Abstract
In humans, prediabetes is characterized by marked increases in plasma insulin and near normal blood glucose levels as well as microvascular dysfunction of unknown origin. Using the extensor digitorum longus muscle of 7-wk inbred male Zucker diabetic fatty rats fed a high-fat diet as a model of prediabetes, we tested the hypothesis that hyperinsulinemia contributes to impaired O2 delivery in skeletal muscle. Using in vivo video microscopy, we determined that the total O2 supply to capillaries in the extensor digitorum longus muscle of prediabetic rats was reduced to 64% of controls with a lower O2 supply rate per capillary and higher O2 extraction resulting in a decreased O2 saturation at the venous end of the capillary network. These findings suggest a lower average tissue Po2 in prediabetic animals. In addition, we determined that insulin, at concentrations measured in humans and Zucker diabetic fatty rats with prediabetes, inhibited the O2-dependent release of ATP from rat red blood cells (RBCs). This inability to release ATP could contribute to the impaired O2 delivery observed in rats with prediabetes, especially in light of the finding that the endothelium-dependent relaxation of resistance arteries from these animals is not different from controls and is not altered by insulin. Computational modeling confirmed a significant 8.3-mmHg decrease in average tissue Po2 as well as an increase in the heterogeneity of tissue Po2, implicating a failure of a regulatory system for O2 supply. The finding that insulin attenuates the O2-dependent release of ATP from RBCs suggests that this defect in RBC physiology could contribute to a failure in the regulation of O2 supply to meet the demand in skeletal muscle in prediabetes.
Keywords: adenosine 5′-triphosphate, erythrocyte, oxygen regulation, microvasculature, systems biology, computational modeling
prediabetes is a precursor to the development of type 2 diabetes. In 2000, it was reported that 25% of overweight adults aged 45–74 yr (12 million persons in the United States) had prediabetes and would be anticipated to develop type 2 diabetes within 10 years (2). One hallmark of prediabetes is the presence of supraphysiological circulating insulin levels. These high-insulin levels are presumably required to overcome insulin resistance, permitting blood glucose to be maintained in the normal or near normal range. Prediabetes progresses to type 2 diabetes when pancreatic β-cells can no longer sustain the level of insulin production needed to compensate for insulin resistance resulting in the rise in blood glucose (3, 6, 37, 41, 47).
Although the increased insulin levels in prediabetes can be thought of as a physiological response in terms of glucose homeostasis, it is also possible that hyperinsulinemia itself has pathophysiological consequences. In support of a role for increased plasma insulin in the development of microvascular dysfunction, several groups have described decreased reactive hyperemia and other indicators of vascular dysfunction in humans with prediabetes and hyperinsulinemia (26, 31, 32, 45, 50). Importantly, in humans with prediabetes, while the microvascular dysfunction did not correlate with plasma glucose, age, body mass index, serum lipids, or blood pressure, this dysfunction did correlate directly with plasma insulin concentration (31, 32). Indeed, even plasma insulin levels within the normal range were shown to negatively correlate with the hyperemic response of healthy individuals (1). Although impaired vasodilator function of resistance arteries has been reported in some animal models of insulin resistance (14, 15), the effects of hyperinsulinemia on the matching of O2 supply with the O2 need in skeletal muscle have not been determined.
Since human red blood cells (RBCs) possess insulin receptors (17, 18, 49), we hypothesized that insulin itself, via the inhibition of ATP release from RBCs, could contribute directly to vascular dysfunction in prediabetes. Insulin, at levels found in humans with prediabetes (38, 40, 42, 44), has been shown to inhibit ATP release from human RBCs in response to an exposure to reduced Po2 (27). Additionally, it was shown that this concentration of insulin impairs the ability of RBCs to stimulate the dilation of isolated perfused skeletal muscle arterioles in the presence of low extraluminal O2 levels (27). These results support the hypothesis that the hyperinsulinemia present in prediabetes could hinder the matching of O2 supply with the O2 need in skeletal muscle, resulting in impaired tissue oxygenation.
Here we characterized microvascular O2 transport in the extensor digitorum longus (EDL) muscle of 7-wk inbred Zucker diabetic fatty (ZDF) rats that express a dysfunctional leptin receptor. Leptin, a small adipose-derived protein hormone, plays a central role in the hypothalamic regulation of food intake (4). When male rats that are homozygous for the defective leptin receptor gene (fa/fa) are fed a high-fat diet, they develop obesity, insulin resistance, and hyperinsulinemia (39, 43). This phenotype is not found in control ZDF rats that are heterozygous for the leptin receptor mutation (fa/+). At 7 wk, inbred fa/fa ZDF rats, but not their controls, demonstrate marked increases in circulating plasma insulin but nonfasting blood glucose is only slightly higher than that in control animals (5, 16), a profile that is characteristic of humans with prediabetes (38, 40, 42, 44). In addition to microvascular studies in intact animals, the effects of insulin on low O2-induced ATP release from prediabetic and control ZDF rat RBCs, as well as on vascular reactivity of isolated resistance arteries obtained from these animals, were determined. Finally, we used a computational model of O2 transport to evaluate the impact of these alterations in erythrocyte physiology on the O2 supply to skeletal muscle.
METHODS
Two sets of animals were studied in this project. The in situ microcirculatory studies were carried out at the University of Western Ontario, whereas the RBC and isolated resistance artery studies were carried out at St. Louis University. Before experimentation, all experimental protocols were approved by the Animal Care and Use Committees of the respective institutions. Inbred male ZDF rats, either leptin receptor knockouts (fa/fa) or their controls (fa/+) (Charles River) were obtained at 6 wk of age. Animals were raised on Purina Rodent Lab diet 5008. All animals were studied at 7 wk of age at the time at which they display hyperinsulinemia but before their development of severe hyperglycemia.
In situ studies of capillary O2 supply.
ZDF rats [10 control (fa/+) and 12 prediabetic (fa/fa)] were anesthetized with pentobarbital sodium (65 mg/kg ip), and their body temperature was maintained with a heat lamp. Arterial and venous blood samples were drawn before the intravital study to determine Po2, Pco2, pH (iSTAT handheld blood gas monitor), blood glucose, and plasma insulin. The animals were mechanically ventilated with 30% O2 (balance N2) and inspired O2 levels (Oxychek), and blood pressure (MicroMed) was continuously monitored and recorded throughout the experiment.
The EDL muscle was prepared for in vivo microscopy as described previously (48). The muscle was transilluminated with a 100-W xenon lamp and viewed through a Nikon inverted microscope equipped with long-working distance ×10 and ×20 objectives and a beam splitter for dual video cameras (DAGE-MTI CCD cameras). Interference filters in a cassette in the beam splitter passed the 420-nm image to one camera and the 431-nm image to the second camera. The two cameras were temporally synchronized and mounted such that video images were in register. Simultaneous real-time capture (640 × 480, 30 frames/second) of video output from both cameras was achieved using a single time-code generator and two separate computers with time code readers and DT3153 frame grab boards. Software running on each computer (Neovision) captured 60-s video sequences and stored the images as uncompressed AVI movie files. Ten fields of view (5 at the arteriolar end and 5 at the venular end of the capillary network) were randomly selected and recorded for 1 min in each animal.
Measurements of O2 transport parameters.
O2 transport measurements were obtained by off-line analysis of AVI movie files (420- and 431-nm wavelengths) as described by Ellis et al. (9, 10) and Japee et al. (35, 36) using custom image analysis software written in MatLab. The computer processed the video sequences to generate images of capillaries in focus and suitable for analysis (36). The capillary segments were selected for analysis, and the software automatically traced the capillary geometry including bends to generate data on capillary diameter, surface area, and volume. Using the location of the capillary, the software extracted light intensity data from each video frame (1,800 frames/30 s) for both the 420- and 431-nm AVI movie files (431-nm images automatically aligned and registered to the 420-nm images). The light intensity values were analyzed frame by frame for hemodynamic data (RBC velocity, capillary hematocrit, and supply rate of RBCs) and RBC hemoglobin O2 saturation data. An index of the convective transport of O2 in the capillaries was calculated from the product of RBC supply rate and RBC oxyhemoglobin saturation values. This product represents the equivalent number of erythrocytes fully saturated with O2 passing through the capillary per second, i.e., 10 RBCs per second at an average oxyhemoglobin saturation of 0.7 is equivalent to seven fully O2 saturated RBCs per second. Perfused capillary density was determined during the playback of each video file by counting the number of capillaries with flowing RBCs during the 30-s sample period that crossed a line placed on the computer monitor perpendicular to the muscle fibers and scaling the results to capillaries per millimeter length. Capillaries were categorized as stopped if there were stationary RBCs during the sample period and intermittent if the flow stopped for a period of time or reversed. Since capillaries without RBCs, i.e., plasma-perfused capillaries, were not detectable, no attempt was made to quantify the total capillary density.
To enable an analysis of the data using our computational model, which is based on a single isolated capillary network, animals (3 control and 2 prediabetic) in which a net arteriolar-venular decrease in capillary O2 saturation was not observed were excluded. It was assumed that an increase in capillary O2 saturation across the network indicated the presence of a nearby arteriole or an additional capillary network that substantially affected O2 transport from the measured network (13). While this eliminated our ability to assess the impact of precapillary losses on tissue O2 supply, it was necessary to provide appropriate O2 transport data for our existing O2 transport model that does not include a diffusional supply of O2 from arterioles. Importantly, there was no significant difference in any of the measured parameters as a result of excluding these animals from the study.
Isolation of rat RBCs.
Rats were anesthetized with pentobarbital sodium (65 mg/kg ip). Heparin was administered via the tail vein, and after 5 min, the abdomen was opened, the abdominal aorta was cannulated, and the animals were exsanguinated. Blood glucose was determined using a handheld glucose meter (Ascencia Breeze 2, Bayer), and the blood was then centrifuged at 500 g at 4°C for 10 min. The plasma, buffy coat, and uppermost RBCs were removed by aspiration and discarded. The RBCs were then washed three times in buffer containing (in mM) 21.0 tris(hydroxymethyl)aminomethane, 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, and 5.5 glucose with 0.5% bovine albumin fraction V (final pH 7.4). Wright stains of RBCs prepared in this fashion revealed fewer than one leukocyte per 50 high-power fields (∼8–10 leukocytes/mm3). Previous studies demonstrate that RBCs prepared in this manner are devoid of platelet contamination (28, 29). Cells were prepared on the day of use.
Determination of ATP release from RBCs in response to exposure to reduced Po2 in the absence and presence of insulin.
Isolated RBCs were diluted to a 20% hematocrit in a Ringer buffer containing (in mM) 4.7 KCl, 2.0 CaCl2, 140.5 NaCl, 1.2 MgSO4, 11 glucose, 23.8 NaHCO3, and 5.5 dextrose with 0.5% BSA with final pH 7.4 at 37°C in the presence of gas containing 6% CO2, balance nitrogen. RBCs were equilibrated for 20 min in a tonometer (model 237, Instrumentation Laboratory) with a normoxic gas mixture containing 15% O2, 6% CO2, balance N2 (Po2, ∼100 mmHg), in the presence of 1 nM insulin or its vehicle (saline). The gas mixture was then changed to one containing 0% O2, 6% CO2, balance N2 (reduced Po2; Po2, ∼15 mmHg). The concentration of ATP released from RBCs was determined during normoxia and following a 10-min exposure of RBCs to reduced Po2 as described in Measurement of ATP. The pH, Po2, and Pco2 were determined during exposure to each gas mixture using a blood gas analyzer (model pHOx, Nova Biomedical).
Measurement of ATP.
ATP was measured by the luciferin-luciferase technique (27). A 200-μl sample of RBC suspension (0.04% hematocrit) was injected into a cuvette containing 100 μl of firefly lantern extract (10 mg/ml distilled water, FLE 250; Sigma) and 100 μl of a solution of synthetic d-luciferin (50 mg/100 ml distilled water; Sigma). The light emitted was detected using a luminometer (Turner Designs). A standard curve was obtained for each experiment. ATP concentration was normalized to 4 × 108 RBCs/ml.
Measurement of total intracellular ATP of RBCs.
A known number of RBCs, determined by direct counting using a hemocytometer was lysed in distilled water. ATP was measured as described in Measurement of ATP, and the values were normalized to ATP concentration per RBC.
Measurement of free hemoglobin.
To exclude the presence of significant hemolysis in studies where the release of ATP was measured, samples were centrifuged at 500 g at 4°C for 10 min, and the presence of free hemoglobin in the supernatant was determined by light absorption at a wavelength of 405 nm. If increases in free hemoglobin were detected, the studies were not included to ensure that hemolysis did not influence the measured ATP levels.
Measurement of plasma insulin.
Anticoagulated whole blood was centrifuged for 10 min at 500 g at 4°C. The plasma was removed and EDTA (1 mg/ml) was added. Insulin levels were determined using a kit (80-INSRT-E01, Alpco Diagnostics). A standard curve was performed for each assay.
Measurement of Giα2 in RBC membranes.
Washed RBCs (2 ml) were added to 200 ml of hypotonic buffer containing (in mM) 5 Tris·HCl and 2 EDTA (with pH adjusted to 7.4, 4°C) and stirred vigorously for 20 min at 4°C. The mixture was centrifuged at 23,300 g for 15 min at 4°C. The membrane fraction was resuspended in buffer and washed twice. The protein concentration was determined by bicinchoninic acid assay (Pierce). The membranes were then solubilized in SDS buffer containing 8% SDS, 60% glycerol, 0.25 M Tris·HCl (pH 6.8), 0.004% bromophenol blue, and 400 mM dithiothreitol; boiled; loaded onto a precast 7.5% gel; and subjected to electrophoresis at 150 V for 90 min and transferred to a polyvinylidene difluoride membrane (100 V for 60 min) in buffer containing 25 mM Tris-base, 192 mM glycine, and 10% methanol. The polyvinylidene difluoride membranes were blocked overnight with 5% nonfat dry milk in PBS containing 0.1% Tween-20, immunoblotted with a primary antibody in 1% nonfat dry milk directed against the α-subunit of Giα2 (United States Biological, Swampscott, MA) or β-actin (Sigma, St. Louis), followed by an incubation with a secondary antibody in 1% nonfat dry milk and visualized using enhanced chemiluminescence. Membrane proteins loaded onto gels were standardized by a determination of protein concentration of each preparation and by a determination of the β-actin in each sample. The amounts of Giα2 protein present in individual membrane preparations are reported as the ratio of the density of that protein to β-actin as determined by densitometry.
Isometric tension recording in rat femoral resistance arteries.
Distal branches of rat femoral arteries (200 to 300 μm in diameter) were isolated and placed in cold (4°C) MOPS buffer solution (pH 7.3) containing (in mM) 144 NaCl, 3.0 KCl, 2.5 CaCl2, 1.5 MgSO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, 2.0 MOPS, and 1.21 NaH2PO4. The resistance arteries were carefully cleaned of adipose and connective tissue, and the ring segments (1 to 2 mm in length) were mounted on a two-chamber wire myograph (model 510A, Danish Myo Technology) for measurement of isometric tension. Arteries were maintained at 37°C in the MOPS buffer solution. Arteries were stretched to a resting tension of 2–6 mN and stimulated at least three times with KCl (60 mM). Once KCl contractions were consistent, the arteries were washed and subsequently contracted with concentrations of phenylephrine (1–10 μM) that induced a submaximal contraction of 50–75% of the vessel's maximum KCl contraction. Following confirmation of an intact, functional endothelium, as evidenced by a >40% relaxation to 10 μM acetylcholine, the effect of insulin on endothelium-dependent responses was examined by incubating the vessels with 1 nM insulin for 30 min before contraction with phenylephrine followed by an application of acetylcholine. Acetylcholine-induced relaxation was again assessed after washing the vessels with MOPS buffer to remove insulin.
Computational model of capillary-tissue O2 transport.
Simulations of steady-state O2 transport were performed using an established computational model (23–25) that couples the continuum partial differential equations describing convective transport in the capillaries to those describing O2 diffusion and consumption in the tissue. The model includes both dissolved and hemoglobin-bound O2 in the capillaries. Transport between the blood and tissue is described using a flux boundary condition with mass transfer coefficients calculated previously using a discrete RBC model (8). For all O2 transport simulations, an array of parallel capillaries was used to represent a typical capillary network (21, 22). The placement of the capillaries and the relative distribution of hemodynamic parameters (RBC velocity and hematocrit) were taken from in vivo measurements of a single network in a control animal. In particular, the velocity and hematocrit in normal flow capillaries had coefficients of variation of 0.53 and 0.43, respectively.
Average hemodynamic parameters in normal flow capillaries (80% of perfused capillary density) were set to match measured values. For capillaries with RBC supply rates (proportional to RBC velocity × capillary hematocrit) too high to measure, hematocrit was set to the average measured value and velocity was set to 1,000 μm/s in the control network. Supply rates in the prediabetes network were varied via changes in RBC velocity. Capillary entrance saturations were set based on experimental data, and the tissue O2 consumption rate (M) was adjusted to yield the average measured exit saturation. To solve the transport equations via a standard numerical finite-difference method, the tissue was discretized using a 2-μm spatial step in all three directions and the capillaries were discretized into 15 μm cylindrical segments.
Data analysis.
Because of the small sample size and non-normal distribution in the measured values, a nonparametric analysis (Mann-Whitney) was used to assess statistical significance for the microcirculatory data. For the studies using isolated RBCs or resistance arteries, significance was determined using an analysis of variance (ANOVA). In the event that the F ratio indicated that a change had occurred, a Fisher least significant difference test was performed to identify individual differences. A Student's t-test was used where appropriate. Results were reported as means ± SE unless otherwise indicated.
RESULTS
Mean arterial blood pressure, arterial blood gasses and pH, body weight, blood glucose, and plasma insulin values for control and prediabetic ZDF rats are presented in Table 1. The prediabetic rats were heavier and had elevated nonfasting blood glucose and plasma insulin levels when compared with their age-matched controls (P < 0.05). Although the values for glucose and insulin in the control rats are statistically different between the in situ and erythrocyte study groups, the differences are small. In the prediabetic rats, neither value is different between the two studies.
Table 1.
Systemic arterial blood pressure, arterial blood gasses and pH, body weight, blood glucose, and plasma insulin for Zucker diabetic fatty rats
| Control | Prediabetes | |
|---|---|---|
| In situ studies | ||
| Arterial blood pressure, mmHg | 104 ± 5 | 102 ± 2 |
| Po2, mmHg | 125 ± 4 | 108 ± 4* |
| Pco2, mmHg | 46 ± 2 | 49 ± 1 |
| pH | 7.42 ± 0.01 | 7.44 ± 0.01 |
| Weight, g | 174 ± 3 | 251 ± 5* |
| Glucose, mg/dl | 123 ± 5 | 251 ± 13* |
| Insulin, nmol | 0.2 ± 0.1 | 6.8 ± 1.3* |
| n | 7 | 10 |
| Erythrocyte studies | ||
| Weight, g | 205 ± 7 | 271 ± 10* |
| Glucose, mg/dl | 159 ± 6 | 230 ± 24* |
| Insulin, nmol | 0.4 ± 0.1 | 3.6 ± 0.8* |
| n | 6 | 6 |
Values are means ± SE; n, number of rats.
P < 0.05, different from control.
Microvascular O2 supply.
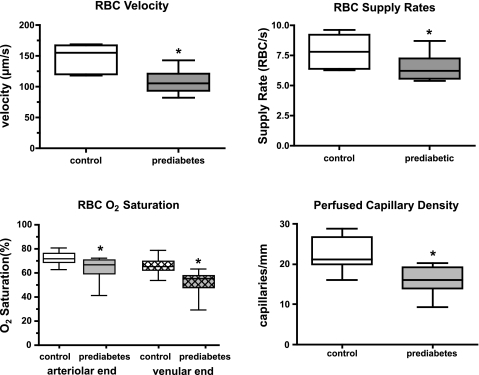
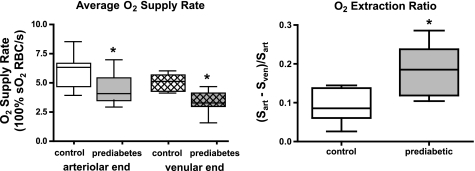
When compared with the control animals, prediabetic rats had significantly lower RBC velocity, RBC supply rate, and average RBC O2 saturation (average of O2 saturations measured at arteriolar and venular ends of the capillary bed) in capillaries of the resting EDL muscle (Fig. 1). RBC O2 saturations at the arteriolar (Sart) and venular ends (Sven) of capillaries were significantly lower (Fig. 1) and O2 extraction across the capillary bed was significantly higher (Fig. 2) in the prediabetic group. The average O2 supply entering each capillary was computed as the product of arteriolar end RBC supply rate and the O2 saturations of these RBCs (supply rate × Sart) and is expressed as an equivalent number of 100% O2 saturated RBCs. By this measure, the prediabetic animals had a significantly lower O2 supply rate per capillary compared with controls (4.4 ± 1.2 vs. 5.9 ± 1.5 fully O2 saturated RBCs per second, P < 0.05, Fig. 2). In addition, although the number of stopped or intermittent flow capillaries was not significantly different between the groups, there was a significant decrease in perfused capillary density in the prediabetic group that translated into a further reduction in the total O2 supply to the capillary bed with the value in prediabetic rats 64% of the value found in control animals (Fig. 1).
Fig. 1.
In situ capillary data for control (n = 6) and prediabetes (n = 6) groups showing red blood cell (RBC) velocity, RBC supply rate, RBC O2 saturation at the arteriolar and venular end of the capillary bed and the density of perfused capillaries. Box and whisker plots show median, upper and lower quartiles, and maximum and minimum values. *P < 0.05, different from control.
Fig. 2.
The average O2 supply rate was calculated for the arteriolar and venular end of the capillary bed as the product of RBC supply rate × O2 saturation for both control and prediabetes group. The O2 extraction ratio was calculated for the control and prediabetes group from the O2 saturation gradient from arteriolar (Sart) to venular (Sven) end divided by the arteriolar end O2 saturation, i.e., (Sart − Sven)/Sart. Box and whisker plots show median, upper and lower quartiles, and maximum and minimum values. So2, oxyhemoglobin saturation. *P < 0.05, different from control.
Effect of insulin on ATP release in response to exposure of RBCs to reduced Po2.
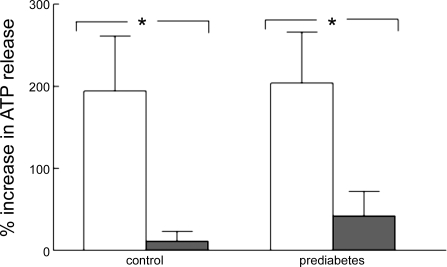
Under normoxic conditions (15% O2; Po2 = 106 ± 1 mmHg; Pco2 = 38 ± 0.7 mmHg; and pH = 7.39 ± 0.01), the amount of ATP released from isolated RBCs did not differ between the control and prediabetes groups [10.3 ± 2.4 and 8.0 ± 2.6 nmol per 4 × 108 RBCs in control (n = 6) and prediabetic rats (n = 6), respectively]. When the Po2 was reduced to 11 ± 1 mmHg (Pco2 = 38 ± 0.5 mmHg; and pH = 7.39 ± 0.01), ATP release increased in both groups (Fig. 3). The incubation of RBCs from both control and prediabetic rats with insulin (1 nM) had no effect on ATP levels measured under normoxic conditions (15% O2) but prevented ATP release when these cells were exposed to reduced O2 (Fig. 3). Total intracellular ATP levels were 2.4 ± 0.5 and 2.2 ± 0.2 mM per RBC in control and prediabetes groups, respectively. Importantly, these levels were not altered by an incubation with insulin, indicating that a decreased ATP release cannot be attributed to insulin-induced reductions in ATP synthesis.
Fig. 3.
Effect of low O2 on ATP release from washed rat RBCs of control (n = 6) and prediabetic (n = 6) Zucker diabetic fatty rats. RBCs were exposed to 15% O2, 6% CO2, balance N2 (P = 105.9 ± 1.3 mmHg), in a tonometer for 30 min, and ATP was measured. The gas was then changed to 0% O2, 6% CO2, balance N2 (Po2 = 11.4 ± 1.3 mmHg), and ATP was measured after 10 min. The percent increase in ATP release from baseline (15% O2) is reported. White bars, washed RBCs in the absence of insulin; black bars, RBCs incubated with insulin (1 nM) for 30 min before ATP determinations. Values are means ± SE. *P < 0.05, different from control.
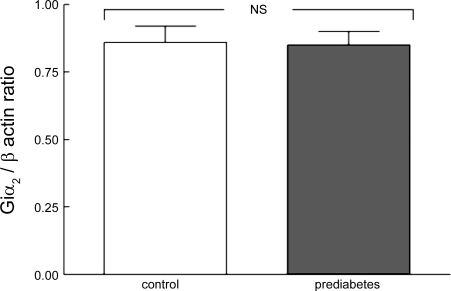
Expression of Giα2 in RBC membranes of prediabetic rats.
Previously, it was reported that the expression of the α-subunit of the heterotrimeric G protein, Gi2, was selectively decreased in RBCs of humans with type 2 diabetes (46). This defect in Giα2 expression was suggested to contribute to a decreased ATP release. As shown in Fig. 4, the expression of this G protein α-subunit in RBC membranes of prediabetic rats was not different from the amounts in age-matched control rats. Thus the mechanism responsible for reduced ATP release in response to the exposure of RBCs to reduced Po2 in prediabetic rats is independent of any alteration in Giα2 expression.
Fig. 4.
Measurement of amounts of α-subunit of the heterotrimeric G protein, Gi2, in RBC membranes of control (n = 7) and prediabetic (n = 7) Zucker diabetic fatty rats. Values represent the ratio of Giα2 to β-actin in the same samples. NS, not statically significant.
Effect of insulin on acetylcholine-induced vasodilation in isolated resistance arteries of prediabetic rats.
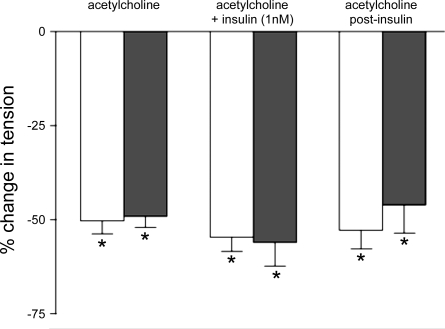
To determine whether insulin, at concentrations present in prediabetic ZDF rats, interferes directly with endothelium-dependent vasodilation, isolated resistance arteries from prediabetic rats and their age-matched controls were incubated with insulin (1 nM) or its vehicle (saline) and the response to acetylcholine was determined. In the absence of insulin, acetylcholine (10 μM) decreased phenylephrine-induced contraction in vessels from control and prediabetic rats by 50.3 ± 3.4 and 54.6 ± 3.8%, respectively (Fig. 5). The results were not altered by the addition of insulin.
Fig. 5.
Effect of prediabetes on acetylcholine-induced relaxation of isolated resistance arteries (200–300 μm) in the presence and absence of insulin. Vessels were incubated with insulin (1 nM) or its vehicle (saline) for 30 min before administration of acetylcholine (10 μM) to phenylephrine-contracted vessels. White bars represent control rats (n = 11), and black represent prediabetic rats (n = 7). Values are means ± SE. *P < 0.05, different from phenylephrine-induced contraction.
Tissue O2 distributions from experiment-based computational model.
Using data from the control rats obtained in this study, we estimated the typical arteriolar-venular length between saturation measurements (300 μm), and an absolute capillary density for the control case was achieved with 19 capillaries placed in a tissue domain of 80 × 331 × 300 μm. For prediabetes, the functional capillary density was effectively reduced by decreasing the RBC supply rate in five randomly selected capillaries to 1% of the baseline supply rate.
The two main cases simulated were control and prediabetes. Based on the results of the in situ experiments, the prediabetes simulation used a lower capillary entrance saturation and lower functional capillary density (Table 2). The prediabetes simulation also used an increased tissue O2 consumption rate, which was required to match the capillary exit saturation measured in situ. Figure 6 shows the locations of functional capillaries (black circles) for control and prediabetes and the calculated tissue Po2 at the venular end of the tissue domain. These simulations predict decreased tissue Po2 in prediabetes (Fig. 7 and Table 2) and increased heterogeneity of tissue oxygenation. This increased heterogeneity can be seen in the broadening of the prediabetes Po2 distribution in Fig. 7, as well as in the increased coefficient of variation (SD/mean) of tissue Po2 from 0.026 to 0.044 (Table 2). Additional prediabetes cases were simulated to estimate the effect of restoring capillary entrance saturation (Sart), RBC supply rate, perfused capillary density, or tissue O2 consumption (M) to control levels (Table 2). We also simulated the effect of increasing supply rate in prediabetes to twice the control level (Fig. 7).
Table 2.
Tissue oxygen transport properties set in computational model and calculated results
| Set |
Calculated |
||||||
|---|---|---|---|---|---|---|---|
| Capillary Entrance Saturation: Sart | Relative RBC Supply Rate | Relative Functional Capillary Density | Relative Tissue O2 Consumption Rate | Mean Tissue Po2, mmHg | CV of Tissue Po2, SD/mean | Capillary Exit Saturation: Sven | |
| Control | 0.715 | 1.00 | 1.00 | 1.00 | 47.9 | 0.026 | 0.652* |
| PD | 0.636 | 0.74 | 0.74 | 1.29 | 39.6 | 0.044 | 0.521* |
| PD + ↑Sart | 0.715 | 0.74 | 0.74 | 1.29 | 45.3 | 0.044 | 0.609 |
| PD + ↑SR | 0.636 | 1.00 | 0.74 | 1.29 | 40.1 | 0.039 | 0.534 |
| PD + ↑CD | 0.636 | 0.74 | 1.00 | 1.29 | 40.3 | 0.039 | 0.533 |
| PD + ↓M | 0.636 | 0.74 | 0.74 | 1.00 | 40.8 | 0.034 | 0.547 |
| PD + ↑Sart + ↑SR | 0.715 | 1.00 | 0.74 | 1.29 | 45.9 | 0.039 | 0.621 |
| PD + ↑Sart + 2·SR | 0.715 | 2.00 | 0.74 | 1.29 | 47.7 | 0.026 | 0.655 |
Sart, arteriolar saturation; Sven, venular saturation; CV, coefficient of variation; PD, prediabetes; PD + ↑Sart, prediabetes properties except entrance O2 saturation set to control level; PD + ↑SR, prediabetes properties except red blood cell (RBC) supply rate set to control level; PD + ↑CD, prediabetes properties except perfused capillary density set to control level; PD + ↓M, prediabetes properties except O2 consumption rate set to control level; PD + ↑Sart + ↑SR, prediabetes properties except entrance O2 saturation and RBC supply rate set to control levels; PD + ↑Sart +2·SR, prediabetes properties except entrance O2 saturation set to control level and RBC supply rate set to 2 times control level.
Capillary exit saturation was set for control and prediabetes by adjusting the O2 consumption rate. The resulting control O2 consumption rate was 6.4 × 10−5 ml O2·ml−1·s−1.
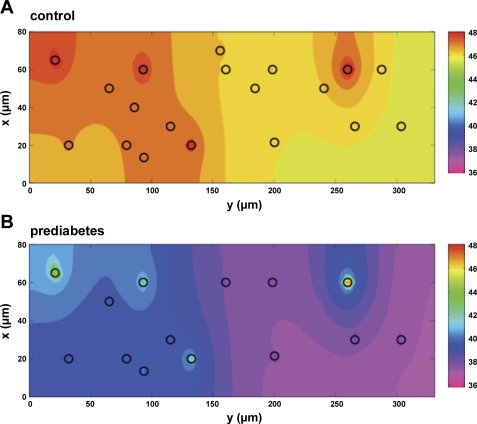
Fig. 6.
Color contour plots of calculated tissue Po2 at the venular exit of the capillary bed (z = 300 μm). Control Po2 in this plane (A) is 46.6 ± 0.6 mmHg (mean ± SD). Decreased O2 supply rate and capillary density in prediabetes resulted in a lower exit plane Po2 of 37.9 ± 1.0 mmHg (B). Black circles indicate RBC-perfused capillaries delivering O2 to the tissue (19 in control and 14 in prediabetes). Color spectrum scale bars (right) represent tissue Po2 levels (in mmHg).
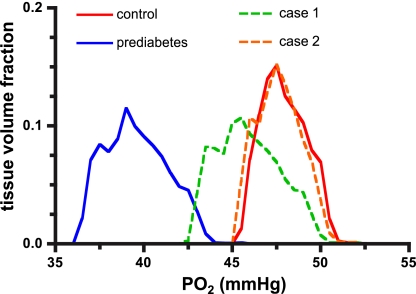
Fig. 7.
Calculated Po2 distributions for tissue volume surrounding array of parallel capillaries. Relative to control (red line), prediabetes (blue line) results in a shift of the Po2 distribution to the left (decreased mean Po2) and broadening (increased heterogeneity) of the Po2 distribution curve. In prediabetes, increasing the entrance O2 saturation and RBC supply rate to control levels (case 1, dashed green line) does not fully restore the Po2 distribution to control. A further increase in RBC supply rate to twice control levels (case 2, dashed orange line) is required to restore tissue oxygenation.
DISCUSSION
In humans with prediabetes, blood glucose is maintained at or near the normal range despite insulin resistance by virtue of significantly elevated plasma insulin levels. It has been shown that, during this prediabetic period, vascular reactivity is abnormal in humans, although the responsible mechanisms have not been defined. At the age of 7 wk, male ZDF rats (fa/fa) fed a high-fat diet (Purina 5008), but not their genetic controls (fa/+), develop a pattern that mimics prediabetes in humans with respect to blood glucose and plasma insulin levels (39, 43). Here we used this model of prediabetes to demonstrate that O2 transport in skeletal muscle is compromised in these animals. In addition, we provide support for the hypothesis that this defect in O2 delivery is related to the inability of RBCs to release ATP in response to exposure to reduced Po2. Finally, we have developed a computational model to examine the mechanisms responsible for decreased O2 delivery to skeletal muscle in prediabetic ZDF rats.
O2 transport in situ.
The total O2 supply to capillaries in the EDL muscle in prediabetic rats was reduced to 64% of that measured in control rats. The reduced O2 supply was due to a combination of factors including reduced RBC supply rate, because of reduced RBC velocity, and lower RBC O2 saturations at the entrance to capillaries. The increase in O2 extraction ratio across the capillary bed and the lower venular RBC O2 saturations in the prediabetic rats are consistent with the findings of lower RBC velocities in the capillaries, resulting in longer transit times and lower capillary density. When these data were incorporated into a computational model, it was determined that the O2 consumption in the model needed to be increased by 29% to equate the O2 extraction in the simulation and the measured O2 extraction, suggesting that O2 consumption in the EDL muscle may be higher in prediabetes. In terms of tissue oxygenation, the simulation indicates that the mean tissue Po2 is 8.3 mmHg lower in prediabetes (Table 2) and that the entire distribution of tissue Po2 values is shifted to lower values (Fig. 7), indicative of tissue hypoxia relative to control animals. These results support our hypothesis that the regulation of O2 supply is impaired in prediabetes. If the O2 regulatory system were functional, one could expect that the RBC supply would have increased sufficiently to maintain the RBC O2 saturation in the capillaries and the distribution of tissue Po2 values near the levels found in control animals. Our hypothesis is that high-insulin levels in prediabetes interfere with the release of ATP from erythrocytes exposed to low Po2, which would provide a mechanism by which blood flow regulation could be impaired.
Regulation of O2 supply.
There have been a number of mechanisms proposed to account for the increase in blood flow that occurs in response to tissue hypoxia (7, 19, 20, 30, 33). One possibility under active investigation is that the RBC senses a decrease in O2 levels in the tissue and, via the release of ATP, signals the vasculature to increase O2 supply to the region of tissue in need through the initiation of a conducted vasomotor response (12). The magnitude of the ATP release from RBCs has been shown to be O2 saturation dependent in the physiological range (34). Thus, if O2 saturation falls anywhere along the microvascular tree, e.g., in response to increased O2 metabolism or loss of capillaries delivering O2, ATP released from the RBCs would increase and stimulate an increase in blood flow and O2 supply to where it is needed to restore tissue oxygenation. For this proposed system to function, the endothelium must be intact, the arterioles must be able to dilate, and RBCs must be able to release the appropriate amount of ATP. Other regulatory mechanisms (Po2 sensing in the arteriolar wall or metabolic signaling from tissue) likely contribute to increasing O2 supply under higher metabolic conditions.
Consequence of high plasma insulin levels.
It has been reported that insulin at levels observed in prediabetes in humans attenuates the O2-dependent release of ATP from human RBCs (27). Importantly, if insulin is removed by washing, the same RBCs release ATP when exposed to reduced Po2 in amounts not different from cells not treated with insulin. It has been reported that the inhibition of ATP release from RBCs is mediated by the stimulation of phosphodiesterase 3 activity, resulting in an increased hydrolysis of cAMP, thus decreasing the level of this critical second messenger in the pathway for ATP release (28, 29). When RBCs from both prediabetic and control rats were washed to remove insulin, these cells released ATP in response to exposure to low O2. However, when the same cells were incubated with insulin at levels similar to that measured in prediabetic rats, an O2-dependent release of ATP was prevented (Fig. 3). It is important to note that RBCs from patients with clinical type 2 diabetes also have impaired O2-dependent ATP release, which has been attributed to the decreased expression of the α-subunit of the heterotrimeric G protein, Gi2. To establish that a defect in Giα2 expression was not responsible for the impaired O2-dependent ATP release associated with prediabetes, we measured its expression in prediabetic and control rats. Figure 4 demonstrates that RBCs from the prediabetes group have normal Giα2 expression, supporting a different mechanism for the impairment in O2-dependent ATP release in prediabetes. This is consistent with the capacity of RBCs from prediabetic rats to release ATP in the absence of insulin and further implicating insulin in the inhibition of ATP release.
Another mechanism by which insulin could interfere with the regulation of O2 supply to skeletal muscle is via direct effects on resistance vessels. In these studies we determined that resistance arteries isolated from both control rats and rats with prediabetes, when contracted with phenylephrine, display the same degree of relaxation to acetylcholine (Fig. 5) as has been reported previously for prediabetic ZDF rats (39, 43). Importantly, the addition of insulin did not alter the response in either group. Thus, in our study, the altered regulation of O2 supply in skeletal muscle in 7-wk-old ZDF rats with prediabetes cannot be attributed to either the differences in the endothelium-dependent relaxation of resistance arteries or the direct effects of insulin on these vessels.
The in situ experimental data and the O2 transport modeling results both demonstrate an impaired oxygenation in our rat model of prediabetes. These results, coupled with data indicating that insulin attenuates the release of ATP from RBCs exposed to reduced Po2 and the observation that endothelial function is intact in prediabetic ZDF rats and unaffected by insulin, suggest a cause-and-effect relationship. In addition, previously published data in isolated arterioles indicated that healthy human erythrocytes in the presence of insulin were unable to increase vessel diameter when exposed to reduced extraluminal Po2 (27), which provides additional support for the hypothesis that elevated insulin levels contribute to impaired oxygenation of skeletal muscle in prediabetes. Although the defect is significant, it should not be sufficient to cause injury to the resting tissue, i.e., the simulation does not indicate anoxia since minimum Po2 values are above 35 mmHg (Fig. 7). However, it is likely that there is a reduced capacity to further increase aerobic activity that could contribute to the microvascular dysfunction reported in humans with prediabetes.
If increased precapillary O2 losses in prediabetes are partially due to low tissue Po2 levels, then one could speculate that an increased O2 consumption would further increase these precapillary losses, thus causing an even larger drop in tissue Po2. With a functional O2 regulatory system, blood flow should increase to restore tissue Po2 levels and hence limit the precapillary O2 losses. However, our results suggest that this would not occur in prediabetes or at least would be severely attenuated. Other regulatory mechanisms associated with metabolism would likely prevent catastrophic microvascular collapse, but without the ability to regulate O2 supply, the tissue could be exposed to prolonged periods of tissue hypoxia.
Although a failure in the regulatory system could account for the observed differences between the control and prediabetes data, we explored other aspects that might have contributed to the differences using our mathematical model. The density of continuously perfused capillaries in the prediabetes groups was 71% of control (P < 0.05). The lower perfused capillary density was not due to an increase in stopped or intermittent flow capillaries although it could be due to an anatomical loss of capillaries that we were unable to measure in this study. It is also possible that the lower RBC supply rate in the capillary bed may have contributed to greater perfusion heterogeneity resulting in more capillaries perfused only with plasma (11). We evaluated the contribution of the lower perfused capillary density, resulting in reduced surface area for exchange and greater diffusion distances in the prediabetes group, to the lower tissue Po2 values relative to the control group. Table 2 (row labeled PD + ↑CD) shows that increasing perfused capillary density to control levels, with all other prediabetes parameters unchanged, had a small effect on increasing mean tissue Po2 from 39.6 to 40.3 mmHg or 8.4% of the difference between prediabetes and control. Using the same approach, we tested the impact of the lower RBC supply rate in prediabetes by simulating the prediabetes case with the control RBC supply rate. The effect was even less with mean tissue Po2 increased by only 0.5 mmHg (Table 2, PD + ↑SR). Since the simulation required that we increase O2 consumption by 29%, we determined its contribution to the lower tissue Po2 levels. Reducing the O2 consumption in the prediabetes simulation to control levels (all parameters except O2 consumption were set to prediabetes values) increased the tissue Po2 by only 1.2 mmHg (Table 2, PD + ↓M). We conclude from these simulations that changes in capillary density, RBC supply rate, and O2 consumption had little impact on the reduced tissue oxygenation in prediabetes.
It is apparent from the simulations that the lower entrance O2 saturation is the primary factor responsible for the significant 8.3-mmHg decrease in mean tissue Po2 in the prediabetes group. Increasing entrance O2 saturation to control levels in the prediabetes simulation increases the mean tissue Po2 to 45.3 mmHg (simulation results in Table 2, PD + ↑Sart). The lower entrance O2 saturation suggests a greater than normal precapillary loss of O2 from the arteriolar tree and/or an increased heterogeneity of O2 supply among arterioles. Greater precapillary losses could be due to a combination of lower tissue Po2 with an increased Po2 gradient for diffusion from the arterioles to nearby tissue, capillaries, and venules and longer transit times for the exchange in the arteriolar tree as suggested by lower RBC velocities measured in the capillaries. An increased heterogeneity of O2 supply among arterioles could occur if the O2 regulatory system is impaired in prediabetes and unable to match O2 supply with demand (12). A diffusion of O2 from arteriole to venule and an oversupply of O2 in some arterioles act as functional O2 shunts that divert O2 from the tissue. Under these conditions, precapillary losses due to O2 diffusion to tissue or capillaries would support tissue metabolism. From this study we cannot determine how much of the precapillary O2 losses may have supported tissue oxygenation. However, the results presented here would suggest that if arteriolar blood flow had increased in response to the decrease in tissue Po2, the increased RBC supply rate and reduced arteriolar transit times would likely have reduced the precapillary losses and the heterogeneity of arteriolar perfusion, resulting in an increase in the capillary entrance O2 saturations.
Taken together, these results suggest that the impaired tissue oxygenation in prediabetes could be due to a dysfunctional O2 regulatory system rather than a loss of capillaries or increased O2 consumption. In addition, the simulations enabled us to determine the extent to which the O2 regulatory system would have had to increase the RBC supply rate to restore tissue oxygenation and venular O2 saturation to control levels in the prediabetes group. Figure 7 shows the effect of increasing the O2 supply in the prediabetes simulation to control levels (case 1) and to twice control levels (case 2). A twofold increase in RBC supply rate with Sart at control levels would be sufficient to match the tissue Po2 distribution (Fig. 7, case 2) and return venous O2 saturations (65%: Table 2, PD + ↑Sart + 2·SR) to values normally found in control animals. A twofold increase in O2 supply to skeletal muscle is well within physiological limits. This result supports the idea that an impairment in the function of the O2 regulatory system could have contributed to the lower tissue Po2 levels observed in prediabetes.
Conclusions.
Our multiscale systems biology approach has demonstrated that high-insulin levels impair the ability of rat RBCs to release ATP in response to low Po2, which could contribute to the impairment in tissue oxygenation observed in this rat model of prediabetes. The O2 transport simulation using experimental data from the animals used in this study enabled the direct interpretation of the role each parameter played in impaired tissue oxygenation. The results confirmed that impaired O2 supply was the primary factor for the defect in tissue oxygenation. However, there are unanswered questions that need to be addressed in future experiments and simulations. If the O2 regulatory system is challenged with increased O2 consumption or reduced tissue Po2 levels, will it respond by increasing the O2 supply in prediabetes? Our in situ results under resting conditions together with our in vitro data strongly imply that the O2 regulatory system will be unable to respond in prediabetes.
GRANTS
This research was funded by National Heart, Lung, and Blood Institute Grants R33-HL-089094 and HL-089125.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
We thank J. L. Sprague for inspiration.
Present address of M. Hanson: Department of Biophysics, Medical College of Wisconsin, Milwaukee, WI.
REFERENCES
- 1.Ardigo D, Franzini L, Valtuena S, Monti LD, Reaven GM, Zavaroni I. Relation of plasma insulin levels to forearm flow-mediated dilatation in healthy volunteers. Am J Cardiol 97: 1250–1254, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Benjamin SM, Valdez R, Geiss LS, Rolka DB, Narayan KM. Estimated number of adults with prediabetes in the US in 2000: opportunities for prevention. Diabetes Care 26: 645–649, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bock G, Dalla Man C, Campioni M, Chittilapilly E, Basu R, Toffolo G, Cobelli C, Rizza R. Pathogenesis of pre-diabetes: mechanisms of fasting and postprandial hyperglycemia in people with impaired fasting glucose and/or impaired glucose tolerance. Diabetes 55: 3536–3549, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392: 398–401, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Corsetti JP, Sparks JD, Peterson RG, Smith RL, Sparks CE. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis 148: 231–241, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Dinneen SF, Maldonado D, 3rd, Leibson CL, Klee GG, Li H, Melton LJ, 3rd, Rizza RA. Effects of changing diagnostic criteria on the risk of developing diabetes. Diabetes Care 21: 1408–1413, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Duling BR. Oxygen sensitivity of vascular smooth muscle. II. In vivo studies. Am J Physiol 227: 42–49, 1974 [DOI] [PubMed] [Google Scholar]
- 8.Eggleton CD, Vadapalli A, Roy TK, Popel AS. Calculations of intracapillary oxygen tension distributions in muscle. Math Biosci 167: 123–143, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Ellis CG, Ellsworth ML, Pittman RN. Determination of red blood cell oxygenation in vivo by dual video densitometric image analysis. Am J Physiol Heart Circ Physiol 258: H1216–H1223, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Ellis CG, Ellsworth ML, Pittman RN, Burgess WL. Application of image analysis for evaluation of red blood cell dynamics in capillaries. Microvasc Res 44: 214–225, 1992 [DOI] [PubMed] [Google Scholar]
- 11.Ellis CG, Wrigley SM, Groom AC. Heterogeneity of red blood cell perfusion in capillary networks supplied by a single arteriole in resting skeletal muscle. Circ Res 75: 357–368, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology 24: 107–116, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellsworth ML, Pittman RN. Arterioles supply oxygen to capillaries by diffusion as well as by convection. Am J Physiol Heart Circ Physiol 258: H1240–H1243, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Eringa EC, Bakker W, Smulders YM, Serne EH, Yudkin JS, Stehouwer CD. Regulation of vascular function and insulin sensitivity by adipose tissue: focus on perivascular adipose tissue. Microcirculation 14: 389–402, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab 293: E1134–E1139, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Etgen GJ, Oldham BA. Profiling of Zucker diabetic fatty rats in their progression to the overt diabetic state. Metabolism 49: 684–688, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Gambhir KK, Archer JA, Bradley CJ. Characteristics of human erythrocyte insulin receptors. Diabetes 27: 701–708, 1978 [DOI] [PubMed] [Google Scholar]
- 18.Gherzi R, Andraghetti G, Adezati L, Cordera R. Insulin receptor regulation in human mature red cells in vitro. Horm Res 22: 270–275, 1985 [DOI] [PubMed] [Google Scholar]
- 19.Gladwin MT. Role of the red blood cell in nitric oxide homeostasis and hypoxic vasodilation. Adv Exp Med Biol 588: 189–205, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Gladwin MT, Crawford JH, Patel RP. The biochemistry of nitric oxide, nitrite, and hemoglobin: role in blood flow regulation. Free Radic Biol Med 36: 707–717, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Goldman D, Bateman RM, Ellis CG. Effect of decreased O2 supply on skeletal muscle oxygenation and O2 consumption during sepsis: role of heterogeneous capillary spacing and blood flow. Am J Physiol Heart Circ Physiol 290: H2277–H2285, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Goldman D, Bateman RM, Ellis CG. Effect of sepsis on skeletal muscle oxygen consumption and tissue oxygenation: interpreting capillary oxygen transport data using a mathematical model. Am J Physiol Heart Circ Physiol 287: H2535–H2544, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Goldman D, Popel AS. A computational study of the effect of capillary network anastomoses and tortuosity on oxygen transport. J Theor Biol 206: 181–194, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Goldman D, Popel AS. A computational study of the effect of vasomotion on oxygen transport from capillary networks. J Theor Biol 209: 189–199, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Goldman D, Popel AS. Computational modeling of oxygen transport from complex capillary networks. Relation to the microcirculation physiome. Adv Exp Med Biol 471: 555–563, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Haffner SM, Stern MP, Hazuda HP, Mitchell BD, Patterson JK. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA 263: 2893–2898, 1990 [DOI] [PubMed] [Google Scholar]
- 27.Hanson MS, Ellsworth ML, Achilleus D, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Insulin inhibits low oxygen-induced ATP release from human erythrocytes: implication for vascular control. Microcirculation 16: 424–433, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanson MS, Stephenson AH, Bowles EA, Sprague RS. Insulin inhibits human erythrocyte cAMP accumulation and ATP release: role of phosphodiesterase 3 and phosphoinositide 3-kinase. Exp Biol Med (Maywood) 235: 256–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol 295: H786–H793, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hester RL. Uptake of metabolites by postcapillary venules: mechanism for the control of arteriolar diameter. Microvasc Res 46: 254–261, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Jaap AJ, Hammersley MS, Shore AC, Tooke JE. Reduced microvascular hyperaemia in subjects at risk of developing type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 37: 214–216, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Jaap AJ, Shore AC, Tooke JE. Relationship of insulin resistance to microvascular dysfunction in subjects with fasting hyperglycaemia. Diabetologia 40: 238–243, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Jackson WF. Arteriolar oxygen reactivity: where is the sensor? Am J Physiol Heart Circ Physiol 253: H1120–H1126, 1987 [DOI] [PubMed] [Google Scholar]
- 34.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Japee SA, Ellis CG, Pittman RN. Flow visualization tools for image analysis of capillary networks. Microcirculation 11: 39–54, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Japee SA, Pittman RN, Ellis CG. A new video image analysis system to study red blood cell dynamics and oxygenation in capillary networks. Microcirculation 12: 489–506, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kanauchi M, Kanauchi K, Inoue T, Kimura K, Saito Y. Surrogate markers of insulin resistance in assessing individuals with new categories “prehypertension” and “prediabetes”. Clin Chem Lab Med 45: 35–39, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Reaven GM. Isolated impaired fasting glucose and peripheral insulin sensitivity: not a simple relationship. Diabetes Care 31: 347–352, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Lesniewski LA, Donato AJ, Behnke BJ, Woodman CR, Laughlin MH, Ray CA, Delp MD. Decreased NO signaling leads to enhanced vasoconstrictor responsiveness in skeletal muscle arterioles of the ZDF rat prior to overt diabetes and hypertension. Am J Physiol Heart Circ Physiol 294: H1840–H1850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissen M, Isomaa B, Forsen B, Homstrom N, Saloranta C, Taskinen MR, Groop L, Tuomi T. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54: 166–174, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes 52: 1475–1484, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Mori Y, Hoshino K, Yokota K, Itoh Y, Tajima N. Japanese IGT subjects with high insulin response are far more frequently associated with the metabolic syndrome than those with low insulin response. Endocrine 29: 351–355, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Oltman CL, Richou LL, Davidson EP, Coppey LJ, Lund DD, Yorek MA. Progression of coronary and mesenteric vascular dysfunction in Zucker obese and Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol 291: H1780–H1787, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Psyrogiannis A, Habeos I, Kyriazopoulou V. Insulin sensitivity and Lp(alpha) concentrations in normoglycemic offspring of type 2 diabetic parents. Lipids Health Dis 2: 8, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandeman DD, Pym CA, Green EM, Seamark C, Shore AC, Tooke JE. Microvascular vasodilatation in feet of newly diagnosed non-insulin dependent diabetic patients. BMJ 302: 1122–1123, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprague RS, Stephenson AH, Bowles EA, Stumpf MS, Lonigro AJ. Reduced expression of G(i) in erythrocytes of humans with type 2 diabetes is associated with impairment of both cAMP generation and ATP release. Diabetes 55: 3588–3593, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, Kochba I, Rudich A; Israeli Diabetes Research Group Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med 353: 1454–1462, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Tyml K, Budreau CH. A new preparation of rat extensor digitorum longus muscle for intravital investigation of the microcirculation. Int J Microcirc Clin Exp 10: 335–343, 1991 [PubMed] [Google Scholar]
- 49.Ward GM, Harrison LC. Structure of the human erythrocyte insulin receptor. Diabetes 35: 101–105, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Wiernsperger N. Defects in microvascular haemodynamics during prediabetes: contributor or epiphenomenon? Diabetologia 43: 1439–1448, 2000 [DOI] [PubMed] [Google Scholar]