Abstract
Therapeutic hypothermia (TH) cardioprotection has recently been associated with increased Akt signaling in a rat model of cardiac arrest. However, it is not known whether Akt is required for this beneficial effect of TH. We used a mouse model of cardiac arrest demonstrating TH cardioprotection to study the response of mice deficient in an Akt1 allele. We hypothesized that Akt1 mediates TH cardioprotection and that decreases in Akt1 content would diminish such protection. Adult C57BL/6 wild-type (WT) mice underwent an 8-min cardiac arrest. After 6 min, the mice were randomized to normothermia (WTNT, 37°C) or TH (WTTH, 30°C). Following cardiopulmonary resuscitation and the return of spontaneous circulation (ROSC), the animals were hemodynamically monitored for 240 min (R240). At R240, cardiac tissue Akt content and phosphorylation were assayed. Studies were repeated in Akt1 heterozygous (Akt1+/−) mice. As a result, baseline characteristics and ROSC rates were equivalent across groups. At R240, WTTH mice exhibited lower heart rate, larger stroke volume, and higher cardiac output than WTNT animals (P < 0.05). Cardioprotection in WTTH at R240 was associated with increased cardiac Akt phosphorylation at Ser473 and Thr308 compared with that in WTNT (P < 0.05). TH-associated alterations in Akt phosphorylation, stroke volume, heart rate, and cardiac output were abrogated in Akt1+/− animals. In conclusion, TH improves post-ROSC cardiac function and increases Akt phosphorylation in WT, but not Akt1+/−, mice. The Akt1 isoform appears necessary for TH-mediated cardioprotection.
Keywords: sudden cardiac death, asystole, cardiopulmonary resuscitation, therapeutic hypothermia, protein kinase B
sudden cardiac arrest is a leading contributor to cardiovascular disease mortality with only 5–7% of out-of-hospital cardiac arrest victims surviving hospital discharge (27). During the first minutes to hours following the return of spontaneous circulation (ROSC), patients often exhibit severe and lethal myocardial dysfunction than can occur in the absence of underlying focal coronary artery occlusion (8, 16, 25, 38, 41). The mechanisms of post-ROSC myocardial depression are likely related to factors associated with ischemia-reperfusion (I/R) injury including substrate depletion, acidosis, oxidative stress, mitochondrial injury, alterations in intracellular calcium handling and in circulating cytokines, and the posttranslational modification of contractile and mitochondrial proteins (6, 20, 23, 24, 28).
The induction of mild to moderate therapeutic hypothermia (TH) has been demonstrated to improve survival and neurological outcomes in comatose survivors of out-of-hospital cardiac arrest, in part, by limiting cardiac dysfunction during the immediate post-ROSC period (3, 40). Researchers from our laboratory and others have previously demonstrated that intra-arrest hypothermia attenuates cardiac dysfunction following cardiac arrest though the mechanism of this protection is poorly understood (1, 18, 47). The serine/threonine kinase Akt (PKB), which has three isoforms in the mammalian genome (Akt1, Akt2, and Akt3), has emerged as a central mediator of contractile function within the heart in the setting of I/R injury (33). In addition, Akt has been shown to be activated by a variety of cardioprotective interventions including growth factors and hypothermia (18, 29, 48). However, it is unknown whether Akt activation is an essential feature of TH or merely an epiphenomenon. To address this question, we used a mouse model of cardiac arrest to assess the effect of TH on the phosphorylation state of Akt and to study the effect of decreased Akt1 expression on postarrest cardiac dysfunction and TH protection (33).
METHODS
Animal preparation and cardiac arrest protocol.
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago and have been detailed in our prior work (1, 47). Briefly, mice were anesthetized with 80 μg/g of ketamine (Phoenix Scientific, St. Joseph, MO) and 12 μg/g xylazine (Ben Venue Laboratories, Bedford, OH) with periodic redosing of 20–30% of the initial ketamine dose as required to maintain surgical anesthesia. Rectal temperature was monitored and maintained at 37 ± 0.5°C throughout the surgical preparation. Mice were orally intubated and mechanically ventilated with a tidal volume of 12.5 μl/g, a respiratory rate of 110 breaths/min, an inspired oxygen fraction of 1.0, and a positive end-expiratory pressure of 2 cmH2O. An ultraminiature pressure-volume (P-V) catheter (SPR-839, P/N 840-8111, Millar Instruments, Houston, TX) was inserted into the left ventricle for hemodynamic measurements, a microcatheter (BioTime, Berkeley, CA) was inserted into the left jugular vein for fluid administration, and needle probes were placed to provide three-lead ECG.
Following this surgical preparation, animals were monitored for 50 min from the time of anesthesia induction; asystolic cardiac arrest was then induced in mice with mean arterial pressures (MAPs) >80 mmHg and a partial pressure of end-tidal CO2 of >35 mmHg by the intravenous administration of 0.08 mg/g potassium chloride solution (Sigma, St. Louis, MO). After 8 min of arrest, resuscitation was attempted with chest compressions, mechanical ventilation, and scheduled fluid administration as previously described (47). ROSC was defined as the return of sinus rhythm with a MAP of >40 mmHg lasting at least 5 min. Resuscitation efforts were terminated after 5 min or upon hemodynamic evidence of initial ROSC. Successfully resuscitated animals were monitored hemodynamically on mechanical ventilation for up to 240 min during which time they received scheduled intravenous injections of 0.9% saline at a rate of 100 μl/h. Early protocol termination was triggered immediately for death because of cardiovascular collapse as defined by a MAP of <40 mmHg for at least 5 min.
Experimental groups.
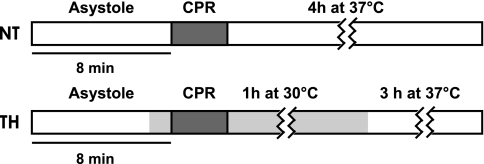
Following cardiac arrest induction (Fig. 1), adult female wild-type (WT) mice (C57BL/6, Taconic Farms, Germantown, NY) were randomized to either continued normothermia (NT, 37 ± 0.5°C) or intra-arrest hypothermia (TH, 30 ± 0.5°C) induced after 6 min as previously described (1, 47). At 60 min post-ROSC, TH animals were rewarmed over 20 min to 37 ± 0.5°C. This TH protocol has been previously shown to improve cardiac function during the first 120 min following ROSC as well as 3-day neurologically intact survival in WT mice (1, 47). This randomization protocol was later repeated in a cohort of Akt1 heterozygous (Akt1+/−) mice (B6.129P2-Akt1tm1Mbb/J, Jackson Laboratories, Bar Harbor, ME). Akt1+/− mice have a normal phenotype. Akt1−/− mice were not used as they have significantly lower body mass than WT mice and can demonstrate altered myocardial fiber size and other heart defects (9, 11).
Fig. 1.
Schematic of experimental protocol. Thick horizontal lines show duration of cardiac arrest, dark gray box represents cardiopulmonary resuscitation (CPR) interval, and light gray area represents interval of therapeutic hypothermia (TH, 30 ± 0.5°C). At 1 h post-return of spontaneous circulation (ROSC), animals were rewarmed to 37 ± 0.5°C. NT, normothermia.
Physiological signal acquisition and processing.
Continuous physiological signals were digitally acquired using PowerLab Chart (ADInstruments, Colorado Springs, CO). Continuous left ventricular P-V data were acquired from the P-V catheter via the Millar Instruments P-V conductance system (MPCU-200, Millar Instruments). Volume data were initially acquired as a conductance voltage signal and converted into relative volume units using the built-in electronic calibration settings on the MPCU-200 and then later converted into true volume units (in μl) using a linear relationship derived from a calibration system described by the manufacturer. Parametric indexes of left ventricular function were derived from P-V catheter data using Millar PVAN 3.0 analytic software.
Tissue Western blot methods.
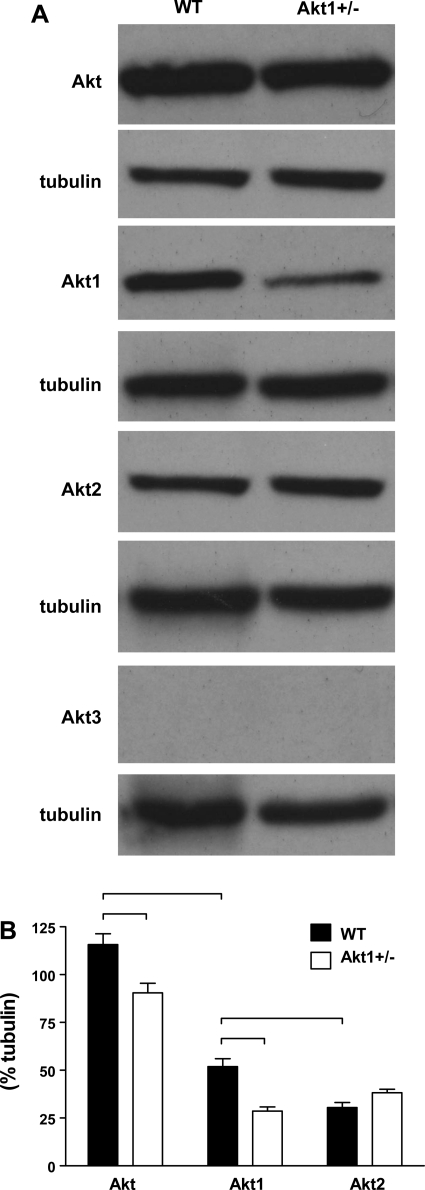
Western blot analysis was used to measure whole heart total Akt content and phosphorylation at the phospho (p)-Akt (Ser473) and p-Akt (Thr308) sites in a subset of WT and Akt1+/− animals (n = 3 to 5 animals) surviving the entire 240-min post-ROSC period. Following death under anesthesia, hearts were harvested and snap frozen in liquid nitrogen and stored at −80°C for subsequent analysis. For comparison, hearts were also obtained from sham arrest WT and Akt1+/− mice (n ≥ 3) after 240 min of continued sedation and hemodynamic monitoring without arrest. Naïve animals, WT (n = 4) and Akt1+/− (n = 12), were euthanized to characterize the relative expression of the total Akt, Akt1, Akt2, and Akt3 isoforms in the heart.
For protein isolation, the hearts were pulverized under liquid nitrogen into a fine powder, and proteins were extracted in ice-cold lysis buffer (Cell Signaling, Danvers, MA) on ice for 10 min. Protein concentrations were determined using the Bradford protein assay (Bio-Rad, Hercules, CA). Proteins (25 μg) were separated on 10% gels by SDS-PAGE, transferred to a nitrocellulose membrane, and subjected to immunoblotting with appropriate primary antibody (Cell Signaling) overnight at 4°C. After being washed and incubated with appropriate secondary antibody, the bands were visualized using chemiluminescent detection. Densitometry analysis of Western blots was carried out using Quantity One Software (Bio-Rad, Richmond, CA).
Statistical methods.
All statistical computations were performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA) with significance levels at P > 0.05 except where noted. Continuous data were described by group means and standard deviation except where noted. Baseline characteristics were compared using the two-tailed Student's t-test. ROSC rates were compared by Fisher exact testing. Kaplan-Meier survival analysis was performed using log-rank (Mantel-Cox) testing. Densitometric and serial hemodynamic comparisons were made by the nonparametric Kruskal-Wallis ANOVA by ranks test with post hoc Dunn's testing.
RESULTS
Survival and hemodynamics in WT mice.
A total of 23 of 25 (92%) WT mice underwent successful instrumentation, cardiac arrest, and randomization to WTNT or WTTH intervention groups. Baseline characteristics including weight, heart rate, and partial pressure of end-tidal CO2 were identical between groups (Table 1). In addition, baseline left ventricular performance indexes including maximum pressure (LVPmax), maximum rate of change in left ventricular pressure (dP/dtmax), diastolic relaxation time constant (τ), stroke volume, and cardiac output were statistically indistinguishable.
Table 1.
WT group baseline and resuscitation period characteristics
| WTNT | WTTH | |
|---|---|---|
| n | 13 | 10 |
| Baseline | ||
| Body weight, g | 31.6 ± 2.3 | 32.0 ± 2.2 |
| Heart rate, beats/min | 292 ± 50 | 285 ± 20 |
| LVPmax, mmHg | 99.0 ± 11.4 | 101 ± 11.6 |
| dP/dtmax, mmHg/ms | 7.3 ± 1.2 | 8.2 ± 1.1 |
| τ, ms | 9.0 ± 1.2 | 9.0 ± 1.2 |
| Stroke volume, μl | 12.0 ± 2.1 | 12.4 ± 1.4 |
| Cardiac output, ml/min | 3.2 ± 0.5 | 3.5 ± 0.30 |
| PetCO2, mmHg | 32.3 ± 4.88 | 31.7 ± 7.16 |
| CPR | ||
| CC rate, beats/min | 342 ± 59 | 301 ± 41 |
| DBP, mmHg | 15.6 ± 3.6 | 19.3 ± 4.4 |
| PetCO2, mmHg | 20.4 ± 3.2 | 14.8 ± 3.6* |
| ROSC, n (%) | 9 (90) | 8 (80) |
| Time to ROSC†, s | 178 ± 61 | 141 ± 51 |
Values are means ± SD; n, number of mice. WT, wild-type; NT, normothermia; TH, therapeutic hypothermia; LVPmax, maximum left ventricular pressure; dP/dtmax, maximum rate of change in left ventricular pressure; τ, diastolic relaxation time constant; PetCO2, partial pressure end-tidal CO2; CC rate, chest compression rate; DBP, diastolic blood pressure during cardiopulmonary resuscitation (CPR); ROSC, return of spontaneous circulation.
P < 0.05, statistically significant difference by Student's t-test.
Calculated for subset of animals achieving ROSC.
During resuscitation, measures of cardiopulmonary resuscitation (CPR) quality including chest compression rate and aortic diastolic blood pressure were equivalent between the intervention groups (Table 1). As previously reported in this model, partial pressure of end-tidal CO2 during CPR was significantly lower in WTTH versus WTNT groups (14.8 ± 3.6 vs. 20.4 ± 3.2 mmHg, P < 0.05) (47). The rate of initial ROSC and the time until ROSC were equivalent between groups.
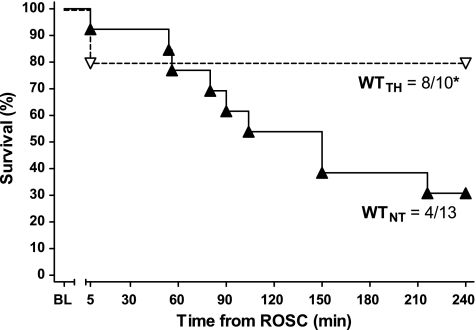
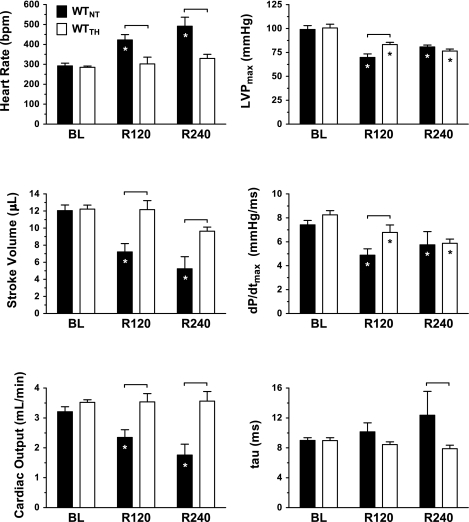
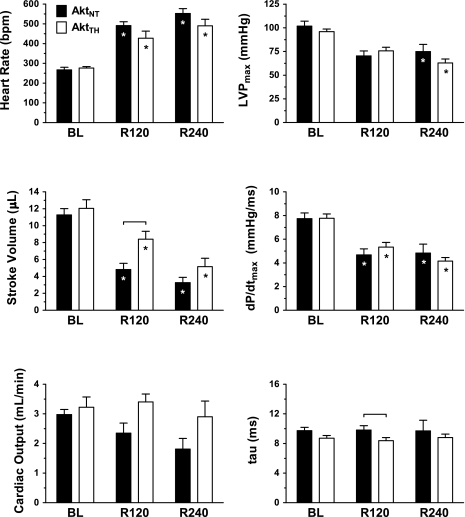
TH conferred a significant short-term survival benefit (Fig. 2) in WTTH compared with WTNT (80% vs. 31%, P < 0.05) at 240 min post-ROSC (R240). When compared with WTNT, WTTH animals displayed lower heart rate and higher stroke volume (Fig. 3) at 120 min (R120) post-ROSC, which were sustained at R240 (P < 0.05). These differences translated into persistent group differences in cardiac output in WTTH versus WTNT at R120 (2.4 ± 0.3 vs. 3.5 ± 0.3 ml/min, respectively, P < 0.05) and at R240 (3.6 ± 0.3 vs. 1.8 ± 0.4 ml/min, P < 0.05). TH-treated animals also exhibited significantly improved LVPmax and dP/dtmax relative to NT at R120 (P < 0.05). However, treatment-related differences in LVPmax and dP/dtmax did not persist in the subgroup of animals surviving to R240.
Fig. 2.
Kaplan-Meier plot of NT wild-type (WTNT) and TH wild-type (WTTH) survival following cardiac arrest. *P < 0.05, TH conferred statistically significant survival benefit at 240 min post-ROSC relative to NT group (Mantel-Cox log-rank). BL, baseline.
Fig. 3.
Indexes of left ventricular function in WT animals. Heart rate [in beats/min (bpm)], left ventricular maximum pressure (LVPmax), stroke volume, maximum rate of change in left ventricular pressure (dP/dtmax), cardiac output, and diastolic relaxation time constant (τ) are displayed at BL before arrest and at 120 min (R120) and 240 min (R240) post-ROSC. *P < 0.05, significant difference relative to BL. P < 0.05 (denoted by bracket), significant difference between treatment groups.
Cardiac Akt activation in WT mice.
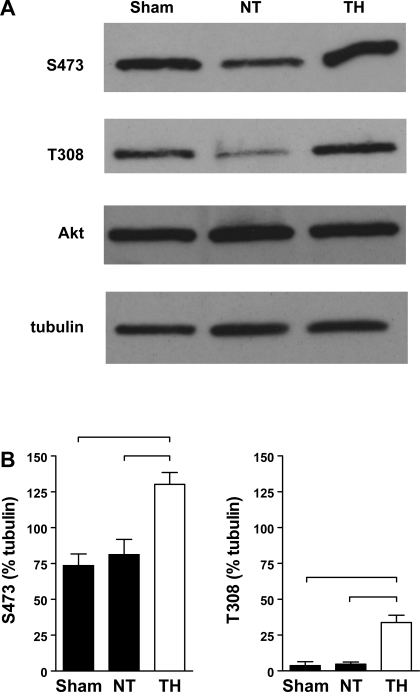
Akt activation after cardiac arrest resuscitation was assessed by phosphorylation. Whole heart lysates from sham-operated WT animals exhibited basal Akt protein expression as well as significant phosphorylation at the p-Akt (Ser473) site and minimal phosphorylation at the p-Akt (Thr308) site (Fig. 4). At R240, the phosphorylation at p-Akt (Thr308) and p-Akt (Ser473) was significantly elevated in the TH group relative to both the sham and NT groups. Heart total Akt protein content did not vary significantly between groups.
Fig. 4.
A: Akt phosphorylation at phospho (p)-Akt [Ser473 (S473)] and p-Akt [Thr308 (T308)] in WT hearts at R240. Western blot analysis of whole heart lysates collected from WTNT, WTTH, and normothermic WT sham-arrested animals at 240 min. B: densitometry, expressed as a percentage of β-tubulin, revealed significant differences in phosphorylation (P < 0.05, denoted by bracket).
Cardiac Akt expression in WT versus Akt1+/− mice.
Evaluation of the relative expression of the three mammalian isoforms of Akt in naïve WT mice (Fig. 5) revealed a significantly higher expression of Akt1 compared with Akt2 (P < 0.05) and no measurable expression of Akt3. This result was consistent with prior reports of Akt1 being the most abundant Akt isoform found in the heart (33). When compared with that of naïve WT mice, the expression of total Akt and Akt1 in naïve Akt1+/− mice was significantly less (P < 0.05). A small, nonsignificant increase in Akt2 expression was noted in the hearts of Akt1+/− animals compared with that in WT mice. Thus there did not appear to be any significant compensation by non-Akt1 isoforms in the heart as a result of partial Akt1 deletion.
Fig. 5.
A: protein expression of Akt isoforms in whole heart lysates from naïve WT and Akt1+/− animals. B: densitometric analysis of Western blots revealed significant strain-related differences in total Akt, and Akt1 expression (P < 0.05, denoted by bracket). Akt3 was not detected in whole heart lysates.
Survival and hemodynamics in Akt1+/− mice.
For the Akt1+/− group, 18 of 22 (82%) mice underwent successful instrumentation, cardiac arrest, and randomization to Akt1NT+/− or Akt1TH+/− groups. As with the WT groups, baseline characteristics, CPR quality indexes, ROSC rates, and time until ROSC were identical between the intervention groups (Table 2). In contrast to those in the WT mice, survival rates to R240 were equivalent between the Akt1NT+/− (5 of 8, 65%) and Akt1TH+/− (7 of 10, 70%) groups. During the post-ROSC period, both Akt1+/− groups (Fig. 6) displayed significant increases in heart rate and decreases in LVPmax, stroke volume, and dP/dtmax relative to baseline (P < 0.05). TH treatment-related differences in heart rate, LVPmax, dP/dtmax, or τ were not observed in Akt1+/− animals. In contrast to WT animals, TH-related improvements in stroke volume dissipated by R240 in Akt1+/− animals. Similarly, while there was a trend toward an increased cardiac output at R120 and R240 in Akt1 TH+/− vs. Akt1 NT+/−, this trend did not reach significance.
Table 2.
Akt1+/− group baseline and resuscitation period characteristics
| Akt1NT+/− | Akt1TH+/− | |
|---|---|---|
| n | 8 | 10 |
| Baseline | ||
| Body weight, g | 30.9 ± 4.9 | 30.5 ± 3.1 |
| Heart rate, beats/min | 267 ± 34 | 276 ± 23 |
| LVPmax, mmHg | 101.8 ± 14.0 | 95.9 ± 8.8 |
| dP/dtmax, mmHg/ms | 7.7 ± 1.3 | 7.7 ± 1.2 |
| τ, ms | 9.7 ± 1.2 | 8.7 ± 1.1 |
| Stroke volume, μl | 11.3 ± 2.0 | 12.1 ± 3.2 |
| Cardiac output, ml/min | 3.0 ± 0.5 | 2.9 ± 0.7 |
| PetCO2, mmHg | 37.1 ± 5.68 | 37.8 ± 5.00 |
| CPR | ||
| CC rate, beats/min | 345 ± 44 | 358 ± 36 |
| DBP, mmHg | 21.4 ± 8.8 | 22.4 ± 7.8 |
| PetCO2, mmHg | 24.5 ± 5.6 | 20.8 ± 4.7 |
| ROSC, n (%) | 8 (100) | 8 (80) |
| Time to ROSC*, s | 137 ± 46 | 147 ± 59 |
Values are means ± SD; n, number of mice.
Calculated for subset of animals achieving ROSC.
Fig. 6.
Indexes of left ventricular function in Akt1+/− animals. Heart rate, LVPmax, stroke volume, dP/dtmax, cardiac output, and τ are displayed at BL before arrest and at R120 and R240. *P < 0.05, significant difference relative to BL. P < 0.05 (denoted by bracket), significant difference between treatment groups.
Cardiac Akt activation in Akt1+/− mice.
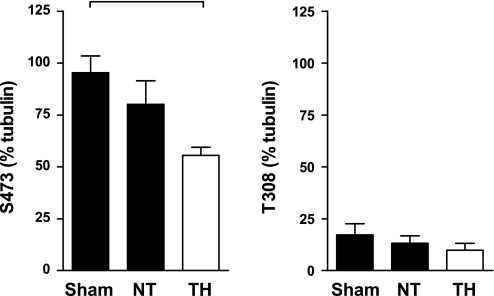
In the Akt1+/− animals (Fig. 7), phosphorylation at the p-Akt (Ser473) and p-Akt (Thr308) sites was apparent in sham-operated animals and was unchanged at 240 min following ROSC in NT animals. In contrast to WT animals, TH-treated animals exhibited a significant decrease in phosphorylation at p-Akt (Ser473) by 240 min following ROSC with no significant change at p-Akt (Thr308). Total Akt protein levels did not vary significantly between groups (data not shown).
Fig. 7.
Akt phosphorylation at p-Akt (Ser473) and p-Akt (Thr308) in Akt1+/− hearts at R240 compared with normothermic Akt1+/− sham-arrested mice. P < 0.05 (denoted by bracket), densitometry (expressed as a percentage of β-tubulin) revealed significant differences.
DISCUSSION
In the present study, we demonstrate that TH, induced before resuscitation from cardiac arrest and continued 1 h after ROSC, results in cardioprotection that lasts for hours following ROSC and rewarming. Furthermore, we demonstrate that such cardioprotection is associated with increased cardiac Akt activity as measured by increased phosphorylation. Finally, our results suggest that the loss of an Akt1 allele, resulting in decreased cardiac Akt1 protein content, disrupts this pattern of TH cardioprotection and associated phosphorylation.
Effect of TH post-ROSC cardiac dysfunction in WT and Akt1+/− mice.
Victims of cardiac arrest frequently exhibit severe myocardial dysfunction during the first minutes to hours following ROSC, which negatively impacts outcomes and can occur in the absence of underlying focal coronary artery occlusion (8, 16, 25, 37, 38, 41). In agreement with this clinical literature, as well as several large animal models of cardiac arrest (15, 21, 22), both WT and Akt1+/− mice displayed severe cardiac dysfunction following a brief period of whole body ischemia. Our results in WT mice are also consistent with past reports in our model and other's demonstrating that TH induces improvement in several indexes of left ventricular function (18, 47). We add to this literature by demonstrating that TH-associated improvements in systolic function, such as LVPmax and dP/dtmax, may dissipate during the first few hours following ROSC. We further add to this literature by demonstrating that in Akt1+/− animals, TH did not significantly alter heart rate or measures of systolic function including LVPmax and dP/dtmax. This difference in treatment effect implies that Akt1 may play an important role in mediating this aspect of hypothermic protection.
Our study is also one of the first to suggest that intra-arrest TH induces improvements in diastolic relaxation, stroke volume, and cardiac output (18, 47) and the first to document that such improvements may persist for up to 3 h following rewarming. Interestingly, Akt1TH+/− animals displayed only transient improvement in stroke volume relative to Akt1NT+/− animals, suggesting a role that TH may preserve stroke volume through an Akt1-dependent mechanism.
Akt-dependent mechanisms of TH cardioprotection.
The mechanisms of such post-ROSC myocardial depression and TH protection are not well described but are likely related to factors associated with I/R injury including substrate depletion, acidosis, oxidative stress, mitochondrial injury, and alterations in intracellular calcium handling (6, 12, 20, 23, 24, 28). Our results suggest that the serine/threonine kinase Akt (PKB), which has emerged as a central molecule within signal transduction pathways connecting extracellular growth factors and stressors with a variety of intracellular mechanisms related to cardiomyocyte growth and survival (14), may also play a protective signaling role in TH cardioprotection following ROSC. Consistent with this theory, increased levels of heart p-Akt have also been associated with erythropoietin-induced preservation of left ventricular function in a rat model of asphyxia-induced cardiac arrest (17, 19). Consistent with our results, the overexpression of Akt in the heart has been shown to be cardioprotective following I/R injury (14). However, no work has been done to manipulate Akt1 content specifically in the context of cardiac arrest. Our results add to this literature by demonstrating, for the first time, that a partial reduction in Akt1 expression can affect the hemodynamic response post-ROSC in NT and TH animals. Such manipulation would impact the expression of Akt1 in both the heart as well as in peripheral tissues, such as skeletal muscle, that produce factors affecting heart function (34).
TH-induced enhancement of Akt signaling during the post-ROSC period, as demonstrated in our WT mice, could protect the heart via both direct cardiomyocyte and endothelial effects. At the level of the cardiomyocyte, treatment-related differences in ventricular chronotropy, contractility, and lusitropy suggest that such protection may involve calcium regulation at the level of sarcolemmal or sarcoplasmic reticulum or potentially via protective interactions involving localization to the mitochondrial outer membrane (7). Specifically, Akt is thought to mediate signals from a variety of receptors including insulin receptors, estrogen receptors, and G protein-coupled receptors such as β-adrenergic receptors (26, 46). Furthermore, Akt signaling pathways have been recently implicated in the fine tuning of intracellular calcium concentrations through effectors including L-type calcium channels, ryanodine receptors (RyR), and sarcoplasmic reticulum calcium-ATPase pumps. In addition to altering contractility and lusitropy, Akt-mediated alterations in intracellular calcium handling could theoretically alter sinoatrial automaticity and β-adrenergic signaling and thus explain the differences in the chronotropic response to cardiac arrest, resuscitation, and hypothermia noted between our experimental groups. At the level of the endothelium, we have also recently reported that enhanced Akt activation during oxidant stress in human microvascular endothelial cells is necessary for the restoration of barrier function (13). Though not measured in the present in vivo study, such reduced cardiac Akt activity could result in greater microvascular dysfunction in NT and Akt1+/− animals following ROSC.
Hypothermic modulation of Akt signaling.
Hypothermic adaptations are thought to upregulate a variety of cell survival signaling pathways at the transcriptional as well as the posttranslational regulatory level (29–32, 39). Our results suggest that TH sustains Akt activity following rewarming and are consistent with recent evidence from a rat model of asphyxial arrest, which suggests that the induction of TH following ROSC may increase Akt phosphorylation at 2 h post-ROSC in treated animals (18). TH has also been shown to preserve p-Akt (Ser473) levels during cerebral ischemia and to attenuate the decline in Akt kinase activity in the brain following reperfusion (48). Other work in isolated hearts suggests that moderate (30°C), but not mild (34°C), hypothermia during 120 min of index ischemia exhibits a preserved expression of Akt1 as well as several stress response proteins such as hypoxia-inducible factor-1α and heme oxygenase-1 and nuclear transcriptional factors such as peroxisome proliferator-activated receptor-β and peroxisome proliferator-activated receptor-γ coactivator-1α that are important to the regulation of a number of mitochondrial genes involved in oxidative metabolism (29). Our findings that TH increased p-Akt at 240 min following ROSC are consistent with these studies and extend this work into a mouse model of cardiac arrest.
In the heart, constitutive activity of Akt1, the dominant isoform within the mammalian heart, is enhanced or preserved by a variety of growth factors that signal via phosphoinositide 3-kinases through the phosphorylation of the serine [p-Akt (Ser473)] and threonine [p-Akt (Thr308)] sites in the COOH-terminal (regulatory) and NH2-terminal (kinase) domains, respectively (2, 33). While the mechanism for TH preservation of Akt phosphorylation is unknown, it could potentially alter the relative balance of kinase or phosphatase activity. Alternatively, TH could modulate Akt activity through the activation of heat shock/cold shock proteins or the modulation of reactive oxygen species-sensitive signaling pathways (10, 35, 39). The observed reduction in Akt phosphorylation in Akt1+/− mice could reflect a change in the relative stoichiometry of Akt, kinases, and phosphatases including protein phosphatase 2 in these animals.
Consistent with reports by others, our work demonstrates that among the three Akt isoforms, Akt1 is predominantly expressed in the heart (33). To our knowledge, no reports exist regarding the relative Akt1 and Akt2 isoform concentrations in the heart in Akt1+/− mice. Our current work suggests that there is a significant myocardial reduction of Akt1 in these animals without compensatory increases in Akt2 or Akt3. To our knowledge, this is the first report to suggest that a partial reduction in Akt1 expression can affect outcome in a whole animal model of cardiac arrest. In addition, our results suggest that a partial reduction of Akt1 abrogates a TH-induced increase in Akt activity following ROSC.
Akt activation strategies and the translational challenges of intra-arrest TH.
Intra-arrest cooling has been successfully translated into a few large animal models of cardiac arrest (5, 36, 45) and a recent study of out-of-hospital cardiac arrest (4). In addition, induced temperatures of 32–34°C before reperfusion with percutaneous coronary intervention have been reported in one study of cardiac arrest survivors with evidence of acute myocardial infarction (43). Despite these successful demonstration projects, the rapid induction of hypothermia in cardiac arrest patients before ROSC introduces additional thermodynamic, physiological, and implementation challenges and complexity to the earliest links in the chain of survival (42, 45). Accordingly, the adjunct use of pharmacological TH mimetic agents could conceivably improve TH protection in postcardiac arrest patients. Our results suggest that Akt1 is a myocardial target of TH that could hold promise in this regard.
Limitations.
The interpretation of our results is somewhat limited by the use of a partial, rather than complete, Akt1 knockout mouse with reduced, rather than ablated, Akt1 expression. However, this experimental design decision was made given the knowledge that mice with a homozygous disruption of the Akt1 gene (Akt1−/−) exhibit 50% perinatal mortality, growth retardation, and decreased life span, which poses a significant technical, physiological, and financial barrier to using these animals in our adult mouse model of cardiac arrest (9, 11). Also, while we did not observe an elevated expression of Akt2 or Akt3 isoforms in the Akt1+/− group, it is possible that these mice may develop compensation by other proteins involved in Akt1 signaling within the heart. Finally, this study was designed to focus on cardiovascular events immediately following ROSC. Accordingly, a generalization of our study results to long-term outcomes is limited. Finally, recent studies have also suggested subtle differences in the degree of myocardial dysfunction following electrically induced, as well as ischemia-induced, models of ventricular fibrillation arrest (44). Thus additional work is warranted to test the conclusions from our KCl model of arrest in other cardiac arrest etiologies.
Conclusions.
TH preserves post-ROSC cardiovascular function and increases Akt activity in WT mice. The response to TH is altered dramatically in Akt1-deficient mice, suggesting that TH modulates Akt-dependent cardioprotective pathways.
GRANTS
The project was supported by National Heart, Lung, and Blood Institute Grants K08-HL-091184 (to D. G. Beiser), RO1-HL-68951 (to T. L. Vanden Hoek), and RO1-HL-79641 and RO1-HL-84643 (to K. J. Hamann). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
REFERENCES
- 1.Abella B, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker L. Intra-arrest cooling improves outcomes in a murine cardiac arrest Model. Circulation 109: 2786–2791, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15: 6541–6551, 1996 [PMC free article] [PubMed] [Google Scholar]
- 3.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 346: 557–563, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Bernard SA, Rosalion A. Therapeutic hypothermia induced during cardiopulmonary resuscitation using large-volume, ice-cold intravenous fluid. Resuscitation 76: 311–313, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Boddicker KA, Zhang Y, Zimmerman MB, Davies LR, Kerber RE. Hypothermia improves defibrillation success and resuscitation outcomes from ventricular fibrillation. Circulation 111: 3195–3201, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev 79: 609–634, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Catalucci D, Condorelli G. Effects of Akt on cardiac myocytes: location counts. Circ Res 99: 339–341, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Chang WT, Ma MH, Chien KL, Huang CH, Tsai MS, Shih FY, Yuan A, Tsai KC, Lin FY, Lee YT, Chen WJ. Postresuscitation myocardial dysfunction: correlated factors and prognostic implications. Intensive Care Med 33: 88–95, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Connor KM, Subbaram S, Regan KJ, Nelson KK, Mazurkiewicz JE, Bartholomew PJ, Aplin AE, Tai YT, Aguirre-Ghiso J, Flores SC, Melendez JA. Mitochondrial H2O2 regulates the angiogenic phenotype via PTEN oxidation. J Biol Chem 280: 16916–16924, 2005 [DOI] [PubMed] [Google Scholar]
- 11.DeBosch B, Treskov I, Lupu TS, Weinheimer C, Kovacs A, Courtois M, Muslin AJ. Akt1 is required for physiological cardiac growth. Circulation 113: 2097–2104, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation 120: 897–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dossumbekova A, Berdyshev EV, Gorshkova I, Shao Z, Li C, Long P, Joshi A, Natarajan V, Vanden Hoek TL. Akt activates NOS3 and separately restores barrier integrity in H2O2-stressed human cardiac microvascular endothelium. Am J Physiol Heart Circ Physiol 295: H2417–H2426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazmuri RJ, Weil MH, Bisera J, Tang W, Fukui M, McKee D. Myocardial dysfunction after successful resuscitation from cardiac arrest. Crit Care Med 24: 992–1000, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez MM, Berg RA, Nadkarni VM, Vianna CB, Kern KB, Timerman S, Ramires JA. Left ventricular systolic function and outcome after in-hospital cardiac arrest. Circulation 117: 1864–1872, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Grmec S, Strnad M, Kupnik D, Sinkovic A, Gazmuri RJ. Erythropoietin facilitates the return of spontaneous circulation and survival in victims of out-of-hospital cardiac arrest. Resuscitation 80: 631–637, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Hsu CY, Huang CH, Chang WT, Chen HW, Cheng HJ, Tsai MS, Wang TD, Yen ZS, Lee CC, Chen SC, Chen WJ. Cardioprotective effect of therapeutic hypothermia for postresuscitation myocardial dysfunction. Shock 32: 210–216, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Huang CH, Hsu CY, Chen HW, Tsai MS, Cheng HJ, Chang CH, Lee YT, Chen WJ. Erythropoietin improves the postresuscitation myocardial dysfunction and survival in the asphyxia-induced cardiac arrest model. Shock 28: 53–58, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Idris AH, Roberts LJ, 2nd, Caruso L, Showstark M, Layon AJ, Becker LB, Vanden Hoek T, Gabrielli A. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med 33: 2043–2048, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Kern KB, Berg RA, Hilwig RW, Larson DF, Gaballa MA. Myocardial cytokine IL-8 and nitric oxide synthase activity during and after resuscitation: preliminary observations in regards to post-resuscitation myocardial dysfunction. Resuscitation 77: 401–409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kern KB, Hilwig RW, Rhee KH, Berg RA. Myocardial dysfunction after resuscitation from cardiac arrest: an example of global myocardial stunning. J Am Coll Cardiol 28: 232–240, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Kleber AG. Consequences of acute ischemia for the electrical and mechanical function of the ventricular myocardium. A brief review. Experientia 46: 1162–1167, 1990 [DOI] [PubMed] [Google Scholar]
- 24.Kloner RA, Jennings RB. Consequences of brief ischemia: stunning, preconditioning, and their clinical implications: part 1. Circulation 104: 2981–2989, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Laurent I, Monchi M, Chiche JD, Joly LM, Spaulding C, Bourgeois B, Cariou A, Rozenberg A, Carli P, Weber S, Dhainaut JF. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol 40: 2110–2116, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Morisco C, Condorelli G, Trimarco V, Bellis A, Marrone C, Sadoshima J, Trimarco B. Akt mediates the cross-talk between beta-adrenergic and insulin receptors in neonatal cardiomyocytes. Circ Res 96: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Nichol G, Stiell IG, Laupacis A, Pham B, De Maio VJ, Wells GA. A cumulative meta-analysis of the effectiveness of defibrillator-capable emergency medical services for victims of out-of-hospital cardiac arrest. Ann Emerg Med 34: 517–525, 1999 [PubMed] [Google Scholar]
- 28.Niemann J, Garner D, Lewis R. Tumor necrosis factor-alpha is associated with early postresuscitation myocardial dysfunction. Crit Care Med 32: 1753–1758, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Ning XH, Chi EY, Buroker NE, Chen SH, Xu CS, Tien YT, Hyyti OM, Ge M, Portman MA. Moderate hypothermia (30°C) maintains myocardial integrity and modifies response of cell survival proteins after reperfusion. Am J Physiol Heart Circ Physiol 293: H2119–H2128, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Ning XH, Xu CS, Song YC, Childs KF, Xiao Y, Bolling SF, Lupinetti FM, Portman MA. Temperature threshold and modulation of energy metabolism in the cardioplegic arrested rabbit heart. Cryobiology 36: 2–11, 1998 [DOI] [PubMed] [Google Scholar]
- 31.Ning XH, Xu CS, Song YC, Xiao Y, Hu YJ, Lupinetti FM, Portman MA. Hypothermia preserves function and signaling for mitochondrial biogenesis during subsequent ischemia. Am J Physiol Heart Circ Physiol 274: H786–H793, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Ning XH, Xu CS, Song YC, Xiao Y, Hu YJ, Lupinetti FM, Portman MA. Temperature threshold and preservation of signaling for mitochondrial membrane proteins during ischemia in rabbit heart. Cryobiology 36: 321–329, 1998 [DOI] [PubMed] [Google Scholar]
- 33.O'Neill BT, Abel ED. Akt1 in the cardiovascular system: friend or foe? J Clin Invest 115: 2059–2064, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ouchi N, Oshima Y, Ohashi K, Higuchi A, Ikegami C, Izumiya Y, Walsh K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J Biol Chem 283: 32802–32811, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posen Y, Kalchenko V, Seger R, Brandis A, Scherz A, Salomon Y. Manipulation of redox signaling in mammalian cells enabled by controlled photogeneration of reactive oxygen species. J Cell Sci 118: 1957–1969, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Riter HG, Brooks LA, Pretorius AM, Ackermann LW, Kerber RE. Intra-arrest hypothermia: both cold liquid ventilation with perfluorocarbons and cold intravenous saline rapidly achieve hypothermia, but only cold liquid ventilation improves resumption of spontaneous circulation. Resuscitation 80: 561–566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Bailen M, Aguayo de Hoyos E, Ruiz-Navarro S, Diaz-Castellanos MA, Rucabado-Aguilar L, Gomez-Jimenez FJ, Martinez-Escobar S, Moreno RM, Fierro-Roson J. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation 66: 175–181, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Schoenenberger RA, von Planta M, von Planta I. Survival after failed out-of-hospital resuscitation. Are further therapeutic efforts in the emergency department futile? Arch Intern Med 154: 2433–2437, 1994 [PubMed] [Google Scholar]
- 39.Shao ZH, Chang WT, Chan KC, Wojcik KR, Hsu CW, Li CQ, Li J, Anderson T, Qin Y, Becker LB, Hamann KJ, Vanden Hoek TL. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol 292: H1995–H2003, 2007 [DOI] [PubMed] [Google Scholar]
- 40.The Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 346: 549–556, 2002 [DOI] [PubMed] [Google Scholar]
- 41.Trzeciak S, Jones AE, Kilgannon JH, Milcarek B, Hunter K, Shapiro NI, Hollenberg SM, Dellinger P, Parrillo JE. Significance of arterial hypotension after resuscitation from cardiac arrest. Crit Care Med 37: 2895–2903, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Vanden Hoek TL, Kasza KE, Beiser DG, Abella BS, Franklin JE, Oras JJ, Alvarado JP, Anderson T, Son H, Wardrip CL, Zhao D, Wang H, Becker LB. Induced hypothermia by central venous infusion: saline ice slurry versus chilled saline. Crit Care Med 32: S425–S431, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 36: 1780–1786, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Xu T, Tang W, Ristagno G, Sun S, Weil MH. Myocardial performance index following electrically induced or ischemically induced cardiac arrest. Resuscitation 76: 103–107, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Yannopoulos D, Zviman M, Castro V, Kolandaivelu A, Ranjan R, Wilson RF, Halperin HR. Intra-cardiopulmonary resuscitation hypothermia with and without volume loading in an ischemic model of cardiac arrest. Circulation 120: 1426–1435, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Yano N, Ianus V, Zhao TC, Tseng A, Padbury JF, Tseng YT. A novel signaling pathway for beta-adrenergic receptor-mediated activation of phosphoinositide 3-kinase in H9c2 cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H385–H393, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, Hoek TL, Becker LB. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation 77: 242–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao H, Shimohata T, Wang JQ, Sun G, Schaal DW, Sapolsky RM, Steinberg GK. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 25: 9794–9806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]