Abstract
We previously demonstrated that several epoxyeicosatrienoic acids (EETs) produce reductions in myocardial infarct size in rats and dogs. Since a recent study demonstrated the release of opioids in mediating the antinociceptive effect of 14,15-EET, we hypothesized that endogenous opioids may also be involved in mediating the cardioprotective effect of the EETs. To test this hypothesis, we used an in vivo rat model of infarction and a rat Langendorff model. In the infarct model, hearts were subjected to 30 min occlusion of the left coronary artery and 2 h reperfusion. Animals were treated with 11,12-EET or 14,15-EET (2.5 mg/kg) alone 15 min before occlusion or with opioid antagonists [naloxone, naltrindole, nor-binaltorphimine (nor-BNI), and d-Phe-Cys-Tyr-d-Trp-Om-Thr-Pen-Thr-NH2 (CTOP), a nonselective, a selective δ, a selective κ, and a selective μ receptor antagonist, respectively] 10 min before EET administration. In four separate groups, antiserum to Met- and Leu-enkephalin and dynorphin-A-(1–17) was administered 50 min before the 11,12-EET administration. Infarct size expressed as a percent of the area at risk (IS/AAR) was 63.5 ± 1.2, 45.3 ± 1.0, and 40.9 ± 1.2% for control, 11,12-EET, and 14,15-EET, respectively. The protective effects of 11,12-EET were abolished by pretreatment with either naloxone (60.5 ± 1.8%), naltrindole (60.8 ± 1.0%), nor-BNI (62.3 ± 2.8%), or Met-enkephalin antiserum (63.2 ± 1.7%) but not CTOP (42.0 ± 3.0%). In isolated heart experiments, 11,12-EET was administered to the perfusate 15 min before 20 min global ischemia followed by 45 min reperfusion in control hearts or in those pretreated with pertussis toxin (48 h). 11,12-EET increased the recovery of left ventricular developed pressure from 33 ± 1 to 45 ± 6% (P < 0.05) and reduced IS/AAR from 37 ± 4 to 20 ± 3% (P < 0.05). Both pertussis toxin and naloxone abolished these beneficial effects of 11,12-EET. Taken together, these results suggest that the major cardioprotective effects of the EETs depend on activation of a Gi/o protein-coupled δ- and/or κ-opioid receptor.
Keywords: nor-binaltorphimine, infarct size, area at risk
arachidonic acid is metabolized to various biologically active eicosanoids by cyclooxygenase, lipoxygenase, and the cytochrome P450 (CYP) monooxygenase pathways (21). We and others have recently found that activation of the CYP epoxygenase pathway by overexpressing CYP2J2 or knocking out soluble epoxide hydrolase (sEH) (19,20) or by administering exogenous epoxyeicosatrienoic acids (EETs) (19) results in an improvement in the recovery of contractile function in isolated perfused mouse hearts (19, 20) subjected to global ischemia and reperfusion and a reduction in infarct size in intact canine, rat, and mouse hearts (10, 15, 16, 19). Although it is assumed that the beneficial effects of CYP2J2 overexpression or sEH knockouts are the result of an increase in endogenous EETs, there is no direct evidence that the protective actions of these two interventions are actually the result of the actions of EETs on a specific EET membrane receptor since one has not been identified.
Nevertheless, there have been several recent reports (2, 5, 24) which suggest that EETs may be acting directly or indirectly on several G protein-coupled receptors. Evidence for a direct action of the EETs on a G protein-coupled receptor has recently been demonstrated by Behm and colleagues (2), in which they demonstrated that the EETs selectively bound to a thromboxane receptor (TP). They found that 14,15-EET blocked the vasoconstrictor effect of the TP agonist U-46619 in mice with knockout of the large-conductance Ca2+-activated K+ channel and the transient receptor potential cation channel subfamily V member 4 cation channels in aorta. These two channels have previously been thought to be responsible for the vasodilator effect of the EETs and dihydroxyepoxyeicosatrienoic acids (DHETs) (2).
An indirect effect of EETs to produce an antinociceptive effect via the activation of opioid receptors in the rat ventrolateral periaqueductal gray (vlPAG) has recently been demonstrated by Terashvilli et al. (24). The rat tail-flick response was shown to be blocked by 14,15-EET microinjected in the vlPAG. However, the tail-flick inhibition produced by 14,15-EET was blocked by intra-vlPAG pretreatment with antiserum against β-endorphin or Met-enkephalin, by the μ-opioid receptor antagonist d-Phe-Cys-Tyr-d-Trp-Om-Thr-Pen-Thr-NH2 (CTOP), or the δ-opioid receptor antagonist naltrindole but not by the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) or dynorphin antiserum. These effects were shown to be independent of 14,15-EET specific binding to the μ- or δ-opioid receptor directly, and it was hypothesized that EET activates β-endorphin and Met-enkephalin to produce antinociception in rats. Thus, based on these studies and previous work from several laboratories (4, 13, 17, 28) that have shown an important effect of opioids to produce cardioprotection in many models of ischemia-reperfusion, we hypothesized that EETs may be releasing or activating opioid receptors in the heart to produce a part of their anti-infarct effect during ischemia and/or reperfusion. To test this hypothesis, we used an in vivo rat infarct model and a rat Langendorff model and measured the cardioprotective effects of 11,12- and 14,15-EET in the absence and presence of nonselective and selective opioid antagonists and antisera for several endogenous opioid peptides known to be released in the rat heart (25, 27, 28) during stress.
MATERIALS AND METHODS
All experiments conducted in this study were in accordance with the Position of the American Heart Association on Research and Animal Use adopted by the American Heart Association and approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin. The Medical College of Wisconsin is accredited by the American Association of Laboratory Animal Care.
Materials.
11,12- and 14,15-EETs were synthesized in the laboratory of Dr. J. R. Falck. 11,12-EET and 14,15-EET were dissolved in a vehicle composed of 95% ethanol, polyethylene glycol 200, and 1 N sodium hydroxide (5:5:1). Naloxone, naltrindole, nor-BNI, and CTOP were obtained from Tocris Biosciences (Ellisville, MO). Pertussis toxin was obtained from Sigma (St. Louis, MO). All opioids were dissolved in DMSO and diluted with deionized water so that DMSO was <1%. All drug and antibody solutions were made up fresh daily.
Intact rat preparation.
The surgical procedure for the intact rat preparation has been previously described in detail (10). Briefly, male Sprague-Dawley rats weighing 250–300 g were fasted overnight, anesthetized with Inactin (100 mg/kg ip; Sigma), and ventilated with room air supplemented with 100% oxygen. Body temperature was maintained at 37 ± 1°C with a heating pad. Arterial blood pH, Pco2, and Po2 were monitored at selected intervals by an AVL automatic blood gas system and maintained within normal physiological limits (pH 7.35–7.45, Pco2 30–40 mmHg, and pO2 85–120 mmHg) by adjusting the respiration rate and oxygen flow or by intravenous administration of 1.5% sodium bicarbonate if necessary. Hemodynamics, heart rate, and coronary blood flow were monitored throughout the experiment. A cannula was placed in the jugular vein and carotid artery to administer drugs and measure peripheral hemodynamics, respectively.
Experimental preparation and protocol.
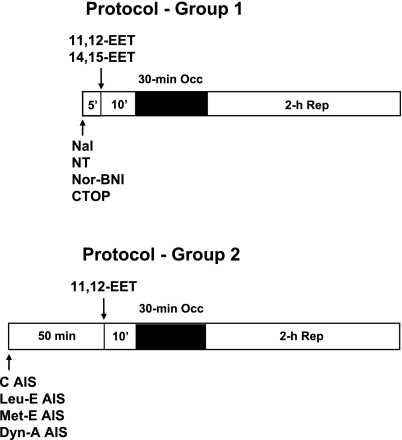
Rats were sequentially assigned to 14 groups for the different treatments. Typically, 8–10 rats were included in each group of experiments. At 15 min before the 30-min left anterior descending artery (LAD) occlusion period, 11,12-EET (2.5 mg/kg) or 14,15-EET (2.5 mg/kg) was administered by intravenous injection for 1–2 min as shown in Fig. 1. The 2.5 mg/kg dose was chosen based on preliminary experiments in which 1.0 mg/kg of 11,12- and 14,15-EETs was only modestly effective at reducing infarct size expressed as a percent of the area at risk (IS/AAR), and 5 mg/kg was similar in efficacy in reducing IS/AAR as 2.5 mg/kg. In the opioid antagonist studies, either naloxone (3.0 mg/kg), naltrindole (5.0 mg/kg), nor-BNI (0.3 mg/kg), or CTOP (0.1 mg/kg) was administered at doses previously used in our laboratory alone or 10 min before EET administration. The antisera (25 mg/kg iv) to the various endogenous opioid peptides were administered 50 min before EET administration (8, 24). In all groups, hemodynamic measurements and blood gas analyses were performed at baseline and at 15 min into the 30-min occlusion period and at the end of 2 h of reperfusion.
Fig. 1.
Protocols used to study the role of opioids in epoxyeicosatrienoic acid (EET)-induced cardioprotection in rat hearts. In protocol 1, 11,12- or 14,15-EET were given alone 10 min before a 30-min occlusion (Occ) period and 2 h reperfusion (Rep) or in rats pretreated with a nonselective opioid antagonist (naloxone, Nal) or selective opioid antagonists naltrindole (NT, δ-antagonist), nor-binaltorphimine (Nor-BNI, κ-antagonist), and d-Phe-Cys-Tyr-d-Trp-Om-Thr-Pen-Thr-NH2 (CTOP, μ-antagonist). The opioid antagonists were given 5 min before 11,12- or 14,15-EET. In protocol 2, antisera (AIS) control (C) and AIS to Leu- and Met-enkephalin and dynorphin-A-(1–17) (Dyn-A) were administered 50 min before 11,12-EET administration, which was given 10 min before coronary occlusion.
Infarct size determination.
Infarct size was determined as previously described (10). Briefly, at the end of the 2-h reperfusion period, the LAD was reoccluded. To determine the anatomic area at risk (AAR) and the nonischemic area, 1 ml of Patent blue dye was injected in the jugular vein, and the heart was arrested by an intravenous injection of potassium chloride. The heart was then immediately removed, and the left ventricle (LV) was dissected and sliced into serial transverse sections 1–2 mm in width. The nonstained ischemic area and the blue-stained normal area were separated and incubated with 1% 2,3,5-triphenyltetrazolium chloride (Sigma) in 0.1 mol/l phosphate buffer, pH 7.4 at 37°C for 15 min. After incubation overnight in 10% formaldehyde, the noninfarcted and infarcted tissues within the AAR were separated and determined gravimetrically under a dissecting microscope. Infarct size was expressed as a percentage of the AAR.
Antiserum studies.
In four separate groups of intact rat experiments, selective polyclonal rabbit antisera for the major endogenous opioid peptides were given 50 min before 11,12-EET and 60 min before the 30-min ischemic period. The antibodies were a kind gift from Dr. Hsiang-en Wu (Department of Anesthesiology at the Medical College of Wisconsin) and included antisera against Leu-enkephalin, Met-enkephalin, and dynorphin A-(1–17). Control antiserum was also included. The antisera were dissolved in 0.9% sterile saline. The dose (25 mg/kg) and time of optimal administration were based on previous studies using similar antisera (8, 24).
Langendorff heart studies.
The surgical procedure for the Langendorff rat heart preparation has been previously described in detail (1). Isolated rat hearts were perfused in a retrograde manner with bicarbonate buffer at constant perfusion pressure (98 mmHg) with a balloon inflated in the LV. End-diastolic pressure was set to 5 mmHg, and developed pressure was recorded during steady-state conditions (1). Hearts were allowed to beat spontaneously. Hearts were kept in temperature-controlled chambers to maintain myocardial temperature at 37°C during periods of ischemia and perfusion. Hearts were subjected to 30 min of regional no-flow ischemia and 3 h of reperfusion. 11,12-EET alone or in combination with naloxone was added to the coronary perfusate as needed in separate groups. In a different group of experiments, animals were pretreated with pertussis toxin (10 μg/kg ip) 48 h before heart removal. Pertusis toxin-treated hearts were then treated with vehicle or 11,12-EET administered in the perfusate 10 min before no-flow, global ischemia and throughout the 20-min ischemic period. At the completion of the experiment, we tested the bradycardic effects of a known Gi/o agonist, adenosine, vs. the vehicle control, to test for adequacy of Gi/o blockade by the pertussis toxin dose.
Statistical analysis.
All values are expressed as means ± SE (n = 8–16/group). Differences between groups in hemodynamics were compared by using a two-way ANOVA followed by a Tukey's post hoc test. Differences between groups in AAR and infarct size were compared by one-way ANOVA. Differences between groups were considered significant if P < 0.05.
RESULTS
Hemodynamics in the in vivo studies.
There were 140 rats that were used in the in vivo infarct studies of which 133 rats survived the protocol. Three rats (2 in control group and 1 in naltrindole alone) died of intractable ventricular fibrillation, and four rats died of severe hypotension (1 in the 11,12-EET, 1 in the 14,15-EET, 1 in the naloxone alone, and 1 in the nor-BNI + 11,12-EET group). In the 14 surviving groups, there were no differences in baseline hemodynamics, and there were only three groups where the heart rates were significantly lower during ischemia (naltrindole alone and naltrindole + 11,12-EET) and at reperfusion (naltrindole alone). There were no other changes in heart rate or mean arterial pressure in any of the other groups compared with their corresponding control points in time (Table 1). These results suggest that any changes observed in IS/AAR were unlikely to be the result of differences in peripheral hemodynamics between groups.
Table 1.
Hemodynamic values
| CON | Vehicle or Drug | REP (2 h) | |
|---|---|---|---|
| Heart rate, beats/min | |||
| Control | 378 ± 4 | 383 ± 6 | 369 ± 7 |
| 11,12-EET | 368 ± 4 | 369 ± 4 | 345 ± 6 |
| 14,15-EET | 366 ± 7 | 367 ± 7 | 347 ± 8 |
| Naloxone | 353 ± 10 | 358 ± 10 | 320 ± 11* |
| Naloxone + 11,12-EET | 388 ± 8 | 390 ± 8 | 365 ± 14 |
| Naltrindole | 352 ± 9 | 322 ± 6† | 318 ± 8† |
| Naltrindole + 11,12-EET | 379 ± 7 | 346 ± 8* | 344 ± 5 |
| Nor-BNI | 372 ± 3 | 376 ± 4 | 347 ± 4 |
| Nor-BNI + 11,12-EET | 385 ± 4 | 385 ± 6 | 378 ± 8 |
| CTOP | 373 ± 7 | 375 ± 9 | 360 ± 9 |
| CTOP + 11,12-EET | 380 ± 7 | 380 ± 4 | 363 ± 5 |
| Control AIS | 357 ± 6 | 348 ± 7 | 357 ± 4 |
| Leu-E AIS | 391 ± 14 | 383 ± 11 | 370 ± 7 |
| Met-E AIS | 353 ± 8 | 347 ± 7 | 338 ± 9 |
| Dyn-A AIS | 388 ± 9 | 382 ± 15 | 364 ± 10 |
| Mean arterial pressure, mmHg | |||
| Control | 131 ± 4 | 115 ± 7 | 83 ± 4 |
| 11,12-EET | 128 ± 2 | 126 ± 3 | 89 ± 3 |
| 14,15-EET | 134 ± 5 | 135 ± 6 | 85 ± 4 |
| Naloxone | 115 ± 6 | 117 ± 6 | 77 ± 5 |
| Naloxone + 11,12- EET | 120 ± 6 | 117 ± 7 | 86 ± 4 |
| Naltrindole | 120 ± 7 | 107 ± 8 | 80 ± 4 |
| Naltrindole + 11,12-EET | 115 ± 3 | 108 ± 6 | 77 ± 3 |
| Nor-BNI | 127 ± 5 | 129 ± 4 | 76 ± 8 |
| Nor-BNI + 11,12-EET | 124 ± 4 | 121 ± 5 | 90 ± 4 |
| CTOP | 122 ± 6 | 119 ± 7 | 87 ± 6 |
| CTOP + 11,12-EET | 118 ± 5 | 114 ± 6 | 80 ± 5 |
| Control AIS | 125 ± 5 | 100 ± 4 | 75 ± 4 |
| Leu-K AIS + 11,12-EET | 123 ± 5 | 105 ± 4 | 84 ± 5 |
| Met-E AIS + 11,12-EET | 117 ± 5 | 104 ± 4 | 76 ± 6 |
| Dyn-A AIS + 11,12-EET | 122 ± 3 | 107 ± 5 | 82 ± 6 |
All values are means ± SE; n = 8–15 rats. CON, control; REP, reperfusion; EET, epoxyeicosatrienoic acid; Nor-BNI, nor-binaltorphimine; CTOP, d-Phe-Cys-Tyr-d-Trp-Om-Thr-Pen-Thr-NH2; AIS, antibody inhibitory serum; Dyn-A, dynorphin-A-(1–17).
P < 0.01 vs. control group at same treatment time.
P < 0.001 vs. control group at same treatment time.
Infarct size data in in vivo experiments.
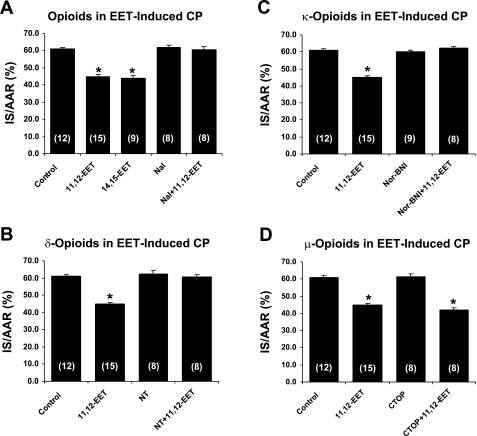
The data concerning infarct size are summarized in Table 2 and Figs. 2 and 3. There were no statistically significant differences in left ventricular weight, AAR, infarct weight, or AAR as a percent of left ventricular weight (AAR/LV) between groups. However, both 11,12- and 14,15-EET produced significant (P < 0.01) decreases in IS/AAR. Interestingly, these decreases in IS/AAR produced by 11,12-EET were completely blocked by pretreatment with naloxone, a nonselective opioid antagonist, by naltrindole, a selective δ-opioid receptor antagonist, and by nor-BNI, a selective κ-opioid receptor antagonist but not by CTOP, a selective μ-opioid receptor antagonist. Similar results were obtained with 14,15-EET and naloxone pretreatment (65.0 ± 1.2, n = 8).
Table 2.
Infarct size data
| Groups | n | LV, g | AAR, g | IS, g | AAR/LV, % | IS/AAR, % |
|---|---|---|---|---|---|---|
| Control | 12 | 0.739 ± 0.017 | 0.287 ± 0.016 | 0.174 ± 0.010 | 38.6 ± 1.6 | 61.0 ± 0.8 |
| 11,12-EET | 15 | 0.699 ± 0.016 | 0.301 ± 0.010 | 0.136 ± 0.005 | 42.8 ± 1.3 | 44.3 ± 0.9* |
| 14,15-EET | 9 | 0.767 ± 0.023 | 0.355 ± 0.016 | 0.155 ± 0.007 | 45.5 ± 1.7 | 44.0 ± 1.8* |
| Naloxone | 8 | 0.729 ± 0.040 | 0.309 ± 0.026 | 0.191 ± 0.016 | 42.1 ± 2.0 | 61.8 ± 0.9 |
| Naloxone + 11,12-EET | 8 | 0.626 ± 0.023 | 0.250 ± 0.017 | 0.151 ± 0.011 | 40.0 ± 2.7 | 60.5 ± 1.8 |
| Naltrindole | 8 | 0.694 ± 0.022 | 0.306 ± 0.019 | 0.190 ± 0.012 | 44.0 ± 2.2 | 62.4 ± 1.9 |
| Naltrindole + 11,12-EET | 8 | 0.739 ± 0.022 | 0.303 ± 0.016 | 0.184 ± 0.009 | 40.9 ± 1.7 | 60.8 ± 1.0 |
| Nor-BNI | 9 | 0.745 ± 0.015 | 0.286 ± 0.011 | 0.172 ± 0.006 | 38.6 ± 1.4 | 60.3 ± 0.8 |
| Nor-BNI + 11,12-EET | 8 | 0.794 ± 0.019 | 0.303 ± 0.020 | 0.189 ± 0.013 | 38.2 ± 2.4 | 62.3 ± 0.8 |
| CTOP | 8 | 0.663 ± 0.016 | 0.268 ± 0.014 | 0.165 ± 0.009 | 40.2 ± 1.3 | 61.5 ± 1.5 |
| CTOP + 11,12-EET | 8 | 0.676 ± 0.037 | 0.318 ± 0.033 | 0.135 ± 0.017 | 46.5 ± 1.1 | 42.0 ± 1.3* |
| Control AIS | 8 | 0.735 ± 0.027 | 0.293 ± 0.021 | 0.180 ± 0.013 | 40.1 ± 1.5 | 61.3 ± 1.5 |
| Leu-E AIS + 11,12-EET | 8 | 0.737 ± 0.013 | 0.345 ± 0.019 | 0.164 ± 0.010 | 46.8 ± 2.7 | 47.5 ± 0.8* |
| Met-E AIS + 11,12-EET | 8 | 0.699 ± 0.021 | 0.297 ± 0.021 | 0.188 ± 0.015 | 42.2 ± 1.7 | 63.1 ± 1.6 |
| Dyn-E AIS + 11,12-EET | 8 | 0.718 ± 0.010 | 0.329 ± 0.014 | 0.153 ± 0.010 | 45.7 ± 1.8 | 46.3 ± 1.2* |
All values are means ± SE; n, no. of rats. LV, left ventricle; AAR, area at risk; IS, infarct size.
P < 0.001 vs. control group.
Fig. 2.
Effect of various opioid antagonists [naloxone (A), naltrindole (B), nor-BNI (C), or CTOP (D)] on 11,12-EET-induced infarct size reduction in intact rat hearts. All of the antagonists had no effect on the infarct size expressed as %area at risk (IS/AAR) by themselves, but all completely blocked the effect of 11,12-EET with the exception of CTOP, the selective μ-antagonist. CP, cardioprotection. All values are means ± SE. No. of rats is in parentheses. *P < 0.01 vs. the control group.
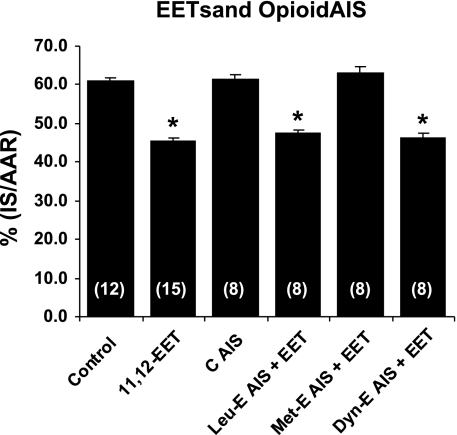
Fig. 3.
Effect of pretreating hearts with control antisera (C AIS) and antisera to various endogenous opioid peptides administered 50 min before 11,12-EET administration. Antisera to Leu-enkephalin (Leu-E-AIS), Met-enkephalin (Met-E-AIS), and dynorphin (Dyn-E-AIS) were administered at 25 mg/kg. Only Met-E AIS blocked the effect of 11,12-EET. All values are means ± SE. No. of rats is in parentheses. *P < 0.01 vs. the control group.
To further determine the type of endogenous opioid peptide that might be involved, we pretreated rats with control antiserum and selective rabbit antisera to Leu-enkephalin, Met-enkephalin, and dynorphin A-(1–17) 60 min before the 30-min ischemic period or 50 min before treatment with 11,12-EET. The different antisera had no hemodynamic effects (Table 1) and did not block the protective effect of 11,12-EET with the exception of Met-enkephalin antiserum, which completely abolished the protective effect of 11,12-EET (Table 2 and Fig. 3). These results suggest that 11,12-EET is acting via the activation of δ- and/or κ-receptors and may be releasing the δ endogenous peptide Met-enkephalin to at least partially elicit its cardioprotective effect in the rat heart.
Isolated rat Langendorff experiments.
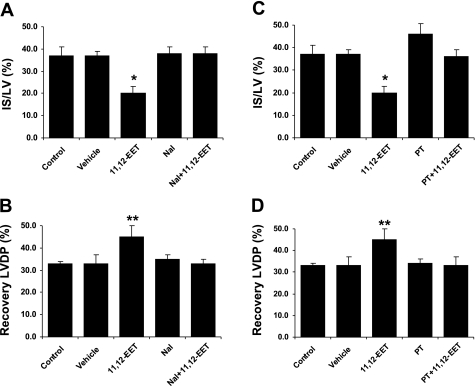
To further test the hypothesis that opioid receptors are mediating the cardioprotective effects of the EETs in rat hearts, we performed additional experiments in the isolated perfused rat heart at a constant perfusion pressure. In the first series, we perfused the hearts during ischemia and throughout reperfusion with 11,12-EET (1 μM) alone or in combination with the nonselective opioid antagonist naloxone (1 μM) and measured changes in left ventricular developed pressure (LVDP) and infarct size (IS/LV) as end points of cardioprotection. 11,12-EET produced an improvement in the recovery of LVDP (33 ± 4 to 45 ± 6%, P < 0.05) and produced a reduction in IS/LV (37 ± 2 to 20 ± 3%, P < 0.05). These beneficial effects were completely blocked by coinfusion with naloxone (Fig. 4), which indicates that an opioid receptor is involved in the isolated heart as well as in vivo. Because it is well-known that the δ-opioid receptor is Gi/o coupled, in the next series of experiments, we pretreated a group of rats with 10 μg/kg of pertussis toxin 48 h before heart isolation and then perfused the hearts with 11,12-EET. Similar to the naloxone experiments, the pertussis toxin completely blocked the cardioprotective effect of 11,12-EET (Fig. 4), which suggests that a Gi/o-coupled receptor is mediating the protective effect of 11,12-EET in the isolated rat heart, most likely the δ-opioid receptor.
Fig. 4.
Effect of pertussis toxin (PT), a Gi/o inhibitor, and naloxone, a nonselective opioid antagonist, on 11,12-EET-induced cardioprotection in the Langendorff perfused heart. 11,12-EET (1 μM) produced a significant reduction in left ventricular developed pressure (LVDP) and infarct size (IS/LV) (A) and a significant increase in the recovery of LVDP (B). Both of these effects were blocked by the concomitant administration with 3 μM naloxone or by pretreating hearts with pertussis toxin (10 μg/kg) for 48 h (C and D). All values are means ± SE (n = 6–8 rats/group). *P < 0.01 and **P < 0.001 vs. the control group.
DISCUSSION
The results of the present study clearly suggest that the cardioprotective effects of 11,12- and 14,15-EET are the result of activation of the δ-opioid receptor in the rat heart by the release of the δ-active peptide Met-enkephalin. There is also evidence that the κ-opioid receptor may mediate part of the EET effect, since the κ-opioid receptor antagonist nor-BNI blocked the cardioprotective effect. However, it is not clear how the κ-opioid receptor is involved, since the antiserum to the endogenous κ ligand dynorphin A-(1–17) did not block 11,12-EET-induced cardioprotection.
Interestingly, the present results have similarities to a recently published paper of Terashvilli et al. (24). These investigators reported that the antinociceptive effect of 14,15-EET was the result of activation of two opioid ligands and receptors, β-endorphin acting on the μ-opioid receptor and Met-enkephalin acting on the δ-opioid receptor. In the present study, the cardioprotective effect has a broader spectrum and includes both 11,12- and 14,15-EET. In addition, these two compounds did not appear to act through the μ-opioid receptor in the heart but primarily via the δ-opioid receptor since CTOP, a selective μ-receptor antagonist, was ineffective at blocking 11,12-EET-induced cardioprotection. Because the δ-opioid receptor is known to be a Gi/o protein-coupled receptor that we showed was sensitive to blockade by pertussis toxin further supports the importance of the δ-opioid receptor in mediating the cardioprotective effects of the EETs.
Recent work, including the present study, suggest that the EETs may be exerting actions on multiple receptor systems to mediate their numerous physiological effects such as vasodilation and cardioprotection (14). In one instance, Behm and coworkers (2), using tissue obtained from rats and mice, found that all EET and DHET regioisomers tested functioned as thromboxane (TP) receptor antagonists in rat aorta. Similar results were also observed in nonvascular tissue. Binding studies performed with 14,15-EET and the TP receptor antagonist SQ-29548 indicated that the EETs and DHETs were specifically binding to the TP receptor and produced vasodilation by this interesting mechanism.
A more recent paper published by Chaudhary et al. (5) is more closely related to the present study in several aspects and deals with mechanisms by which EETs produce cardioprotection. Chaudhary and coworkers tested the hypothesis that B-type natriuretic peptide (BNP) may be involved in EET-induced cardioprotection in mice with the sEH or Ephx2 gene knocked out (sEH null mice). In those experiments, the mouse hearts obtained from wild-type and sEH null mice were perfused in the Langendorff mode and subjected to 30 min of global no-flow ischemia and 40 min of reperfusion, and the recovery of contractile function (LVDP) was monitored as the end point for cardiac protection. In the sEH null perfused hearts, the significant increases in prepro-BNP mRNA and BNP protein were associated with a better recovery of LVDP during the 40 min of reperfusion than that observed in wild-type controls. Perfusion with the putative EET receptor antagonist 14,15-epoxyeicosa-5(Z)-enoic acid (EEZE) and the natriuretic peptide receptor type-A antagonist A-71915 partially prevented the beneficial effects observed in the sEH null mice and the beneficial effects produced by exogenously administered recombinant full-length mouse BNP(rBNP) and 11,12-EET. In addition, these investigators found that there was an increased expression of phosphorylated protein kinase Cϵ and protein kinase B (Akt) in wild-type mice perfused with rBNP or 11,12-EET and a decrease in total glycogen synthase kinase-3β in these same treated mice. Interestingly, wortmannin, a phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor, only blocked the beneficial effects of 11,12-EET but not rBNP. These data suggest that there are differences in the signaling pathways activated by these two systems. Based on the present results, opioids may be a strong candidate as the mediator of the BNP-insensitive component responsible for EET-induced cardioprotection in the mouse heart.
The fact that abolishing opioid signaling in the rat heart completely blocked EET-induced infarct size reduction suggests that a species difference may exist in the rat and mouse and that opioids play a greater role in the rat in terms of cardioprotection. It is also possible that changes in the recovery of contractile function and infarct size reduction may have divergent signaling pathways, although this has not been tested.
Although the present results strongly support a role for opioids in mediating the cardioprotective effects of EETs in the rat heart, the actual mechanism by which endogenous Met-enkephalin and possibly other opioid peptides mediate their effects remains unknown. It seems most likely to hypothesize that the EETs are producing effects on the synthesis or release of stored opioids from the heart, since there is a large amount of preproenkephalin (ppENK) in the rat myocardium (25). In fact, the left ventricular myocardium has the most ppENK mRNA than in any other tissues in the rat (25). As far as receptors are concerned, only κ and δ receptors are present in the intact adult rat heart, which agrees with our present findings (26) in which there was no role for the μ receptor in EET-induced cardioprotection.
That the EETs are acting to protect the heart via activation of a Gi/o-coupled receptor seems very likely based on the present study where we showed that pertussis toxin abolished the cardioprotective effects of 11,12-EET. The studies of Behm et al. (2) implicating TP receptors in the vasodilatory effects of the EETs, those of Chaudhary et al. (5) showing a role for a BNP receptor in mediating the cardioprotective effects in mice, and the present study implicating opioid receptors in mediating the anti-infarct effects of EETs all suggest that a Gi/o-coupled receptor or multiple Gi/o-coupled receptors are involved. Yang et al. (27) have recently characterized a radiolabeled EET agonist, 20-[125I]14,15-EE8ZE, that binds with high affinity to U937 membranes. This compound also stimulated cAMP production in U937 membranes and relaxed bovine coronary arteries, an effect blocked by 14,15-EEZE, the EET antagonist. These results led the investigators to suggest that there is a high abundance of EET binding sites in the U937 cell membrane and that is likely to be a G protein-coupled receptor, perhaps a Gs in this cell.
Another piece of evidence which suggests that EETs and opioids are acting on similar signaling pathways comes from the preconditioning (IPC) and postconditioning (POC) field. Many previous papers have shown that opioids are key mediators in both IPC and POC, and a very recent publication from our laboratory in dogs has shown that EETs are important mediators of both IPC and POC (12), similar to what has been shown previously with opioids (28, 29). Similarly, both EETs and opioids share similar signaling pathways such as Gi/o proteins (22), transactivation of epidermal growth factor receptor (9, 23), reactive oxygen species (10), the sarcolemmal ATP-activated K+ (KATP) channel (10), the mito KATP channel (3, 11, 19), and members of the reperfusion injury salvage kinase pathway such as PI 3-kinase (3, 7), Akt (3, 7), glycogen synthase kinase 3β (20, 21), and several members of the MAP kinase pathways (19). All of these pathways may converge on the mitochondrial permeability transition pore to inhibit its opening during reperfusion (20). Thus it is tempting to suggest that these two systems may share similar sites of action, perhaps via cross talk, since it has been clearly shown that opioids also cross talk with adenosine (6), another important trigger of IPC, POC, and β-adrenergic receptor signaling (18).
In summary, it is becoming more obvious that enhancing the EET system by numerous methods, including sEH inhibitors, overexpression of EETs in transgenic animals, or by exogenous administration of novel EET analogs, may be a new therapeutic target for reducing ischemia-reperfusion injury. A major advantage of this approach may be the broad spectrum of beneficial effects resulting from activating the EET system, which appears to turn on a number of cardioprotective and anti-inflammatory pathways such as the opioids, BNP, and antagonizing TP receptors. The further observation that the EETs mimic the effects of both IPC and POC adds further credence to the possibility that EET mimetics may lead to an important new treatment of patients with ischemic heart disease.
DISCLOSURES
No conflicts of interest are declared by the authors.
ACKNOWLEDGMENTS
This work was supported National Institutes of Health (NIH) Grants HL-08311 (G. J. Gross), HL-74314 (G. J. Gross), and HL-54075 (J. E. Baker). Partial support was provided by NIH Grant GM-31278 and the Robert Welch Foundation (J. R. Falck).
REFERENCES
- 1.Baker JE, Su Jidong Fu X, Hsu A, Gross GJ, Tweddell JS, Hogg N. Nitrite confers protection against myocardial infarction: role of xanthine oxidoreductase, NADPH oxidase and KATP channels. J Mol Cell Cardiol 43: 437–444, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behm DJ, Ogbonna A, Wu C, Burns-Kurtis CL, Douglas SA. Epoxyeicosatrienoic acids function as selective, endogenous antagonists of native thromboxane receptors: identification of a novel mechanism of vasodilation. J Pharmacol Exp Ther 328: 231–239, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Bodiga S, Zhang R, Jacobs DE, Larsen BT, Tampo A, Manthati VL, Kwok WM, Zeldin DC, Falck JR, Gutterman DD, Jacobs ER, Medhora MM. Protective actions of epoxyeicosatrienoic acid: Dual targeting of cardiovascular PI3K and KATP channels. J Mol Cell Cardiol 46: 978–988, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Z, Liu L, Van Winkle DM. Activation of δ- and κ-opioid receptors by opioid peptides protects cardiomyocytes via KATP channels. Am J Physiol Heart Circ Physiol 285: H1032–H1039, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chaudhary KR, Batchu SN, Das D, Suresh MR, Falck JR, Graves JP, Zeldin DC, Seubert JM. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated post-ischaemic recovery of heart contractile function. Cardiovasc Res 83: 362–370, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol 103: 203–215, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol 294: H724–H735, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellenberger EA, Lucas HL, Russo JM, Mueller JL, Barrington PL, Tseng LF, Quock RM. An opioid basis for early phase isoflurane-induced hypotension in rats. Life Sci 73: 2591–2602, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Forster K, Kuno A, Solenkova N, Felix SB, Kreig T. The δ-opioid receptor agonist DADLE at reperfusion protects the heart through activation of pro-survival kinases via EGF receptor transactivation. Am J Physiol Heart Circ Physiol 293: H1604–H1608, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Gross GJ, Hsu A, Nithipatikom K. Mechanisms by which epoxyeicosatrienoic acids (EETs) elicit cardioprotection in rat hearts. J Mol Cell Cardiol 42: 687–691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross GJ, Gauthier KM, Moore J, Falck JR, Hammock BD, Campbell WB, Nithipatikom K. Effects of the selective EET antagonist, 14,15-EEZE, on cardioprotection produced by exogenous or endogenous EETs in the canine heart. Am J Physiol Heart Circ Physiol 294: H2838–H2844, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gross GJ, Gauthier KM, Moore J, Campbell WB, Falck JR, Nithipatikom K. Evidence for a role of epoxyeicosatrienoic acids in mediating ischemic preconditioning and postconditioning in dog. Am J Physiol Heart Circ Physiol 297: H47–H52, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzume K, Wolff RA, Amakawa K, Kuzume K, Van Winkle DM. Sustained exogenous administration of Met5-enkephalin protects against infarction in vivo. Am J Physiol Heart Circ Physiol 285: H2463–H2470, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Lu T, Ye D, Wang X, Seubert JM, Graves JP, Bradbury JA, Zeldin DC, Lee HC. Cardiac and vascular KATP channels in rats are activated by endogenous epoxyeicosatrienoic acids through different mechanisms. J Physiol 575.2: 627–644, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, Alkayed NJ, Van Winkle DM. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol 295: H2128–H2134, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids in cardioprotection: ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol 291: H537–H542, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Peart JN, Gross GJ. Adenosine and opioid receptor-mediated cardioprotection in the rat: evidence for cross talk between receptors. Am J Physiol Heart Circ Physiol 285: H81–H89, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Pepe S, van den Brink OWV, Lakatta EG, Xiao RP. Cross-talk of opioid peptide receptor and β-adrenergic receptor signaling in the heart. Cardiovasc Res 63: 414–422, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res 95: 506–514, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, Goralski K, Carey MA, Luria A, Newman JW, Hammock BD, Falck JR, Roberts H, Rockman HA, Murphy E, Zeldin DC. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res 99: 442–450, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat 82: 50–59, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng JZ, Wong NS, Wang HX, Wong TM. Pertussis toxin, but not tyrosine kinase inhibitors, abolishes effects of U-50488H on [Ca+]i in myocytes. Am J Physiol Cell Physiol 272: C650–C654, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol 292: C996–C1012, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Terashvilli M, Tseng LF, Hsiang-en W, Narayanan J, Hart LM, Falck JR, Pratt PF, Harder DR. Antinciception produced by 14,15-epoxyeicosatrienoic acid is mediated by the activation of β-endorphin and met-enkephalin in the rat ventrolateral periaqeductal gray. J Pharmacol Exp Ther 326: 614–622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weil J, Eschenhagen T, Fliege G, Mittman C, Orthey E, Scholz H. Localization of preproenkephalin mRNA in rat heart: selective gene expression in left ventricular myocardium. Am J Physiol Heart Circ Physiol 275: H378–H385, 1998 [DOI] [PubMed] [Google Scholar]
- 26.Wittert G, Hope P, Pyle D. Tissue distribution of opioid gene expression in the rat. Biochem Biophys Res Commun 218: 877–881, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Yang W, Tuniki VR, Anjaiah S, Hillard CJ, Campbell WB. Characterization of epoxyeicosatrienoic acid binding site in U937 membranes using a novel radiolabeled agonist, 20-125I-14,15-epoxyeicosa-8Z-enoic acid. J Pharmacol Exp Ther 324: 1019–1027, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Younes A, Pepe S, Barron BA, Spurgeon HA, Lakatta EG, Caffrey JL. Cardiac synthesis, processing, and coronary release of enkephalin-related peptides. Am J Physiol Heart Circ Physiol 279: H1989–H11998, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Younes A, Pepe S, Yoshishige D, Caffrey JL, Lakatta EG. Ischemic preconditioning increases the bioavailability of cardiac enkephalins. Am J Physiol Heart Circ Physiol 289: H1652–H1661, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Zatta AJ, Kin H, Yoshishige D, Jiang R, Wang Reeves JG N, Mykytenko J, Guyton RA, Zhao ZQ, Caffrey JL, Vinten-Johansen J. Evidence that cardioprotection by postconditioning involves preservation of myocardial opioid content and selective opioid receptor activation. Am J Physiol Heart Circ Physiol 294: H1444–H1451, 2008 [DOI] [PubMed] [Google Scholar]