Abstract
We tested the hypothesis that physical activity can attenuate the temporal decline of ACh-induced endothelium-dependent relaxation during type 2 diabetes mellitus progression in the Otsuka Long-Evans Tokushima fatty (OLETF) rat. Sedentary OLETF rats exhibited decreased ACh-induced abdominal aortic endothelium-dependent relaxation from 13 to 20 wk of age (20–35%) and from 13 to 40 wk of age (35–50%). ACh-induced endothelium-dependent relaxation was maintained in the physically active OLETF group and control sedentary Long-Evans Tokushima Otsuka (LETO) group from 13 to 40 wk of age. Aortic pretreatment with NG-nitro-l-arginine (l-NNA), indomethacin (Indo), and l-NNA + Indo did not alter the temporal decline in ACh-induced endothelium-dependent relaxation. Temporal changes in the protein expression of SOD isoforms in the aortic endothelium or smooth muscle did not contribute to the temporal decline in ACh-induced endothelium-dependent relaxation in sedentary OLETF rats. A significant increase in the 40-wk-old sedentary LETO and physically active OLETF rat aortic phosphorylated endothelial nitric oxide (p-eNOS)-to-eNOS ratio was observed versus 13- and 20-wk-old rats in each group that was not seen in the 40- versus 13- and 20-wk-old sedentary OLETF rats. These results suggest that temporal changes in the antioxidant system, EDHF, and cycloxygenase metabolite production in sedentary OLETF rat aortas do not contribute to the temporal decline in sedentary OLETF rat aortic ACh-induced endothelium-dependent relaxation seen with type 2 diabetes mellitus progression. We also report that physical activity in conjunction with aging in the OLETF rat results in a temporal increase in the aortic endothelial p-eNOS-to-eNOS ratio that was not seen in sedentary OLETF rats. These results suggest that the sustained aortic ACh-induced endothelium-dependent relaxation in aged physically active OLETF rats may be the result of an increase in active aortic eNOS.
Keywords: superoxide dismutase, phosphorylated endothelial nitric oxide synthase, Otsuka Long-Evans Tokushima fatty rat
the incidence of type 2 diabetes mellitus (T2DM) is rising in parallel with the presently expanding worldwide obesity epidemic. According to the American Heart Association and Centers for Disease Control and Prevention, the diabetic and obese population in the United States has more than doubled in the last 15 yr (2, 3, 6). Macro- and microangiopathy are the putative primary causes of morbidity and mortality in subjects with T2DM, and loss of the regulatory role of the vascular endothelium is believed to be an initiating factor in the development of T2DM vascular disease (10). It is well documented that endothelial cell dysfunction occurs in T2DM, which is generally defined as a decrease in release of relaxing factors and/or an increase in release of constricting factors from the endothelium (for reviews, see Refs. 7, 16, and 46).
The Otsuka Long-Evans Tokushima fatty (OLETF) rat is a model of T2DM and obesity selectively bred to maintain a spontaneous mutation of the cholecystokinin-1 receptor, resulting in a within-meal feedback defect for satiety that consequently makes them hyperphagic. OLETF rats are known to spontaneously develop obesity, insulin resistance, hyperinsulinemia, hyperglycemia, dyslipidemia, and moderate hypertension (28, 29), all of which are also major risk factors for metabolic syndrome (15, 61) and could lead to the development of T2DM. OLETF rats are known to exhibit impaired ACh-induced endothelium-dependent relaxation (EDR) relative to their nonmutated controls [Long-Evans Tokushima Otsuka (LETO) rats] in the aorta (22, 25) and renal (25), mesenteric (33, 34), and basilar arteries (35). Previous OLETF vascular studies have reported improved ACh-induced EDR (31) and decreased superoxide formation (30) after aortic incubation with SOD1, one of the three SOD isoforms that scavenges superoxide (12). ACh-induced EDR in the aorta has also been shown to improve after incubation with a SOD1 mimetic in OLETF rats (35); however, SOD protein isoform expression in the vasculature of OLETF rat has not been examined. Impaired ACh-induced EDR in the OLETF rat aorta is thought to be independent of basal nitric oxide (NO) release and endothelial NO synthase (eNOS) protein expression as neither have been observed to differ between diabetic OLETF and nondiabetic control LETO rat aortas (31, 43). However, Matsumoto et al. (36) recently showed in mesenteric arteries that the Ser1177-phosphorylated eNOS (p-eNOS)-to-eNOS ratio (p-eNOS/eNOS ratio) was lower in OLETF rats than in LETO rats at 60–65 wk of age. Additionally, ACh-stimulated phosphorylation of eNOS at Ser1177 is significantly compromised and contributes in part to endothelial dysfunction in rodent models of T2DM (51, 59). However, it is not known whether there are temporal alterations in the OLETF aortic p-eNOS/eNOS ratio during T2DM progression.
The importance of a physically active lifestyle for maintaining endothelial health and the prevention of cardiovascular disease that accompanies T2DM is becoming established in humans (for reviews, see Refs. 5, 40, and 42). Physical activity has been shown to improve ACh-induced EDR in the thoracic aorta and mesenteric arteries from physically active OLETF rats compared with sedentary OLETF rats (43, 49), but observations were not made throughout the progression of T2DM. Little is also known about whether physical activity maintains the vascular SOD antioxidant system and the p-eNOS/eNOS ratio compared with a sedentary lifestyle during the progression of T2DM. Studies in aortas from diabetic and nondiabetic rodent models have shown increases in SOD isoform protein expression in response to exercise (9, 44), and regular physical activity/exercise is also known to be a powerful stimulus for the phosphorylation of eNOS in humans (17) and rodents (19, 60). A study (36) on OLETF rats has shown that the mesenteric artery p-eNOS/eNOS ratio is lower in OLETF rats versus LETO rats. However, in all these studies observations were made only at a single age and not throughout the progression of T2DM. In addition, studies using the OLETF rat, a T2DM rat model, to examine whether physical activity alone (i.e., voluntary running on running wheels) maintains the vascular SOD antioxidant system and p-eNOS/eNOS ratio during the progression of T2DM are nonexistent.
The primary aim of this study was to evaluate whether a primary preventative therapy such as regular physical activity can attenuate the temporal decline of ACh-induced EDR during the progression of T2DM. Our hypothesis was that a sedentary lifestyle contributes to the reduction in ACh-induced EDR in the sedentary OLETF aorta associated with T2DM disease progression due to a decreased bioavailability of NO resulting from the downregulated expression of endothelial SOD isoform(s) and a reduction in the p-eNOS/eNOS ratio. It was also hypothesized that physical activity would maintain ACh-induced EDR in part by temporally preserving endothelial SOD isoform(s) protein expression and the p-eNOS/eNOS ratio in the OLETF rat aorta.
METHODS
Animals and experimental design.
The animal protocol was approved by the Institutional Animal Care and Use Committee of the University of Missouri. OLETF and LETO male rats at 4 wk of age were kindly supplied by the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). Upon arrival, male OLETF and LETO rats were immediately assigned to either physically active or sedentary groups to be killed at 13, 20, or 40 wk of age. Nondiabetic LETO rats with functional cholecystokinin-1 receptor gene expression were used as controls for OLETF rats. Physically active OLETF groups were cage confined with access to voluntary running wheels equipped with a Sigma Sport BC 606 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA), and sedentary groups were cage confined without running wheel access. Voluntary wheel running was selected to simulate the more natural activity state and lifestyle of the animal. All rats had ad libitum access to water and rat chow (Formulab 5008, Purina Mills, St Louis, MO) and were housed 1 rat/cage in a room with a 06.00–18.00 h light:18.00–06.00 h dark cycle at 20–22°C, which was maintained throughout the experimental period.
At 13, 20, and 40 wk of age (n = 5–8 rats/group), rats were killed with running wheels locked 53 h (53WL) before death. Based on previous unpublished data from this laboratory, 53WL was not expected to alter the beneficial long-term effects of regular physical activity on aortic vasomotor function as the beneficial effects of physical activity on endothelial function have been shown to last up to 2 days after the cessation of regular physical activity in rats (18). Additionally, 0-h postphysical activity reveals a transient decrease in endothelial function (18), further supporting the rationale for the time point of 53WL. 53WL also permitted us to examine the effects of a regular physically active lifestyle and not the acute effects after a recent bout of physical activity. Additionally, rats were fasted 5 h before death for accurate serum measurements.
Rats were deeply anesthetized with pentobarbital sodium (100 mg/kg) for tissue removal and terminated via heart exsanguination. Before heart exsanguination, blood was collected using a hypodermic syringe for the determination of plasma glucose and insulin levels to evaluate the maintenance of glycemic control in all rats at each experimental time point. Rat body weights were measured before anesthesia, and wet weights of the hearts were taken after exsanguinations using a standard laboratory balance. Heart weight-to-body weight ratios were determined as a measure of the extent of physical activity as well as citrate synthase activity (CSA) in the red portion of the gastrocnemius muscle (methods described below).
Dual-energy X-ray absorptiometry.
Whole body composition was measured using a Hologic QDR-1000/w dual-energy X-ray absorptiometry machine calibrated for rats while rats were under anesthesia, as performed previously at this institution (32).
Assessment of vascular function.
The vessel functional experimental protocols described here and some drug concentrations are similar to previous experiments conducted in this laboratory (23). To evaluate changes in the temporal profile of vascular function during T2DM disease progression in the OLETF rat, at 13, 20, and 40 wk of age the abdominal aortas were dissected and cleaned of connective and adipose tissue after rats had been killed via heart exsanguination. The abdominal aorta was then cut into 3-to 4-mm-long vessel rings with the first ring being cut at the distal abdominal aorta; from there, the remaining rings were cut moving proximal up the vessel. Cut rings were photographed on an Olympus video microscope for ring morphological characteristic measurements using Image-J software. Rings were then mounted on wire feet connected to isometric force transducers and submerged in 20-ml water baths containing physiological Krebs solution maintained at 37°C for 1 h to allow for equilibration. Aortic rings were stretched to a length that produced maximal force stimulated by 60 mM KCl. Once that length was set, two separate maximal constrictions induced by 80 mM KCl were conducted on all rings to analyze vessel contractility. Aortic vasomotor function was investigated with cumulative concentration-response curves of vasoactive agents conducted in the following order: ACh (half log-concentration increments ranging from 1 e−10 to 1 e−4 M) and sodium nitroprusside (SNP; whole log-concentration increments ranging from 1 e−10 to 1 e−4 M). A submaximal concentration of phenylephrine (3 e−7 M) was used to preconstrict all vessels before ACh and SNP relaxation curves. We used a pharmacological approach to examine the contribution of different endothelial signaling pathways in EDR. We used NG-nitro-l-arginine (l-NNA) to inhibit eNOS, the enzyme that produces NO. We used indomethacin (Indo) to inhibit cyclooxygenase (COX), the enzyme responsible for prostacyclin and prostaglandin production. We used combined treatment with l-NNA and Indo to block both COX and NOS, leaving only the non-NOS, non-COX pathways to induce EDR (likely EDHF). All cumulative concentration-response curves were conducted on four aortic rings that were pretreated as follows: control/untreated (in Krebs solutions only), 20-min preincubation with l-NNA (3 e−4 M in Krebs solution), 20-min preincubation with Indo (5 e−6 M in Krebs solution), and 20-min preincubation with both Indo and l-NNA (in Krebs solution).
Immunoblots.
After exsanguination, the thoracic aorta was dissected and stripped of connective and adipose tissue. The aorta was opened from end to end using a scalpel, and endothelial cells were scraped off into ice-cold Laemmli buffer and stored at 80°C for SOD1, SOD2, SOD3, eNOS, and Ser1177 p-eNOS immunoblot analysis. Segments of the thoracic aorta that had been scraped free of endothelial cells were also stored in Laemmli buffer at −80°C for future vascular smooth muscle cell SOD1, SOD2, eNOS, and p-eNOS immunoblot analysis. Immunoblot analysis for protein expression in vascular smooth muscle and endothelial cells was done following methods used previously in this laboratory (39). Briefly, a NanoOrange protein assay was used to determine the sample protein content (in μg/μl) using a Tecan Safire Plate reader (model A-5082). Samples containing equal amounts of aortic proteins (10 μg) were loaded onto a 15-well 4–12% bis-Tris gel, subjected to electrophoresis, and then transferred to a polyvinylidene difluoride membrane. Membranes were blocked in a 5% nonfat milk-Tris-buffered saline-Tween 20 (TBST) solution and incubated overnight with a primary antibody against SOD1 (19 kDa, 1:5,000), SOD2 (25 kDa, 1:1,000), SOD3 (35 kDa, 1:500), eNOS (140 kDa, 1:1,000), or p-eNOS (140 kDa, 1:250). Next, membranes were incubated with a horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibody in a 5% nonfat milk-TBST solution. For the analysis of p-eNOS expression, the detection of phosphoprotein was followed by membrane stripping in stripping buffer at 50°C for 30 min followed by the detection of total eNOS expression. Blots for all proteins were then incubated in Pierce SuperSignal West Dura visualization reagent for 5 min, and a Kodak image station (4000R) was used to visualize and quantify protein band densities. All Western blot data were normalized to 13-wk values, allowing us to compare temporal changes in vascular protein. Data were not obtained regarding SOD3 protein expression because SOD3 antibodies did not detect any protein. This is consistent with reports (4, 27) showing that SOD3 is virtually undetectable and/or absent in rat uterine, lung, and vascular tissue.
Citrate synthase activity.
The red portion of the gastrocnemius muscle was dissected and frozen in liquid nitrogen to be stored at −80°C for an assay of CSA as a measure of the adaptive response to physical activity on skeletal muscle oxidative capacity, as previously described by this laboratory (39) using the methods of Srere (50).
Serum and plasma NO metabolite measurements.
Serum glucose (Sigma), triglycerides (Sigma), and insulin (Linco Research) were measured using commercially available kits according to the manufacturer's instructions. Hemoglobin A1c (HbA1c) concentrations were determined as previously described by our group (32). The plasma NO metabolite (nitrite + nitrate; NOx) concentration was determined via VCl3-induced reduction of NOx to NO and its subsequent reaction with O3, producing chemiluminescence (model NOA 280i, Sievers) as has been previously done in this laboratory (41).
Chemicals, solutions, and drugs.
The Krebs-bicarbonate buffer solution contained (in mM) 131.5 NaCl, 5.0 KCl, 1.2 NaH2PO4, 1.2 MgCl2, 2.5 CaCl2, 11.2 glucose, 20.8 NaHCO3, 0.003 propranolol, and 0.025 EDTA. The solution was aerated with 95% O2-5% CO2 (pH 7.4) and maintained at 37°C. The stripping buffer for immunoblots contained 62.5 mM Tris base, 2% SDS, and 100 mM 2-mercaptoethanol (pH 6.7). ACh, SNP, l-NNA, and Indo were purchased from Sigma, and all other chemicals were purchased from Sigma or Fisher Scientific. All equipment used for gels and immunoblot transfer was purchased from Invitrogen. Polyclonal antibodies for SOD1, SOD2, and SOD3 were purchased from Stressgen, and eNOS and p-eNOS were purchased from BD Transduction. Anti-rabbit and anti-mouse secondary antibodies were purchased from Sigma and GE Healthcare, respectively. SuperSignal visualization reagent was purchased from Pierce ThermoScientific.
Statistics.
Differences between groups regarding immunoblot data, logEC50 values, serum and plasma measurements, heart weight, body weight, heart weight-to-body weight ratio, percent body fat, CSA, food consumption, and ring characteristics were determined via one-way ANOVA using GraphPad Prism version 5.0a. Significant main effects (P < 0.05) were followed up with Fisher least-significant-difference post hoc comparisons.
The analysis of concentration-response curves was performed using the Mixed procedure in SAS version 9 to allow for heterogeneous variances across doses. The statistical model used was a two-factor study with age being a between-subjects factor and drug concentration being a within-subjects factor. Residual plots were examined to check on the assumption of normally distributed error terms. There were some instances of convergence problems when running the mixed model for certain combinations of group and treatment. In these cases, a series of nonparametric analyses was run to look for differences in age groups. Specifically, the Kruskal-Wallis test was used at each concentration level separately. When results were significant, pairwise comparisons were performed to determine which age groups significantly differed from others. Given the large number of tests that were ran, the false discovery rate adjustment for multiple tests was used. Differences with false discovery rate-adjusted P values of <0.05 (P < 0.05) were considered significant.
RESULTS
Animal characteristics.
Animal characteristics are shown in Table 1. Consistent with our previous reports (32, 47), the average daily voluntary running distance in the physically active OLETF rats was ∼9 km/day at 13 wk, ∼7 km/day at 20 wk, and ∼4 km/day at 40 wk (data not shown). Body masses and body fat percentages were significantly greater in sedentary OLETF animals compared with sedentary LETO rats and physically active OLETF rats at all ages studied. A higher heart weight-to-body weight ratio, a marker of exercise training, was found higher in physically active OLETF rats at all ages compared with sedentary LETO rats and sedentary OLETF rats. In addition, CSA in the red gastrocnemius muscle increased in all groups from 13 to 20 wk but returned back to 13-wk levels by 40 wk and was significantly greater in physically active OLETF rats compared with sedentary OLETF rats in only the 20-wk-old group. Average food consumption per week relative to body weight was significantly greater in physically active OLETF rats compared with sedentary LETO rats at each age and significantly greater than sedentary OLETF rats at 13 and 20 wk, suggesting that observations in the hyperphagic physically active OLETF rats are not due to the consumption of less calories.
Table 1.
Animal characteristics
| Percent Body Fat | Body Weight, g | Heart Weight-to-Body Weight Ratio, mg/g | Red Gastrocnemius Muscle Citrate Synthase Activity | Food Consumption, g·wk−1·g body wt−1 | |
|---|---|---|---|---|---|
| Sedentary LETO rats | |||||
| 13 wk old | 10.0 ± 0.5 | 380.8 ± 5.9 | 3.4 ± 0.1 | 207.1 ± 20.6 | 0.36 ± 0.01 |
| 20 wk old | 15.5 ± 1.1* | 477.9 ± 6.6* | 2.9 ± 0.1* | 270.8 ± 31.2* | 0.33 ± 0.01 |
| 40 wk old | 22.4 ± 1.3*§ | 557.4 ± 15*§ | 2.6 ± 0.1*§ | 160.6 ± 26.4§ | 0.30 ± 0.01* |
| Sedentary OLETF rats | |||||
| 13 wk old | 21.7 ± 1.8†‡ | 495.4 ± 15.7†‡ | 3.1 ± 0.1‡ | 157.9 ± 7.9 | 0.38 ± 0.01‡ |
| 20 wk old | 30.4 ± 0.8*†‡ | 607.0 ± 10.5*†‡ | 2.7 ± 0.07*‡ | 253.4 ± 20.7*‡ | 0.35 ± 0.01‡ |
| 40 wk old | 30 ± 4.0*†‡ | 685.2 ± 34.6*§†‡ | 2.7 ± 0.1*‡ | 112.3 ± 10.7§ | 0.47 ± 0.05*†§ |
| Physically active OLETF rats | |||||
| 13 wk old | 7.4 ± 0.6 | 368.0 ± 10.8 | 4.0 ± 0.1† | 200.6 ± 17.3 | 0.55 ± 0.02† |
| 20 wk old | 11.4 ± 1.9* | 434.7 ± 10.5* | 3.6 ± 0.1*† | 334.7 ± 32.3*† | 0.52 ± 0.02† |
| 40 wk old | 17.3 ± 1.9*§ | 544.5 ± 15.8*§ | 3.5 ± 0.1*† | 158.5 ± 17.6§ | 0.43 ± 0.01†§ |
Values are means ± SE; n = 5–8 rats/group. Values were obtained from 5-h fasted rats. LETO rats, Long-Evans Tokushima Otsuka rats; OLETF rats, Otsuka Long-Evans Tokushima fatty rats. Significant differences between groups or ages are denoted with the following symbols:
P < 0.05 vs. 13 wk within the respective group,
P < 0.05 vs. sedentary LETO rats at the respective age,
P < 0.05 vs. physically active OLETF rats at the respective age, and
P < 0.05 vs. 20 wk within the respective group.
Serum and plasma NOx measurements.
Serum and plasma NOx measurements are shown in Table 2. Sedentary OLETF rats displayed insulin resistance by 13 and 20 wk of age, as shown by elevated levels of postprandial serum glucose at 13 wk and serum insulin at 20 wk compared with physically active OLETF rats and sedentary LETO rats. By 40 wk, sedentary OLETF animals developed overt T2DM with a 60% drop in serum insulin and an approximately twofold increase in HbA1c, whereas physically active OLETF rats maintained normal glycemic control to the level of nonobese, nonhyperphagic sedentary LETO rats at all ages examined. In addition, daily voluntary wheel running maintained serum triglyceride levels in physically active OLETF rats to the level of sedentary LETO rats, which was significantly reduced compared with sedentary OLETF rats at 13, 20, and 40 wk of age. The serum glucose, HbA1c, insulin, and triglyceride data collectively illustrate the importance of regular physical activity in the maintenance of glycemic control and circulating lipid levels in these hyperphagic rats. Additionally, the percent body fat data in conjunction with serum glucose, HbA1c, insulin, and triglyceride data further illustrate the importance of regular physical activity in the prevention of metabolic syndrome in these hyperphagic rats.
Table 2.
Serum and plasma measurements
| Glucose, mg/dl | Hemoglobin A1c, % | Insulin, ng/ml | Triglycerides, mg/dl | NOx, mM/ml Media | |
|---|---|---|---|---|---|
| Sedentary LETO rats | |||||
| 13 wk old | 268.3 ± 26.1 | 4.7 ± 0.1 | 8.4 ± 1.1 | 36.8 ± 4.7 | 12.8 ± 1.3 |
| 20 wk old | 331.2 ± 37.9 | 4.6 ± 0.1 | 9.0 ± 0.8 | 42.7 ± 4.3 | 12.2 ± 1.4 |
| 40 wk old | 261.3 ± 20.1 | 4.6 ± 0.1 | 10.7 ± 0.7 | 42.3 ± 3.1 | 10.4 ± 0.7 |
| Sedentary OLETF rats | |||||
| 13 wk old | 393.5 ± 34.7†‡ | 5.4 ± 0.1†‡ | 10.4 ± 1.2 | 114.7 ± 18.2†‡ | 7.5 ± 0.8† |
| 20 wk old | 541.5 ± 57.7*†‡ | 5.3 ± 0.1†‡ | 12.5 ± 0.7†‡ | 177.1 ± 24.8*† | 7.3 ± 1.0† |
| 40 wk old | 665.9 ± 41.8*†‡§ | 8.8 ± 0.7*†‡§ | 5.0 ± 1.2*†‡§ | 253.4 ± 32.8*†‡§ | 6.4 ± 0.3†‡ |
| Physically active OLETF rats | |||||
| 13 wk old | 256.8 ± 26.3 | 4.8 ± 0.1 | 8.3 ± 1.4 | 52.7 ± 5.7 | 5.4 ± 0.5† |
| 20 wk old | 380.3 ± 52.7 | 4.7 ± 0.1 | 9.6 ± 1.1 | 71.3 ± 10.8 | 6.1 ± 0.8† |
| 40 wk old | 301.5 ± 16.6 | 4.8 ± 0.1 | 11.7 ± 0.8 | 81.0 ± 9.7 | 10.3 ± 0.4*§ |
Values are means ± SE; n = 5–8 rats/group for serum measurements and 4 rats/group for plasma nitric oxide metabolite (NOx) measurements. Values were obtained from 5-h fasted rats. Significant differences between groups or ages are denoted with the following symbols:
P < 0.05 vs. 13 wk within the respective group,
P < 0.05 vs. sedentary LETO rats at the respective age,
P < 0.05 vs. physically active OLETF rats at the respective age, and
P < 0.05 vs. 20 wk within the respective group.
Significant temporal differences in plasma NOx were only observed in the physically active OLETF group, where NOx was significantly higher in 40-wk-old (10.3 ± 0.4 nM/ml media) compared with 13-wk-old (5.4 ± 0.5 nM/ml media) and 20-wk-old (7.3 ± 1.0 nM/ml media) physically active OLETF rats. Within-group comparisons of plasma NOx revealed that control LETO rats had significantly higher NOx plasma levels than sedentary OLETF rats at each age and significantly higher plasma NOx levels than physically active OLETF rats at 13 and 20 wk of age. Physically active OLETF rat plasma NOx levels returned to control LETO rat levels by 40 wk. Physically active OLETF rats at 40 wk (10.3 ± 0.4 nM/ml media) had significantly higher NOx levels than sedentary OLETF rats at 40 wk (6.4 ± 0.3nM/ml media).
Vessel characteristics.
No differences were found in vessel characteristics (outer diameter, inner diameter, vessel length, and preconstriction induced by phenylephrine; data not shown) between rat groups at each age or between ages in each rat group. In addition, no differences were found in specific tension responses elicited by 80 mM KCl between ages in each rat group or between rat groups at each age except that 13-wk-old physically active OLETF rats (0.8 ± 0.13 g/mm2) had significantly (P < 0.05) less specific tension than 13-wk-old sedentary LETO rats (1.4 ± 0.6 g/mm2).
Relaxation responses to ACh.
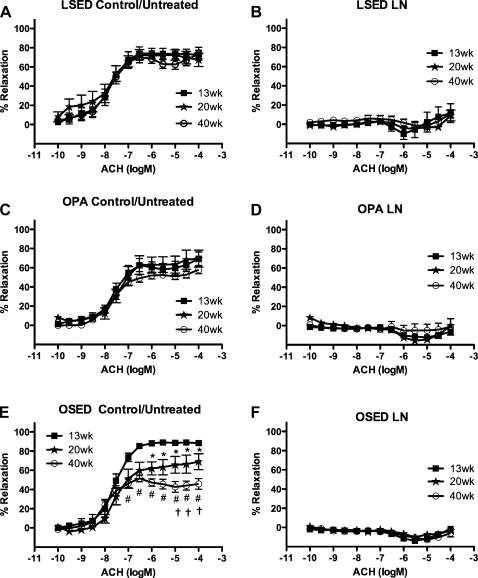
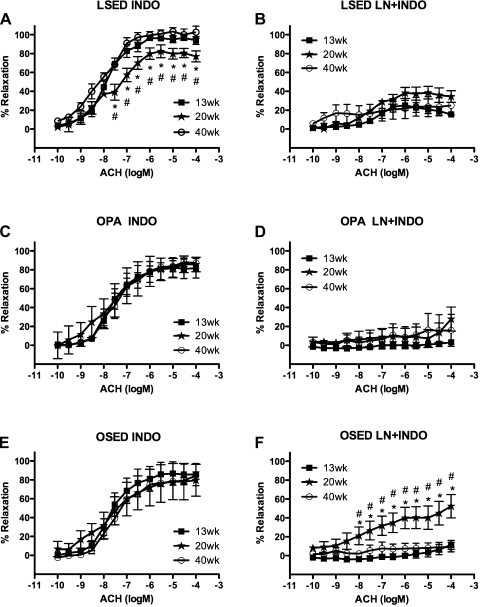
Temporal alterations in ACh-induced aortic responses of sedentary LETO rats or physically active OLETF rats were not observed among 13-, 20-, and 40-wk-old sedentary LETO rat or 13-, 20-, and 40-wk-old physically active OLETF rat control/untreated aortic rings (Fig. 1, A and C). In contrast, rat aortic rings from 13 to 20 wk in sedentary OLETF rats exhibited a significant decrease (20–25%) of the relaxation response to ACh (1 e−6–1 e−4 M), and this response continued to decrease (35–50%) from 13 to 40 wk (1 e−7–1 e−4 M) in sedentary OLETF rat aortic rings (Fig. 1E). Pretreatment with an inhibitor of eNOS, l-NNA, blocked the aortic ring relaxation responses to ACh at each age equally in each rat group (Fig. 1, B, D, and F). Pretreatment with Indo, an inhibitor of the enzyme COX that is responsible for prostacyclin and prostaglandin production, revealed that 20-wk-old sedentary LETO rat aortic rings exhibited significantly less ACh relaxation than 13- and 40-wk-old sedentary LETO rat aortic rings (3 e−8–1 e−4 M; Fig. 2A). No significant differences in Indo-pretreated aortic ring relaxation responses to ACh were observed among 13-, 20-, and 40-wk-old physically active OLETF rats (Fig. 2C) or 13-, 20-, and 40-wk-old sedentary OLETF rats (Fig. 2E). Combined pretreatment of aortic rings with l-NNA and Indo did not result in any significant differences among 13-, 20-, and 40-wk-old sedentary LETO rats (Fig. 2B) or 13-, 20-, and 40-wk-old physically active OLETF rats (Fig. 2D). Pretreatment of 20-wk-old sedentary OLETF rat aortic rings with l-NNA + Indo failed to completely block ACh relaxation compared with aortic rings from 13- and 40-wk-old sedentary OLETF rats (1 e−8–1 e−4 M; Fig. 2F), suggesting the presence of non-NOS-, non-COX-induced relaxation. In aortic rings that had been denuded, meaning that the endothelial cell layer was scraped off with forceps (n = 3–4), ACh-induced aortic ring relaxation responses were equally abolished at 13, 20, and 40 wk within each rat group, suggesting that the denuded rings were essentially free of endothelial cells (data not shown). Additionally, no differences were found among the logEC50 values at each age within each rat group for the ACh concentration-response curves in the presence or absence of inhibitors (data not shown).
Fig. 1.
Concentration-response curves for the ACh-induced relaxation of isolated abdominal aortic rings obtained from sedentary Long-Evans Tokushima Otsuka sedentary (LETO) rats (LSED), physically active Otsuka Long-Evans Tokushima fatty (OLETF) rats (OPA), and sedentary OLETF rats (OSED) at 13, 20, and 40 wk of age. Data were obtained in the absence (A, C, and E) or presence (B, D, and F) of NG-nitro-l-arginine [L-NNA (LN); 3 e−4 M]. See methods for more details. Data are means ± SE; n = 6–8 rats per group or treatment combination. *P < 0.05, 20 vs. 13 wk; #P < 0.05, 40 vs. 13 wk; †P < 0.05, 40 vs. 20 wk.
Fig. 2.
Concentration-response curves for the ACh-induced relaxation of isolated abdominal aortic rings obtained from LSED, OPA, and OSED rats at 13, 20, and 40 wk of age. Data were obtained in the presence of indomethacin (Indo; 5 e−6 M; A, C, and E) or combined l-NNA + Indo (B, D, and F). See methods for more details. Data are means ± SE; n = 6–8 rats per group or treatment combination. *P < 0.05, 20 vs. 13 wk; #P < 0.05, 20 vs. 40 wk.
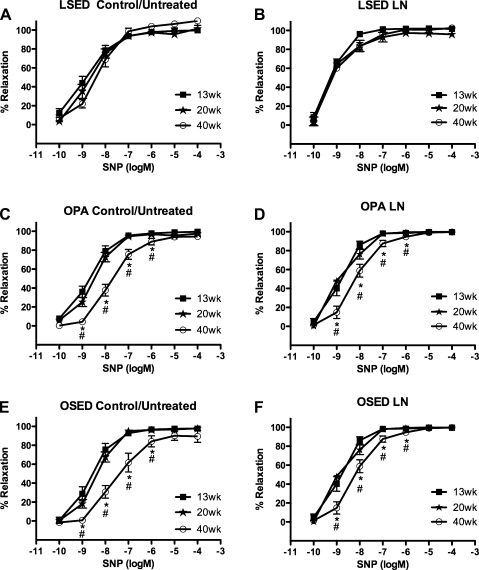
Relaxation responses to SNP.
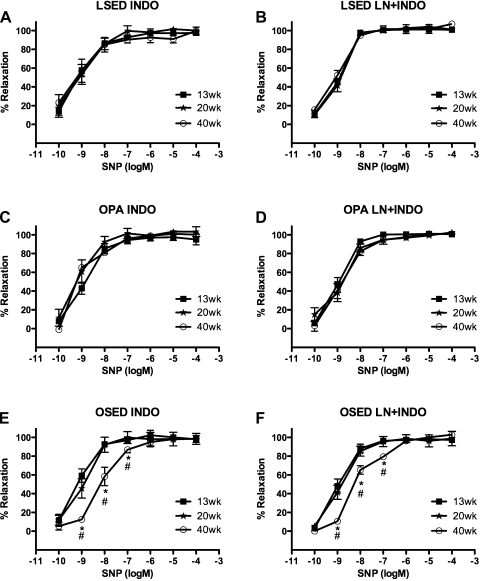
No significant differences in the relaxation response to SNP were observed among 13-, 20-, and 40-wk-old sedentary LETO rat control/untreated aortic rings (Fig. 3A). In contrast, physically active OLETF rat control/untreated aortic rings at 40 wk (−7.75 ± 0.14 logEC50) showed decreased sensitivity to SNP, as demonstrated by a significant rightward shift in the SNP concentration-response curve compared with those of 13-wk-old (−8.65 ± 0.15 logEC50) and 20-wk-old (−8.48 ± 0.11 logEC50) physically active OLETF rats (Fig. 3C). Sedentary OLETF rat control/untreated aortic rings at 40 wk (−7.60 ± 0.15 logEC50) also demonstrated a significant rightward shift in the SNP concentration-response curve compared with those of 13-wk-old (−8.61 ± 0.19 logEC50) and 20-wk-old (−8.37 ± 0.11 logEC50) sedentary OLETF rats (1 e−9–1 e−6 M; Fig. 3E). Pretreatment with l-NNA did not affect SNP-induced relaxation responses in the 13-, 20-, and 40-wk-old LETO rat groups (Fig. 3B). The rightward shift in SNP-induced relaxation responses persisted after pretreatment with l-NNA at 40 wk (−8.23 ± 0.15 logEC50) compared with 13-wk (−8.88 ± 0.15 logEC50) and 20-wk (−8.90 ± 0.13 logEC50) responses in physically active OLETF rats (Fig. 3C). Similarly, in sedentary OLETF rats, the rightward shift in SNP-induced relaxation responses persisted after pretreatment with l-NNA at 40 wk (−7.70 ± 0.06 logEC50) compared with 13-wk (−8.82 ± 0.09 logEC50) and 20-wk (−8.49 ± 0.19 logEC50) sedentary OLETF responses (Fig. 3D). There were no significant differences among 13-, 20-, and 40-wk sedentary LETO rat aortic responses (Fig. 4A) or 13-, 20-, and 40-wk physically active OLETF rat aortic responses to SNP after treatment with Indo (Fig. 4C). These results in physically active OLETF aortas suggest that Indo abolished the rightward shift in SNP relaxation responses in 40-wk-old physically active OLETF rat aortic rings. Sedentary OLETF rat aortic rings pretreated with Indo continued to exhibit a significant rightward shift in the SNP dose-response relaxation curve at 40 wk (−8.13 ± 0.15 logEC50) compared with 13-wk-old (−9.29 ± 0.16 logEC50) and 20-wk-old (−9.15 ± 38 logEC50) sedentary OLETF rats (Fig. 4E). Combined pretreatment of aortic rings with l-NNA + Indo had effects similar to the effects of Indo pretreatment alone in control LETO rats and physically active OLETF rats (Fig. 4, B and D). Sedentary OLETF rat aortic responses to SNP after combined pretreatment of aortic rings with l-NNA + Indo continued to result in a significant rightward shift in 40-wk-old (−8.24 ± 0.07 logEC50) sedentary OLETF rat responses compared with 13-wk-old (−9.10 ± 0.18 logEC50) and 20-wk-old (−8.94 ± 0.14 logEC50) sedentary OLETF rat responses. In aortic rings that had been denuded (n = 3–4), SNP-induced aortic ring relaxation responses were equally unaffected by denudation at 13, 20, and 40 wk within each rat group (data not shown). Unless otherwise noted, no differences were found between logEC50 values at each age within each rat group for the SNP concentration-response curves in the presence or absence of inhibitors (data not shown).
Fig. 3.
Concentration-response curves for the sodium nitroprusside (SNP)-induced relaxation of isolated abdominal aortic rings obtained from LSED, OPA, and OSED rats at 13, 20, and 40 wk of age. Data were obtained in the absence (A, C, and E) or presence (B, D, and F) of l-NNA (3 e−4 M). See methods for more details. Data are means ± SE; n = 6–8 rats per group or treatment combination. *P < 0.05, 40 vs. 13 wk; #P < 0.05, 40 vs. 20 wk.
Fig. 4.
Concentration-response curves for the SNP-induced relaxation of isolated abdominal aortic rings obtained from LSED, OPA, and OSED rats at 13, 20, and 40 wk of age. Data were obtained in the presence of Indo (5 e−6 M; A, C, and E) or combined l-NNA + Indo (B, D, and F). See methods for more details. Data are means ± SE; n = 6–8 rats per group or treatment combination. *P < 0.05, 40 vs. 13 wk; #P < 0.05, 40 vs. 20 wk.
Aortic vascular smooth muscle cell protein expression of SOD1 and SOD2.
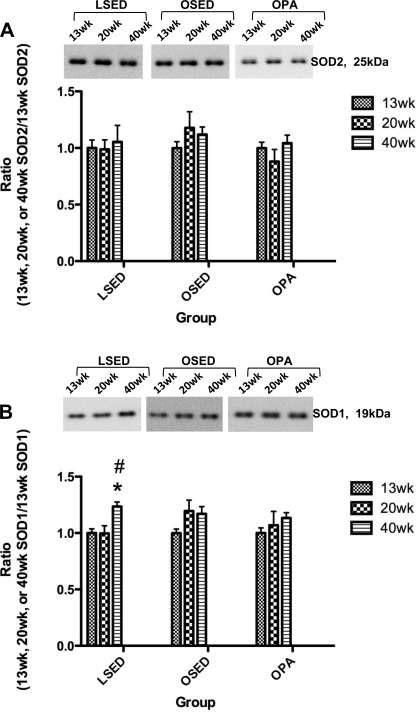
Immunoblot analysis revealed no significant differences among 13-, 20-, and 40-wk thoracic aorta smooth muscle SOD2 protein expression in any rat group (Fig. 5A). SOD1 thoracic aorta smooth muscle protein expression in 40-wk-old sedentary LETO rats was higher compared with 13- and 20-wk-old sedentary LETO animals (Fig. 5B), but no differences among 13-, 20-, and 40-wk-old sedentary OLETF rats or 13-, 20-, and 40-wk-old physically active OLETF thoracic aorta smooth muscle SOD1 protein levels were observed (Fig. 5B).
Fig. 5.
Analysis of SOD2 (A) and SOD1 (B) protein expression in thoracic aorta segments scraped free of endothelial cells obtained from LSED, OSED, and OPA rats at 13, 20, and 40 wk of age. Top: representative Western blots for SOD2 and SOD1 in each rat group at each age. Bottom: bands were quantified as described in methods. Ratios were calculated for the optical density of each SOD isoform at 13, 20, or 40 wk of age over that of 13-wk-old rats in each respective group. Values are means ± SE of 6–8 determinations. *P < 0.05 vs. 13 wk in each respective group; #P < 0.05 vs. 20 wk in each respective group.
Aortic endothelial cell protein expression of SOD1, SOD2, eNOS, and p-eNOS.
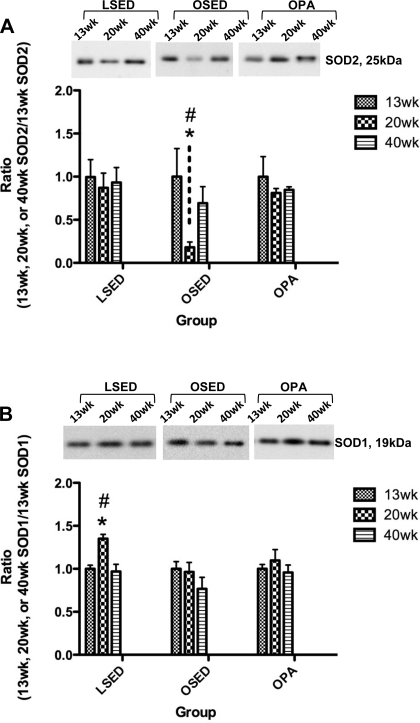
Immunoblot analysis revealed no significant differences among 13-, 20-, and 40-wk-old thoracic aorta endothelial SOD2 protein expression in sedentary LETO and physically active OLETF rat groups (Fig. 6A). Thoracic aorta endothelial SOD2 protein levels in 20-wk-old sedentary OLETF rats were significantly lower compared with 13- and 40-wk-old sedentary OLETF rats (Fig. 6A).
Fig. 6.
Analysis of SOD2 (A) and SOD1 (B) protein expression in endothelial cells scraped from segments of the thoracic aorta obtained from LSED, OSED, and OPA rats at 13, 20, and 40 wk of age. Top: representative Western blots for SOD2 and SOD1 in each rat group at each age. Bottom: bands were quantified as described in methods. Ratios were calculated for the optical density of each SOD isoform at 13, 20, or 40 wk of age over that of 13-wk-old rats in each respective group. Values are means ± SE of 6–8 determinations. *P < 0.05 vs. 13 wk in each respective group; #P < 0.05 vs. 40 wk in each respective group.
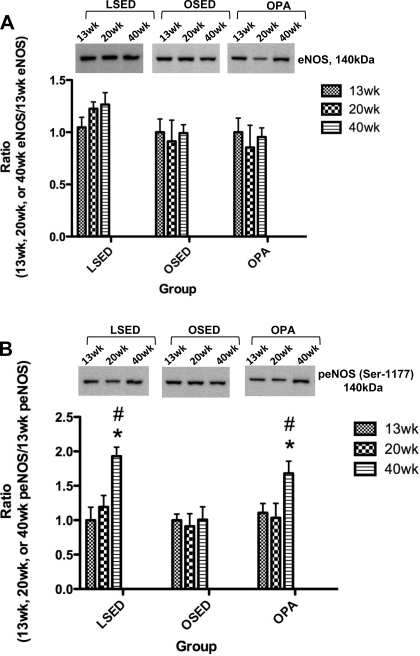
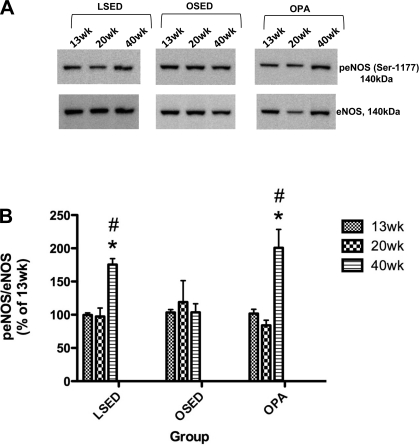
Thoracic aorta endothelial SOD1 protein expression in 20-wk-old sedentary LETO rats was significantly higher than in 13- and 40-wk-old sedentary LETO animals, whereas no significant differences among 13-, 20-, and 40-wk thoracic aorta endothelial SOD1 protein expression in sedentary OLETF and physically active OLETF rat groups were observed (Fig. 6B). Thoracic aorta endothelial eNOS protein expression was not significantly different among 13-, 20-, and 40-wk-old rats within any group (Fig. 7A). Thoracic aorta endothelial protein levels of p-eNOS (Ser1177) at 40 wk was significantly greater compared with 13- and 20-wk-old rats in the sedentary LETO and physically active OLETF rat groups; however, no differences were found in sedentary OLETF rat thoracic aorta endothelial p-eNOS (Ser1177) expression at 13, 20, and 40 wk (Fig. 7B). Examination of the p-eNOS/eNOS ratios shown in Fig. 8B showed that relative to the total pool of available eNOS in the thoracic aorta endothelium, the percentage of p-eNOS at Ser1177 was significantly greater in 40-wk-old rats compared with 13- and 20-wk-old rats in the sedentary LETO and physically active OLETF rat groups. Concurrent with this temporal increase in p-eNOS at Ser1177 in 40-wk-old physically active OLETF rats was a significantly augmented plasma NOx concentration in 40-wk-old physically active OLETF rats compared with 13- and 20-wk-old physically active OLETF rats (Table 2). This temporal increase in plasma NOx was not observed in sedentary LETO or sedentary OLETF groups (Table 2) and demonstrates the importance of physical activity as a stimulus to return plasma NOx levels in OLETF rats to levels similar to those of LETO control rats.
Fig. 7.
Analysis of endothelial nitric oxide synthase (eNOS; A) and phosphorylated eNOS (p-eNOS; Ser1177; B) protein expression in endothelial cells scraped from segments of the thoracic aorta obtained from LSED, OSED, and OPA rats at 13, 20, and 40 wk of age. Top: representative Western blots for eNOS and p-eNOS in each rat group at each age. Bottom: bands were quantified as described in methods section. Ratios were calculated for the optical density of eNOS or p-eNOS at 13, 20, or 40 wk over that of 13-wk-old rats in each respective group. Values are means ± SE of 6–8 determinations. *P < 0.05 vs. 13 wk in each respective group; #P < 0.05 vs. 20 wk in each respective group.
Fig. 8.
A: representative Western immunoblots of p-eNOS (Ser1177; top) and eNOS (bottom) protein expression in endothelial cells scraped from segments of the thoracic aorta obtained from LSED, OSED, and OPA rats at 13, 20, and 40 wk of age. B: analysis of eNOS and p-eNOS (Ser1177) protein expression in endothelial cells scraped from segments of the thoracic aorta. Ratios were calculated for the optical density of eNOS or p-eNOS at 13, 20, or 40 wk over that of 13-wk-old rats in each respective group and are expressed as the ratio of p-eNOS to eNOS. Values are means ± SE of 6–8 determinations. *P < 0.05 vs. 13 wk in each respective group; #P < 0.05 vs. 20 wk in each respective group.
DISCUSSION
The purpose of this study was to evaluate whether physical activity can attenuate the temporal decline in ACh-induced EDR in the OLETF rat abdominal aorta during the progression to overt T2DM. Our hypothesis was that a sedentary lifestyle contributes to a reduction in ACh-induced EDR in the OLETF aorta during T2DM disease progression due to decreased bioavailability of NO resulting from the downregulated expression of endothelial SOD isoform(s) and a reduction in the Ser1177 p-eNOS/eNOS ratio. We hypothesized that physical activity would maintain ACh-induced EDR in part by preserving endothelial SOD protein content and the p-eNOS/eNOS ratio. We found that physical activity indeed preserved ACh-induced EDR in the physically active OLETF abdominal aorta, whereas ACh-induced EDR progressively decreased in sedentary OLETF rats in association with the development of T2DM. Contrary to our hypothesis, it does not appear that the temporal decrease in ACh-induced EDR in sedentary OLETF rats was due to the downregulated expression of endothelial SOD1 and SOD2 isoforms during the progression to T2DM as there were no significant differences in the protein content of SOD1 and SOD2 isoforms in sedentary OLETF rats across ages. Furthermore, contrary to our hypothesis, the p-eNOS/eNOS ratio was maintained by sedentary OLETF rats during T2DM progression. However, in LETO rats and in physically active OLETF rats, there was a significant increase in the p-eNOS/eNOS ratio in the aortic endothelium at 40 wk of age that was not seen in the sedentary OLETF group at 40 wk of age. These results illustrate the importance of physical activity in elevating the active state of eNOS in the OLETF rat aorta during the time of progression to the insulin-dependent stage of T2DM.
A primary finding in the present report, as indicated by the results shown in Fig. 1, A, C, and E, supports our hypotheses that a sedentary lifestyle contributes to a temporal reduction in ACh-induced EDR in the OLETF abdominal aorta during T2DM disease progression and that physical activity acts to temporally maintain ACh-induced EDR in the OLETF abdominal aorta during T2DM progression. The findings reported in this study are the first to show that during the transition from insulin resistance to overt T2DM there is a concurrent temporal decline in ACh-induced EDR in the OLETF abdominal aorta. Additionally, at 13 wk of age, sedentary OLETF rats were insulin resistant (Table 2) before the observed decline in ACh-induced EDR, suggesting that the decline in ACh-induced EDR is a consequence of T2DM progression rather than a contributing factor to the development of T2DM. The temporal decline in ACh-induced EDR in the sedentary OLETF abdominal aorta and T2DM progression was prevented by physical activity in physically active OLETF rats. These data indicate the importance of regular physical activity in maintaining endothelial function and the prevention of T2DM and obesity in the OLETF rat.
The temporal maintenance of aortic endothelial function in physically active OLETF rats did not appear to be mediated via temporal alterations in SOD2 or SOD1 expression within aortic smooth muscle (Fig. 5, A and B) or endothelial cells (Fig. 6, A and B). Previous studies (9, 44) using rodents that have shown exercise-induced increases SOD2 and SOD1 protein expression differed from ours in that a motorized treadmill was used, whereas in the present study voluntarily running wheels were used. Additionally, mesenteric arteries were used in one study (9) to examine OLETF SOD isoform protein expression rather than the thoracic aorta, which was used in the present study. We (48) have also previously shown in the porcine aorta that treadmill exercise training induces increases in SOD1 expression, and others (24) have shown that exposure of human aortic endothelial cells to high laminar shear stress, as observed during exercise, induces increases in SOD1 expression. It is possible that voluntarily running does not reach the same intensity stimulus as treadmill exercise training, which signals changes in aortic SOD isoform expression in endothelium and/or smooth muscle. Future studies should use a more rigorous and formal exercise training program to fully elucidate whether aortic SOD isoform expression can indeed be enhanced by more vigorous exercise during T2DM progression in the OLETF rat. On the other hand, 20-wk-old sedentary OLETF rats demonstrated a significant temporal reduction in SOD2 expression compared with 13-wk-old sedentary OLETF rats that returned to 13-wk-old levels in 40-wk-old sedentary OLETF rats. Perhaps the initial decrease in ACh-induced EDR in the 20-wk-old sedentary OLETF aorta was due to this temporal reduction in SOD2 not seen in sedentary LETO rats or physically active OLETF rats, as exercise-induced increases in SOD2 activity have been implicated to contribute to beneficial cardiac (57) and aortic (58) adaptations in rodents.
Alterations in aortic eNOS protein expression do not appear to account for the temporal maintenance of endothelial function in physically active OLETF and sedentary LETO rat abdominal aortas (Fig. 7A). Additionally, the temporal loss of ACh-induced EDR in sedentary OLETF rats does not appear to be due to altered eNOS protein content (Fig. 7A). Previous studies (31, 43) in OLETF rats have yielded similar results, where neither aortic eNOS protein expression nor basal NO production were different between diabetic OLETF and nondiabetic LETO controls. On the other hand, Ser1177 p-eNOS levels at 40 wk in both sedentary LETO and physically active OLETF rat groups were significantly higher than those in 13- and 20-wk-old rats in each respective group (Fig. 7B). Further analysis of the p-eNOS/eNOS ratio shown in Fig. 8, A and B, revealed that relative to the total pool of available eNOS in the thoracic aorta endothelium, the amount of p-eNOS was significantly greater in 40-wk-old rats compared with 13- and 20-wk-old rats in the sedentary LETO and physically active OLETF rat groups. This is the first report to demonstrate that physical activity in an obese T2DM model results in temporal increases in both aortic p-eNOS protein expression and the p-eNOS/eNOS ratio with a similar concurrent increase in plasma NOx concentration back to nondiabetic LETO control levels (Table 2). The temporal increase in p-eNOS at 40 wk in sedentary LETO and physically active OLETF rat aortas could serve as a compensatory mechanism associated with aging in these rats that is not present in sedentary diabetic OLETF rats. Matsumoto et al. reported similar reductions in p-eNOS protein content in the mesenteric arteries of sedentary OLETF rats compared with LETO control rats (33) and recently showed that OLETF rats aged similarly to those in the present study had significantly lower p-eNOS/eNOS ratios than LETO rats (36). Our findings are also in agreement with recent studies in humans (17) and rodents (19, 60) that demonstrated that exercise training resulted in a marked increase in the phosphorylation of eNOS at Ser1177. Collectively, these findings suggest that in physically active OLETF and LETO rats, p-eNOS expression increases with age in the aortic endothelium, perhaps due to changes in the hemodynamic signals produced by aging and physical activity levels in OLETF rats. The reason that sedentary OLETF rat aortic endothelium p-eNOS expression does not increase similarly with aging may be related to physical activity levels, obesity, and/or T2DM progression. These conclusions are also in agreement with the current literature, which suggests that the mechanical stimulus of chronically elevated levels of systemic shear stress in response to regular physical activity in OLETF rats compared with sedentary OLETF rats would account, in part, for the normalized p-eNOS levels seen in physical active OLETF rats. Reports on the influence of shear stress from in vivo human laboratories (13, 14, 52) and from laboratories that use cultured endothelial cells (20, 21, 55, 56) highlight the importance of episodic increases in shear rate for maintaining normal endothelial function via the eNOS/p-eNOS signaling pathway. However, additional studies are needed to fully elucidate the exact aging effect that interacts with physical inactivity in sedentary OLETF rats.
Hyperphagia and physical inactivity in sedentary OLETF rats resulted in increased percent body fat (Table 1) and elevated glucose, HbA1c, and triglyceride levels (Table 2) compared with sedentary LETO rats and physically active OLETF rats at each age during T2DM disease progression. Increased adiposity and elevated triglycerides are well-established risk factors for endothelial dysfunction, independent of T2DM (53, 54). These factors, which are present in metabolic syndrome (15, 61), likely contributed to the inability of sedentary OLETF rats to temporally maintain ACh-induced EDR in their abdominal aortas in addition to the lack of an increase in the aortic p-eNOS/eNOS ratio and significantly lower NO production. Conversely, the absence of a genetic predisposition to T2DM in sedentary LETO rats and regular physical activity in physically active OLETF rats resulted in a lower percent body fat (Table 1) and serum parameters as well as significantly higher NO production (Table 2), thereby preventing the development of T2DM and metabolic syndrome in OLETF rats. Taken together, these data indicate that physical activity in OLETF rats likely contributed directly or indirectly to their ability to temporally maintain ACh-induced EDR in their abdominal aortas via an increase in the aortic p-eNOS/eNOS ratio and NO production.
Our results suggest that a NOS- and COX-independent mediator partially compensated for the initial decrease in ACh-induced EDR in the 20-wk-old sedentary OLETF rat aorta. This interpretation was inferred from the results in rings pretreated with the NOS inhibitor l-NNA plus the COX inhibitor Indo (l-NNA + Indo; Fig. 2F). When used in combination, l-NNA and Indo blocked the two well-established EDR signaling pathways resulting from NOS and COX activity. Data obtained during this treatment of the aortas demonstrated that from 13 to 20 wk sedentary OLETF rat abdominal aortas exhibited a significant temporal increase in endothelium NOS- and COX-independent-mediated relaxation signaled by ACh (i.e., increased contribution from another mediator of EDR, perhaps EDHF), which returned to 13-wk levels by 40 wk. The temporal increase in EDHF-dependent ACh-induced EDR coincided with a 20–35% decrease in ACh-induced EDR from 13 to 20 wk in sedentary OLETF rats (Fig. 1E). Temporal changes in EDHF-dependent ACh-induced EDR were not observed in sedentary LETO or physically active OLETF rat groups, and the maximal EDHF-dependent ACh-induced EDR achieved was 10–20% less than that of sedentary OLETF rats (Fig. 2, B and D). Several studies in OLETF rats appear to conflict with our findings. Matsumoto et al. (33, 34) reported that at 60 wk of age OLETF rat mesenteric arteries and Minami et al. (25, 43) reported that at 35–40 wk of age in OLETF thoracic aortas that EDHF-dependent ACh-induced EDR responses are significantly depressed/absent compared with LETO controls. However, these studies did not make comparisons of vascular function within OLETF rat groups while the animals aged, as we did in the present study; instead, observations were made at a single age and not throughout the progression of T2DM. Further studies are warranted to investigate EDHF-dependent ACh-induced EDR responses in the OLETF rat using the same vessels from animals of the same age and that examine ACh-induced EDR responses during the progression of T2DM to better comprehend these conflicting results.
There was a significant temporal decrease in the sensitivity of the abdominal aorta to SNP from 20 to 40 wk in physically active OLETF rats and sedentary OLETF rats (Fig. 3, C and D); however, no such change in sensitivity was seen in 40-wk-old sedentary LETO animals (Fig. 3A). This is the first report where decreased sensitivity to NO was observed in the OLETF rat vasculature. Treatment with Indo returned this decrease in sensitivity in 40-wk-old physically active OLETF rats back to 13- and 20-wk-old levels (Fig. 4C) but not in 40-wk-old sedentary OLETF rats (Fig. 4E). This difference between the responses of physically active OLETF rats and sedentary OLETF rats likely indicates that COX-generated metabolites contribute to the decreased NO sensitivity in 40-wk-old physically active OLETF rat aortas. COX enzymes are known to metabolize arachidonic acid (8), producing metabolites that have been shown to enhance mesenteric artery and skeletal muscle arteriole vascular smooth muscle contractility in rat T2DM models (11, 45). COX metabolites have also been shown to contribute to depressed ACh-induced relaxation in OLETF mesenteric arteries (36) via increases in the production of vasoconstrictor COX-derived metabolites. Collectively, these studies and our data suggest that overproduction of COX metabolites may contribute to the temporal decline in physically active OLETF abdominal aorta smooth muscle sensitivity to SNP. These are similar findings to those demonstrated by Matsumoto et al. (33), where endothelium-dependent contractions mediated by COX-derived constrictor prostaglandins potentially contributed to alterations in the vascular responsiveness of sedentary OLETF rats. Presumably, pretreatment with l-NNA, Indo, and l-NNA + Indo should block the well-established EDR pathways resulting from COX and eNOS activity. However, the continued depression seen in 40-wk-old sedentary OLETF rat abdominal aorta sensitivity to SNP in light of l-NNA, Indo, and l-NNA + Indo pretreatment (Figs. 3F and 4, E and F) makes it difficult to establish the exact cause of this decline in aortic function. Studies have shown that molecular mechanisms such as diminished expression and/or activity of vascular smooth muscle cell guanylyl cyclase (1, 26) or adenylyl cyclase (37, 38) contribute to impaired vasodilatory responsiveness in a sedentary lifestyle and T2DM progression, similar to sedentary OLETF rats in this study.
In conclusion, we have shown that a sedentary lifestyle in the OLETF rat results in a temporal decrease of abdominal aorta ACh-induced EDR and that ACh-induced EDR is temporally maintained in OLETF rats that engage in regular physical activity. Our data also indicate that temporal alterations in aortic SOD isoforms are not the putative underlying mechanisms for the temporal decline in sedentary OLETF rat ACh-induced aortic EDR. However, the lack of an increase in the aortic p-eNOS/eNOS ratio and plasma NOx concentration in 40-wk-old sedentary OLETF rats that was observed in physically active OLETF rats and sedentary LETO rats at 40 wk likely contributed to the observed decline in sedentary OLETF rat abdominal aortic ACh-induced EDR. This is the first report showing that regular physical activity on voluntary running wheels in conjunction with aging in the OLETF rat results in a temporal increase in aortic p-eNOS expression compared with sedentary OLETF rats. Therefore, we propose an important role for p-eNOS in temporally maintaining OLETF aortic endothelial function that can be exploited by the prevention of T2DM and obesity through regular physical activity.
GRANTS
This work was partially supported by the College of Veterinary Medicine and Department of Internal Medicine of the University of Missouri as well as by National Institutes of Health Grants HL-36088 (to M. H. Laughlin) and F32-DK-83182 (to R. S. Rector). This work was also supported with resources and the use of facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, MO.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
OLETF and LETO rats were a generous gift of Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors thank Whitney Collins, Ann Melloh, Pam Thorne, Miles Tanner, and Grace Uptergrove for technical assistance. The authors also acknowledge Dr. Richard Madsen for conducting statistical analysis of the data presented in this article.
REFERENCES
- 1.Abiru T, Watanabe Y, Kamata K, Miyata N, Kasuya Y. Decrease in endothelium-dependent relaxation and levels of cyclic nucleotides in aorta from rabbits with alloxan-induced diabetes. Res Commun Chem Pathol Pharmacol 68: 13–25, 1990 [PubMed] [Google Scholar]
- 2.American Heart Association. Diabetes Mellitus–Statistics. (online). http://www.americanheart.org/presenter.jhtml?identifier=3000944 [29 March 2010]
- 3.American Heart Association. Overweight and Obesity–Statistics. (online). http://www.americanheart.org/presenter.jhtml?identifier=3000947 [29 March 2010]
- 4.Carlsson LM, Marklund SL, Edlund T. The rat extracellular superoxide dismutase dimer is converted to a tetramer by the exchange of a single amino acid. Proc Natl Acad Sci USA 93: 5219–5222, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caspersen CJ, Fulton JE. Epidemiology of walking and type 2 diabetes. Med Sci Sports Exerc 40: S519–S528, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Diabetes Data & Trends. National Diabetes Fact Sheet. (online). http://apps.nccd.cdc.gov/ddtstrs/FactSheet.aspx [29 March 2010]
- 7.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood 91: 3527–3561, 1998 [PubMed] [Google Scholar]
- 8.Coleman RA, Smith WL, Narumiya S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev 46: 205–229, 1994 [PubMed] [Google Scholar]
- 9.de Moraes C, Davel AP, Rossoni LV, Antunes E, Zanesco A. Exercise training improves relaxation response and SOD-1 expression in aortic and mesenteric rings from high caloric diet-fed rats. BMC Physiol 8: 12, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH, Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdei N, Bagi Z, Edes I, Kaley G, Koller A. H2O2 increases production of constrictor prostaglandins in smooth muscle leading to enhanced arteriolar tone in Type 2 diabetic mice. Am J Physiol Heart Circ Physiol 292: H649–H656, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol 24: 1367–1373, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Green D, Cheetham C, Reed C, Dembo L, O'Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retrograde flows during cycle ergometry. J Appl Physiol 93: 361–368, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Green DJ, Bilsborough W, Naylor LH, Reed C, Wright J, O'Driscoll G, Walsh JH. Comparison of forearm blood flow responses to incremental handgrip and cycle ergometer exercise: relative contribution of nitric oxide. J Physiol 562: 617–628, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement Executive summary. Cardiol Rev 13: 322–327, 2005 [PubMed] [Google Scholar]
- 16.Hadi HA, Suwaidi JA. Endothelial dysfunction in diabetes mellitus. Vasc Health Risk Manag 3: 853–876, 2007 [PMC free article] [PubMed] [Google Scholar]
- 17.Hambrecht R, Adams V, Erbs S, Linke A, Krankel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 107: 3152–3158, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Haram PM, Adams V, Kemi OJ, Brubakk AO, Hambrecht R, Ellingsen O, Wisloff U. Time-course of endothelial adaptation following acute and regular exercise. Eur J Cardiovasc Prev Rehabil 13: 585–591, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Harris MB, Mitchell BM, Sood SG, Webb RC, Venema RC. Increased nitric oxide synthase activity and Hsp90 association in skeletal muscle following chronic exercise. Eur J Appl Physiol 104: 795–802, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison DG, Sayegh H, Ohara Y, Inoue N, Venema RC. Regulation of expression of the endothelial cell nitric oxide synthase. Clin Exp Pharmacol Physiol 23: 251–255, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Harrison DG, Widder J, Grumbach I, Chen W, Weber M, Searles C. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med 259: 351–363, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Juliet PA, Kano-Hayashi H, Tsunekawa T, Dingqunfang D, Sumi D, Matsui-Hirai H, Fukatsu A, Iguchi A. NADPH oxidase inhibitor, apocynin, restores the impaired endothelial-dependent and -independent responses and scavenges superoxide anion in rats with type 2 diabetes complicated by NO dysfunction. Diabetes Obes Metab 7: 334–343, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Ingram DG, Newcomer SC, Price EM, Eklund KE, McAllister RM, Laughlin MH. Chronic nitric oxide synthase inhibition blunts endothelium-dependent function of conduit coronary arteries, not arterioles. Am J Physiol Heart Circ Physiol 292: H2798–H2808, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inoue N, Ramasamy S, Fukai T, Nerem RM, Harrison DG. Shear stress modulates expression of Cu/Zn superoxide dismutase in human aortic endothelial cells. Circ Res 79: 32–37, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Kagota S, Yamaguchi Y, Nakamura K, Kunitomo M. Altered endothelium-dependent responsiveness in the aortas and renal arteries of Otsuka Long-Evans Tokushima Fatty (OLETF) rats, a model of non-insulin-dependent diabetes mellitus. Gen Pharmacol 34: 201–209, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Kamata K, Miyata N, Kasuya Y. Impairment of endothelium-dependent relaxation and changes in levels of cyclic GMP in aorta from streptozotocin-induced diabetic rats. Br J Pharmacol 97: 614–618, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlsson K, Marklund SL. Extracellular superoxide dismutase in the vascular system of mammals. Biochem J 255: 223–228, 1988 [PMC free article] [PubMed] [Google Scholar]
- 28.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima fatty) rat: a new NIDDM rat strain. Diabetes Res Clin Pract 24, Suppl: S317–S320, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima fatty (OLETF) strain. Diabetes 41: 1422–1428, 1992 [DOI] [PubMed] [Google Scholar]
- 30.Kim IJ, Kim YK, Son SM, Hong KW, Kim CD. Enhanced vascular production of superoxide in OLETF rat after the onset of hyperglycemia. Diabetes Res Clin Pract 60: 11–18, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Kim YK, Lee MS, Son SM, Kim IJ, Lee WS, Rhim BY, Hong KW, Kim CD. Vascular NADH oxidase is involved in impaired endothelium-dependent vasodilation in OLETF rats, a model of type 2 diabetes. Diabetes 51: 522–527, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Laye MJ, Rector RS, Warner SO, Naples SP, Perretta AL, Uptergrove GM, Laughlin MH, Thyfault JP, Booth FW, Ibdah JA. Changes in visceral adipose tissue mitochondrial content with type 2 diabetes and daily voluntary wheel running in OLETF rats. J Physiol 587: 3729–3739, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matsumoto T, Kakami M, Noguchi E, Kobayashi T, Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol 293: H1480–H1490, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto T, Kobayashi T, Kamata K. Mechanisms underlying the impaired EDHF-type relaxation response in mesenteric arteries from Otsuka Long-Evans Tokushima fatty (OLETF) rats. Eur J Pharmacol 538: 132–140, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto T, Kobayashi T, Wachi H, Seyama Y, Kamata K. Vascular NAD(P)H oxidase mediates endothelial dysfunction in basilar arteries from Otsuka Long-Evans Tokushima fatty (OLETF) rats. Atherosclerosis 192: 15–24, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto T, Noguchi E, Ishida K, Kobayashi T, Yamada N, Kamata K. Metformin normalizes endothelial function by suppressing vasoconstrictor prostanoids in mesenteric arteries from OLETF rats, a model of type 2 diabetes. Am J Physiol Heart Circ Physiol 295: H1165–H1176, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Diabetes-related changes in cAMP-dependent protein kinase activity and decrease in relaxation response in rat mesenteric artery. Am J Physiol Heart Circ Physiol 287: H1064–H1071, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto T, Wakabayashi K, Kobayashi T, Kamata K. Functional changes in adenylyl cyclases and associated decreases in relaxation responses in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ Physiol 289: H2234–H2243, 2005 [DOI] [PubMed] [Google Scholar]
- 39.McAllister RM, Jasperse JL, Laughlin MH. Nonuniform effects of endurance exercise training on vasodilation in rat skeletal muscle. J Appl Physiol 98: 753–761, 2005 [DOI] [PubMed] [Google Scholar]
- 40.McAllister RM, Laughlin MH. Vascular nitric oxide: effects of physical activity, importance for health. Essays Biochem 42: 119–131, 2006 [DOI] [PubMed] [Google Scholar]
- 41.McAllister RM, Newcomer SC, Pope ER, Turk JR, Laughlin MH. Effects of chronic nitric oxide synthase inhibition on responses to acute exercise in swine. J Appl Physiol 104: 186–197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGavock JM, Eves ND, Mandic S, Glenn NM, Quinney HA, Haykowsky MJ. The role of exercise in the treatment of cardiovascular disease associated with type 2 diabetes mellitus. Sports Med 34: 27–48, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y, Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis 162: 85–92, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Moien-Afshari F, Ghosh S, Khazaei M, Kieffer TJ, Brownsey RW, Laher I. Exercise restores endothelial function independently of weight loss or hyperglycaemic status in db/db mice. Diabetologia 51: 1327–1337, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol 144: 953–960, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rask-Madsen C, King GL. Mechanisms of disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 3: 46–56, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima fatty (OLETF) rats. J Physiol 586: 4241–4249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rush JW, Turk JR, Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol 284: H1378–H1387, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto S, Minami K, Niwa Y, Ohnaka M, Nakaya Y, Mizuno A, Kuwajima M, Shima K. Effect of exercise training and food restriction on endothelium-dependent relaxation in the Otsuka Long-Evans Tokushima fatty rat, a model of spontaneous NIDDM. Diabetes 47: 82–86, 1998 [DOI] [PubMed] [Google Scholar]
- 50.Srere PA. Citrate synthase. Methods Enzymol 13: 3–5, 1969 [Google Scholar]
- 51.Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, Jones D, Cooksey RC, Birnbaum MJ, McClain DA, Zhang QJ, Gale D, Wilson LJ, Abel ED. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res 104: 1085–1094, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 53: 986–992, 2009 [DOI] [PubMed] [Google Scholar]
- 53.Thompson MA, Henderson KK, Woodman CR, Turk JR, Rush JW, Price E, Laughlin MH. Exercise preserves endothelium-dependent relaxation in coronary arteries of hypercholesterolemic male pigs. J Appl Physiol 96: 1114–1126, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH. Arterial endothelial function in a porcine model of early stage atherosclerotic vascular disease. Int J Exp Pathol 86: 335–345, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uematsu M, Ohara Y, Navas JP, Nishida K, Murphy TJ, Alexander RW, Nerem RM, Harrison DG. Regulation of endothelial cell nitric oxide synthase mRNA expression by shear stress. Am J Physiol Cell Physiol 269: C1371–C1378, 1995 [DOI] [PubMed] [Google Scholar]
- 56.Weber M, Hagedorn CH, Harrison DG, Searles CD. Laminar shear stress and 3′ polyadenylation of eNOS mRNA. Circ Res 96: 1161–1168, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med 189: 1699–1706, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young CG, Knight CA, Vickers KC, Westbrook D, Madamanchi NR, Runge MS, Ischiropoulos H, Ballinger SW. Differential effects of exercise on aortic mitochondria. Am J Physiol Heart Circ Physiol 288: H1683–H1689, 2005 [DOI] [PubMed] [Google Scholar]
- 59.Zecchin HG, Priviero FB, Souza CT, Zecchin KG, Prada PO, Carvalheira JB, Velloso LA, Antunes E, Saad MJ. Defective insulin and acetylcholine induction of endothelial cell-nitric oxide synthase through insulin receptor substrate/Akt signaling pathway in aorta of obese rats. Diabetes 56: 1014–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Zhang QJ, McMillin SL, Tanner JM, Palionyte M, Abel ED, Symons JD. Endothelial nitric oxide synthase phosphorylation in treadmill-running mice: role of vascular signalling kinases. J Physiol 587: 3911–3920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zieve FJ. The metabolic syndrome: diagnosis and treatment. Clin Cornerstone 6, Suppl 3: S5–S13, 2004 [DOI] [PubMed] [Google Scholar]