Abstract
Using neonatal rat ventricular myocytes, we previously reported that the expression of a dominant negative form of the c-Fos proto-oncogene (AFos) inhibited activator protein 1 activity and blocked the induction of the pathological gene profile stimulated by phenylephrine (PE) while leaving growth unaffected. We now extend these observations to the adult rat ventricular myocyte (ARVM) to understand the relationship between gene expression, growth, and function. Ventricular myocytes were isolated from adult rats and infected with adenovirus expressing β-galactosidase (control) or AFos. The cells were subsequently treated with PE, and protein synthesis, gene program, calcium transients, and contractility were evaluated. As seen with the neonatal rat ventricular myocytes, in control cells PE stimulated an increase in protein synthesis, induced the pathological gene profile, and exhibited both depressed contractility and calcium transients. Although ARVMs expressing AFos still had PE-induced growth, pathological gene expression as well as contractility and calcium handling abnormalities were inhibited. To determine a possible mechanism of the preserved myocyte function in AFos-expressing cells, we examined phospholamban (PLB) and sarco(endo)plasmic reticulum calcium-ATPase proteins. Although there was no change in total PLB or sarco(endo)plasmic reticulum calcium-ATPase expression in response to PE treatment, PE decreased the phosphorylation of PLB at serine-16, an observation that was prevented in AFos-expressing cells. In conclusion, although PE-induced growth was unaffected in AFos-expressing ARVMs, the expression of the pathological gene profile was inhibited and both contractile function and calcium cycling were preserved. The inhibition of functional deterioration was, in part, due to the preservation of PLB phosphorylation.
Keywords: α-adrenergic, activator protein 1, hypertrophy, myocyte
evidence for the involvement of the dimeric transcription factor activator protein 1 (AP-1) in a number of disease states is extensive, and its role in pathological myocardial growth and heart failure is also mounting (4). AP-1 possesses transcriptional specificity owing to three regulatory mechanisms (2, 25, 26). First, AP-1 requires homo- and/or heterodimerization within, but not limited to, AP-1 family members. Whereas Jun members (c-Jun, JunB, and JunD) can form both homo- and heterodimers, Fos members (c-Fos, FosB, Fra-1, and Fra-2) can only form heterodimers with Jun members, giving the seven AP-1 members up to 17 potential dimer combinations. Second, AP-1 members possess different baseline and temporal expression patterns. Jun is expressed at low levels basally indicating an essential need in cellular homeostasis. Fos members are, however, immediately and transiently transcribed in response to specific stimuli and bind with Jun members to determine the stimuli-specific balance of homo- and heterodimers within the AP-1 expression matrix. Third, different AP-1 dimers have different nuclear binding affinity with c-Fos/c-Jun heterodimers having the highest affinity to the consensus AP-1 binding site known as the 12-O-tetradecanoyl phorbol 13-acetate-response element. When we combine these characteristics, AP-1 achieves its regulatory specificity by a temporally specific balance of various homo- and heterodimer formations within the AP-1 dimerization matrix. Furthermore, it is clear that there is a hierarchy of transcriptional activation within the AP-1 family members, an observation that is confirmed by exploring the effects of a number of transgenic mouse models. Indeed, the overexpression of the naturally occurring dominant negative AP-1 monomer JunD attenuates phenylephrine (PE)-mediated myocyte hypertrophy (8) and the JunD-deficient mouse accentuates the pathological effects of aortic banding (9), suggesting that preferential JunD expression confers a “protective” effect. In contrast, c-Jun appears to be essential for many of the pathological features of endothelin-1 and PE stimulation (12, 20), indicating that c-Jun may favor the “pathological” phenotype. Specifically, c-Jun−/− and JunB−/− animals are embryolethal (11), whereas c-Fos−/− mice (18, 33), although displaying developmental alterations, remain viable. These findings confirm the notion that while c-Jun and JunB are necessary for basal cellular homeostasis, c-Fos likely serves a vital but more modulating role. Although it is clear that all AP-1 members serve an important role in transcriptional activation and subsequent biological effects, it is our belief that c-Fos is indispensable for determining the stimuli-specific balance of the AP-1 matrix particularly within the cardiac myocyte context. Using neonatal rat ventricular myocytes (NRVMs), we previously reported that abrogating the activity of c-Fos, another member of the AP-1 complex, resulted in the inhibition of PE-mediated pathological gene expression without an inhibition of myocyte hypertrophy (10). Our findings showed that pathological gene expression and myocyte growth were not obligate processes in response to a pathological stimulus and suggested that within the AP-1 dimerization matrix, c-Fos plays a more modulatory role in hypertrophy signaling.
We now expand on our previous report to determine the relationship between cardiomyocyte hypertrophy, gene expression, and single myocyte function. To that end, we abrogate the activities of c-Fos with the dominant negative reagent AFos in which an appended NH2-terminal acidic sequence to the intact leucine zipper region renders DNA binding ineffective (19) and stabilizes the AFos/AP-1 dimer ∼3,000-fold relative to the endogenous AP-1 dimer. We now speculate that inhibiting the activity of c-Fos with AFos will not only inhibit the pathological gene expression in response to 48 h of α-adrenergic stimulation but will also abrogate the functional deterioration that accompanies this pathological stimulus. To examine whether AP-1 can affect the contractile consequences of prolonged α-adrenergic stimulation, we infected adult rat ventricular myocytes (ARVMs) with AFos and treated them with PE for 48 h. The gene expression, protein levels and phosphorylation changes, fractional cell shortening, and calcium levels were examined to determine how AFos expression affected myocyte function in a pathological model.
METHODS
Cell culture.
ARVMs were obtained from adult female Sprague-Dawley rats (250–350 g) as described previously (22). Animals were studied according to the University of Colorado Institutional Animal Care and Use Committee and the National Institutes of Health guidelines. Ketamine and xylazine were administered according to the weight of the animal. Hearts were rapidly removed and retrograde perfused with perfusion buffer containing (in mM) 120.4 NaCl, 14.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 4.6 NaHCO3, 10 sodium-HEPES, 30 taurine, 10 2,3-butanedione monoxime, and 5.5 glucose (pH 7.4) for 8 min at 37°C. The perfusion solution with collagenase II (2 mg/ml, Worthington) was then infused for 22 min for enzymatic digestion. The heart was then cut into small pieces, and the cells were dispersed by shaking for an additional 10 min in fresh perfusion solution with collagenase II (2 mg/ml, Worthington). The slurry was filtered through a sterile 150-nm mesh, allowed to settle (20 min), and resuspended in DMEM (GIBCO), layered over 60 μg/ml bovine serum albumin (Sigma) to separate ventricular myocytes from nonmyocytes. ARVMs were then plated at a density of 100 to 150 cells/mm2 on 60-mm, 35-mm plastic culture dishes (Becton-Dickinson) or ×40 22-mm glass coverslips (Fisher) precoated with laminin (1 μg/cm2, Invitrogen). After 1 h, myocytes were washed with serum-free DMEM to remove cells that were not attached. Myocytes were studied in serum-free DMEM supplemented with albumin (2 mg/ml), 2,3-butanedione monoxime (1 mg/ml), l-carnitine (2 mmol/l), creatine (5mmol/l), penicillin-streptomycin (100 μg/ml), triiodothyronine (1 pmol/l), and taurine (5 mmol/l).
Adenoviral constructs.
The adenoviral construct for hemagglutinin (HA)-tagged AFos (AFos) has been described previously (19). Briefly, an acidic extension is appended to the leucine zipper dimerization region to abolish the nuclear binding capacity of the c-Fos protein. The intact leucine zipper region allows dimerization with other AP-1 partners. The acidic extension stabilizes the AFos/AP-1 dimer ∼3,000-fold, making AFos a potent dominant negative reagent. Adenoviral infection was performed at a multiplicity of infection of 100 on culture day 0 and incubated for 48 h. An adenovirus expressing β-galactosidase (β-Gal) was used as control.
Analysis of AFos expression and immunohistochemistry.
Total cell protein extract was used for Western blot analysis of AFos expression (sc-53516, Santa Cruz), and qualitative AFos expression was visualized by immunohistochemistry. For these latter studies, the myocytes were permeabilized by incubation for 1 h in blocking buffer composed of 0.1% (wt/vol) Triton X-100, 0.2% Tween-20, 1% bovine serum albumin, and 10% (vol/vol) normal goat serum in phosphate-buffered saline. Immunostaining was performed with anti-HA rat monoclonal IgG (sc-53516, Santa Cruz) using biotinylated secondary antibody immunodetection kit (M.O.M. Basic Kit, Vector) and visualized with streptavidin-conjugated Alexa Fluor 546 (S11225, Molecular Probes).
Determination of cardiomyocyte hypertrophy.
β-Gal- or AFos-infected myocyte cultures were treated with PE (20 μmol/l) or its vehicle on day 2 and incubated in serum-free medium containing [3H]leucine for an additional 48 h. The incorporation of [3H]leucine into newly synthesized protein allowed for the quantification of hypertrophy. Bradford Assay (Bio-Rad) was used to determine the total protein content as an independent measure of cardiomyocyte hypertrophy.
Determination of cardiomyocyte gene expression.
The expression of α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC), atrial natriuretic factor, brain natriuretic factor (BNP), and α-skeletal actin (sACT) was determined by real-time reverse transcription polymerase chain reaction. To perform these assays, total RNA was extracted from myocytes with TRIzol (GIBCO). First-strand cDNA was synthesized using Superscript II reverse transcriptase (Invitrogen) and random hexamer primers. The induction of each gene was quantified using SYBR green dye with gene-specific primer sets and Applied Biosystems 7500 Real-Time PCR System as described previously (28).
Determination of cardiomyocyte contractility.
Myocytes were plated on coverslip chambers and treated with virus and agonist as above. For experimental determinations, each chamber was incubated for 20 min in Tyrode buffer [containing (in mmol/l) 137 NaCl, 4 KCl, 1 MgCl2, 10 HEPES, 0.33 NaH2PO4, 1.8 calcium, 5.5 glucose, and 0.5 probenecid (pH 7.4)] containing 0.5 μM membrane-permeant fura-2 AM (Molecular Probes). Chambers were washed in Tyrode buffer for 20 min at room temperature with gentle shaking. Myocytes were paced at 1 Hz by a stimulator for 2 min to reach a steady state before measurements were made. Myocyte length was monitored from a red-light bright-field image, and cell length was measured by video edge-detection system (IonOptix). The fluorescence signal was measured by a photomultiplier for 10 s. Fluorescence data were corrected for background fluorescence.
Determination of cardiomyocyte protein expression.
Whole cell protein extracts were harvested in Laemmli buffer with 2% β-mercaptoethanol and diluted to yield equivalent concentrations of 1 μg/μl. Total protein (10–20 μg) was resolved via SDS-PAGE (Bio-Rad Criterion Precast Gel, 7.5 or 12%) and transferred to a 0.2- or 0.45-μm polyvinylidene difluoride membrane (Millipore). Membranes were blocked for 1 h at room temperature in 5% milk (Carnation) or 1% bovine serum albumin (Sigma) dissolved in Tris-buffered saline with 0.1% Tween (TBST). Primary antibody was incubated overnight at 4°C (see below for list of primary antibodies). Horseradish peroxidase-conjugated goat anti-mouse, goat anti-rabbit, or donkey anti-goat secondary antibodies were used at a 1:25,000 dilution at room temperature for 1 h (Nos. 115-035-164, 111-035-144, and 705-035-147 respectively; Jackson Immunoresearch). Calnexin was used to standardize for loading. Direct protein staining was performed to confirm MHC Western blot analysis results. For these studies, samples were run on a 6% acrylamide gel overnight at 4°C and stained with blue-silver stain, an adaptation of the Neuhoff dye recipe. Samples (20, 10, 5, and 2.5 μg), serially diluted from stock samples (1 μg/μl), were loaded for dilutional immunoassay. Bands were quantified using ImageJ, and protein quantification was determined by methods described previously (32).
The primary antibodies were as follows: 1) sarco(endo)plasmic reticulum calcium-ATPase 2a (SERCA2a; MA3–919, Affinity Bioreagent) and ab2861 (Abcam) at 1:1,000 and 1:10,000 dilutions, respectively, in TBST; 2) α-MHC (BA-G5 hybridoma specific to rat cardiac α-MHC; HB-276, ATCC) at 1:10,000 dilution in TBST; 3) β-MHC (M8421, Sigma) at 1:25,000 dilution in TBST; 4) phospholamban (PLB; 05-205, Upstate Biotechnology) at 1:1,000 dilution in TBST; 5) phosphorylated PLB (serine-16; 07-052, Upstate Biotechnology) at 1:1,000 dilution in TBST; 6) HA antibody (sc-53516, Santa Cruz Biotechnology) at 1:500 dilution for immunostaining; and 7) calnexin (ab13504, Abcam) at 1:10,000 dilution in TBST.
Data analysis and statistics.
Values are means ± SE values and were compared by one-way ANOVA and the Newman-Keuls posttest. Probability values of P < 0.05 were considered significant.
RESULTS
Evaluation of AFos expression.
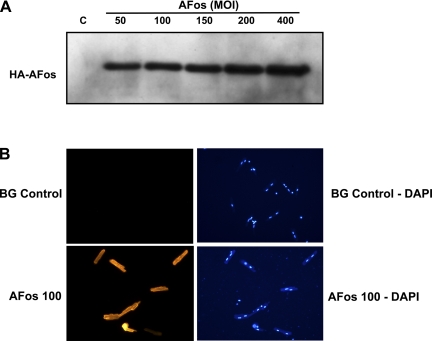
To determine the appropriate conditions for adenoviral infection of ARVM cultures, myocytes were infected with AFos (50–400 multiplicity of infection) for Western blot analysis (Fig. 1A). The efficiency of AFos infection was determined from the ratio of infected (fluorescence image) over total rod-shaped myocytes (phase image) (data not shown). Fluorescence images showed both cytoplasmic and nuclear AFos expression. The corresponding 4,6-diamino-2-phenolindole stain shows viable myocytes with a binuclear staining pattern (Fig. 1B). The specificity of AFos interaction with AP-1 constituents was confirmed with electrophoretic mobility shift assay where AP-1 oligonucleotide and c-Jun antibody were used to show specific binding to the AP-1 consensus sequence (sc-2501; 5′-CGC TTG ATG ACT CAG CCG GAA-3′) and a nuclear factor-kB oligonucleotide (sc2505; Santa Cruz) was used as a nonspecific competitor (data not shown).
Fig. 1.
Determination of AFos expression in the adult rat ventricular myocytes. A: myocytes were infected with AFos in increasing MOI (50–400 MOI) for Western blot analysis and compared with β-galactosidase (β-Gal; BG) control (C) [100 multiplicity of infection (MOI)]. Anti-hemagglutinin (HA) antibody diluted 1:1,000 shows increasing signal corresponding to increasing expression of AFos. B: myocytes were infected with β-Gal (100 MOI) vs. AFos in increasing MOI. 4,6-Diamino-2-phenolindole (DAPI) stain shows viable myocytes with binuclear expression pattern with corresponding immunofluorescence in rod-shaped myocytes.
α-Adrenergic-mediated myocyte hypertrophy is not affected by AFos expression.
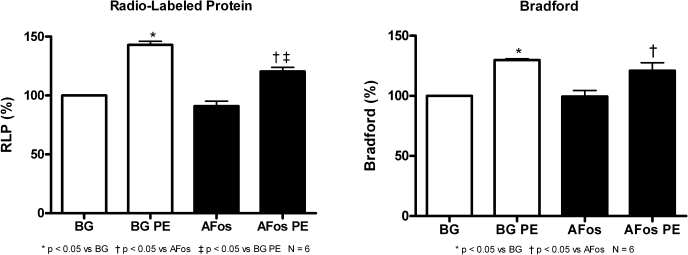
Previously, we showed in NRVMs that a prolonged PE treatment led to a significant increase in newly synthesized proteins, total RNA, and myocyte size, and this increase in agonist-induced growth was not affected with AFos expression (10). We found similar results in ARVMs where PE (20 μmol/l) treatment significantly increased both newly synthesized and total protein (P < 0.05 vs. β-Gal) as measured by both [3H]leucine and Bradford Assays, respectively (Fig. 2). This PE growth effect was also seen in the AFos-expressing myocytes. Specifically, the treated-to-control ratios of newly synthesized protein and Bradford (means ± SE) for β-Gal/PE vs. β-Gal were 1.43 ± 3.1 and 130 ± 1.2 and for AFos/PE vs. AFos were 132.4 ± 3.8 and 121.6 ± 6.5, respectively.
Fig. 2.
α-Adrenergic-mediated myocyte hypertrophy is not affected by AFos expression. Myocytes were infected with β-Gal (100 MOI) or AFos (100 MOI) on culture day 0 and treated with phenylephrine (PE, 20 μmol/l) or vehicle on day 2 for 48 h. Myocytes were harvested on culture day 4 for protein synthesis analysis. Data shown are compiled from N = 6 experiments. Radio-labeled protein (RLP) assay indicates newly synthesized protein, and Bradford indicates total protein content as independent measures of myocyte growth.
The c-Fos transcription factor is crucial for PE-induced pathological gene expression.
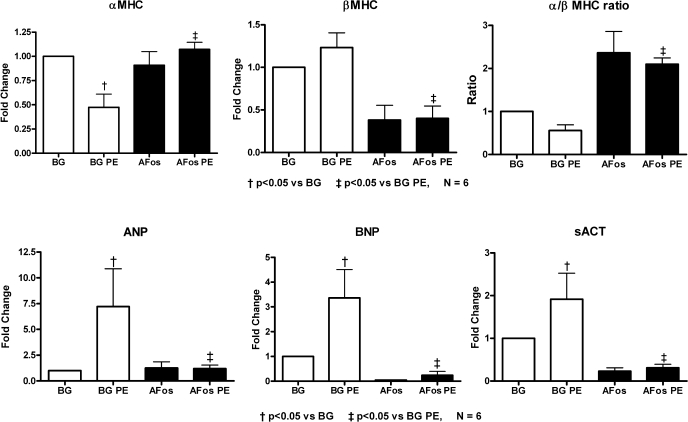
Our previous data with cultured NRVMs indicated that AFos expression inhibited the PE-mediated induction of the pathological gene profile, and similar results were also seen in ARVMs (Fig. 3). Specifically, PE treatment of ARVMs led to a decreased expression of α-MHC, whereas that for β-MHC, BNP, and sACT were increased. Myocytes-expressing AFos showed an inhibition of this pattern, i.e., the upregulation of α-MHC to baseline levels and the suppression of BNP, sACT, and β-MHC. Similar to our NRVM gene expression findings, β-MHC and BNP transcription were reduced in response to AFos expression at baseline (10). It is known that both of these genes possess an AP-1 response element in their promoter regions, and our data suggest that these genes are under the strong control of AP-1 for transcriptional activation.
Fig. 3.
The c-Fos transcription factor is crucial for PE-induced pathological gene expression. Myocytes were infected with β-Gal (100 MOI) or AFos (100 MOI) on culture day 0 and treated with PE (20 μmol/l) or vehicle on day 2 for an additional 48 h. Myocytes were harvested on culture day 4, and RNA was isolated with TRIzol. The expression of α-myosin heavy chain (α-MHC), β-myosin heavy chain (β-MHC), atrial natriuretic factor (ANP), brain natriuretic factor (BNP), and α-skeletal actin (sACT) was determined by real-time reverse transcription polymerase chain reaction. Data were normalized to β-Gal control. Summary data from N = 6 experiments are shown.
Inhibiting c-Fos transcriptional factor prevents PE-mediated myocyte contractile dysfunction and prolongation of the calcium transient.
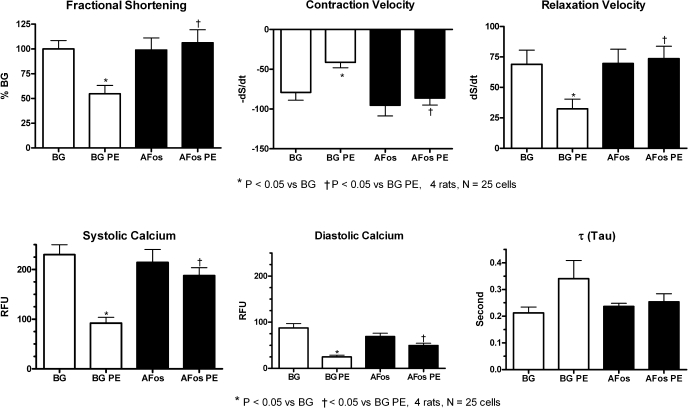
We next investigated the effects of PE on contraction and calcium cycling in ARVMs to determine whether inhibiting the pathological gene expression affected cardiomyocyte function. As shown in Fig. 4, PE treatment led to an overall deterioration of both contraction and calcium cycling in the β-Gal-expressing control myocytes. Specifically, fractional shortening, contraction and relaxation velocities, and systolic and diastolic calcium levels were all depressed with PE treatment in the β-Gal group. Although not statistically significant, we also saw a prolongation of τ, the exponential time decay of calcium handling. AFos-expressing myocytes did not display this PE-mediated contractile dysfunction, and both contractile function and calcium cycling values remained at control values. There was no significant difference between AFos- and β-Gal-expressing myocytes at baseline.
Fig. 4.
Inhibiting c-Fos transcription factor prevents PE-mediated dysfunction of myocyte contraction and calcium handling. Myocytes were infected with β-Gal (100 MOI) or AFos (100 MOI) on culture day 0 and treated with PE (20 μmol/l) or vehicle on day 2 for additional 48 h. At the end of the treatment protocol, myocytes were incubated in 0.5 μmol/l of fura-2 AM for 20 min and washed as described in methods. Myocytes were perfused in Tyrode buffer and paced at 1 Hz for 5 min before measuring contractile parameters (fractional shortening, contraction, and relaxation velocities) and calcium transients [systolic and diastolic calcium and exponential time decay of calcium handling (τ)]. Individual parameters of calcium handling and contractile function are shown. A maximum of 5 myocytes per coverslip were studied with a total of 25 myocytes per treatment groups from 4 experiments. RFU, relative fluorescence units.
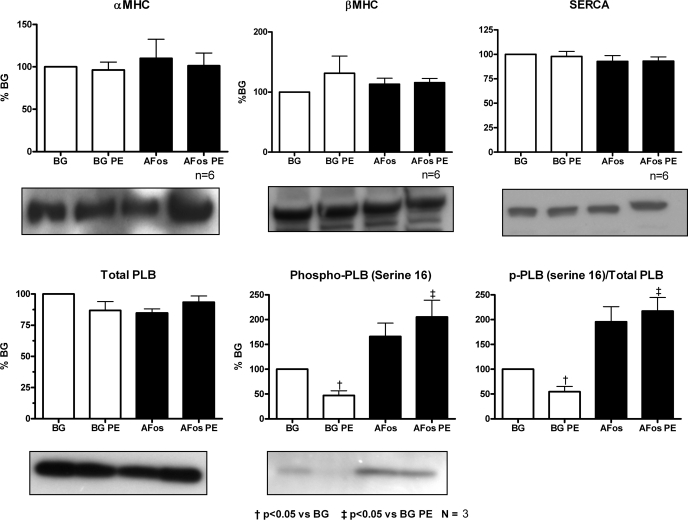
AFos expression in ARVMs reverses PE-mediated dephosphorylation of PLB on serine-16.
To elucidate the possible mechanism of the contractile dysfunction seen in PE-treated myocytes, we looked at the expression and phosphorylation status of key proteins regulating sarcoplasmic reticulum (SR) calcium handling and contractility (Fig. 5). Although the expression of neither SERCA nor PLB were changed with either AFos expression or PE treatment, PE led to a decrease in the phosphorylation of PLB at the serine-16 site in the β-Gal control group. AFos expression significantly inhibited this PE-induced dephosphorylation event. Given our gene expression data where PE treatment caused alterations in MHC RNA transcription, the expression of α- and β-MHC proteins were also investigated. We did not see a significant change in α- and β-MHC protein expression with either AFos expression or with PE treatment over the duration of the experiment. There was, however, a nonsignificant trend toward an increased β-MHC protein expression in the PE-treated β-Gal control myocytes that was not seen in the AFos group. Suspecting that Western blot analysis may not be sensitive enough to detect a subtle change in MHC protein expression, we quantified the MHC isoform abundance by electrophoresis with direct protein staining and densitometry. Confirming our Western blot results, we did not see a change in the expression of α-MHC or β-MHC with either PE treatment or AFos expression by this method (data not shown).
Fig. 5.
AFos expression in adult rat ventricular myocytes reverses PE-mediated dephosphorylation of phospholamban (PLB) on serine-16. Myocytes were infected with β-Gal (100 MOI) or AFos (100 MOI) on culture day 0 and treated with PE (20 μmol/l) or vehicle on day 2 for an additional 48 h. Western blot analysis was performed on whole cell lysates with antibodies specific for α-MHC (n = 6), β-MHC (n = 6), sarco(endo)plasmic reticulum calcium-ATPase (SERCA; n = 6), PLB (n = 3), and phosphorylated phospholamban (p-PLB; n = 3). The bands were quantified by densitometry and corrected for loading with calnexin. Data were normalized to the β-Gal control.
DISCUSSION
The major finding of this report is that the functional deterioration of cardiomyocyte contraction and calcium handling that result from PE treatment can be inhibited by an abrogation of the activity of c-Fos with AFos expression. To explain this finding, the expression and modification of key proteins involved in SR calcium cycling were investigated. Here we report that 48 h of PE treatment led to the dephosphorylation of PLB at serine-16 which corresponded to the deterioration of cardiomyocyte contraction and calcium handling. AFos inhibited this dephosphorylation event and prevented the functional deterioration. We did not see, however, a change in the levels of either SERCA or PLB protein in PE-treated myocytes. Taken together, the decrease in phosphorylation of PLB without changes in the expression of SERCA and PLB protein level is associated with an overall decreased activity of SR calcium transport. This functional deterioration of SR calcium transport is consistent with our functional data showing depressed contractility and overall calcium handling at the single myocyte level.
Our data linking myocyte functional deterioration with dephosphorylation of Ser16-PLB have been established in both human heart failure and rodent heart failure models in vivo (21, 23, 24). Although it is known that the regulation of PLB phosphorylation is, in large part, a β-adrenergic process (34), contributions to PLB modification by the α-adrenergic cascade are not well understood and deserve more attention. Indeed, a clear distinction between the necessity of AP-1 transcription factors in transducing the hypertrophic effects of α-adrenergic, but not β-adrenergic, stimulation has been recently made (29), giving credence to the specificity of the AFos effect in our model. Independent of the signaling mechanisms, our data connecting reduced PLB phosphorylation with prolonged α-adrenergic stimulation are consistent with those seen with long-term β-adrenergic stimulation where adult rats treated with 4 days of isoproterenol infusion exhibited both a significant decrease in PLB protein phosphorylation as well as a decreased myocyte contractility (27). Whereas the long-term effects of α- and β-adrenergic stimulation appear to be similar, acute exposures of α- and β-adrenergic stimulation yield different results. Although an acute course of isoproterenol treatment led to an increased phosphorylation of Ser16-PLB, elevated calcium transient, and augmented cardiomyocyte contractility, similar α-adrenergic stimulation did not exert any change in the PLB protein density or level of phosphorylation (14, 15, 30, 31). To our knowledge, this is the first report to investigate the deleterious functional effects of prolonged PE treatment and changes in PLB phosphorylation, and further work is needed to clarify the involvement of prolonged α-adrenergic stimulation in Ser16-PLB phosphorylation in ARVMs.
Contrary to our expectation from the RNA results, there were no differences seen in the expression of MHC proteins with PE treatment, and no differences were noted between the β-Gal and AFos groups. The disparity between MHC RNA and protein expression is consistent with the long half-lives of MHC proteins, reported to be ∼5.5 days (3, 17). The time course of our experimental protocol that included 2 days of adenoviral infection followed by 2 days of PE treatment was likely not long enough to result in altered MHC isoform abundance. We did not pursue longer experimental courses to investigate whether MHC protein levels would follow the changes in RNA levels as the biological effects of PE were already present during our experimental protocol. Taken together, our data suggest that the dysregulation of calcium handling, rather than an altered abundance of the contractile proteins examined here, accounts for the decrement in myocyte performance seen with prolonged PE treatment.
The process of pathological cardiomyocyte growth, in and of itself, may not lead to contractile dysfunction. Using the model of NRVMs in primary culture, we previously showed that the pathological gene program could be successfully separated from myocyte growth (10) and have now extended this finding to ARVMs. We now present evidence that the expression of a more physiological gene pattern and a qualitative change in PLB, a key regulatory protein involved in contraction-excitation coupling, contribute to the inhibition of the contractile dysfunction that follows 48 h of PE treatment. This finding confirms our hypothesis that improving the genetic and protein makeup of the myocyte can lead to the preservation of myocyte function even in the setting of what would otherwise be considered “pathological” myocardial growth as measured by cell size or protein content alone. Taken in this context, myocardial hypertrophy may be an adaptive process to overcome a real or perceived stress, and the resultant myocyte function is based on the qualitative nature of the hypertrophy as seen by the alteration of key regulatory proteins involved in excitation-contraction coupling. Although it has been accepted that the presence of myocardial hypertrophy itself is a risk factor for increased cardiovascular morbidity and mortality (13), accumulating data indicate that the qualitative nature of hypertrophy is, in fact, the determining factor in the outcome (23). In certain disease states, such as acute myocardial infarction, the regional loss of cardiomyocytes due to cell death leads to a compensatory myocardial hypertrophy in unaffected regions. This adaptive hypertrophy may be a necessary response to meet the overall hemodynamic demand on the heart. The ultimate progression to pump failure is due to the dysregulation and dysfunction of the sarcomere. In light of the accumulating evidence and findings presented here, treatment strategies do not necessarily need to be directed at curbing the hypertrophy itself but rather directed toward altering the qualitative nature of the hypertrophy. Indeed, numerous studies have shown that RNA and protein profiles that are more physiological, as assessed by an α-MHC-to-β-MHC ratio that favors the α-subtype and the phosphorylation state of various proteins such as PLB, are associated with salutary effects on myocardial function (1, 16). In this same way we show here that suppressing the induction of the fetal gene program and maintaining a phosphorylation state of PLB that promotes SR calcium cycling correlate with improved cardiomyocyte function. Perhaps the most provocative aspect of our observation is that the effect we describe is initiated by altering the function of a single proto-oncogene c-Fos, a member of the AP-1 family of transcription factors.
Certainly, the involvement of AP-1 in both clinical heart failure and in animal models of pathological growth has been established; c-Jun levels are elevated in human cardiomyopathies (4), and AP-1 activity is decreased with blockade of the angiotensin-aldosterone axis (35). Interestingly, a novel anti-AP-1 agent, iguratimod, was successfully tested in a rheumatoid arthritis cohort in preparation for a long-term safety trial (6, 7). Although the gene expression findings presented here in response to inhibiting the transcriptional activity of c-Fos were not necessarily unexpected, the functional consequences of this effect and the characterization of the mechanism(s) behind the posttranslational modification of proteins are novel. An important next “proof of concept” step would be to show that abrogating the activity of c-Fos in animal model(s) of pathological myocardial growth could inhibit or prevent the expression of the pathological phenotype. In this regard, it is noteworthy that the expression of AFos in a chemical-induced skin carcinogenesis model was associated with a reversal of neoplastic transformation (5). It is tempting to speculate that the conditional expression of AFos in the heart under circumstances known to be associated with contractile dysfunction and progression to heart failure might also be associated with a return toward a more physiological gene profile and an improvement in global cardiac function. To that end, a better understanding of the involvement of AP-1 and the pathways involved in its signaling may offer novel therapeutic targets for the devastating syndrome of heart failure.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-059428 (to C. S. Long) and F32-HL-087479 (to M. Y. Jeong).
DISCLOSURES
None.
ACKNOWLEDGMENTS
We are grateful to Dr. P. M. Buttrick for editorial comments and to G. M. Jones for technical assistance.
REFERENCES
- 1.Chen G, Zhou X, Nicolaou P, Rodriguez P, Song G, Mitton B, Pathak A, Zachariah A, Fan GC, Dorn GW, 2nd, Kranias EG. A human polymorphism of protein phosphatase-1 inhibitor-1 is associated with attenuated contractile response of cardiomyocytes to beta-adrenergic stimulation. FASEB J 22: 1790–1796, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chinenov Y, Kerppola TK. Close encounters of many kinds: Fos-Jun interactions that mediate transcription regulatory specificity. Oncogene 20: 2438–2452, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Everett AW, Prior G, Zak R. Equilibration of leucine between the plasma compartment and leucyl-tRNA in the heart, and turnover of cardiac myosin heavy chain. Biochem J 194: 365–368, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frantz S, Fraccarollo D, Wagner H, Behr TM, Jung P, Angermann CE, Ertl G, Bauersachs J. Sustained activation of nuclear factor kappa B and activator protein 1 in chronic heart failure. Cardiovasc Res 57: 749–756, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Gerdes MJ, Myakishev M, Frost NA, Rishi V, Moitra J, Acharya A, Levy MR, Park SW, Glick A, Yuspa SH, Vinson C. Activator protein-1 activity regulates epithelial tumor cell identity. Cancer Res 66: 7578–7588, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M. Long-term safety study of iguratimod in patients with rheumatoid arthritis. Mod Rheumatol 17: 10–16, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Hara M, Abe T, Sugawara S, Mizushima Y, Hoshi K, Irimajiri S, Hashimoto H, Yoshino S, Matsui N, Nobunaga M, Nakano S. Efficacy and safety of iguratimod compared with placebo and salazosulfapyridine in active rheumatoid arthritis: a controlled, multicenter, double-blind, parallel-group study. Mod Rheumatol 17: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Hilfiker-Kleiner D, Hilfiker A, Castellazzi M, Wollert KC, Trautwein C, Schunkert H, Drexler H. JunD attenuates phenylephrine-mediated cardiomyocyte hypertrophy by negatively regulating AP-1 transcriptional activity. Cardiovasc Res 71: 108–117, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Hilfiker-Kleiner D, Hilfiker A, Kaminski K, Schaefer A, Park JK, Michel K, Quint A, Yaniv M, Weitzman JB, Drexler H. Lack of JunD promotes pressure overload-induced apoptosis, hypertrophic growth, and angiogenesis in the heart. Circulation 112: 1470–1477, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Jeong MY, Kinugawa K, Vinson C, Long CS. AFos dissociates cardiac myocyte hypertrophy and expression of the pathological gene program. Circulation 111: 1645–1651, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev 7: 1309–1317, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Kim-Mitsuyama S, Izumi Y, Izumiya Y, Namba M, Yoshida K, Wake R, Yoshiyama M, Iwao H. Dominant-negative c-Jun inhibits rat cardiac hypertrophy induced by angiotensin II and hypertension. Gene Ther 13: 348–355, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322: 1561–1566, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Lindemann JP. Alpha-adrenergic stimulation of sarcolemmal protein phosphorylation and slow responses in intact myocardium. J Biol Chem 261: 4860–4867, 1986 [PubMed] [Google Scholar]
- 15.Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. Beta-adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem 258: 464–471, 1983 [PubMed] [Google Scholar]
- 16.Lowes BD, Gilbert EM, Abraham WT, Minobe WA, Larrabee P, Ferguson D, Wolfel EE, Lindenfeld J, Tsvetkova T, Robertson AD, Quaife RA, Bristow MR. Myocardial gene expression in dilated cardiomyopathy treated with beta-blocking agents. N Engl J Med 346: 1357–1365, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Martin AF, Rabinowitz M, Blough R, Prior G, Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem 252: 3422–3429, 1977 [PubMed] [Google Scholar]
- 18.Okada S, Wang ZQ, Grigoriadis AE, Wagner EF, von Ruden T. Mice lacking c-fos have normal hematopoietic stem cells but exhibit altered B-cell differentiation due to an impaired bone marrow environment. Mol Cell Biol 14: 382–390, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. A dominant negative to activation protein-1 (AP1) that abolishes DNA binding and inhibits oncogenesis. J Biol Chem 272: 18586–18594, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Omura T, Yoshiyama M, Yoshida K, Nakamura Y, Kim S, Iwao H, Takeuchi K, Yoshikawa J. Dominant negative mutant of c-Jun inhibits cardiomyocyte hypertrophy induced by endothelin 1 and phenylephrine. Hypertension 39: 81–86, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Sande JB, Sjaastad I, Hoen IB, Bokenes J, Tonnessen T, Holt E, Lunde PK, Christensen G. Reduced level of serine(16) phosphorylated phospholamban in the failing rat myocardium: a major contributor to reduced SERCA2 activity. Cardiovasc Res 53: 382–391, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Satoh N, Suter TM, Liao R, Colucci WS. Chronic alpha-adrenergic receptor stimulation modulates the contractile phenotype of cardiac myocytes in vitro. Circulation 102: 2249–2254, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Schmitt JP, Kamisago M, Asahi M, Li GH, Ahmad F, Mende U, Kranias EG, MacLennan DH, Seidman JG, Seidman CE. Dilated cardiomyopathy and heart failure caused by a mutation in phospholamban. Science 299: 1410–1413, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca2+-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol 31: 479–491, 1999 [DOI] [PubMed] [Google Scholar]
- 25.Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol 4: E131–E136, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Shaulian E, Karin M. AP-1 in cell proliferation and survival. Oncogene 20: 2390–2400, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Stein B, Bartel S, Kokott S, Krause EG, Schlichtmann T, Schmitz W, Scholz H. Effects of isoprenaline on force of contraction, cAMP content, and phosphorylation of regulatory proteins in hearts from chronic beta-adrenergic-stimulated rats. Ann NY Acad Sci 752: 230–233, 1995 [DOI] [PubMed] [Google Scholar]
- 28.Sucharov CC, Dockstader K, McKinsey TA. YY1 protects cardiac myocytes from pathologic hypertrophy by interacting with HDAC5. Mol Biol Cell 19: 4141–4153, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taimor G, Schluter KD, Best P, Helmig S, Piper HM. Transcription activator protein 1 mediates α- but not β-adrenergic hypertrophic growth responses in adult cardiomyocytes. Am J Physiol Heart Circ Physiol 286: H2369–H2375, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Talosi L, Edes I, Kranias EG. Intracellular mechanisms mediating reversal of β-adrenergic stimulation in intact beating hearts. Am J Physiol Heart Circ Physiol 264: H791–H797, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Talosi L, Kranias EG. Effect of alpha-adrenergic stimulation on activation of protein kinase C and phosphorylation of proteins in intact rabbit hearts. Circ Res 70: 670–678, 1992 [DOI] [PubMed] [Google Scholar]
- 32.Walker JS, Walker LA, Etter EF, Murphy RA. A dilution immunoassay to measure myosin regulatory light chain phosphorylation. Anal Biochem 284: 173–182, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature 360: 741–745, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Wegener AD, Simmerman HK, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem 264: 11468–11474, 1989 [PubMed] [Google Scholar]
- 35.Yoshiyama M, Omura T, Takeuchi K, Kim S, Shimada K, Yamagishi H, Teragaki M, Akioka K, Iwao H, Yoshikawa J. Angiotensin blockade inhibits increased JNKs, AP-1 and NF-kappa B DNA-binding activities in myocardial infarcted rats. J Mol Cell Cardiol 33: 799–810, 2001 [DOI] [PubMed] [Google Scholar]