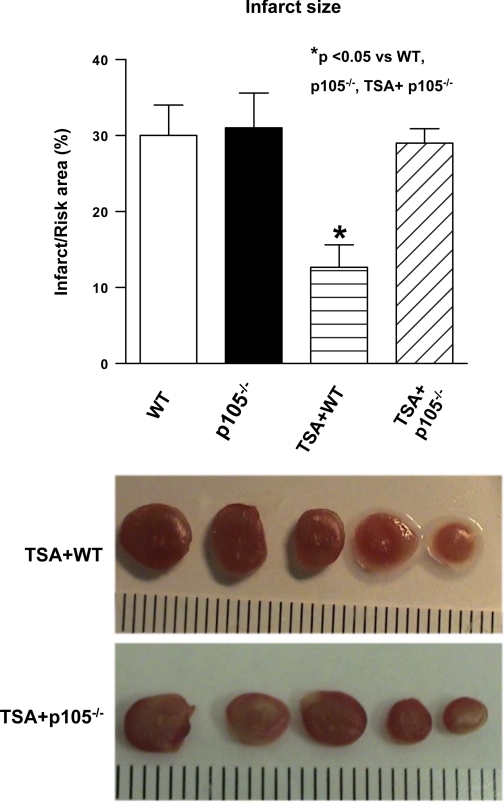
Myocardial infarct size, an index of irreversible myocardial injury, was measured. The infarct size in wild-type mice was also not significantly different from that in NF-κB p105−/− mice. However, following the TSA treatment, the infarct size in the wild-type B6.129 + TSA group was 13 ± 3%, which was increased to 29 ± 2% in TSA-treated NF-κB p105−/− (P < 0.05; Fig. 4). The data suggest that the reduction of infarct size by inhibition of HDAC requires NF-κB p50.
Fig. 4.
Effect of TSA on myocardial infarct infarction in WT and NF-κB p105−/− mice. Values are means ± SE (n = 5–7). Representative sections of a heart demonstrating reduction of postischemic infarct size 30 min after treatment with TSA. At the end of experimental protocol as described in Materials and Methods, hearts were sliced and stained with 2,3,5-triphenyltetrazolium chloride followed by fixation in formalin. Viable areas are stained brick red, whereas infarcted areas are gray or white. *P < 0.05 vs. WT, p105−/−, and TSA + p105−/− groups.
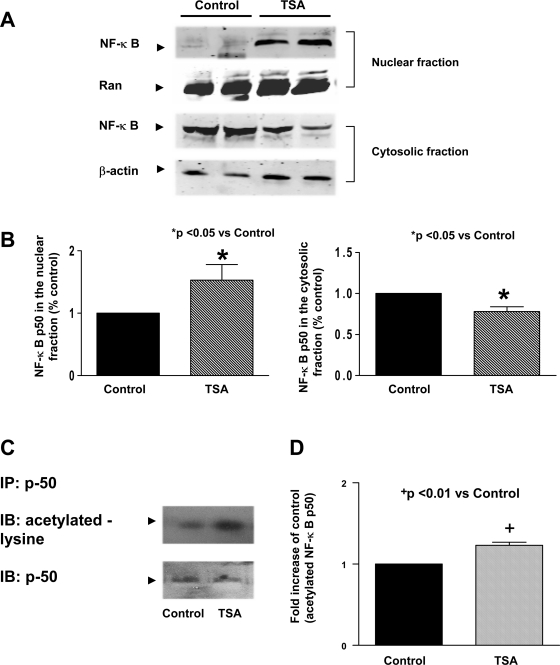
NF-κB p50 is acetylated and activated through HDAC inhibition.
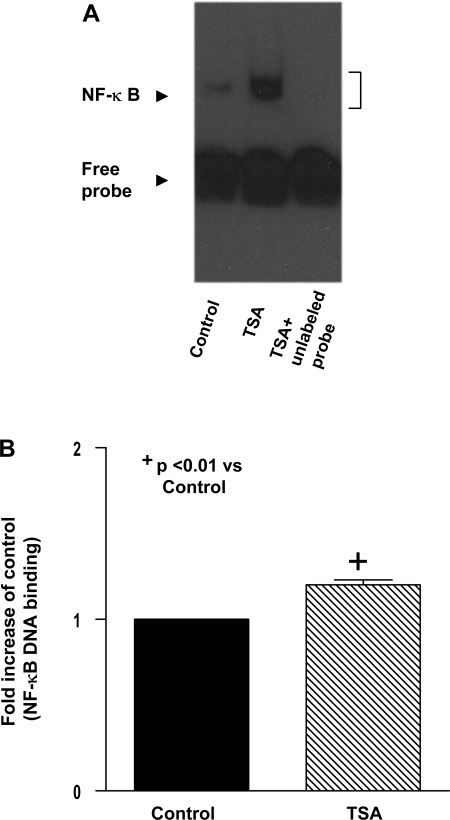
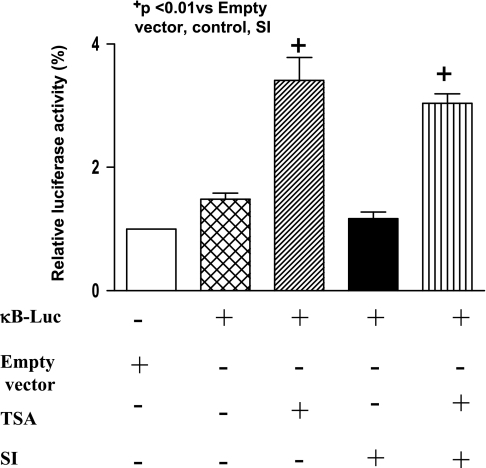
We examined the effect of TSA treatment on the accumulation of NF-κB p50. As shown in Fig. 5, A and B, TSA treatment in wild-type mice led to a significant increase in NF-κB p50 in nuclei, which is accompanied by the decrease in NF-κB p50 in cytosolic fractions, indicating an augmentation of NF-κB p50 nuclear in nuclei following hyperacetylation. To determine whether NF-κB p50 could be acetylated, we immunoprecipitated NF-κB p50 from cardiac tissue and probed the blots with an acetylated-lysine antibody. As shown in Fig. 5, C and D, NF-κB p50 was acetylated at lysine residues after HDAC inhibition. Notably, EMSA shows that NF-κB DNA binding activity was low in the control group, which increased rapidly following TSA treatment (Fig. 6A). The densitometric analysis shows a significant difference in the NF-κB binding activity between the HDAC treatment and control group (Fig. 6B), suggesting a direct link between acetylation of NF-κB p50 and its DNA binding activity. To further quantify the HDAC inhibition-mediated transcriptional regulation of NF-κB, we performed the transient luciferase assays. Inhibition of HDAC with TSA significantly increased the luciferase activity of NF-κB reporter construct in either the presence or the absence of simulated ischemia (Fig. 7), supporting the notion that HDAC inhibition results in an effective activation of NF-κB.
Fig. 5.
A: representative Western blot showing the effect of TSA on the contents of NF-κB p50 in myocardial nuclear and cytosolic fractions. B: quantification of NF-κB p50 contents in nuclei (left) and cytosolic fraction (right) after TSA stimulation. C: immunoprecipitation (IP) of myocardial protein with NF-κB p50 and immunoblot (IB) of precipitated samples with acetylated lysine antibody. D: quantification of acetylated NF-κB p50 contents in nuclei after TSA stimulation. Densitometric signal was normalized to the control group and expressed as a percentage. Results are means ± SE (n = 6/group). *P < 0.05, +P < 0.01 vs. control.
Fig. 6.
A: increased NF-κB DNA binding activity following TSA treatment. Description of experimental group is provided in Materials and Methods. Nuclear extracts were prepared and incubated with the biotin-labeled NF-κB oligonucleotide probe to determine the binding activity using electromobility gel shift assay. Competition reactions were performed by addition of 100-fold excess unlabeled double-stranded oligonucleotide to the reaction mixture in the TSA-treated samples. B: quantification of NF-κB DNA binding activity following TSA treatment. Densitometric signal was normalized to the control group and expressed as a percentage. Results are means ± SE (n = 6/group). +P < 0.01 vs. control.
Fig. 7.
Induction of κB promoter activity by HDAC inhibition with TSA treatments. H9c2 cells were transiently transfected with the 2×κB luciferase reporter construct. Twenty-four hours after transfection, cells were treated with or without TSA and luciferase activities were determined 3 times and results were normalized to the basal levels (empty vector). Results are means ± SE (n = 3/group). +P < 0.01 vs. empty vector.
HDAC inhibition enhances the survival of H9c2 cardiomyoblasts via NF-κB p50.
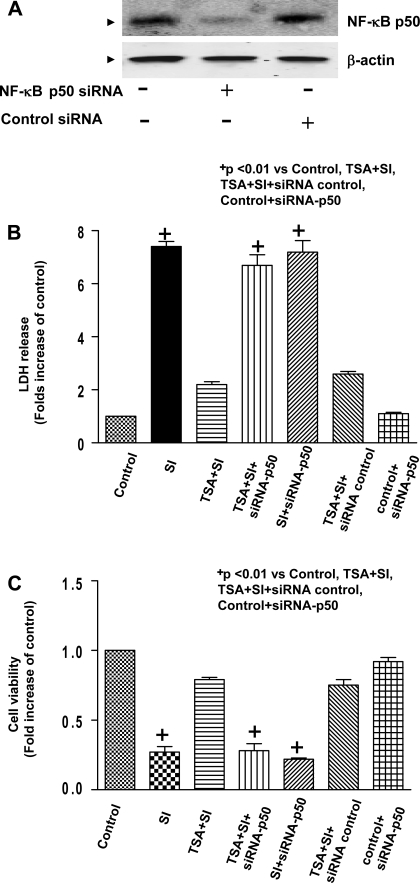
We next determined cell necrosis and viability of H9c2 cardiomyoblasts in combination of genetic silencing. As shown in Fig. 8A, Western blot exhibits the reduction of expression of NF-κB p50 in H9c2 cardiomyoblast cells transfected with NF-κB p50 siRNA but not control scrambled siRNA, suggesting that targeted siRNA effectively knocked down NF-κB p50. As shown in Fig. 8B, compared with control, simulated ischemic stimulus led to the increased LDH leakage, which significantly decreased in H9c2 cardiomyoblast cells treated with 50 nM of TSA. However, genetic knockdown of NF-κB p50 with siRNA remarkably abolished the reduction of LDH leakage by HDAC inhibition, but the scrambled siRNA did not show any effect on LDH leakage. In the absence of TSA treatments, there was no difference in LDH leakage between the SI and SI + NF-κB p50 siRNA groups. Additionally, in the absence of both TSA treatment and SI, transfection of NF-κB p50 siRNA did not cause the LDH leakage compared with the control. Furthermore, simulated ischemia decreased cell viability, which was profoundly prevented by HDAC inhibition. Strikingly, the improvement in cell viability of HDAC inhibition was absent with knockdown of NF-κB p50 with specific siRNA (Fig. 8C).
Fig. 8.
A: H9c2 cardiomyoblast cells were transiently transfected with NF-κB p50 siRNA (500 nmol/l) or nonspecific siRNA (500 nmol/l) for 24 h to prepare protein lysates and following immunoblotting assay. B: LDH release of H9c2 myocytes in culture medium at the end of reperfusion (n = 4/group). C: cell viability was measured using the MTT cell viability assay (n = 5/group). H9c2 cardiomyoblast cells were exposed to simulated ischemia (potassium cyanide and 2-deoxy-d-glucose), and description of simulated ischemia is provided in Materials and Methods. The HDAC inhibitor TSA (50 nmol/l) was maintained in culture medium. NF-κB p50 and negative control siRNA transfections are described in Materials and Methods. SI, simulated ischemia. Results are means ± SE, and values are expressed as percentages of control values. +P < 0.01 vs. control, TSA + SI, TSA + SI + siRNA control, and control + siRNA-p50.
DISCUSSION
Salient findings.
HDAC inhibition has been recently discovered as a novel signaling pathway to confer myocardial protection following I/R. In the present study, we have shown that the genetic deletion of NF-κB p50 eliminated cardioprotection elicited by HDAC inhibition. Inhibition of HDAC resulted in the increase in NF-κB p50 in the nuclei of the myocardium. Immunoprecipitation assay reveals that NF-κB p50 was acetylated at lysine residues following HDAC inhibition, which was associated with a subsequent increase in NF-κB p50 DNA binding activity and NF-κB activation. With the use of siRNA gene silencing and well-established in vitro cell culture model, HDAC inhibition increased the resistance of H9c2 cardiomyoblast cells to simulated ischemia, which was indicated by the reduction of cell necrosis and the increase in cell viability. Genetic suppression of NF-κB p50 with siRNA mitigated the beneficial effect of HDAC inhibition on cell viability and necrosis. Taken together, the present study is the first direct demonstration to reveal that acetylation and activation of NF-κ B p50 constitute a novel major pathway of HDAC inhibition to elicit cardioprotection.
The class II HDACs are abundantly expressed in the heart. TSA acts as a potent inhibitor of HDAC and is rapidly inactivated in vivo following intraperitoneal administration to mice (39). The deacetylase-TSA structure reveals that TSA binds to the deacetylase catalytic core to form a tubular pocket, a zinc-binding site, and a two Asp-His charge-relay system to inhibit the histone acetylase (9). TSA at 50 nM demonstrated a significant increase in acetylated histone 4 and inhibition of HDAC activity (1, 40), providing a basis of HDAC inhibitor concentration employed in cultured H9c2 cardiomyoblasts. In addition, we (10, 25, 33) have conducted a well-established pharmacological preconditioning approach, since preconditioning stimuli have been demonstrated as powerful approaches to protecting the heart and hold promising clinical implications. Our previous studies (42, 43) show that a short period of pharmacological preconditioning stimuli effectively induces a delayed cardioprotection after 24 h of treatments, confirming that these triggers activate downstream signaling pathways to confer a late phase preconditioning. In this regard, we assessed the molecular signaling components 30 min after the pharmacological treatments and characterized the ventricular functional parameters in a delayed window. The treatment of the heart with TSA resulted in an improvement in recovery of ventricular function as well as the reduction of myocardial infarct size in wild-type mice (40). This is consistent with the observation in which suppression of HDAC activity in the heart or knockdown of specific HDAC in myocytes alters the response to ischemic injury and reduces infarct size as well as prevents cellular injury (12). Similarly, the beneficial effects of HDAC inhibition have also been reported in the pressure overload in mice and cultured neonatal cardiomyocytes (13, 17, 18). In addition, TSA has been demonstrated to induce tumor-selective apoptosis in acute myeloid leukemia and acute promyelocytic leukemia (16). This evidence suggests that HDAC inhibition serves as an important and common protective signaling pathway. Although HDAC in cardiovascular implication has recently garnered significant attention of late, the precise downstream signaling in which HDAC inhibition produces cardioprotection is unclear.
Stimulation of NF-κB has been reported to be essential in protecting the heart against ischemia injury (20, 23, 38, 42, 43, 46). Targeted deletion of NF-κB p50 markedly increases the extent of expansive remodeling and aggravated systolic dysfunction in the infarcted heart (32). NF-κB has been shown to be acetylated following inhibition of HDAC with TSA (2, 4). This is consistent with another investigation that p300 overexpression resulted in increased NF-κB p50 acetylation that was reduced by HAT mutation in RAW 264.7 cells (6). These observations suggest a potential link between inhibition of HDAC and activation of NF-κB. Strikingly, immunoprecipitation of NF-κB p50 demonstrated that NF-κB p50 was acetylated at lysine residues following HDAC inhibition in the myocardium in our study. In addition, NF-κB p50 acetylation was closely related with the increase in NF-κB p50 DNA binding activity and activation. This implicates acetylation as a critical determinant to activate NF-κB p50. It will be interesting to conduct future studies to decipher the deep mechanisms of how NF-κB p50 is acetylated in ischemic myocardium. Furthermore, this study provides the direct evidence that disruption of NF-κB p50 eliminated the cardioprotection following HDAC inhibition, demonstrating a pivotal role of NF-κB p50 in modulating myocardial protection by HDAC inhibition. The protective effect of HDAC inhibition is related to the acetylation and activation of the p50 subunit of NF-κB. We observed that TSA treatments caused an increase in coronary effluents in postischemic heart, which might be related to the protective effects of HDAC inhibition. However, the improvement in coronary effluents by HDAC inhibition was absent with disruption of NF-κB p50, which suggests that downstream signaling of NF-κB p50 might be responsible for this event. However, we did not find significant differences in the ventricular functional recovery as well as myocardial infarct between wild-type and p105−/− mice without receiving TSA treatment. This suggests that the existence of abundant acetylated p50 plays a major role to confer the heart against ischemic injury. H9c2 cardiomyoblast cells have been widely used as a powerful in vitro model to study I/R injury (36). Using the in vitro H9c2 myocytes, we conducted the experiment of the simulated ischemia, which is a well-established approach to study cellular injury (7, 36). NF-κB p50 protein dramatically decreased in H9c2 cardiomyoblasts transfected with siRNA against NF-κB p50 rather than the scrambled siRNA, confirming interfering RNA effectively silences NF-κB p50. HDAC inhibition augmented the cell viability and reduced cell necrosis in response to ischemic stress, which were abrogated in H9c2 cardiomyoblast cells in which NF-κB p50 gene was suppressed with siRNA, indicating that NF-κB p50 is attributable to the survival of myocytes by HDAC inhibition. Therefore, our study provides strong evidence supporting that a cascade consisting of HDAC inhibition-acetylation of NF-κB p50 and their functional couplings are essential in the genesis of myocardial protection. Further investigation is necessary to elucidate how specific class II HDAC inhibition in hearts to mediate NF-κB p50 to orchestrate cardioprotection. In addition, whether or not the transcriptional profiling of HDAC inhibition in NF-κB p50-deficient mice and/or another subunit of NF-κB p65 also involves the protective effect of HDAC inhibition is a subject matter that merits further investigation.
Summary.
In the present investigation, we have revealed a novel mechanism by which acetylated NF-κB p50 following HDAC inhibition is essential to induce cardioprotection. HDAC inhibition caused the acetylation of NF-κB p50, which was related with a subsequent activation of NF-κB p50. These data suggest a direct cause and effect relationship between HDAC inhibition and NF-κB p50 acetylation and activation. We have proven the central role of this novel pathway by demonstrating the abrogation of cardioprotection by targeted deletion of NF-κB p50 in mice. Furthermore, using the in vitro H9c2 cardiomyoblasts culture and siRNA gene silencing, we revealed that the reduction of cell necrosis and increase in viability following HDAC inhibition were mitigated with genetic knockdown of NF-κB p50. To the best of our knowledge, this is the first study providing direct evidence of mediating NF-κB p50 by HDAC inhibition to achieve cardioprotective effects. Our investigation not only provides new insight into our understanding of mechanisms of myocardial I/R but also suggests that a therapeutic strategy targeting this signaling pathway could be a novel and useful approach to the protection of the ischemic myocardium.
GRANTS
The work is supported by the National Heart, Lung, and Blood Institute Grant R01-HL-089405 and American Heart Association-National Center Grant 0735458N (to T. C. Zhao).
DISCLOSURE
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, Rindt H, Gorczynski RJ, Olson EN. Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors. J Biol Chem 278: 28930–28937, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol 21: 7065–7077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauerle PA, Baltimore D. NF-κB: ten years after. Cell 87: 13–20, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Chen LF, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappa B action regulated by reversible acetylation. Science 293: 1653–1657, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell 103: 263–267, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Deng WG, Wu KK. Regulation of inducible nitric oxide synthase expression by p300 and p50 acetylation. J Immunol 171: 6581–6588, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Engelbrecht AM, Niesler C, Page C, Lochner A. p38 and JNK have distinct regulatory functions on the development of apoptosis during simulated ischaemia and reperfusion in neonatal cardiomyocytes. Basic Res Cardiol 99: 338–350, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Fischle W, Emiliani S, Hendzel MJ, Nagase T, Nomura N, Voelter W, Verdin E. A new family of human histone deacetylases related to Saccharomyces cerevisiae HDA1. Biol Chem 274: 11713–11720, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature 401: 188–193, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Fryer RM, Auchampach JA, Gross GJ. Therapeutic receptor targets of ischemic preconditioning. Cardiovasc Res 55: 520–525, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grozinger CM, Hassig CA, Schreiber SL. Three proteins define a class of human histone deacetylases related to yeast Hda1p. Proc Natl Acad Sci USA 96: 4868–4873, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ. Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice. FASEB J 22: 3549–3560, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo P, Latronico MV, Gallo P, Grimaldi S, Borgia F, Todaro M, Jones P, Gallinari P, De Francesco R, Ciliberto G, Steinkühler C, Esposito G, Condorelli G. Inhibition of class I histone deacetylase with an apicidin derivative prevents cardiac hypertrophy and failure. Cardiovasc Res 80: 416–424, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Hansen JC, Tse C, Wolffe AP. Structure and function of the core histone N-termini: more than meets the eye. Biochemistry 37: 17637–17644,1998 [DOI] [PubMed] [Google Scholar]
- 15.Hassig CA, Tong JK, Fleischer TC, Owa T, Grable PG, Ayer DE, Schreiber SL. A role for histone deacetylase activity in HDAC1-mediated transcriptional repression. Proc Natl Acad Sci USA 95: 3519–3524, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Insinga A, Monestiroli S, Ronzoni S, Gelmetti V, Marchesi F, Viale A, Altucci L, Nervi C, Minucci S, Pelicci PG. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat Med 11: 71–76, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Kee HJ, Sohn IS, Nam KI, Park JE, Yin Z, Ahn Y, Jeong MH, Bang YJ, Kim N, Kim JK, Kim KK, Epstein JA, Kook H. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin II infusion and aortic banding. Circulation 113: 51–95, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Kong Y, Tannous P, Lu G, Berenji K, Rothermel BA, Olson EN, Hill JA. Suppression of class I and II histone deacetylases blunts pressure-overload cardiac hypertrophy. Circulation 113: 2579–25788, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TM, Lin MS, Chang NC. Inhibition of histone deacetylase on ventricular remodeling in infarcted rats. Am J Physiol Heart Circ Physiol 293: H968–H977, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. PKC epsilon modulates NF-κ B and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol 279: H1679–H1689, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Li CF, Browder W, Kao RL. Early activation of transcription factor NF-κ B during ischemia in perfuse rat heart. Am J Physiol Heart Circ Physiol 276: H543–H552, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 389: 251–260, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Maulik N, Sato M, Price BD, Das DK. An essential role of NFkappaB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Lett 429: 365–369, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, Maeda K, Sawa Y, Kaneda Y, Higaki J, Ogihara T. In vivo transfection of cis element “decoy” against nuclear factor κ B binding site prevents myocardial infarction. Nat Med 3: 894–899, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemicmyocardium. Circulation 74: 1124–1136, 1986 [DOI] [PubMed] [Google Scholar]
- 26.Ren M, Leng Y, Jeong M, Leeds PR, Chuang DM. Valproic acid reduces brain damage induced by transient focal cerebral ischemia in rats: potential roles of histone deacetylase inhibition and heat shock protein induction. J Neurochem 89: 1358–1367, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Sanderson L, Taylor GW, Aboagye EO, Alao JP, Latigo JR, Coombes RC, Vigushin DM. Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin A after intraperitoneal administration to mice. Drug Metab Dispos 32: 1132–1138, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κ B. Annu Rev Cell Biol 10: 405–455, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Strahl BD, Allis CD. The language of covalent histone modifications. Nature 403: 41–45, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Tantini B, Fiumana E, Cetrullo S, Pignatti C, Bonavita F, Shantz LM, Giordano E, Muscari C, Flamigni F, Guarnieri C, Stefanelli C, Caldarera CM. Involvement of polyamines in apoptosis of cardiac myoblasts in a model of simulated ischemia. J Mol Cell Cardiol 40: 775–782, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Turner BM. Histone acetylation and an epigenetic code. Bioessays 22: 836–845, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Timmers L, van Keulen JK, Hoefer IE, Meijs MF, van Middelaar B, den Ouden K, van Echteld CJ, Pasterkamp G, de Kleijn DP. Targeted deletion of nuclear factor kappaB p50 enhances cardiac remodeling and dysfunction following myocardial infarction. Circ Res 104: 699–706, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Van Wylen DM, Thornton JD, Downey JM. The natural history of preconditioning: cardioprotection depends on duration of transient ischemia and time to subsequent ischemia. Coron Artery Dis 2: 613–619, 1991 [Google Scholar]
- 34.Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet 19: 286–923, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Vigushi DM, Coombes RC. Targeted histone deacetylase inhibition for cancer therapy. Curr Cancer Target 4: 205–218, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Vitadello M, Penzo D, Petronilli V, Michieli G, Gomirato S, Menabò R, Di Lisa F, Gorza L. Overexpression of the stress protein Grp94 reduces cardiomyocyte necrosis due to calcium overload and simulated ischemia. FASEB J 17: 923–925, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Wang AH, Bertos NR, Vezmar M, Pelletier N, Crosato M, Heng HH, Th'ng J, Han J, Yang XJ. HDAC4, a human histone deacetylase related to yeast HDA1, is a transcriptional corepressor. Mol Cell Biol 19: 7816–7827, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, Qiu Y, Li JJ, Bolli R. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res 84: 1095–1109, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 265: 17174–17179, 1990 [PubMed] [Google Scholar]
- 40.Zhao TC, Cheng G, Zhang LX, Tseng YT, Padbury JF. Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury. Cardiovasc Res 76: 473–481, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Zhao T, Parikh P, Bhashyam S, Bolukoglu H, Poornima I, Shen YT, Shannon RP. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther 317: 1106–1113, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zhao TC, Kukreja RC. Late preconditioning elicited by activation of adenosine A(3) receptor in heart: role of NF- kappa B, iNOS and mitochondrial K(ATP) channel. J Mol Cell Cardiol 34: 263–277, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Zhao TC, Kukreja RC. Protein kinase C-delta mediates adenosine A3 receptor-induced delayed cardioprotection in mouse. Am J Physiol Heart Circ Physiol 285: H434–H441, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Ishida A, Fujita N, Kitazawa R, Tsuruo T. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J Biol Chem 277: 26217–26224, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Wu TR, Cai S, Welte T, Chin YE. Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol Cell Biol 13: 4505–4512, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao TC, Taher MM, Valerie KC, Kukreja RC. p38 Triggers late preconditioning elicited by anisomycin in heart: involvement of NF-kappaB and iNOS. Circ Res 89: 915–922, 2001 [DOI] [PubMed] [Google Scholar]