Abstract
Adenosine via an adenosine A1 receptor (A1R) is a negative feedback inhibitor of adrenergic stimulation in the heart, protecting it from toxic effects of overstimulation. Stimulation of the A1R results in the activation of Gi protein, release of free Gβγ-subunits, and activation/translocation of PKC-ε to the receptor for activated C kinase 2 protein at the Z-line of the cardiomyocyte sarcomere. Using an anti-Gβγ peptide, we investigated the role of these subunits in the A1R stimulation of phospholipase C (PLC), with the premise that the resulting diacylglycerol provides for the activation of PKC-ε. Inositol 1,4,5-triphosphate release was an index of PLC activity. Chlorocyclopentyl adenosine (CCPA), an A1R agonist, increased inositol 1,4,5-triphosphate production by 273% in mouse heart homogenates, an effect absent in A1R knockout hearts and inhibited by anti-Gβγ peptide. In a second study, p38 MAPK and heat shock protein 27 (HSP27), found by others to be associated with the loss of myocardial contractile function, were postulated to play a role in the actions of A1R. Isoproterenol, a β-adrenergic receptor agonist, increased the Ca2+ transient and sarcomere shortening magnitudes by 36 and 49%, respectively. In the rat cardiomyocyte, CCPA significantly reduced these increases, an action blocked by the p38 MAPK inhibitor SB-203580. While CCPA significantly increased the phosphorylation of HSP27, this action was inhibited by isoproterenol. These data indicate that the activation of PKC-ε by A1R results from the activation of PLC via free Gβγ-subunits released upon A1R-induced dissociation of Giαβγ. Attenuation of β-adrenergic-induced contractile function by A1R may involve the activation of p38 MAPK, but not HSP27.
Keywords: Gβγ subunits, antiadrenergic, rodent, contractility
adenosine is released endogenously in the heart that is stressed by hypoxia, ischemia, or adrenergic stimulation (13, 15). In the interstitium, adenosine activates cell surface receptors that initiate signal cascades within the cardiomyocyte, ultimately reducing the cardiotoxic manifestations of the initial insult (16, 43). For many years, study has focused on the antiadrenergic action of adenosine, or the ability of this nucleoside to protect the heart against excessive stimulation (34) by norepinephrine released during ischemia (36). In manifesting this action, adenosine A1 receptors (A1R) attenuate β-adrenergic catecholamine-elicited increases in Gs protein cycling (17), adenylyl cyclase activity (33), cAMP formation (5), activation of protein kinase A (PKA) (6), protein phosphorylation (12), myocardial Ca2+ transient magnitude (14), and ventricular contractility (6, 16).
Adenosine also reduces the responsiveness of the myocardium to catecholamine stimulation by a mechanism independent of changes in adrenergic-stimulated cyclase activity. This adenoprotection by the A1R agonist chlorocyclopentyl adenosine (CCPA) has been demonstrated by us to involve the translocation of PKC-ε to receptor for activated C kinase 2 (RACK2) in rat and mouse myocardium (18, 28). Overexpression of PKC-ε was found to enhance the attenuation by CCPA of adrenergic-induced contractile responses (28), whereas inhibition of phospholipase C (PLC) with U-73122 reduced this CCPA-induced attenuation (18). These data would suggest that, in addition to the antiadrenergic action involving the reduction of cAMP levels, adenosine provides adenoprotection by a pathway involving PLC and the activation/translocation of PKC-ε to RACK2 located at the Z-line of the sarcomere (18). However, questions still remain concerning the identity of the stimulatory mediator between the A1R and the PLC, as well as events occurring downstream from the activated PKC-ε/RACK2 complex.
A1R are Gi protein-coupled receptors that manifest their antiadrenergic action through the activation of Gαi-subunits that attenuate Gs protein cycling in the adrenergic-stimulated heart (17). The release of Gβγ dimers with A1R stimulation would be expected. Gβγ-subunits have been implicated by others in the activation of PLC (32, 46). Furthermore, activation of PKC with subsequent activation of p38 MAPK has been associated with the reduction of force development by myocardium (3, 23, 25), suggesting that the attenuation of adrenergic responses by adenosine may involve the downstream p38 MAPK pathway initiated by PLC. One objective of the present study was to investigate whether the Gβγ-subunit released from Gi protein in response to A1R stimulation mediates the activation of PLC. Another objective was to determine whether the phosphorylation/activation of heat shock protein 27 (HSP27) occurring in response to p38 MAPK activation (27) results in response to the activation of A1R.
MATERIALS AND METHODS
Animals used in this study were maintained and used in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals prepared by the Institute of Laboratory Animal Resources, National Research Council (DHEW Publication National Institutes of Health no. 85-23, revised 1996), and the study was approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Medical School. Sprague-Dawley rats and wild-type and A1R knockout C57BL/6 mice (UMass colony) of 3–4 mo of age were housed in rooms with 12:12-h light-dark cycles and fed rodent chow and water ad libitum. Colony-bred mice were genotyped by GeneTyper (New York, NY).
Isolated heart preparations.
Mouse hearts were isolated and perfused as described previously by our laboratory (40). Hearts removed from mice were perfused via an aortic cannula at a constant rate with physiological saline (PS) containing the following (in mM): 118 NaCl, 4.7 KCl, 2.5 CaCl2, 25 NaHCO3, 1.2 KH2PO4, 1.2 MgSO4, and 10 glucose, with the pH maintained at 7.4 by gassing the PS with 95% O2/5% CO2. Flow rates ranged from 2.8 to 4.2 ml/min, producing perfusion pressures ranging from 75 to 90 mmHg. Coronary perfusion pressures were measured by a pressure transducer attached to a side tube immediately above the aorta. Hearts were paced at 420 contractions per min. A water-filled polyethylene balloon was inserted into the left ventricular lumen via the left atrium and attached to a polygraph via a cannula. Balloons were inflated to achieve a maximal developed force and maintained at constant volume thereafter to allow the hearts to develop tension against a load. After instrumentation, hearts were allowed 15 min to stabilize before the experimental protocols were initiated. Agents were infused into the aortic cannula to achieve the desired PS concentrations. On termination of the experimental periods, hearts were frozen with aluminum clamps cooled in liquid N2 and stored in liquid N2 until assayed.
Isolated ventricular myocyte preparation.
Cardiomyocytes were enzymatically isolated from rat hearts, as previously described by our laboratory (18). The myocytes studied displayed no spontaneous contractions, reversibly contracted with electrical stimulation, and were prepared for study as previously reported (8). Briefly, myocytes were placed in a 506-μl chamber mounted on an inverted microscope stage and were superfused with a solution containing (in mM) 136.4 NaCl, 4.7 KCl, 1.0 CaCl2, 10 HEPES, 1.0 NaHCO3, 1.2 MgSO4, 1.2 KH2PO4, 10 glucose, 0.6 ascorbate, and 1.0 pyruvate (pH 7.4). Cells were stimulated to contract at 0.2 Hz with platinum wire electrodes.
Determination of myocyte contractile function and calcium transients.
Contractile function of isolated cardiomyocytes was assessed by determining unloaded sarcomere shortening using an IonOptix contractility system (Milton, MA). This system allowed recording of the maximum changes in sarcomere length and maximum velocities of sarcomere shortening and relaxation (8). Concomitantly, intracellular Ca2+ transients reflecting changes in intracellular levels of free calcium were recorded as previously described (8). Briefly, quiescent cardiomyocytes were incubated in the chamber with 2 μM fura-2 acetoxymethyl ester (fura-2 AM) for 30 min, followed by a 10-min washout period with fura-2-free superfusion fluid. Cardiomyocytes were subsequently exposed to alternating 340- and 380-nm wavelengths, and corresponding emissions were captured at 510 nm.
Immunoblotting.
One hundred milligrams of frozen mouse heart were homogenized (PRO200 homogenizer, PRO Scientific, Oxford, CT) in 200 μl of homogenization buffer containing 20 mM HEPES, 0.3 mM MgCl2, and 0.2 mM EDTA (pH 7.4). A sample was removed for protein determination using a bicinchoninic acid protein assay (Pierce) and bovine serum albumin as a standard. The remainder was supplemented with 25% β-mercaptoethanol, 300 mM Tris, and 0.5% bromophenol blue. After boiling for 3–5 min, samples were centrifuged for clarification, and the supernatant was resolved using 10% SDS-PAGE. Resolved proteins were transferred to nitrocellulose membranes and blotted against primary rabbit anti-phospho-HSP27 or anti-HSP27. The secondary antibody was goat anti-rabbit conjugated to horseradish peroxidase. The chemiluminescence was monitored with X-ray film and Western Lightning reagents (PerkinElmer). Film densities were quantified using UN-SCAN-IT software (Silk Scientific, Orem, UT).
Protocol 1: Gβγ-subunit A1R signal mediator.
To determine if Gβγ-subunits mediate A1R activation of PLC, the following experiments were conducted in the absence or presence of 50 μM anti-βγ peptide to sequester the free Gβγ-subunits. Inositol 1,4,5-triphosphate (IP3) levels were measured in the treated myocardial samples. An elevation of IP3 levels was used as an index of the activation of PLC. Mouse heart samples were prepared by homogenization in a buffer containing 25 mM HEPES, 3 mM MgCl2, 0.01 mM EGTA, and protease inhibitors (pH 7.4). The homogenate was centrifuged at 2,000 rpm for 20 min at 4°C, and the pellet was resuspended in an incubation buffer containing 25 mM HEPES, 3 mM MgCl2, 0.01 mM EGTA, 100 mM NaCl, 10 μM GTP, 10 μM GDP, and protease inhibitors (pH 7.4). Protein concentrations were determined as described above. The resuspended heart samples (495 μl) were incubated in the absence or presence of 1 μM CCPA at 30°C for 5 min before the reaction was stopped with the addition of 33% perchloric acid. In experiments where free Gβγ-subunits were inhibited, anti-βγ peptide was added 5 min before the administration of CCPA. The samples were subsequently centrifuged at 14,000 rpm for 5 min at 4°C. The supernatant was then neutralized with 10 M KOH and centrifuged again for 5 min with the supernatant analyzed for IP3 content. IP3 was determined using the Amersham d-myo-IP3 [3H] Biotrak Assay System. The assay is based on the relative binding of [3H]IP3 and nonradioactive sample-generated IP3 to a protein that binds IP3. IP3 production levels are reported as picomoles per microgram protein per minute incubation.
Protocol 2: p38 MAPK involvement in the A1R-induced reduction of adrenergic inotropism.
After rat cardiomyocytes were loaded with fura-2 AM and washed, they were stimulated with the β-adrenergic receptor agonist isoproterenol (ISO) at 1 nM for 3 min in the presence or absence of 1 μM CCPA , an A1R agonist, and/or 1 μM SB-203580, a p38 MAPK inhibitor. The A1R agonist, and p38 MAPK inhibitor were each present in the myocyte medium for 5 min before the administration of the ISO. Cardiomyocytes were continuously stimulated to contract after fura-2 AM loading to allow recording of Ca2+ transients and sarcomere shortening.
Protocol 3: A1R stimulation and HSP27 phosphorylation.
Isolated and perfused wild-type mouse hearts were allowed to stabilize for 15 min subsequent to instrumentation. After stabilization, hearts were treated with 1 μM CCPA for 5 or 45 min. To verify the involvement of receptor activation, experiments were repeated after a 5-min administration of the A1R antagonist dipropylcyclopentyl adenosine or utilizing hearts from A1R knockout mice. In other experiments, the p38-MAPK inhibitor SB-203580 was administered at 2.64 μM beginning 5 min before A1R stimulation. In additional experiments, hearts were treated with ISO at 10 nM for 1.0 min in the absence or presence of 1 μM CCPA, which was administered beginning 5 min before the ISO.
Statistical methods.
Data were analyzed using StatMost (Dataxiom, Los Angeles, CA). After applying one-way ANOVA, additional analysis was conducted using Student-Newman-Keuls post hoc test. A P value of <0.05 was taken to indicate a statistically significant difference. All data are presented as means ± SE.
Materials.
Progenitors for the A1RKO−/− mice were obtained as a generous gift from Dr. Jurgen Schnermann of the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD). Homozygous knockout animals used in the present study were offspring of heterozygous (+/−) breeders. Buffer salts were obtained from Fisher Scientific (Fair Lawn, NJ). Anti-Gβγ peptide was purchased from AnaSpec (San Jose, CA). Phospho-HSP27 (Ser82) and HSP27 antibodies were obtained from Cell Signaling (Danvers, MA) and Millipore (Billerica, MA), respectively. ISO and CCPA were purchased from Sigma Chemical (St. Louis, MO). SB-203580 was obtained from Calbiochem (La Jolla, CA). All gel electrophoretic reagents were obtained from Bio-Rad (Richmond, CA).
RESULTS
The Gβγ-subunit as a signal mediator between A1R and PLC.
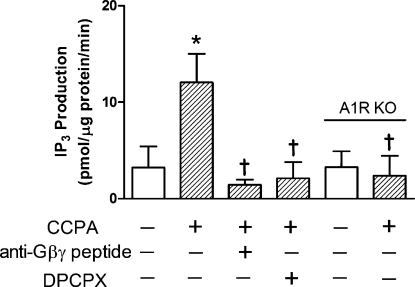
Our laboratory has previously reported that inhibition of PLC attenuates the A1R-induced reduction in adrenergic contractile effects (18). To characterize the involvement of Gβγ-subunits in the activation of PLC by A1R, we employed a membrane-permeable peptide to block the Gβγ signaling. This anti-Gβγ peptide is composed of a membrane-permeable sequence conjugated to a 28-amino acid peptide known to interact with and inhibit free Gβγ-subunits (2). In the absence of agents, myocardial IP3 production reached 3.23 ± 2.18 pmol·μg protein−1·min−1 (Fig. 1). This value was significantly increased 273% upon administration of the A1R agonist CCPA at 1 μM. Anti-Gβγ peptide inhibited the stimulatory action of CCPA. The CCPA response was not present in myocardium obtained from A1R knockout mice or from hearts treated with the A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX). This indicates that the increase in IP3 observed with CCPA treatment resulted solely from A1R activation of PLC.
Fig. 1.
Effect of chlorocyclopentyl adenosine (CCPA) and anti-Gβγ peptide on inositol 1,4,5-triphosphate (IP3) produced in homogenates of hearts obtained from wild-type and adenosine A1 receptor (A1R) knockout (KO) mice. CCPA and peptide were administered at 1 and 50 μM, respectively. The A1R antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) was administered at 100 nM. Values are means ± SE for 3–4 hearts. *Mean is significantly different from the agent-free value (P < 0.05). †Means are significantly different from the CCPA-alone value (P < 0.05).
Ca2+ transients and sarcomere shortening.
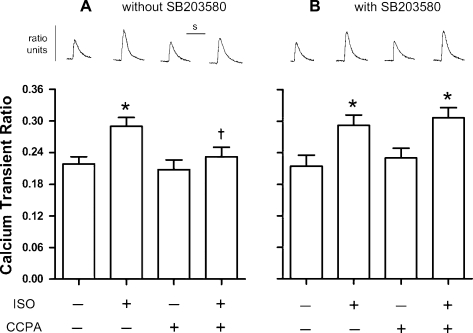
To determine the involvement of p38 MAPK in the A1R-induced reduction of adrenergic responses, β-adrenergic and A1R effects on Ca2+ transient generation and stimulation of unloaded sarcomere shortening were investigated before and after inhibition of p38 MAPK with SB-203580. Changes in Ca2+ transient magnitudes were affected by ISO and CCPA (Fig. 2). In the presence and absence of SB-203580, ISO increased the Ca2+ transient magnitudes by 36–37% from control values that ranged from 0.214 to 0.218. In the absence of SB-203580, CCPA attenuated the response to β-adrenergic stimulation with the increase in transient magnitude reaching only 11% (Fig. 2A). SB-203580 blocked the action of A1R, as indicated by an increase in transient magnitude, reaching 33% in the presence of ISO (Fig. 2B). These data indicate that the activation of p38 MAPK by CCPA appears to play a role in the A1R-induced attenuation of ISO-enhanced Ca2+ transient magnitudes.
Fig. 2.
Effect of p38 MAPK inhibition with SB-203580 (SB) on changes in rat ventricular cardiomyocyte Ca2+ transient ratio magnitudes elicited by isoproterenol (ISO) and CCPA. β-Adrenoceptors were stimulated with 1 nM ISO for 3 min in the absence or presence of the A1R agonist CCPA at 1.0 μM. Experiments were conducted in the absence (A) or presence (B) of the p38 MAPK inhibitor SB (1 μM). Representative calcium transients are depicted above the corresponding bars. Horizontal bar (s) indicates 1 s. Values are means ± SE for 7 (without SB) or 11 (with SB) cardiomyocytes. *Means are significantly different from the appropriate ISO-free control (P < 0.05). †Mean is significantly different from the ISO value in the absence of CCPA (P < 0.05).
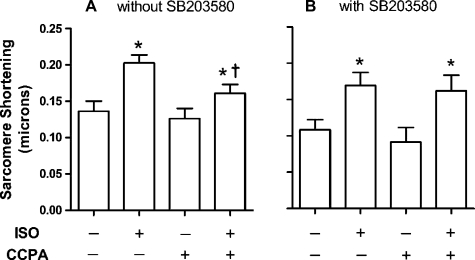
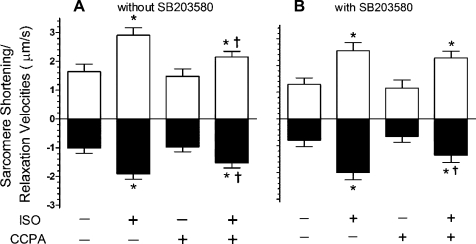
In the absence of SB-203580, ISO at 1 nM for 3 min yielded a significant 49% increase in sarcomere shortening compared with the control value of 0.136 ± 0.014 μm (Fig. 3A). While A1R stimulation alone had no significant effect on shortening, the administration of 1 μM CCPA significantly reduced the ISO response to 28%. Inhibition of p38 MAPK with SB-203580 resulted in the loss of CCPA-induced attenuation of adrenergic effect (Fig. 3B). In the presence of 1 μM SB-203580, ISO increased sarcomere shortening by 56% compared with an agent-free control value of 0.109 ± 0.015 μm. CCPA had no significant effect on sarcomere shortening in the absence or presence of β-adrenergic stimulation by ISO, as evidenced by the adrenergic-stimulated 76% increase in sarcomere shortening. Similar results were observed upon measurement of sarcomere shortening velocities (Fig. 4). ISO induced 77 and 87% increases in the velocities of shortening and relaxation, respectively (Fig. 4A). CCPA at a concentration that had no significant effect alone reduced the ISO-elicited increases in velocities to 45 and 31% for shortening and relaxation, respectively. Inhibition of p38 MAPK with SB-203580 was found to inhibit the effect of CCPA on ISO-elicited shortening velocity (Fig. 4B). ISO-stimulated shortening velocities were 97% greater than control values in the presence or absence of CCPA. ISO-stimulated velocities of relaxation remained significantly reduced by CCPA. Thus the activation of p38 MAPK by CCPA appears to play a role in the A1R-induced attenuation of increases in sarcomere shortening and velocity of shortening caused by ISO, but not in the attenuation of the increase in velocity of relaxation.
Fig. 3.
Effect of SB-203580 on changes in rat cardiomyocyte sarcomere shortening elicited by ISO and CCPA. β-Adrenoceptors were stimulated with 1 nM ISO for 3 min in the absence or presence of the A1R agonist CCPA at 1.0 μM. Experiments were conducted in the absence (A) or presence (B) of the p38 MAPK inhibitor SB (1 μM). The mean resting sarcomere length was 1.76 ± .010 (SE) μm for 19 cells. Values are means ± SE for 7 (without SB) or 11 (with SB) cardiomyocytes. *Means are significantly different from the appropriate ISO-free control (P < 0.05). †Mean is significantly different from the ISO value in the absence of CCPA (P < 0.05).
Fig. 4.
Effect of SB-203580 on changes in rat cardiomyocyte sarcomere maximum shortening and relaxation velocities elicited by ISO and CCPA. One nanomole of ISO was administered for 3 min in the absence or presence of the A1R agonist CCPA at 1.0 μM. Experiments were conducted in the absence (A) or presence (B) of the p38 MAPK inhibitor SB (1 μM). Positive values indicate the velocity of shortening. Negative values indicate the velocity of relaxation. Values are means ± SE for 7 (without SB) or 11 (with SB) cardiomyocytes. *Means are significantly different from the appropriate ISO-free control (P < 0.05). †Means are significantly different from the ISO value in the absence of CCPA (P < 0.05).
Phosphorylation of HSP27 and CCPA.
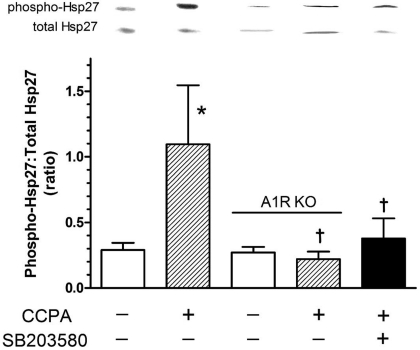
Activation of p38 MAPK is associated with reduced myocardial force development (3, 23, 25) and the translocation of HSP27 to myofilaments (3). Inhibition of activated p38 MAPK reverses these effects (3). Because activation of p38 MAPK appears to play a role in the A1R-mediated attenuation of β-adrenergic signaling (Figs. 3 and 4), and others have shown that the phosphorylation of HSP27 occurs in response to p38 MAPK activation (27), the influence of A1R activation on HSP27 phosphorylation was investigated. Mouse hearts were exposed to 1 μM CCPA to determine if A1R stimulation resulted in an enhanced phosphorylation of HSP27. A 5-min exposure of the myocardium to CCPA resulted in a 278% increase in the ratio of phospho-HSP27 to total HSP27 (Fig. 5). This response was not present in A1R knockout hearts, indicating that this response was due solely to the stimulation of A1R receptors. Treatment of the hearts with SB-203580, a p38 MAPK inhibitor, reduced the CCPA response by 65%, indicating that p38 MAPK participates in the adenosine-initiated signaling pathway, resulting in the phosphorylation of HSP27.
Fig. 5.
Effect of CCPA and SB-203580 on the phosphorylation of heat shock protein 27 (HSP27) in the isolated heart obtained from wild-type and A1R KO mice. CCPA (1.0 μM) was administered for 5 min in the presence or absence of SB (0.265 μM). Representative film densities are indicated above the corresponding bars for Western blots utilizing general (total HSP27) and phospho-specific antibodies (phospho-HSP27). Density ratios are depicted for each respective bar graph. Values are means ± SE of 4–10 hearts and are the ratio of phosphorylated HSP27 to total HSP27. *Mean is significantly different from the agent-free value (P < 0.05). †Means are significantly different from the wild-type CCPA-alone value (P < 0.05).
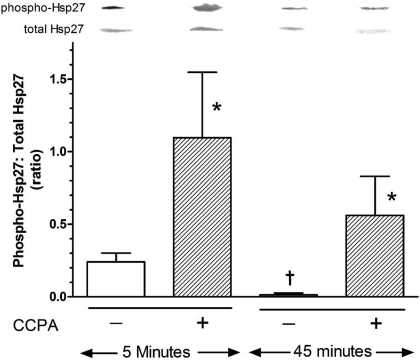
The effect of duration of A1R stimulation was explored by stimulating the hearts for 5 or 45 min with 1 μM CCPA. At 5 min subsequent to the 15-min stabilization period for the hearts, the ratio of phospho-HSP27 to total HSP27 was determined to be 0.240 ± 0.061 (Fig. 6). Five minutes of stimulation by CCPA increased this value by 355%. Fifteen minutes of additional time after the initial stabilization period saw a 94% decrease in the ratio to a value approaching zero. Administration of CCPA during this 15-min period resulted in only a 41-fold increase of the HSP27 phosphorylation ratio. The level of phosphorylation achieved was 49% less than that observed with only 5 min of CCPA.
Fig. 6.
Effect of exposure time on CCPA-induced phosphorylation of HSP27 in the isolated, perfused mouse heart. Hearts were perfused in the absence or presence of CCPA (1.0 μM) for 5 or 45 min before freeze-clamping and analysis. See legend of Fig. 5 for details. Values are means ± SE of 4–9 hearts. *Means are significantly different from the appropriate agent-free value (P < 0.05). †Mean is significantly different from the corresponding 5-min value (P < 0.05).
Phosphorylation of HSP27 with the administration of β1-adrenergic receptor and A1R agonists.
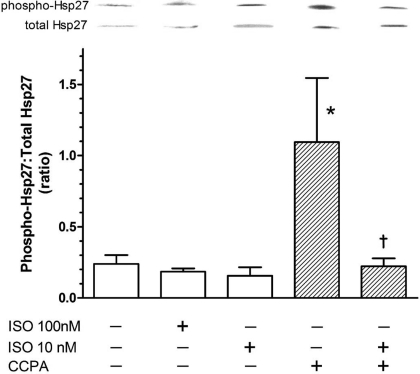
It has been reported previously that β-adrenergic stimulation by norepinephrine at high concentrations (4) or by ISO at prolonged exposure times (45) elicits the phosphorylation of p38 MAPK, suggesting that HSP27 also may be phosphorylated in response to stimulation by ISO. Experiments were conducted with this β-adrenergic receptor agonist, together with CCPA. ISO at 10 and 100 nM alone did not influence the phosphorylation of HSP27 (Fig. 7). It is of particular interest that ISO administered 4 min into a 5-min administration of CCPA reversed the enhancement of HSP27 phosphorylation observed with CCPA alone. The ratio of phosphorylated vs. total HSP27 observed with CCPA alone was reduced by 80% when ISO was administered at this time point. These data suggest that, at lower concentrations, ISO inhibits the activation of HSP27 by A1R.
Fig. 7.
Effect of ISO and CCPA on the phosphorylation of HSP27 in the isolated mouse heart. Hearts were perfused in the absence or presence of ISO (1 min) at 10 or 100 nM. When CCPA was administered, this A1R agonist was infused for 5 min alone or 4 min before and during the ISO administration. Control and CCPA-alone values are repeated from Fig. 2 for comparison. See legend of Fig. 5 for details. Values are means ± SE of 3–9 hearts. *Mean is significantly different from the agent-free value (P < 0.05). †Mean is significantly different from the CCPA-alone value (P < 0.05).
DISCUSSION
This report provides evidence that a signaling cascade initiated by A1R-induced activation of PLC involves Gβγ-subunits released on activation of the A1R-associated Gi protein. We also extend our laboratory's previous studies (18, 28) by providing evidence that activation of an A1R-initiated signaling pathway that includes PKC-ε and p38 MAPK may play a role in the A1R-induced inhibition of adrenergic-elicited increases in contractile and calcium transient activity. The role of HSP27 activation in the A1R-induced attenuation of adrenergic effects is uncertain.
Recent studies from this laboratory have indicated that A1R stimulation of the isolated rat ventricular cardiomyocyte elicits the translocation of PKC-ε to the vicinity of the t-tubules (28). These observations were extended with the demonstration that PKC-ε migrates to the ventricular RACK2 protein within 3 min of A1R stimulation (18). In the latter report, it was also shown that the reduction in β-adrenergic stimulated sarcomere shortening by the A1R agonist CCPA was inhibited by the PLC inhibitor U-73122, indicating that the signal cascade initiated by A1R involved PLC. However, it was left undetermined how A1R activation in the heart influenced PLC activity and what signal cascades were activated in response to the RACK2-bound PKC-ε.
The present study utilized an anti-Gβγ peptide that attenuated the CCPA-induced accumulation of IP3, a metabolic product of PLC activity. Activation of the G protein-coupled A1R is known to stimulate the displacement of GDP by GTP at the Gi protein α-subunit, resulting in its activation. The heterotrimeric Gi protein αβγ-complex subsequently dissociates, thereby freeing the Gαi subunit important to the modulation of β-adrenergic stimulated adenylyl cyclase activity (17) and the heterodimeric complex Gβγ, which has been implicated in the modulation of phosphoinositide-specific PLC activity (11). Recently, it was reported in a study of the regulation of norepinephrine release that adenosine A2A receptors elicited the activation of PLC in rat tail arteries with Gβγ-subunits as the mediator (19). In our ventricular myocardial preparations, stimulation of A1R with CCPA as an agonist resulted in a significant enhancement of IP3 accumulation, as has been previously observed by others (23). The administration of anti-Gβγ peptide, which binds to the Gβγ dimer, thereby preventing it from activating the PLC, attenuated this increase, suggesting that this heterodimeric complex released upon G protein activation mediated the activation of PLC by A1R. The IP3 response elicited by A1R was not present in the A1R knockout heart. Taken together, these data would suggest that A1R activation of the cardiomyocyte elicits free Gβγ-subunits that stimulate PLC, elevating cellular levels of diacylglyerol, which, in turn, results in the activation and translocation of PKC-ε to the RACK2 protein (18, 28).
Events that occur subsequent to the PKC-ε/RACK2 association appear more complex. In studies by others using “skinned” rat cardiomyocytes, use of the A1R agonist phenylisopropyl adenosine, before increasing sarcolemmal permeability with Triton, reduced the unloaded sarcomere shortening velocity, as induced with an elevated calcium level (23). Inhibition of PKC attenuated the action of phenylisopropyl adenosine, suggesting that this kinase mediated events that reduced the shortening velocity. PKC has been found to be involved with MAPK activation in cardiomyocytes (30). The cascade is thought to include the phosphorylation/activation of p38 MAPK by MAPK kinase (MKK) 3 or MKK6 (31) and the subsequent phosphorylation/activation of HSP27 by p38-activated MAPK-activated protein kinase (MAPKAPK) 2 and MAPKAPK3 (39, 44). It is to be noted, however, that HSP27 may also be directly phosphorylated by activated PKD with a PKC intermediate (9, 10) and independently of p38 MAPK (10).
The results of a number of studies draw attention to the central role of p38 MAPK in the induced depression of contractile function as a response to PKC activation. Vahebi et al. (42) postulated that the decrease in tension generation by skinned mouse heart papillary fiber bundles on activation of p38 resulted from dephosphorylation of α-tropomyosin and troponin I. The use of arsenite to enhance the phosphorylation/activation of p38 MAPK resulted in a decrease in the calcium-induced active tension developed by cardiomyocytes “skinned” subsequent to the arsenite administration (3). HSP27 was also found to translocate to the myofilament fraction in these preparations (3). Use of the p38 MAPK inhibitor SB-203580 attenuated both the loss of active tension and HSP27 translocation. The translocation of HSP27 has not always been observed with p38 activation (42). In other studies, overexpression of MKK3 and MKK6 in cardiomyocytes was found to activate p38 MAPK and result in a decrease in the maximal velocity of shortening and relaxation of isolated cardiomyocytes (25) and a negative inotropy in intact hearts (24). These studies were all conducted in the absence of adrenergic stimulation. While these data suggest that p38 MAPK was central to the contractile depression, the phosphorylation/translocation of HSP27 may have initiated the modification of myofilaments, thereby reducing tension development. Alternately, the translocation of HSP27 may simply have been coincidental with the loss of tension.
In the present study, the magnitude of calcium transients was significantly elevated by adrenergic stimulation, an effect that was reduced by CCPA in an SB-203580-sensitive fashion. This suggests that adrenergic-induced increases in cellular calcium levels are reduced in response to p38 MAPK activation by A1R. However, it must be noted that others have found no effect of MKK3-activated p38 MAPK on the calcium transient magnitude, leading them to the conclusion that the decreased maximal velocity of shortening and relaxation resulted instead from a reduced myofilament responsiveness to calcium (25). Alternately, Chen et al. (3) found no evidence for a reduced calcium sensitivity. Explanation for these disparities remains to be determined.
The magnitude and velocity of sarcomere shortening were observed in this study to become enhanced in response to ISO (Figs. 3 and 4). These adrenergic responses were not altered with inhibition of p38 MAPK by SB-203580. Inhibition of basal p38 MAPK activity with SB-203580 resulted in a general 21% depression of shorting and relaxation velocities in the absence of agents. If p38 MAPK activity reduces myofilament tension as proposed by others (42), it is not clear how its inhibition would elicit the same result, unless SB-203580 also affected the activities of other involved kinases (see below). The A1R-induced reduction in the adrenergic responses was sensitive to p38 MAPK inhibition, suggesting p38 MAPK may play a role in the adenoprotective action of adenosine. It is interesting that the A1R effect on sarcomere rate of relaxation with adrenergic stimulation was not affected by the inhibition of p38 MAPK, suggesting an independence of this parameter from p38 MAPK activation. Alternative mechanisms not involving p38 MAPK by which adenosine manifests its action may be involved. These include the well-described inhibitory effect of adenosine on adrenergic-stimulated adenylyl cyclase (6, 7) or the adenosine-induced activation of protein phosphatase 2A (26, 41).
It has been reported by others that high levels of adenosine administered to isolated rat hearts resulted in the phosphorylation of p38-MAPK and MAPKAPK2 (20), the latter known to phosphorylate HSP27 (29). In the present study, a 5-min stimulation by CCPA induced an elevation in the amount of total HSP27 that is phosphorylated. This is a direct response to A1R stimulation, as indicated by its absence in the A1R knockout hearts. It is interesting that the level of HSP27 phosphorylation attained after a 45-min exposure to CCPA was much less than with a 5-min exposure. The endogenous level of HSP27 phosphorylation was observed to fall with time in agent-free hearts, suggesting that HSP27 activation occurs during removal of the heart from the animal. Elevated catecholamine release occurring during this period of ischemia (35) might be expected to enhance the endogenous level of activated HSP27 (27). Although a period of 15 min is allowed for stabilization before initiating the experimental protocol, the difference between 5 and 45 min additional perfusion suggests a progressive decrease in stress-related activated HSP27. In any case, the CCPA-elicited increase in phosphorylated HSP27 from the control value was still greater at 5 min than at 45 min, suggesting a decrease in A1R responsiveness with continued stimulation. It is interesting that Marais et al. (27) observed a reduced enhancement of HSP27 phosphorylation with ischemia of progressively longer durations, perhaps suggesting a reduced response to the accumulation of interstitial adenosine released during ischemia (13).
β-Adrenergic stimulation of the heart with ISO at 10 and 100 nM was not observed to activate the HSP27 (Fig. 7). p38 MAPK is a substrate for β-adrenergic-induced PKA activity (45). Indeed, reports of others have shown an enhanced phosphorylation of p38 MAPK (4, 45) and downstream MAPKAPK2 (4) in response to ISO. The phosphorylation of HSP27 would be expected. However, these studies were conducted utilizing 15-min exposures to ISO (45) or 30- to 60-min exposures to 10 μM norepinephrine (4). We utilized 10 nM and 100 nM ISO for only 1 min. Thus it would appear that elevated levels and durations of β-adrenergic-receptor stimulation are required to elicit the phosphorylation of HSP27 in this manner. It is possible that, as proposed by Zheng et al. (45), β-adrenergic-induced activation of p38 MAPK by PKA actually provides a negative feedback to allow a level of protection from overstimulation of the heart by norepinephrine. This effect may only be operative for long-term exposures to excessive levels of adrenergic stimulation.
It was unexpected that 10 nM ISO inhibited the enhanced phosphorylation of HSP27 elicited by CCPA, especially if activated HSP27 is to be considered as a mediator of adenosine-induced attenuation of β-adrenergic stimulation. HSP27 is phosphorylated through pathways involving both p38 (44) and PKD (10), with PKC common to both pathways. Our results may reflect a PKA-induced inhibition of A1R-stimulated PKC-ε in a manner similar to that reported by Haworth et. al. (21). In the latter study, endothelin-1 was observed to induce the phosphorylation of PKD via activation of PKC-ε. This action was inhibited by ISO with PKA as the mediator. In the present study with adrenergic-stimulated hearts, the activation of PKD, p38 MAPK, and HSP27 by A1R-induced stimulation of PKC-ε may be similarly inhibited at the level of PKC-ε by β-adrenergic-induced PKA. In this manner, excessive negative feedback of β-adrenergic stimulation induced by adenosine is prevented. This aspect of multiple feedback interactions deserves future study.
β-Adrenergic receptor stimulation decreased A1R-induced HSP27 phosphorylation (Fig. 7), suggesting that p38 MAPK phosphorylation may as well be depressed by β-adrenergic stimulation. If this was the case, then p38 MAPK would not be a mediator of the antiadrenergic action of A1R. Yet, as discussed earlier, the inhibition of p38 by SB-203580 significantly attenuated the antiadrenergic action of A1R in β-adrenergic receptor-stimulated cardiomyocytes, as determined by the measurement of calcium transient magnitudes and sarcomere shortening (Figs. 2, 3, and 4). SB-203580 has been reported to exhibit nonspecific inhibition of cellular kinases (1), including receptor-interacting protein 2 (RIP-2) (22) and casein kinase (37). It is possible that SB-203580 inhibited the antiadrenergic action of A1R by attenuating the activity of a kinase exclusive of p38 MAPK. RIP-2 phosphorylates p38 MAPK (22), so the inhibition of RIP-2 would simply inhibit p38 MAPK. Casein kinase-2 knockdown using short interfering RNA resulted in an increase in force responses in vascular smooth muscle (38), suggesting that a similar mechanism of force modulation may be present in the myocardium.
In conclusion, it is proposed that adenosine attenuates β-adrenergic responses in the heart by a Gβγ-subunit-mediated activation of PLC. This mechanism is in addition to the well-known antiadrenergic action of adenosine, whereby Gαi reduces β-adrenergic-stimulated PKA levels. The elevation in diacylglycerol levels that result from PLC activation activates PKC-ε. The initiated signal transduction cascade results in the activation of p38 MAPK and HSP27. p38 MAPK, but not HSP27, appears to be involved directly in the adenoprotective action of adenosine in the heart.
GRANTS
This study was made possible by National Heart, Lung, and Blood Institute Grant HL-84160.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchian HH, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J 408: 297–315, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang M, Zhang L, Tam JP, Sanders-Bush E. Dissecting G protein-coupled receptor signaling pathways with membrane-permeable blocking peptides. J Biol Chem 275: 7021–7029, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Rajashree R, Liu Q, Hofmann P. Acute p38 MAPK activation decreases force development in ventricular myocytes. Am J Physiol Heart Circ Physiol 285: H2578–H2586, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Communal C, Colucci WS, Singh K. p38 Mitogen-activated protein kinase pathway protects adult rat ventricular myocytes against β-adrenergic receptor stimulated apoptosis. J Biol Chem 275: 19395–19400, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Dobson JG., Jr Reduction by adenosine of the isoproterenol-induced increase in cyclic adenosine 3′,5′-monophosphate formation and glycogen phosphorylase activity in rat heart muscle. Circ Res 43: 785–792, 1978 [DOI] [PubMed] [Google Scholar]
- 6.Dobson JG., Jr Mechanism of adenosine inhibition of catecholamine-induced elicited responses in heart. Circ Res 52: 151–160, 1983 [DOI] [PubMed] [Google Scholar]
- 7.Dobson JG, Jr, Shea LG, Fenton RA. β-Adrenergic and antiadrenergic modulation of cardiac adenylyl cyclase is influenced by phosphorylation. Am J Physiol Heart Circ Physiol 285: H1471–H1478, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Dobson JG, Jr, Shea LG, Fenton RA. Adenosine A2A and β-adrenergic calcium transient and contractile responses in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 295: H2364–H2372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doppler H, Storz P, Li J, Comb MJ, Toker A. A phosphorylation state-specific antibody recognizes Hsp27, a novel substrate of protein kinase D. J Biol Chem 280: 15013–15019, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Evans IM, Britton G, Zachary IC. Vascular endothelial growth factor induces heat shock protein (HSP) 27 serine 82 phosphorylation and endothelial tubuloenesis via protein kinase D and independent of p38 kinase. Cell Signaling 20: 1375–1384, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Feng J, Roberts MF, Drin G, Scarlata S. Dissection of the steps of phospholipase Cβ2 activity that are enhanced by Gβγ subunits. Biochemistry 44: 2577–2584, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Fenton RA, Dobson JG., Jr Adenosine and calcium alter adrenergic-induced intact heart protein phosphorylation. Am J Physiol Heart Circ Physiol 246: H559–H565, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Fenton RA, Dobson JG., Jr Measurement by fluorescence of interstitial adenosine levels in normoxic, hypoxic and ischemic perfused rat hearts. Circ Res 60: 177–184, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Fenton RA, Moore EDW, Fay FS, Dobson JG., Jr Adenosine reduces the Ca2+ transients of isoproterenol-stimulated rat ventricular myocytes. Am J Physiol Cell Physiol 261: C1107–C1114, 1991 [DOI] [PubMed] [Google Scholar]
- 15.Fenton RA, Dobson JG., Jr Hypoxia enhances isoproterenol-induced increase in heart interstitial adenosine depressing β-adrenergic contractile responses. Circ Res 72: 571–578, 1993 [DOI] [PubMed] [Google Scholar]
- 16.Fenton RA, Galeckas KJ, Dobson JG., Jr Endogenous adenosine reduces depression of cardiac function induced by β-adrenergic stimulation during low flow perfusion. J Mol Cell Cardiol 27: 2373–2383, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Fenton RA, Dobson JG., Jr Adenosine A1 and A2A receptor effects on G-protein cycling in β-adrenergic stimulated ventricular membranes. J Cell Physiol 213: 785–792, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Fenton RA, Komatsu S, Ikebe M, Shea LG, Dobson JG., Jr Adenoprotection of the heart involves phospholipase C-induced activation and translocation of PKC-ε to RACK2 in adult rat and mouse. Am J Physiol Heart Circ Physiol 297: H718–H725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fresco P, Oliveira JMA, Kunc F, Soares AS, Rocha-Pereira C, Goncalves J, Diniz C. A2A adenosine-receptor-mediated facilitation of noradrenaline release in rat tail artery involves protein kinase C activation and βγ subunits formed after α2-adrenoceptor activation. Neurochem Int 51: 47–56, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Haq SEA, Clerk A, Sugden PH. Activation of mitogen-activated protein kinases (p38-MAPKs, SAPKs/JNKs and ERKs) by adenosine in the perfused rat heart. FEBS Lett 434: 305–308, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Haworth RS, Roberts NA, Cuello F, Avkiran M. Regulation of protein kinase D activity in adult myocardium: novel counter-regulatory roles for protein kinase Cε and protein kinase A. J Mol Cell Cardiol 43: 686–695, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Jacquet S, Nishino Y, Kumphune S, Sicard P, Clark JE, Kobayashi KS, Flavell RA, Eickhoff J, Cotten M, Marber MS. The role of RIP2 in p38 MAPK activation in the stressed heart. J Biol Chem 283: 11964–11971, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lester JW, Hofmann PA. Role for PKC in the adenosine-induced decrease in shortening velocity of rat ventricular myocytes. Am J Physiol Heart Circ Physiol 279: H2685–H2693, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, Saffitz J, Chien K, Xiao R- P, Kass DA, Wang B. The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy. Proc Natl Acad Sci USA 98: 12283–12288, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao P, Wang S-Q, Wang S, Zheng M, Zheng M, Zhang S-J, Cheng H, Wang Y, Xiao R-P. p38 Mitogen-activated protein kinase mediates a negative inotropic effect in cardiac myocytes. Circ Res 90: 190–196, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Q, Hofmann PA. Modulation of protein phosphatase 2a by adenosine A1 receptors in cardiomyocytes: role for p38 MAPK. Am J Physiol Heart Circ Physiol 285: H97–H103, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Marais E, Genade S, Salie R, Huisamen B, Maritz S, Moolman JA, Lochner A. The temporal relationship between p38 MAPK and HSP27 activation in ischemic and pharmacological preconditioning. Basic Res Cardiol 100: 35–47, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki K, Komatsu S, Ikebe M, Fenton RA, Dobson JG., Jr Protein kinase C epsilon and the antiadrenergic action of adenosine in rat ventricular myocytes. Am J Physiol Heart Circ Physiol 287: H1721–H1729, 2004 [DOI] [PubMed] [Google Scholar]
- 29.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood LJ, Kato Y, Parry GCN, Han J. PRAK, a novel protein kinase regulated by the p38 MAP kinase. EMBO J 17: 3372–3384, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan J, Singh US, Takahashi T, Oka Y, Palm-Leis A, Herbelin BS, Baker KM. PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J Cell Physiol 202: 536–553, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol 16: 1247–1255, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70: 281–312, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romano FD, MacDonald SG, Dobson JG., Jr Adenosine receptor coupling to adenylate cyclase of rat ventricular myocyte membranes. Am J Physiol Heart Circ Physiol 257: H1088–H1095, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol 17: 291–306, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Schomig A, Dart AM, Dietz R, Mayer E, Kubler W. Release of endogenous catecholamines in the ischemic myocardium of the rat. Part A: Locally mediated release. Circ Res 55: 689–701, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Schomig A, Haass M, Richardt G. Catecholamine release and arrhythmias in acute myocardial ischemia. Eur Heart J 124: F38–F47, 1991 [DOI] [PubMed] [Google Scholar]
- 37.Shanware NP, Williams LM, Bowler MJ, Tibbetts RS. Non-specific in vivo inhibition of CK1 by the pyridinyl imidazone p38 inhibitors SB 203580 and SB 202190. BMB Rep 42: 142–147, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smolock EM, Wang T, Nolt JK, Moreland RS. siRNA knock down of casein kinase 2 increases force and cross-bridge cycling rates in vascular smooth muscle. Am J Physiol Cell Physiol 292: C876–C885, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Stokoe D, Engel K, Campbell DG, Cohen P, Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett 313: 307–313, 1992 [DOI] [PubMed] [Google Scholar]
- 40.Tikh EI, Fenton RA, Dobson JG., Jr Contractile effects of adenosine A1 and A2A receptors in the isolated murine heart. Am J Physiol Heart Circ Physiol 290: H348–H356, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Tikh EI, Fenton RA, Dobson JG., Jr Adenosine A1 and A2A receptor regulation of protein phosphatase 2A in the murine heart. J Cell Physiol 216: 83–90, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Vahebi S, Ota A, Li M, Warren CM, de Tombe PP, Wang Y, Solaro J. p38-MAPK induced dephosphorylation of α-tropomyosin is associated with depression of myocardial sarcomeric tension and ATPase activity. Circ Res 100: 408–415, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Wang J, Drake L, Sajjadi F, Firestein GS, Mullane KM, Bullough DA. Dual activation of adenosine A1 and A2 receptors mediates preconditioning of isolated cardiac myocytes. Eur J Pharmacol 320: 241–248, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Weber NC, Toma O, Wolter KI, Wirthele NM, Schlack W, Preckel B. Mechanisms of xenon- and isoflurane-induced preconditioning–a potential link to the cytoskeleton via the APKAPK-2/HSP27 pathway. Br J Pharmacol 146: 445–455, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng M, Zhang S-J, Zhu W-Z, Ziman B, Kobilka BK, Xiao R-P. β2-Adrenergic receptor-induced p38 MAPK activation is mediated by protein kinase A rather than by Gi or Gβγ in adult mouse cardiomyocytes. J Biol Chem 275: 40635–40640, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Zhou Y, Sondek J, Harden TK. Activation of human phospholipase C-η2 by Gβγ. Biochemistry 47: 4410–4417, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]