Abstract
Previous studies demonstrated that obesity increases inflammation in periaortic adipose tissue and promotes angiotensin II (ANG II)-induced abdominal aortic aneurysms (AAAs). We sought to determine whether weight loss of obese C57BL/6 mice would influence the progression of established AAAs. Male C57BL/6 mice were fed a high-fat diet (HF) for 4 mo and then infused with either saline or ANG II (1,000 ng·kg−1·min−1) for 3 mo. Mice with dilated suprarenal aortas at 28 days of ANG II infusion were designated to groups fed the HF (HF/HF) or a low-fat diet (LF; 10% kcal as fat; HF/LF) to induce weight loss for the last 2 mo of infusions. Suprarenal aortic lumen diameters of obese mice were increased by ANG II infusion at day 28 (day 0: 1.03 ± 0.02; day 28: 1.86 ± 0.14 mm; P < 0.05), but did not progress with continued infusion in HF/HF mice. Moreover, aortic lumen diameters were not different between groups (HF/HF: 1.89 ± 0.15; HF/LF: 1.79 ± 0.18 mm). However, maximal diameters of excised AAAs were decreased with weight loss (HF/HF: 2.00 ± 0.11; HF/LF: 1.55 ± 0.13 mm; P < 0.05) and had reduced adventitial areas (HF/HF: 1.18 ± 0.10; HF/LF: 0.54 ± 0.02 mm2; P < 0.05). Neovascularization of aortic adventitias was strikingly decreased in HF/LF mice (HF/HF: 43 ± 5; HF/LF: 12 ± 2 endothelial cells/adventitial area; P < 0.05). ANG II-induced elevations in adipose mRNA abundance of CD105, an adipose-derived stem cell marker, were abolished with weight loss. These results demonstrate that weight loss limits adventitial expansion of ANG II-induced AAAs. Reduced neovascularization from weight loss may limit progression of AAAs.
Keywords: neovascularization, adipose stem cells
abdominal aortic aneurysms (AAAs) are permanent dilations that lead to death due to rupture (12). Pathology of AAAs includes degradation of aortic elastin, inflammation, and adventitial neovascularization (10, 11, 19, 20, 27). However, the sequence of these events in the formation of AAAs and their specific roles in AAA progression are poorly understood. This lack of knowledge most likely contributes to a paucity of medical therapies for treatment of AAAs.
Many laboratories have demonstrated that infusion of angiotensin II (ANG II) to hypercholesterolemic mice results in AAA formation (8, 9). Risk factors for ANG II-induced AAAs, similar to the human disease, include male gender (15, 17) and obesity (24). Recent studies in our laboratory demonstrated that inflammation in periaortic adipose tissue surrounding abdominal aortas with obesity was associated with enhanced ANG II-induced AAA formation in obese mice (24).
Weight loss has been demonstrated to improve insulin sensitivity and many cardiovascular diseases associated with obesity (13, 29, 32, 34). Moreover, diet-induced weight loss decreased macrophage infiltration and chemoattractant gene expression in adipose tissue, contributing to reductions in systemic inflammation (3, 4). These beneficial effects of weight loss would be anticipated to favorably impact the progression of AAAs. In this study, we examined effects of weight loss in diet-induced obese mice on the progression of established ANG II-induced AAAs. The purpose of this study was to determine whether dietary weight loss as a lifestyle modification influenced the progression of AAAs as a potential therapy for this highly lethal vascular disease.
METHODS
Animals.
Male C57BL/6 mice (8 wk old, Jackson Laboratory, Bar Harbor, ME; n = 120) were fed a high-fat diet (HF; D12492, 60% calories from fat, Research Diets) for 20 wk. At week 20, mice were assigned to groups fed the HF diet for an additional 8 wk (n = 60; HF/HF), or fed a low-fat diet (LF; 12450B, 10% calories from fat, Research Diets; n = 60; HF/LF) to induce weight loss. All mice were housed at 22°C (14:10-h light-dark cycle) with free access to food and water. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
ANG II infusion.
ANG II (1,000 ng·kg−1·min−1) or saline was infused by subcutaneously implanted Alzet minipumps (Model 2004) from weeks 16–28, as described previously (9). Infusions were continued by replacement of minipumps containing fresh saline or ANG II at weeks 20 and 24.
Experimental protocol.
After 4 wk of ANG II infusion, abdominal aortas of ANG II-infused mice were scanned by noninvasive high-frequency ultrasound to detect AAA formation (Supplemental Fig. I; the online version of this article contains supplemental data). Mice identified with AAAs (defined as luminal diameter increase > 30% from baseline) were assigned to the HF/HF or HF/LF groups (n = 15/group) for an additional 8 wk. Only mice with a formed AAA (30% increase in lumen diameter) were included in further data analyses. ANG II-induced AAAs differed in size, as reflected by aortic lumen diameters (range from 1.25 to 2.95 mm). Large and small AAAs were assigned to both HF/HF and HF/LF groups. Saline-infused mice were also assigned to HF/HF or HF/LF groups (n = 15/group) at week 20. At study endpoint, mice were anesthetized (ketamine/xylazine, 100/10 mg/kg ip), and blood was obtained via cardiac puncture. After the right atrium was cut, mice were exsanguinated by perfusion through the left ventricle, and tissues (retroperitoneal fat and the aorta from the heart to ileal bifurcation) were dissected.
In vivo ultrasonic measurements.
Abdominal aortas of mice were visualized with high-frequency ultrasound (Vevo 660, VisualSonics, Toronto, Canada) at weeks 16, 20, 24, and 28, as described previously (1). Lumen diameters were measured on images at maximal dilation. Two investigators experienced in ultrasound and blinded to the experimental design obtained measurements on each mouse (mean of two measurements used for each mouse).
Measurements of serum and plasma components.
Serum cholesterol concentrations were determined in all mice using enzymatic kits (Wako Pure Chemical, Richmond, VA). Lipoprotein cholesterol distribution was performed on a subset of mice (n = 5 per group) by size exclusion chromatography, as described previously (17). Plasma renin concentrations were measured by generation of ANG I in the presence of excess angiotensinogen substrate, as described previously (6). Plasma insulin and adipokine concentrations were determined in ANG II-infused mice using a mouse adipokine lincoPlex multiplex immunoassay kit.
Assessment of aneurysmal pathology.
Aortas were dissected free and fixed overnight in paraformaldehyde, and extraneous tissue, including periaortic adipose tissue, was removed. To quantify AAA severity, we measured the maximal diameter of cleaned suprarenal aortas (average of three measurements per tissue with aorta oriented for maximal diameter) using Image Pro Plus 5.1 (MediaCybernetics, Bethesda, MD) in all mice from each group. To assess tissue pathology, we chose three suprarenal aortas with external diameters close to the mean for each group. Briefly, after fixation and the removal of extraneous tissue, aortas were injected with low-melting-point agarose containing green dye (31). The suprarenal (AAA-containing) segment of each aorta was embedded in OCT and serially sectioned using a cryostat. Each aortic segment included tissue from ∼12 mm above and ∼3 mm below the left renal branch. Aortic segments were sectioned in serial series with nine tissue sections per slide (10-μm sections; each slide representing ∼100-μm distance of the suprarenal aortic segment). Sections were fixed with absolute alcohol and stained using hematoxylin and eosin, and for Factor VIII Related Antigen (Von Willebrand Factor; Signet Laboratories, Dedham, MA; 1:50 dilution; secondary antibody biotinylated rabbit anti-rat, Vector Laboratories, Burlingame, CA, 1:200 dilution). Tissue sections underwent antigen retrieval (steam; 10-min incubation in Antigen Unmasking Solution, Vector Laboratories) before antibody incubations. A peroxidase-based ABC system and red chromagen AEC (both from Vector Laboratories) were used to identify the antigen-antibody reactions. An isotype-matched IgG was used as control, as was omission of primary antibody. Nuclei were counterstained with hematoxylin. For quantification of adventitial area and endothelial cell numbers, three sections per mouse were chosen within the aneurysm region of medial dissection. Area measurements were taken of the adventitia, the lumen, and the media + lumen, using the entire circumference of each aortic section. Adventitial area was quantified using Image Pro Plus 5.1. For quantification of endothelial cell numbers, four fields per section (3 sections per mouse, for a total of 36 fields per experimental group and across a distance of 100 μm) were analyzed for Von Willebrand factor positive immunostaining. Endothelial cell number was normalized to the adventitial surface area for each section.
Whole vessel CD31 immunostaining of vasa vasorum.
After cleaning of paraformaldehyde fixed aortas, they were permeabilized overnight with buffered saline containing 1% bovine serum albumin (BSA) and 0.1% Triton X-100. We performed CD31 immunostaining (35) on n = 3 aortas/diet group using aortas with maximal external diameters close to the mean for each group. Endogenous peroxidase activity was blocked by incubation (1 h) in 0.3% hydrogen peroxide. A monoclonal rabbit anti-mouse CD31 antibody (0.5 μg/ml; Abcam, Cambridge, MA) was incubated overnight at 4°C, with aortas (n = 3) from ANG II-infused mice in each diet group, followed by incubation with a biotinylated goat anti-rabbit IgG secondary antibody (1:500, Vector Laboratories) for 2 h. Positive immunostaining was detected using an avidin-linked peroxidase complex kit (ABC kit, Vector Laboratories), followed by incubation of aortas with 0.05% diaminobenzidine substrate in 50 mM Tris, pH 7.4, and then 0.01% hydrogen peroxide. Color was developed over 5 min in a shaded area. Aortas incubated with secondary antibody only served as controls for specificity of immunostaining.
Preparation of the stromal vascular fraction of adipose tissue.
Segments of retroperitoneal fat, visceral adipose tissue in close proximity to the site of aneurysm formation, were collagenase digested to isolate the stromal vascular fraction (SVF). Briefly, adipose tissue pieces were excised and minced in Kreb's bicarbonate buffer containing 1% BSA. Minced samples were digested with collagenase (1 mg/ml; Worthington, Freehold, NJ) at 37°C for 1 h with shaking. The digested cell suspension was passed through a sterile 100-μm filter and centrifuged (500 g, 10 min) to separate floating adipocytes from the SVF.
Quantification of mRNA abundance by real-time PCR.
Total RNA was extracted from the SVF of abdominal adipose tissue using Trizol. RNA quality (18-to-28S ribosomal RNA ratio) was assessed in three samples per experimental group using a Bio-Rad Experion Automated Electrophoresis System (Bio-Rad, Hercules, CA). Mouse primers were designed using Primer 3 and obtained from Operon (Operon Biotechnologies, Huntsville, AL). Relative quantification of mRNA abundance for each gene of interest was performed using an iCycler (BioRad) based on a standard curve method using the SYBR Green PCR core reagent (Applied Biosystems). Primer sequences are in Supplemental Table I, and data are expressed as ratio of 18S mRNA.
Statistical analyses.
Data are presented as means ± SE. Data were analyzed using two-way ANOVA, with diet (HF/HF, HF/LF) and treatment (saline, ANG II) as between-group factors. If statistical differences existed between experimental groups, Tukey's test was utilized for post hoc analyses. Values of P < 0.05 were considered to be statistically significant. All statistical analyses were performed using SigmaStat (SPSS).
RESULTS
Effects of weight loss in obese mice on metabolic parameters.
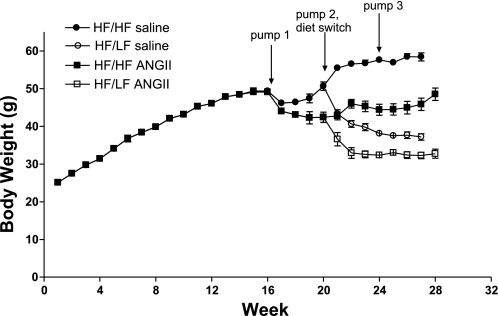
Chronic HF feeding for 16 wk led to significant weight gain (Fig. 1), with an average body weight of ∼50 g (100% gain from baseline) before ANG II infusion. The LF diet promoted significant weight loss in obese mice, as HF/LF mice exhibited decreased final body weights compared with HF/HF mice (HF/LF: 37 ± 1 and 33 ± 1; HF/HF: 58 ± 1 and 49 ± 2 g; P < 0.05, saline and ANG II-infused, respectively). In addition, there was a significant effect of ANG II infusion to decrease body weight compared with saline-infused controls (P < 0.001 compared with saline, Table 1 and Fig. 1). The mass of retroperitoneal adipose tissue was decreased with LF feeding and ANG II infusion (Table 1).
Fig. 1.
Weight loss and angiotensin (ANG) II infusion decreased body weight in obese mice. C57BL/6 mice gained weight over 16 wk of high-fat (HF) feeding. At 16 wk, mice were implanted with pumps infusing either saline or ANG II, with replacement of pumps at weeks 20 and 24. At week 20, mice were assigned to groups fed the HF (HF/HF) or low-fat (LF; HF/LF) diet to induce weight loss. The LF diet promoted weight loss in both saline and ANG II-infused mice. In addition, infusion of ANG II decreased body weight in HF/HF and HF/LF mice. Values are means ± SE from n = 10–15 mice/group.
Table 1.
Characteristics of mice
| Final Body Weight, g | Retroperitoneal Adipose, %body wt | Plasma Renin Concentration, ng/ml | |
|---|---|---|---|
| Saline | |||
| HF/HF | 58 ± 1 | 3.6 ± 0.2 | 4.2 ± 1.6 |
| HF/LF | 37 ± 1* | 1.2 ± 0.1* | 8.8 ± 2.0* |
| ANG II | |||
| HF/HF | 49 ± 2† | 2.5 ± 0.2† | 1.4 ± 0.5 |
| HF/LF | 33 ± 1*† | 0.7 ± 0.1* | 2.0 ± 0.5† |
Values are means ± SE. HF, high-fat diet; LF, low-fat diet; ANG II, angiotensin II.
P < 0.05 compared with HF/HF, within treatment.
P < 0.01 compared with saline, within diet.
Total sera cholesterol concentrations were decreased with weight loss in HF/LF compared with HF/HF mice infused with saline or ANG II (Supplemental Fig. II). In HF/LF mice (saline or ANG II infusion), HDL cholesterol predominated, while HF/HF mice exhibited a proportion of LDL cholesterol (Supplemental Fig. II). Plasma renin concentrations were increased with weight loss in HF/LF compared with HF/HF mice infused with saline or ANG II (Table 1). In addition, plasma renin concentrations were decreased from ANG II infusion in HF/LF, but not HF/HF mice. In saline and ANG II-infused mice, weight loss in HF/LF mice decreased plasma concentrations of leptin and resistin (Supplemental Table II). Plasma insulin concentrations were decreased with weight loss in HF/LF compared with HF/HF mice infused with ANG II, but not saline. Weight loss in HF/LF mice reduced plasma resistin concentrations in saline and ANG II-infused mice. In addition, plasma resistin concentrations were increased by infusions of ANG II in HF/HF and HF/LF mice.
Weight loss limited adventitial expansion and neovascularization of ANG II-induced AAAs.
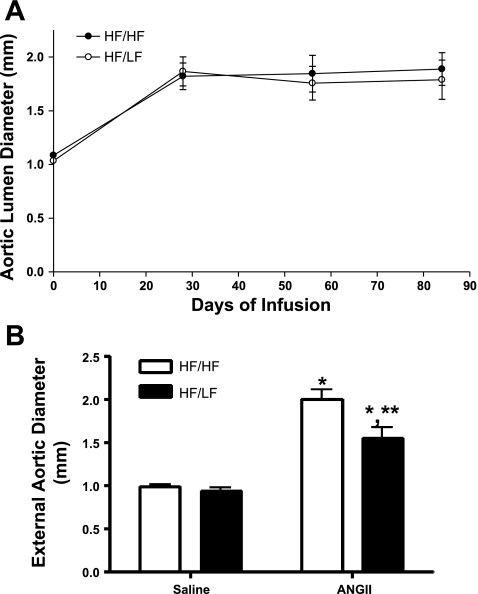
In saline-infused mice, lumen diameters of abdominal aortas were not different between HF/HF and HF/LF mice at study endpoint (HF/HF, 1.10 ± 0.02; HF/LF, 1.02 ± 0.02 mm; P > 0.05). Lumen diameters of suprarenal aortas of mice in each diet group increased following 28 days of ANG II infusion (Fig. 2A). However, there was no change in aortic lumen diameters of HF/HF or HF/LF mice from days 28 through 84 of ANG II infusion. Moreover, lumen diameters were not different between HF/HF and HF/LF mice at study endpoint (1.7 ± 0.2 vs. 1.8 ± 0.2 mm). In saline-infused mice, maximal external diameters were not different between HF/HF and HF/LF mice (Fig. 2B). Infusion of ANG II increased maximal external diameters in both HF/HF and HF/LF mice. However, maximal external diameters were decreased with weight loss in ANG II-infused HF/LF compared with HF/HF mice (Fig. 2B; Supplemental Fig. III). Approximately 10% of HF-fed mice died of aortic rupture during the first 28 days of ANG II infusion. All remaining mice survived the protocol through day 84 of ANG II infusion, regardless of diet group.
Fig. 2.
Weight loss has no effect on aortic lumen diameter, but decreased external diameters of established ANG II-induced abdominal aortic aneurysms (AAAs). A: lumen diameters of suprarenal aortas were measured by ultrasound. ANG II infusion increased lumen diameters at day 28 compared with baseline, but lumen dilation remained constant from day 28 through day 84 of continued ANG II infusions. B: suprarenal external aortic diameters were measured on excised, cleaned tissue. Weight loss decreased external aortic diameters. Values are means ± SE from n = 10–15 mice/group. *P < 0.05 compared with saline, within diet. **P < 0.05 compared with HF/HF, within treatment.
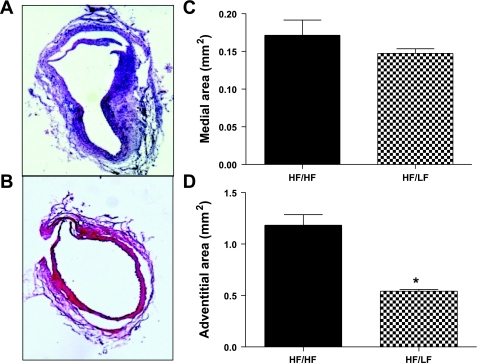
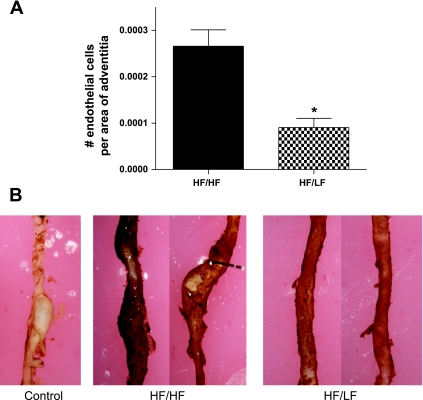
Medial areas of aneurysmal sections from HF/HF and HF/LF mice were similar (Fig. 3). In contrast, adventitial areas were markedly decreased (by 58%) in aneurysmal sections from HF/LF compared with HF/HF mice (Fig. 3). The number of adventitial cells that stained positively for Von Willebrand factor was strikingly decreased (by 64%), with weight loss in aneurysmal sections from HF/LF compared with HF/HF mice (Fig. 4A). Whole vessel staining of vasa vasorum was reduced in aortas from HF/LF mice compared with aortas from HF/HF mice (Fig. 4B).
Fig. 3.
Weight loss reduced adventitial thickening of established ANG II-induced AAAs. A and B: representative AAA sections stained with hematoxylin and eosin from HF/HF (A) and HF/LF (B) mice. C: medial areas were not different in AAA sections from HF/HF and HF/LF mice. D: adventitial areas were decreased with weight loss in AAA sections from HF/LF compared with HF/HF mice. Values are means ± SE from 3 tissue sections from n = 3 mice/group. *P < 0.05 compared with HF/HF.
Fig. 4.
Weight loss markedly reduced adventitial neovascularization of established ANG II-induced AAAs. A: endothelial cell numbers (positive Von Willebrand immunostaining) in adventitias of AAA sections were strikingly reduced with weight loss in HF/LF compared with HF/HF mice. B: whole vessel CD31 immunostaining demonstrates reduced vasa vasorum in aortas from HF/LF compared with HF/HF mice. Specificity of staining is demonstrated by incubation of aorta without primary antibody (Control). Values are means ± SE from 4 fields of 3 sections (B) from n = 3 mice/group. *P < 0.05 compared with HF/HF.
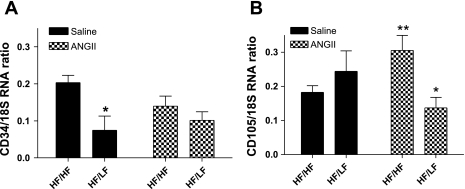
In rats, adipocytes were demonstrated previously to be within 50–100 μm of smooth muscle cells in the aortic media (14). The SVF of adipose tissue contains preadipocytes that are capable of differentiating to mature adipocytes. In addition to formation of new adipocytes, these multipotent, adipose-derived stem cells can differentiate into a variety of cell types, including endothelial cells for neovascularization of adipose tissue (21). Given the close proximity of adipose tissue to the vascular adventitia, we hypothesized that weight loss limited available adipose-derived stem cells for neovascularization of the aortic adventitia, thereby limiting AAA progression with continued ANG II infusion. In SVF from adipose tissue in close proximity to the suprarenal aorta, mRNA abundance of CD34, a marker of bone marrow-derived stem cells, was reduced with weight loss in saline-infused mice (Fig. 5A, P < 0.05). However, while infusion of ANG II had no effect on CD34 mRNA abundance in HF/HF mice, the effect of weight loss to reduce CD34 mRNA abundance was lost in SVF from ANG II-infused mice. Interestingly, infusion of ANG II increased mRNA abundance of the adipose-derived stem cell marker CD105 in SVF from HF/HF mice (Fig. 5B, P < 0.05), and this effect was blunted by weight loss. We also defined effects of weight loss on inflammatory cytokines, inflammatory cell markers, and angiogenic factors in SVF from retroperitoneal adipose tissue of HF/HF and HF/LF mice (Supplemental Table III). Infusion of ANG II had no effect on mRNA abundance of any factors examined in HF/HF mice. Weight loss reduced mRNA abundance of inflammatory cell markers and cytokines in saline-infused mice, but these effects were abolished in SVF from HF/LF mice infused with ANG II.
Fig. 5.
Weight loss reduced ANG II-induced elevations in CD105 mRNA abundance in stromal vascular fraction (SVF) from adipose tissue. A: mRNA abundance of CD34, a bone marrow-derived stem cell marker, is reduced in SVF with weight loss in saline, but not ANG II-infused mice. B: mRNA abundance of CD105, an adipose-derived stem cell marker, is increased by ANG II in SVF from HF/HF, but not HF/LF mice. Values are means ± SE from n = 3–5 mice/group. *P < 0.05 compared with HF/LF within infusion. **P < 0.05 compared with saline within diet.
DISCUSSION
The major findings of this study are that weight loss in diet-induced obese mice limits adventitial expansion of established ANG II-induced AAAs. Reductions in adventitial areas in AAAs from mice experiencing weight loss were associated with a striking reduction in adventitial neovascularization. The SVF from visceral adipose tissue in close proximity to ANG II-induced AAAs exhibited reductions in mRNA abundance of an adipose-derived stromal cell marker with weight loss, suggesting reductions of precursor stem cells for adventitial neovascularization. Collectively, these results suggest that weight loss in obese subjects may serve as a lifestyle modification impacting AAA progression.
Our results do not support progressive elevations in suprarenal aortic lumen dilation with prolonged ANG II infusion in obese C57BL/6 mice. Reductions in body weight in obese mice by infusion of ANG II may have contributed to the lack of progressive lumen dilation. However, a lack of progressive increases in aortic lumen diameter in the present study is consistent with previous findings in apoE−/− mice infused for prolonged periods with ANG II (33). The absence of progressive increases in aortic lumen diameter in ANG II-infused obese mice suggested that weight loss would have to regress an established AAA to have a favorable impact on this experimental measure. Our results demonstrate that weight loss was unable to regress the lumen dilation associated with ANG II-induced AAAs. To our knowledge, only one study has demonstrated that inhibition of JNK in vivo caused regression of established ANG II-induced AAAs (33).
Despite an inability to regress aortic lumen dilations with weight loss, external AAA diameters decreased in obese mice experiencing weight loss. Reductions in AAA diameters with weight loss resulted from decreased adventitial expansion. An interesting finding in the present study was the effect of weight loss to decrease neovascularization of the adventitia and limit AAA expansion. The thinner wall of AAAs in mice experiencing weight loss did not result in aneurysmal rupture, suggesting that the remodeling was beneficial.
Neovascularization is a prominent feature of human AAAs and is thought to contribute to clinical progression of the disease (7, 23). Previous studies demonstrated that AAAs formed from prolonged ANG II infusion (2 mo) to apolipoprotein E-deficient mice exhibited increased neovascularization of the aortic adventitia (26). Recent studies demonstrated that ANG II-induced AAAs are associated with increased expression of vascular endothelial growth factor receptors 1 and 2 in areas of neovessel formation, and that an inhibitor of angiogenesis limited AAA formation (28). The source of stem cells for neovascularization of atherosclerotic plaques or for adventitial neovascularization in large arteries, such as the aorta, has been controversial. However, studies by Bentzon et al. (2), using green fluorescent protein-labeled bone marrow cells administered to apolipoprotein E-deficient mice, demonstrated that smooth muscle cells after plaque disruption were derived entirely from local sources and not from circulating progenitor cells.
Recent studies by our laboratory demonstrated that adipose tissue surrounding abdominal aortas of obese mice exhibited inflammation that was associated with enhanced ANG II-induced AAA formation (24). Results from the present study demonstrate that weight loss reduced the mass of retroperitoneal adipose tissue. Angiogenesis plays a significant role in adipose tissue growth (22, 25, 30), with the large reservoir of adipose-derived stem cells available for new vessel formation to supply nutrients to the expanding tissue. Recent studies demonstrated that ANG II increased differentiation of adipose-derived stem cells to smooth muscle-like cells, supporting a role for ANG II in neovascularization (18). To our knowledge, this is the first study to report that ANG II infusion promotes expression of an adipose-derived stem cell marker in SVF from obese mice, an effect abolished in mice experiencing weight loss. It is unclear whether stem cells residing in adipose tissue within close proximity to the aorta can influence adventitial neovascularization. However, our results support further investigation of this stem cell source for adventitial neovascularization in AAAs.
Similar to previous reports (13, 29, 32, 34), weight loss resulted in a variety of favorable effects, including reductions in serum cholesterol concentrations and more favorable lipoprotein distributions, reduced adipose mass and body weight, and elevations in plasma renin concentrations (indicative of reductions in systemic concentrations of ANG peptides) (5). Moreover, weight loss decreased mRNA abundance of inflammatory cytokines and T-cell markers in retroperitoneal adipose tissue, but elevated anti-inflammatory cytokine expression. Favorable effects of weight loss on serum cholesterol concentrations and plasma cytokines were preserved in ANG II-infused mice and thus may have contributed to remodeling of established AAAs. However, favorable effects of weight loss on adipose mRNA abundance of cytokines and T-cell markers were abolished in mice infused with ANG II, suggesting that these factors were not involved in adventitial remodeling of AAAs.
In conclusion, results from this study demonstrate that weight loss of obese mice remodels established ANG II-induced AAAs by limiting expansion and neovascularization of the aortic adventitia. Reductions in adipose-derived stem cells in visceral adipose tissue in close proximity to the aorta may have contributed to reduced neovascularization and limited adventitial thickening of AAAs. Moreover, favorable changes in serum lipids, adipokines, and inflammatory cytokines may have contributed to adventitial remodeling of ANG II-induced AAAs. These results suggest that the lifestyle modification of weight loss may have favorable impacts on the progression of AAAs.
GRANTS
This work was supported by the National Heart, Lung, and Blood Institute (R01 HL73085 to L. A. Cassis, and P01 HL080100 to L. A. Cassis and A. Daugherty).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Victoria English for excellent technical assistance in measurements of plasma renin concentrations.
REFERENCES
- 1.Barisione C, Charnigo R, Howatt DA, Moorleghen JJ, Rateri DL, Daugherty A. Rapid dilation of the abdominal aorta during infusion of angiotensin II detected by noninvasive high-frequency ultrasonography. J Vasc Surg 44: 372–376, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Bentzon JF, Sondergaard CS, Kassem M, Falk E. Smooth muscle cells healing atherosclerotic plaque disruptions are of local, not blood, origin in apolipoprotein E knockout mice. Circulation 116: 2053–2061, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumié A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clément K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes 54: 2277–2286, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Capel F, Klimcakova E, Viguerie N, Roussel B, Vítková M, Kováciková M, Polák J, Kovácová Z, Galitzky J, Maoret JJ, Hanácek J, Pers TH, Bouloumié A, Stich V, Langin D. Macrophages and adipocytes in human obesity: adipose tissue gene expression and insulin sensitivity during calorie restriction and weight stabilization. Diabetes 58: 1558–1567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. ANG II infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. Am J Physiol Heart Circ Physiol 296: H1660–H1665, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol 27: 380–386, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Choke E, Thompson MM, Dawson J, Wilson WR, Sayed S, Loftus IM, Cockerill GW. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol 26: 2077–2082, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Daugherty A, Cassis L. Chronic angiotensin II infusion promotes atherogenesis in low density lipoprotein receptor −/− mice. Ann N Y Acad Sci 892: 108–118, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Daugherty A, Manning MW, Cassis LA. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J Clin Invest 105: 1605–1612, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dobrin PB, Baker WH, Gley WC. Elastolytic and collagenolytic studies of arteries. Implications for the mechanical properties of aneurysms. Arch Surg 119: 405–409, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 15: 1145–1151, 1995 [DOI] [PubMed] [Google Scholar]
- 12.Gillum RF. Epidemiology of aortic aneurysm in the United States. J Clin Epidemiol 48: 1289–1298, 1995 [DOI] [PubMed] [Google Scholar]
- 13.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E; Pennington CALERIE Team Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: a role in the pathogenesis of atherosclerosis? Arterioscler Thromb Vasc Biol 25: 2594–2599, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Henriques T, Zhang X, Yiannikouris FB, Daugherty A, Cassis LA. Androgen increases AT1a receptor expression in abdominal aortas to promote angiotensin II-induced AAAs in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 28: 1251–1256, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology 145: 3866–3872, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kim YM, Jeon ES, Kim MR, Jho SK, Ryu SW, Kim JH. Angiotensin II-induced differentiation of adipose tissue-derived mesenchymal stem cells to smooth muscle-like cells. Int J Biochem Cell Biol 40: 2482–2491, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 110: 625–632, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Candales A, Holmes DR, Liao S, Scott MJ, Wickline SA, Thompson RW. Decreased vascular smooth muscle cell density in medial degeneration of human abdominal aortic aneurysms. Am J Pathol 150: 993–1007, 1997 [PMC free article] [PubMed] [Google Scholar]
- 21.Miranville A, Heeschen C, Sengenes C, Curat CA, Busse R, Bouloumie A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 110: 349–355, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Nishimura S, Manabe I, Nagasaki M, Hosoya Y, Yamashita H, Fujita H, Ohsugi M, Tobe K, Kadowaki T, Nagai R, Sugiura S. Adipogenesis in obesity requires close interplay between differentiating adipocytes, stromal cells, and blood vessels. Diabetes 56: 1517–1526, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Paik DC, Fu C, Bhattacharya J, Tilson MD. Ongoing angiogenesis in blood vessels of the abdominal aortic aneurysm. Exp Mol Med 36: 524–533, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol 29: 1458–1464, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A 99: 10730–10735, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin II-infused, apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 23: 1621–1626, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Sho E, Sho M, Nanjo H, Kawamura K, Masuda H, Dalman RL. Hemodynamic regulation of CD34+ cell localization and differentiation in experimental aneurysms. Arterioscler Thromb Vasc Biol 24: 1916–1921, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tedesco MM, Terashima M, Blankenberg FG, Levashova Z, Spin JM, Backer MV, Backer JM, Sho M, Sho E, McConnell MV, Dalman RL. Analysis of in situ and ex vivo vascular endothelial growth factor receptor expression during experimental aortic aneurysm progression. Arterioscler Thromb Vasc Biol 29: 1452–1457, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M; Finnish Diabetes Prevention Study Group Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–1350, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology 146: 4545–4554, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Wang YX, Martin-McNulty B, Freay AD, Sukovich DA, Halks-Miller M, Li WW, Vergona R, Sullivan ME, Morser J, Dole WP, Deng GG. Angiotensin II increases urokinase-type plasminogen activator expression and induces aneurysm in the abdominal aorta of apolipoprotein E-deficient mice. Am J Pathol 159: 1455–1464, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long-term effects of modest weight loss in type II diabetic patients. Arch Intern Med 147: 1749–1753, 1987 [PubMed] [Google Scholar]
- 33.Yoshimura K, Aoki H, Ikeda Y, Furutani A, Hamano K, Matsuzaki M. Regression of abdominal aortic aneurysm by inhibition of c-Jun N-terminal kinase in mice. Ann N Y Acad Sci 1085: 74–81, 2006 [DOI] [PubMed] [Google Scholar]
- 34.You T, Nicklas BJ. Chronic inflammation: role of adipose tissue and modulation by weight loss. Curr Diabetes Rev 2: 29–37, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, Naggar JC, Welzig CM, Beasley D, Moulton KS, Park HJ, Galper JB. Simvastatin inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E-knockout mice: possible role of ERK. Arterioscler Thromb Vasc Biol 29: 1764–1771, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.