Abstract
Technologies to increase tissue vascularity are critically important to the fields of tissue engineering and cardiovascular medicine. Currently, limited technologies exist to encourage angiogenesis and arteriogenesis in a controlled manner. In the present study, we describe an injectable controlled release system consisting of VEGF encapsulated in poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs). The majority of VEGF was released gradually over 2–4 days from the NPs as determined by an ELISA release kinetics experiment. An in vitro aortic ring bioassay was used to verify the bioactivity of VEGF-NPs compared with empty NPs and no treatment. A mouse femoral artery ischemia model was then used to measure revascularization in VEGF-NP-treated limbs compared with limbs treated with naked VEGF and saline. 129/Sv mice were anesthetized with isoflurane, and a region of the common femoral artery and vein was ligated and excised. Mice were then injected with VEGF-NPs, naked VEGF, or saline. After 4 days, three-dimensional microcomputed tomography angiography was used to quantify vessel growth and morphology. Mice that received VEGF-NP treatment showed a significant increase in total vessel volume and vessel connectivity compared with 5 μg VEGF, 2.5 μg VEGF, and saline treatment (all P < 0.001). When the yield of the fabrication process was taken into account, VEGF-NPs were over an order of magnitude more potent than naked VEGF in increasing blood vessel volume. Differences between the VEGF-NP group and all other groups were even greater when only small-sized vessels under 300 μm diameter were analyzed. In conclusion, sustained VEGF delivery via PLGA NPs shows promise for encouraging blood vessel growth in tissue engineering and cardiovascular medicine applications.
Keywords: tissue engineering, angiogenesis, arteriogenesis, vascular endothelial growth factor, poly(lactic-co-glycolic acid)
technologies to enhance angiogenesis and arteriogenesis are greatly needed in cardiovascular medicine and tissue engineering. Limited options currently exist to increase tissue vascularity in a controlled manner. The most widely used vasculogenic agent, VEGF, has been previously shown to enhance revascularization in vivo (8, 16, 17). However, human clinical trials have been disappointing because therapeutic effects are achieved only at extremely high doses. This often results in side effects such as hypotension, retinopathy, or progression of malignant tumors (5, 6).
A controlled released system could increase treatment efficacy, thus allowing for a lower dose of growth factor. With the extraordinary expense associated with growth factor therapy, the economic advantages are also attractive. Moreover, the sustained release of VEGF may be necessary for the maintenance of stable neovessels (11). An injectable system is additionally appealing because it obviates the need for the surgical implantation of drug delivery devices, such as scaffolds or osmotic minipumps. An injectable controlled release strategy thus allows for minimally invasive delivery with convenient and infrequent dosing.
Poly(lactic-co-glycolic acid) (PLGA) copolymer is an attractive delivery vehicle because of its excellent biocompatibility, high safety profile, widespread use in medicine, and Federal Drug Administration approval for usage in drug delivery (7, 9). Injectable PLGA delivery systems involve the encapsulation of a growth factor. As the PLGA capsule is hydrolytically degraded over time in vivo or in vitro, growth factor is released into the surrounding region. This method confers a notable advantage over sustained release via gene therapy, which has mutagenic and tumorigenic potential. In addition, the release kinetics of this system can be easily adjusted by altering the ratio of poly(lactic acid) to poly(glycolic acid). Recently, focus has shifted to the encapsulation of bioactive substances with particles of a nanometer scale. Nanoparticle (NP) encapsulation confers several advantages over microparticle encapsulation, including a lower risk of embolization (2, 13).
In this study, a mouse femoral artery ligation model of hindlimb ischemia was used to evaluate the efficacy of VEGF-NPs in inducing vascular growth. Analysis included the use of three-dimensional microcomputed tomography (microCT) angiography, which, to our knowledge, has never been used before to analyze vascular formation stimulated by the sustained delivery of VEGF.
MATERIALS AND METHODS
VEGF-NP fabrication.
VEGF encapsulated PLGA NPs (VEGF-NPs) were fabricated by a modification of the double emulsion (water/oil/water phase) method (7). Briefly, 100 mg of 2% (wt/vol) 50:50 PLGA copolymer (Polysciences, Warrington, PA) was dissolved in 5 ml dichloromethane. Recombinant murine VEGF (Chemicon/Millipore, Billerica, MA) was dissolved in 250 μl of sterilized 0.2% BSA in deionized water. VEGF solution was then added to the preice-cooled PLGA solution. Emulsification of VEGF in PLGA was then performed by homogenization at 4,000 rpm at 13 s/run for three runs. This emulsion was added to poly(vinyl alcohol) solution [0.4% (wt/vol) in sterilized deionized water] and homogenized at 7,000 rpm at 18 s/run for three runs. The double emulsion was diluted in 0.1% poly(vinyl alcohol) solution, and dichloromethane was removed by evaporation under reduced pressure for 3 h. VEGF-NPs in 0.1% poly(vinyl alcohol) solution were collected by centrifugation at 8,500 g for 15 min and washed twice with sterilized deionized water. VEGF-NPs were subsequently lyophilized and stored at −20°C in a desiccator. All steps were conducted under sterile conditions. For size characterization, VEGF-NPs were suspended in 1× PBS and analyzed with a Hitachi H-7500 transmission electron microscope.
Release kinetics.
The release kinetics of VEGF-NPs were characterized in vitro using an ELISA-based assay. VEGF-NPs were mixed with 0.6% SeaPlaque agarose (Cambrex, East Rutherford, NJ) and gelled at 4°C. PBS (1×) was then added above the agarose and incubated at 37°C. On days 1–4, 6, 8, 10, 12, 14, and 21 of incubation under standard tissue culture conditions (37° C, 5% CO2), 1× PBS was collected followed by replacement with fresh PBS. The assay was performed in quadruplicate. Released VEGF concentrations from collected samples were then measured with ELISA (PeproTech, Rocky Hill, NJ). The cumulative VEGF mass release per PLGA NP mass was calculated to determine the effective loading yield (i.e., encapsulation efficiency) of VEGF in NPs.
In vitro bioassay.
To determine the bioactivity of VEGF and VEGF-NPs, a mouse aortic ring assay was performed as previously described (10). Briefly, 1-mm-long aortic rings were harvested from freshly killed 8- to 10-wk-old male C57Bl6/J mice (Jackson Laboratory, Bar Harbor, ME). Explants were embedded in 1.5 mg/ml collagen gels (Serva Chemical) placed inside of agarose wells. In addition to 2.5% murine serum harvested from C57Bl6/J mice, 2.3 mg VEGF-NPs, 2.3 mg saline encapsulated NPs (saline-NPs), or an equivalent volume of saline was added to 6 ml of molecular, cellular, and developmental biology (MCDB) 131 media. Nine replications were performed. On days 4, 6, 8, and 10, the total sprout number and maximum sprout length were recorded using an Olympus inverted microscope.
Hindlimb ischemia surgery and injection.
All surgeries were performed on 7- to 8-wk-old female 129/Sv mice (Charles River Laboratories, Wilmington, MA). Protocols were approved by the Institutional Animal Care and Use Committee of the Georgia Institute of Technology and were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The mouse femoral artery ligation model was used to study collateral vessel formation (1, 3). Anesthesia was induced with 5% isoflurane in 1,000 ml/min O2 and maintained with 2% isoflurane gas in 350 ml/min O2. A 3-cm incision was made over the right medial thigh. The common femoral artery and vein were then ligated at three points: 1) proximal to the superficial epigastric artery, 2) proximal to the bifurcation of the tibial arteries, and 3) at a deep branching radical ∼1 cm distal to the proximal ligation point. The last ligation was required for hemostasis. The length of the artery and vein was then excised between the ligation points. This was followed by six 25-μl saline or treatment/saline injections with a 29-gauge syringe in the ischemic thigh adductor muscles between the ligation points for a total injection volume of 150 μl/mouse. Depending on the assigned group, each animal received 5 μg VEGF (n = 13 for microCT), 2.5 μg VEGF (n = 11 for microCT), 8 mg VEGF-NPs (n = 12 for microCT and n = 3 for histology), 8 mg empty NPs (n = 5 for microCT), or saline (n = 16 for microCT and n = 2 for histology). Based on release kinetics data, 8 mg of VEGF-NPs delivered 375 ng of VEGF. An additional group of mice (n = 7 for microCT) was not injected and was perfused immediately after surgery to establish a baseline. The skin was closed with simple interrupted silk sutures.
Buprenorphine (0.03 mg/kg subcutaneous injection one time upon awakening) was provided for analgesia. Mice were allowed to ambulate freely postoperatively. Within 2 days, nearly all mice were able to use the operated hindlimb. Chewing at sutures was not a noted postoperative behavior. No mice had appreciable postoperative leg swelling, inflammation, or infection. Four days after surgery, animals were killed by a ketamine-xylazine overdose and perfused with heparinized saline and 10% neutral buffered formalin as previously described (3). Mice in the day 0 group were perfused 2 h after surgery.
MicroCT.
Samples were prepared for microCT using a previously described procedure (3). Immediately after standard formalin fixation, mice were perfused with 6 ml of silicone/lead chromate contrast agent (Microfil MV-122, Flow Tech, Carver, MA). The silicone was allowed to polymerize overnight at 4°C. Hindlimbs were separated from the body at the junction of the internal and external iliac arteries, fixed for 48 h in 10% neutral buffered formalin, and treated for 48 h with a formic acid-based solution for the decalcification of bones (Cal Ex II, Fisher Scientific, Pittsburgh, PA). Samples were stored in formalin until being scanned.
High-resolution three-dimensional images were acquired using a microCT imaging system (vivaCT 40, Scanco Medical, Basseldorf, Switzerland). Hindlimbs were scanned using an isotropic voxel size of 30 μm. The appropriate threshold and imaging parameters were selected to specifically include only the perfused vasculature (σ = 1, support = 4, and threshold = 140). System software was used to calculate blood vessel volume, thickness, density, spacing, connectivity, and anisotropy (12). The blood vessel volume was defined as the total volume contained within blood vessels. Volume was measured in voxels, where 1 voxel was set to equal 27,000 μm. Connectivity was defined as the maximal number of branches that can be broken within a structure before it is divided into two separate parts (3). Anisotropy was defined as the degree to which a segmented vascular bed is oriented toward a specific direction. The algorithm for calculating anisotropy and other parameters has been detailed in a previous study (3). Three-dimensional microCT angiograms with color-labeled vessel diameter were also generated to compare overall morphometric differences of collateral vessels between treatment groups.
Vessel volume was expressed as a ratio of the operated limb compared with the unoperated limb. In addition, the day 0 ratio was subtracted from each group so that the 0% baseline represented no new vessel growth. For vessel connectivity, the day 0 connectivity was subtracted from the group so that the 0% baseline represented no improvement in connectivity.
Immunohistochemistry.
After saline/formalin perfusion, hindlimbs were harvested and fixed for 48 h in 10% neutral buffered formalin. A portion of the adductor muscles were excised and cut into 10-μm frozen sections using a cryostat. A biotinylated lectin primary antibody (Vector Labs, Burlingame, CA) was used with steptavidin Texas red quantum dots (Invitrogen, Carlsbad, CA) to mark capillary endothelial cells. Hoechst staining was used as a nuclear counterstain. Samples were also stained with hematoxylin-eosin. Eight sections were chosen at random for qualitative analysis.
Statistical analysis.
Statistical analysis was performed in Minitab 12.23. ANOVA was used to compare differences in means between groups. Differences were considered significant if P < 0.05. Values are means ± SE.
RESULTS
Size characterization, release kinetics, and loading dose.
VEGF-NPs were ∼400 nm in diameter, with most particles falling between 200 and 600 nm. VEGF was released from 50:50 PLGA NPs over 2 wk. Release decreased exponentially from day 0, with the majority occurring over 2 days. By 4 days, 89% was released (Fig. 1). The calculated concentration of VEGF in VEGF-NPs was 52.9 ng/mg. Thus, the estimated dose of VEGF delivered to the mouse hindlimb in an 8 mg VEGF-NP injection over the 4-day experiment was ∼375 ng. The effective loading yield of VEGF during VEGF-NP fabrication was 5.3%.
Fig. 1.
A: transmission electron micrograph of lyophilized 50:50 poly(lactic-co-glycolic acid) (PLGA) VEGF encapsulated nanoparticles (VEGF-NPs). Inset: higher-magnification image. Scale bar = 1 μm. B: release kinetics of 50:50 PLGA VEGF-NPs. By 4 days, 89% was released. Values are means ± SE; n = 4.
Bioassay.
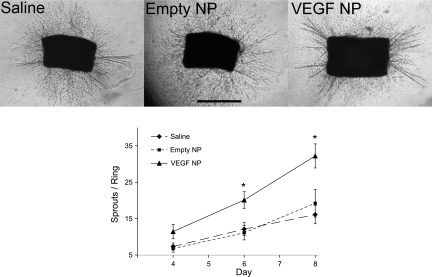
The mouse aortic ring angiogenesis assay demonstrated a significantly increased sprout number in the presence of VEGF-NPs compared with both saline (P = 0.001) and empty NPs (P < 0.05; Fig. 2). This was consistent across the nine replicates. By day 8, rings in the presence of VEGF-NPs contained 32 ± 3 sprouts compared with 19 ± 4 sprouts in the presence of empty NPs (P < 0.05) and 16 ± 3 sprouts in the presence of saline (P = 0.001). The rate of formation followed a gradual exponential curve and was greatest for the VEGF-NP group.
Fig. 2.
Aortic ring angiogenesis assay. Top: representative images of aortic rings in the presence of saline, empty NPs, and VEGF-NPs. Discrete sprouts are indicative of a proangiogenic environment. Bottom: nean number of sprouts per ring over time in the various conditions. Nine replicates were performed. The rate of formation followed a gradual exponential curve and was greatest for the VEGF-NP group. Values are means ± SE; n = 9 for all groups. *Significance between the VEGF-NP group and either of the two control groups at P < 0.05.
MicroCT of hindlimbs.
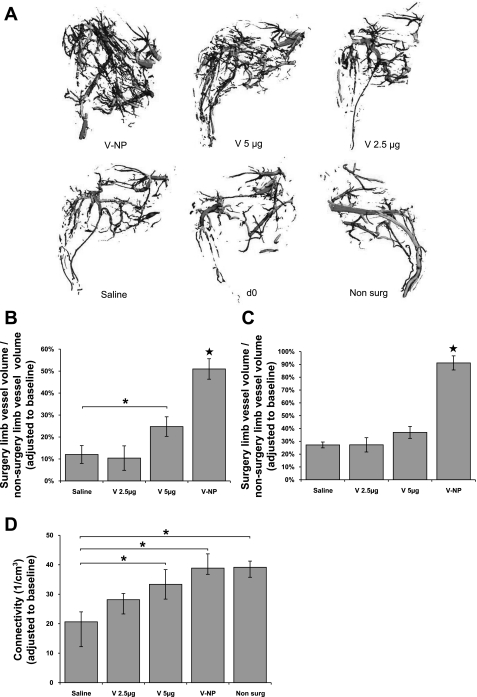
Results from the microCT analysis are shown in Fig. 3. Mice that received VEGF-NP treatment showed a significant increase in total vessel volume compared with mice that received 5 μg VEGF, 2.5 μg VEGF, and saline treatment (all P < 0.001). A significant increase was also observed for mice that received 5 μg VEGF compared with saline; however, the difference was less than half that observed with mice that received VEGF-NPs (Fig. 3B). Differences between mice that received VEGF-NPs and all other groups were even greater when only small-sized vessels under 300 μm diameter were analyzed (Fig. 3C). The value of 300 μm was chosen because it marks the transition between arterioles and arteries and is the general size limit for collateral vessels. A separate in vivo experiment showed no significant differences in vessel growth between saline and empty NP groups. This result was consistent with the in vitro aortic ring assay, which also showed an absence of a baseline effect of the NP delivery vehicle.
Fig. 3.
Limb images and microcomputerized tomography (microCT)-measured parameters. A: representative three-dimensional microCT angiograms of hindlimbs from the different treatment groups [VEGF-NP (V-NP), 5 μg VEGF (V 5ug), 2.5 μg VEGF (V 2.5ug), saline, day 0 (d0), and nonsurgery (Non surg)]. B: total vessel volume in the surgery limb compared with the nonsurgery limb. Day 0 vessel volume was subtracted from each group. C: <300 μm vessel volume in the surgery limb compared with the nonsurgery limb. Day 0 vessel volume was subtracted from each group. D: vessel connectivity. Day 0 connectivity was subtracted from each group. For B–D, n = 16 for the saline group, n = 11 for the 2.5 μg VEGF group, n = 13 for the 5 μg VEGF group, n = 12 for the VEGF-NP group, and n = 69 for the nonsurgery group. Values are means ± SE. ★Significance to all other groups at P < 0.05; *significance between two groups at bracket ends at P < 0.05.
VEGF-NP treatment induced a statistically significant increase in connectivity compared with saline treatment (P < 0.05). Connectivity was defined as the maximal number of branches that can be broken within a structure before it is divided into two separate parts. After an ischemic insult, vessel connectivity decreases dramatically (3). Connectivity was restored to nearly the same level as that of nonoperated limbs. A significant increase was also observed for mice that received 5 μg VEGF compared with saline (P < 0.05); however, this difference was lower than that of mice that received VEGF-NPs. A significant increase was not noted for mice that received 2.5 μg VEGF (P = 0.13; Fig. 3D).
VEGF-NP treatment caused a significant decrease in mean vessel diameter compared with both 5 μg VEGF and saline treatment (P < 0.05). Vessel diameter distribution showed an increase in small vessels under ∼400 μm diameter for all groups compared with day 0 perfusion baseline mice. However, this increase was notably greatest in VEGF-NP-treated mice. No increases in large vessels over 400 μm diameter were observed for any groups compared with the day 0 baseline (Fig. 4).
Fig. 4.
A: distribution of mean vessel diameter of the VEGF-NP group compared with the other experimental and control groups. The x-axis represents vessel diameter, and the y-axis represents the frequency. B: mean vessel diameter for each experimental and control group. n = 16 for the saline group, n = 11 for the 2.5 μg VEGF group, n = 13 for the 5 μg VEGF group, n = 12 for the VEGF-NP group, and n = 7 for the day 0 group. Values are means ± SE. ★Significance to all other groups at P < 0.05; *significance between two groups at bracket ends at P < 0.05.
No statistically significant differences were seen between VEGF-NP treatment, 5 μg VEGF treatment, and saline treatment groups for vessel density or spacing. The degree of anisotropy was also statistically unchanged, indicating that there were no differences in the degree to which vessels were oriented in a specific direction.
Histology of hindlimbs.
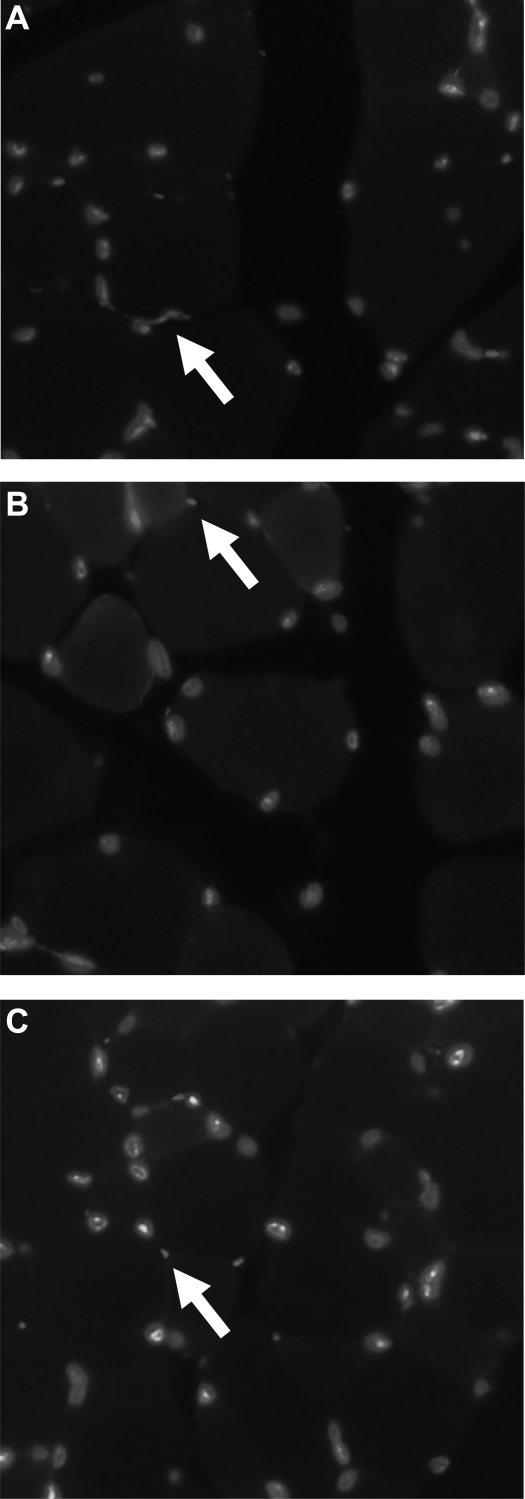
Through immunostaining of endothelial cells, VEGF-NP-treated hindlimbs appeared to have greater vessel numbers qualitatively than saline-treated hindlimbs. The morphometric arrangement between vessels in the surrounding musculature was similar between VEGF-NP-treated hindlimbs, saline-treated hindlimbs, and nonsurgery hindlimbs (Fig. 5).
Fig. 5.
Vessel presence in the ischemic hindlimb musculature (endothelial cells were stained with lectin, and nuclei were counterstained with Hoechst stain). Magnification: ×400. Endothelial cells are denoted with an arrow. A: VEGF-NP group. B: saline group. C: nonsurgery/nonischemic control limb.
DISCUSSION
In the present study, we demonstrated the feasibility of using VEGF-NPs to produce a vigorous revascularization response. VEGF-NP-treated limbs showed a significant increase in total vessel volume compared with 5 μg VEGF, 2.5 μg VEGF, and saline treatment. The difference was even more pronounced when only small- to medium-sized vessels under 300 μm diameter were analyzed. This latter finding agrees with mean vessel diameter data and the vessel diameter distribution data, which showed the greatest peak in vessels at 120 μm. Nearly all vessels that increased in prevalence after VEGF-NP treatment were markedly larger than capillaries (>10 μm diameter). Thus, VEGF-NP treatment did not produce its effect simply by vasodilation of preexisting capillaries.
In addition, VEGF-NP treatment was also the only treatment method that restored blood vessel connectivity to its presurgical value. The increase in connectivity demonstrated that VEGF-NPs caused structural changes to the vascular bed, such as increased anastamoses. This effect would not have been observed if VEGF-NPs merely vasodilated preexisting vessels.
These in vivo data also agree with the in vitro aortic ring angiogenesis assay data. Aortic rings placed in the presence of VEGF-NPs showed significantly more sprouting than aortic rings placed in the presence of empty NPs or saline. A preliminary assay using aortic rings in the presence of unencapsulated VEGF also showed significant sprouting (data not shown). Thus, both the VEGF protein itself as well as fabricated VEGF-NPs were biologically active.
No significant differences were found between VEGF-NP, 5 μg VEGF, and saline treatment for vessel density or spacing. The increase in vessel volume and thickness in the context of an unchanged density or spacing suggests that the treatments did not result in a significant increase in new vessels. Instead, it appears that existing vessels simply became larger or more interconnected with treatment. This supports previous data that ischemia encourages an arteriogenesis pathway (dilation and remolding of existing arterioles) as opposed to an angiogenesis pathway (de novo formation of capillaries) (3, 14). Anisotropy was also unchanged, indicating that VEGF-NP treatment did not change the degree to which vessels were oriented in a specific direction.
For ease of comparison between groups, this study used only female mice. Future studies should replicate the effects in mice of both sexes. This would determine if the increased presence of estrogen or lack of male hormones would have any added vasculogenic effect in females or whether the effect would be equivalent in males.
One potential concern of NP usage is that the NP themselves may cause an immune response, which, in turn, may help to stimulate revascularization. In addition, any organic solvents used in PLGA NP fabrication remaining associated with the particles might further increase the immune response. Both the aortic ring assay and in vivo hindlimb ischemia experiments demonstrated that empty NPs had no significant effect on vasculogenesis. These results demonstrate that the NPs themselves were not responsible for the observed arteriogenic effect of the VEGF-NP group.
Another concern of NP delivery is the possibility of aggregate formation and embolization. Because of the small size of NPs, aggregates would have to be relatively large to produce embolic phenomena. Such aggregates were not seen in in vivo (data not shown). Furthermore, microCT demonstrated an increase in the volume of perfused small vessels, arguing against the occurrence of embolism.
A limitation of NP delivery is the low yield during fabrication. The effective VEGF loading yield during VEGF-NP fabrication was only ∼5%. This value was lower than the yield of 22–70% described for similarly fabricated PLGA NPs (2, 18, 19). However, these assays for measuring loading yield involved mechanically or chemically dissolving NPs encapsulating fluorescent markers immediately after fabrication. It is possible that some of the loaded VEGF was rendered inactive during fabrication or the 21 days of incubation under physiological conditions. While steps were taken to maintain the stability of the protein during fabrication, it is still possible that some degradation/denaturation may have occured. For example, homogenization may produce excessive heat, despite placement of all solutions on ice. Furthermore, hydrolysis of PLGA during the collection phase could produce acidic monomers and oligomers, resulting in VEGF inactivation (13, 20). While potentially underestimating encapsulation efficiency, the effective loading yield described here more accurately reflects the amount of active VEGF that would be present in vivo. A final limitation of the PLGA delivery vehicle is the acidic byproduct of the hydrolysis reaction, which has been shown to be cytotoxic both in vivo and in vitro (15). However, our data showed no evidence of an adverse effect of empty NPs alone.
MicroCT angiography was the primary method of analysis in this study because of its superior ability to quantify three-dimensional vessel growth. Correlation to standard methods of quantitation, such as immunohistochemistry, has been previously demonstrated (3). Furthermore, a previous study (4) has shown that microCT measurements of vessel volume and morphology correspond to functional measures such as blood flow and swim endurance.
When VEGF dosing was taken into account, VEGF-NPs were over an order of magnitude more potent than unencapsulated VEGF in increasing blood vessel volume in vivo. After 4 days of release, a single 8 mg dose of VEGF-NP yielded ∼375 ng of ELISA-detectable VEGF. This is less than 10% of the VEGF dose of the 5-μg injection group. Yet, a significantly increased vasculogenic response was observed in the VEGF-NP-treated mice compared with the 5 μg VEGF group. This finding illustrates the increased efficacy of slow, sustained release of growth factor compared with one-time immediate delivery.
Despite the strong positive effect observed, several methodological hurdles remain before VEGF-NPs can be used clinically. NP fabrication involves use of organic solvents such as poly(vinyl acid), which may remain associated with the NP surface (13). In addition, the effective loading yield is fairly low. Future optimization of the fabrication protocol could greatly increase this figure, making the process even more economically advantageous compared with naked protein delivery. Despite these challenges, this study suggests that sustained release delivery strategies may provide greater therapeutic benefit at lower overall doses than bolus delivery of vasculogenic proteins and thus provide a viable alternative delivery strategy for use in human disease.
GRANTS
This work was funded by the Georgia Tech/Emory Center for the Engineering of Living Tissues, National Science Foundation Grant EEC-9731643, and National Institutes of Health Grants AR-051336, U01-HL-080711, and R01-HL-70531.
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors acknowledge Laura O'Farrell for aid with the surgical technique.
REFERENCES
- 1.Couffinhal T, Silver M, Zheng LP, Kearney M, Witzenbichler B, Isner JM. Mouse model of angiogenesis. Am J Pathol 152: 1667–1679, 1998 [PMC free article] [PubMed] [Google Scholar]
- 2.Davda J, Labhasetwar V. Characterization of nanoparticle uptake by endothelial cells. Int J Pharm 233: 51–59, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Duvall CL, Taylor WR, Weiss D, Guldberg RE. Quantitative microcomputed tomography analysis of collateral vessel development after ischemic injury. Am J Physiol Heart Circ Physiol 287: H302–H310, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Duvall CL, Weiss D, Robinson ST, Alameddine FM, Guldberg RE, Taylor WR. The role of osteopontin in recovery from hind limb ischemia. Arterioscler Thromb Vasc Biol 28: 290–295, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Freedman SB, Isner JM. Therapeutic angiogenesis for coronary artery disease. Ann Intern Med 136: 54–71, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation 107: 1359–1365, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Jain RA. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 21: 2475–2490, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Kofidis T, Nolte D, Simon AR, Metzakis A, Balsam L, Robbins R, Haverich A. Restoration of blood flow and evaluation of corresponding angiogenic events by scanning electron microscopy after a single dose of VEGF in a model of peripheral vascular disease. Angiogenesis 5: 87–92, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Lewis DH. Controlled release of bioactive agents from lactide/glycolide polymers. In: Biodegradable Polymers as Drug Delivery Systems, edited by Chasin M, Langer R. New York: Marcel Dekker, 1990, p. 1–41 [Google Scholar]
- 10.Masson VV, Devy L, Grignet-Debrus C, Bernt S, Bajou K, Blacher S, Roland G, Chang Y, Fong T, Carmeliet P, Foidart JM, Noel A. Mouse aortic ring assay: a new approach of the molecular genetics of angiogenesis. Biol Proc Online 4: 24–31, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 196: 1497–1506, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone 14: 173–182, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Delivery Res 55: 329–347, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol 34: 775–787, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Sung HJ, Meredith C, Johnson C, Galis ZS. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 25: 5735–5742, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, Isner JM, Symes JF. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation 90: II228–II234, 1994 [PubMed] [Google Scholar]
- 17.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest 93: 662–670, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi F, Wu H, Jia GL. Formulation and characterization of poly(d,l-lactide-co-glycolide) nanoparticle containing vascular endothelial growth factor for gene delivery. J Clin Pharm Ther 31: 43–48, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Zambaux MF, Bonneaux F, Gref R, Maincent P, Dellacherie E, Alonso MJ, Labrude P, Vigneron C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J Control Release 50: 31–40, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide). Nat Biotechnol 18: 52–57, 2000 [DOI] [PubMed] [Google Scholar]