Abstract
Therapeutic hypothermia (TH) is a promising cardioprotective treatment for cardiac arrest and acute myocardial infarction, but its cytoprotective mechanisms remain unknown. In this study, we developed a murine cardiomyocyte model of ischemia-reperfusion injury to better determine the mechanisms of TH cardioprotection. We hypothesized that TH manipulates Akt, a survival kinase that mediates mitochondrial protection by modulating reactive oxygen species (ROS) and nitric oxide (NO) generation. Cardiomyocytes, isolated from 1- to 2-day-old C57BL6/J mice, were exposed to 90 min simulated ischemia and 3 h reperfusion. For TH, cells were cooled to 32°C during the last 20 min of ischemia and the first hour of reperfusion. Cell viability was evaluated by propidium iodide and lactate dehydrogenase release. ROS production was measured by 6-carboxy-2′,7′-dichlorodihydrofluorescein diacetate and mitochondrial membrane potential (ΔΨm) by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazoly-carbocyanine iodide (JC-1). Phospho (p)-Akt (Thr308), p-Akt (Ser473), and phosphorylated heat shock protein 27 (p-HSP27) (Ser82) were analyzed by Western blot analysis. TH attenuated reperfusion ROS generation, increased NO, maintained ΔΨm, and decreased cell death [19.3 ± 3.3% (n = 11) vs. 44.7 ± 2.7% (n = 10), P < 0.001]. TH also increased p-Akt during ischemia before reperfusion. TH protection and attenuation of ROS were blocked by the inhibition of Akt and NO synthase but not by a cGMP inhibitor. HSP27, a regulator of Akt, also exhibited increased phosphorylation (Ser82) during ischemia with TH. We conclude that TH cardioprotection is mediated by enhanced Akt/HSP27 phosphorylation and enhanced NO generation, resulting in the attenuation of ROS generation and the maintenance of ΔΨm following ischemia-reperfusion.
Keywords: ischemia-reperfusion, reactive oxygen species, cardiomyocyte
despite initial resuscitation, many cardiac arrest patients die within hours after the return of spontaneous circulation (ROSC) (12). This ischemia-reperfusion (I/R) injury is likely related to significant increases in tissue oxidant stress seen within minutes of cardiopulmonary resuscitation (CPR) and ROSC (19). Recently, it has been demonstrated that significant levels of oxidant stress in the heart following cardiac arrest originate from the mitochondria within minutes of reperfusion (11). In isolated cardiomyocytes, simulated I/R induces mitochondrial reactive oxygen species (ROS) generation, contractile dysfunction, mitochondrial release of cytochrome c, caspase activation, opening of the mitochondrial permeability transition pore, and ultimately cardiomyocyte death (4, 29, 40).
To date, therapeutic hypothermia (TH) is the only treatment currently known to improve survival in the clinical post-cardiac arrest setting (27). Such cooling is now being induced during CPR before ROSC in an attempt to improve cardiovascular function and outcome (6). In addition, following ROSC, cooling is induced in some cardiac arrest survivors while they are transported to the cardiac catheterization laboratory for possible percutaneous coronary intervention of acute myocardial infarction (AMI) (38). While such intra-ischemic cooling induced before reperfusion may be highly cardioprotective, the general use of cooling for AMI (non-cardiac arrest) patients remains unproven and the exact mechanisms of TH cardioprotection remain unknown (32). New insights into how TH protects the ischemic cardiomyocyte during early resuscitation could help optimize and augment TH protection of the heart after cardiac arrest and AMI.
Previous work in our chick cardiomyocyte model of I/R has demonstrated that cardiomyocytes are surprisingly tolerant of ischemia but sustain accelerated injury associated with a mitochondrial ROS burst within 5 min of reperfusion (23, 35). These cells also display significant cardioprotection that is associated with enhanced nitric oxide (NO) generation when TH is induced at end-ischemia and extended until 1 h into reperfusion (29). Possible mediators of this increased NO include Akt, a survival kinase that enhances cardioprotective NO generation from endothelial NO synthase (NOS3) (14). In addition, Akt has multiple targets apart from NOS3 that could decrease caspase activity and mitochondrial ROS generation (25). In this study, we developed a novel murine cardiomyocyte model of I/R to extend our previous findings to mammalian cardiomyocytes. We hypothesized that TH, if initiated before reperfusion, would increase cardiomyocyte survival and attenuate reperfusion ROS via enhanced Akt phosphorylation and NO generation.
MATERIALS AND METHODS
Mouse cardiomyocyte culture.
Primary cultures of mouse ventricular cardiomyocytes were prepared from hearts of 1- to 2-day-old neonatal C57BL6/J mice (Jackson, Bar Harbor, ME) with minor modifications (10). The cells were isolated at 37°C for 8 min with 0.1% trypsin in Hanks’ balanced salt solution (HBSS) without Ca2+ and Mg2+ (pH 7.4). The first cell suspension was discarded, whereas the subsequent suspensions were added to trypsin inhibitor solution in cold HBSS with Ca2+ and Mg2+ (pH 7.4) until all cardiac cells were isolated (5 to 6 cycles). To remove fibroblasts, the isolated cells were preplated for 90 min at 37°C. The resulting supernatants were then centrifuged and plated at a density of 0.6 × 106 on laminin-coated coverslips with MEM supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 50 U/ml penicillin, and 1.5 μM vitamin B12 (Sigma, St. Louis, MO). Myocyte purity was ≈90% as determined by immunofluorescent staining for α-sarcomeric actin and myosin heavy chain (Sigma). Experiments were performed on culture days 6–8. The investigation conforms to the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 85-23, Revised 1996). The procedure for cardiomyocyte isolation was approved by the University of Chicago's Institutional Animal Care and Use Committee.
Perfusion system and perfusate composition.
Synchronously contracting cells on glass coverslips were placed in a 1.2-ml Sykes-Moore perfusion chamber (Penn Century, Philadelphia, PA) as previously described (35). The tubing to these chambers was made of stainless steel (Bellco, Vineland, NJ), and the chambers were sealed with Kynar gaskets to minimize oxygen leaks. The normoxic solution pumped at a perfusion rate of 0.25 ml/min consisted of an oxygenated HBSS with 149 Torr Po2, 40 Torr Pco2, pH 7.4, 4.0 mM [K+], and 5.6 mM glucose. Simulated ischemic HBSS consisted of 20 mM 2-deoxy-glucose (Sigma) and 8.0 mM [K+]. It was bubbled with 80% N2 and 20% CO2 to produce a Po2 of 3–5 Torr, a Pco2 of 144 Torr, and a final pH of 6.8 (35).
Video/fluorescent microscopy.
A Nikon TE 2000-U-inverted phase/epifluorescent microscope was used for cell imaging. A charged-coupled device camera was used to monitor contractions and membrane changes over time in the same field of cells (∼70 × 90 μm). Fluorescent images were acquired from a cooled Cool-SNAP-ES camera (Photometrics, Tuscon, AZ), and changes in fluorescent intensity over time were quantified with MetaMorph software (Molecular Devices, Downington, PA) (30).
Protocols for simulated I/R and TH.
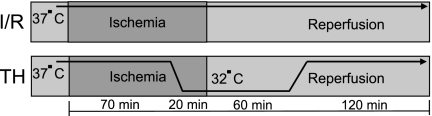
As seen in Fig. 1, cardiomyocytes were equilibrated for 30 min in the perfusion system and then subjected to simulated ischemia for 90 min followed by 3 h reperfusion (I/R) at 37°C. For the TH protocol, the cells were cooled during the last 20 min of ischemia to 32°C within 1–1.5 min and cooling was maintained for 1 h into reperfusion. This protocol was derived from a previous protocol used in our avian cardiomyocyte model (29). Cells were then rewarmed over 10 min to 37°C for another 2 h.
Fig. 1.
Schematic diagram of normothermic ischemia-reperfusion (I/R) protocol and therapeutic hypothermia (TH) protocol. TH was started during the last 20 min of ischemia before reperfusion and continued 1 h into reperfusion. Total ischemia time was 90 min, and total reperfusion time was 3 h.
Viability assay.
Cell viability was assessed with the fluorescent exclusion dye propidium iodide (5 μM; Sigma) as measured at 540 nm excitation and 590 nm emission (35). At the end of 3 h reperfusion, the cells were permeabilized with digitonin (300 μM). Cell death was expressed as the propidium iodide fluorescence relative to the maximal value seen after digitonin exposure at the end of reperfusion (100%).
Measurement of intracellular ROS.
The 6-carboxy-2′,7′-dichloro-dihydrofluorescein diacetate (6-carboxy-H2DCFHDA, 1 μM; Invitrogen) was used to measure intracellular ROS. This nonfluorescent cell permeable dye is oxidized to a highly fluorescent carboxy-dichlorofluorescein (DCF). This carboxylated form is more permeant than the classic H2DCFDA (9). The DCF fluorescence was measured at 488 nm excitation and 520 nm emission and expressed as arbitrary units (AU).
Measurement of intracellular NO.
Intracellular NO production was determined using 4,5-diaminofluorescein diacetate (DAF-2 DA, 1 μM; EMD Biosciences, San Diego, CA) (29). The DAF-2 fluorescence was measured at 488 nm excitation and 520 nm emission. The results were expressed as arbitrary units.
Assessment of mitochondrial membrane potential.
The mitochondrial membrane potential (ΔΨm) was evaluated using the dual-emission mitochondrial potentiometric dye, 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazoly-carbocyanine iodide (JC-1) (Invitrogen) by epifluorescence microscopy. JC-1 is a lipophilic and cationic dye that partitions into healthy mitochondria and aggregates. This potential and concentration-dependent aggregation shifts its emission to red fluorescence (excitation, 530 nm; and emission, 600 nm). Conversely, the depolarization of mitochondria (loss of ΔΨm) causes JC-1 disaggregation, preventing its entry into the mitochondria and leading to diffuse green fluorescence (excitation, 490 nm; and emission, 530 nm). Cells grown on the coverslip were incubated with JC-1 (10 μg/ml) at 37°C for 15 min and then mounted on an Olympus IX71 microscope equipped with an on-stage incubator (20/20 Technologies) for imaging. Tetramethylrhodamine isothiocyanate (TRITC) and fluorescein isothiocyanate (FITC) filter sets (Semrock) were used for collecting repolarized and depolarized mitochondria, respectively. The red and green fluorescence were analyzed with IPLab software (BD Biosciences).
Lactate dehydrogenase release assay.
Lactate dehydrogenase (LDH) activity was measured using LDH cytotoxicity assay kit (BioVision, Mountain View, CA) through the oxidation of lactate to pyruvate which then reacts with tetrazolium salt 2-(4-iodophenyl)-3-(4-nitophenyl)-5-phenyl-2H-tetrazolium chloride to form formazan. The rate of increase in formazan is directly proportional to the LDH activity in the sample. Effluents were collected at 30-min intervals, and a 1% Triton X-100 lysing solution was applied to collect the remaining LDH within the cells. The percentage of LDH release at the end of 3 h reperfusion (released LDH in the effluent) divided by total LDH (released LDH in the effluent + intracellular LDH) were calculated. Absorbance was read at 490 nm using a fluorescent plate reader (Synergy HT, Bio-Tek Instruments, Winooski, VT).
Western blot analysis.
The cells were lysed in buffer containing 1% Triton-100, 20 mM Tris, 137 mM NaCl, 2 mM EDTA, 10% glycerol, 10 mM sodium pyrophosphate, 50 mM NaF, 1 mM NaVO3, 200 mM PMSF, and 1× protease inhibitor cocktail. Protein quantification was performed with a Bradford assay (Bio-Rad, Hercules, CA). Protein lysate (30 μg/lane) was loaded and resolved on a 10% SDS-PAGE gel. It was then transferred to a nitrocellulose membrane. After the membranes were blocked in 5% fat-free milk in TBS-Tween 20, they were probed with antibodies against phosphorylated Akt (Thr308 and Ser473), phosphorylated heat shock protein 27 (HSP27) (Ser82), total Akt, tubulin, and/or total HSP-27. Signals were amplified and visualized with horseradish peroxidase-conjugated secondary antibody and enhanced chemiluminescence (30). Densitometry was performed using ImageJ software (version 1.38, NIH). All antibodies used were from Cell Signaling Technologies (Denver, MA), except for total HSP27, which was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Statistical analysis.
Results are expressed as means ± SE. A field of ∼500 cells was observed in each experiment. To ensure reproducibility, each set of experiments consisted of replicates (n) generated from multiple batches of cells. For comparison among the different treatment groups, Student's t-test and one-way ANOVA were used with post hoc examination by Tukey's test. For serial measurement data, a two-way repeated-measures ANOVA was applied with Tukey's post hoc analysis. A P < 0.05 was considered statistically significant.
RESULTS
Effect of increasing ischemia time on cardiomyocyte death.
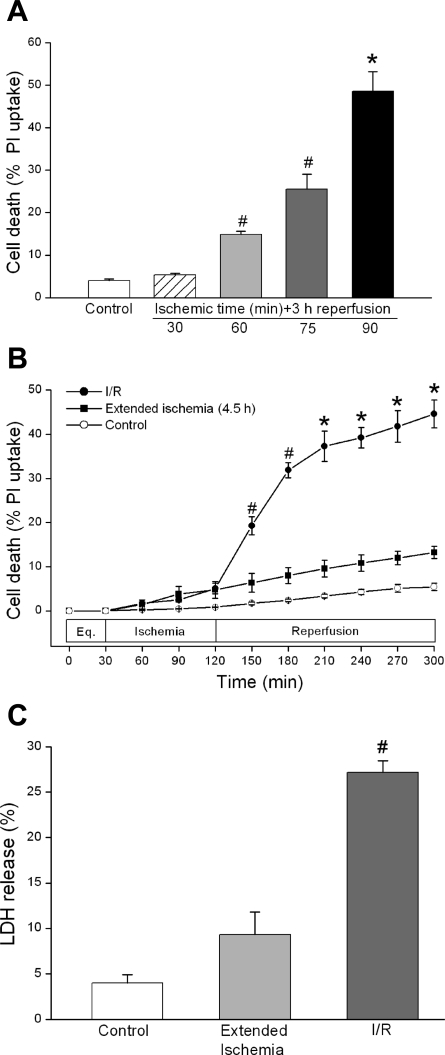
Cardiomyocytes were subjected to 30, 60, 75, or 90 min of simulated ischemia followed by 3 h of reperfusion. Cell death was 6.9 ± 0.7% (n = 4), 14.9 ± 0.7% (n = 4, P < 0.01), 25.5 ± 3.6% (n = 3, P < 0.01), and 48.6 ± 4.6% (n = 4, P < 0.001), respectively. For control cells exposed to 5 h HBSS only, cell death was 4.5 ± 0.6% (n = 3) (Fig. 2A). Ischemia at 90 min was used for all subsequent experiments.
Fig. 2.
Effect of I/R on murine cardiomyocyte death. A: increasing ischemia times resulted in increased cell death during I/R. Ischemia times of 60 min (#P < 0.01, n = 4), 75 min (#P < 0.01, n = 3), and 90 min (*P < 0.001, n = 4), followed by 3 h reperfusion vs. HBSS control (n = 3). PI, propidium iodide. B: reperfusion is associated with increased cell death. *P < 0.001, I (90 min)/R (3 h) (n = 12) vs. extended ischemia (n = 4). Eq, equilibration. C: lactate dehydrogenase (LDH) release also increased during I/R. #P < 0.01, I/R (n = 4) vs. extended ischemia (n = 4). All results are expressed as means ± SE.
Effect of I/R on cell death and LDH release.
Our prior work demonstrated minimal cell death during ischemia and accelerated cell death during reperfusion in chick cardiomyocytes (2, 35). To examine whether a similar I/R injury pattern is seen in murine cardiomyocytes, cells were subjected to either 4.5 h extended ischemia or 90 min ischemia and 3 h reperfusion. As shown in Fig. 2B, I/R resulted in increased cell death compared with either 4.5 h extended ischemia [44.6 ± 3.1% (n = 12) vs. 13.2 ± 1.4% (n = 4), P < 0.001] or 5 h HBSS control [5.4 ± 0.8% (n = 3), P < 0.001]. We further examined I/R injury with the use of LDH, another marker of cellular injury. LDH release in I/R was higher than that seen in cells exposed to 4.5 h extended ischemia or 5 h HBSS [27.2 ± 1.3% (n = 12), 9.5 ± 2.1% (n = 4), and 3.8 ± 0.8% (n = 4), respectively, P < 0.01] (Fig. 2C). Whereas extended ischemia resulted in a level of cell death statistically greater than HBSS alone, reperfusion was associated with a significant acceleration of cell death and injury compared with prolonged ischemia.
TH protects against I/R.
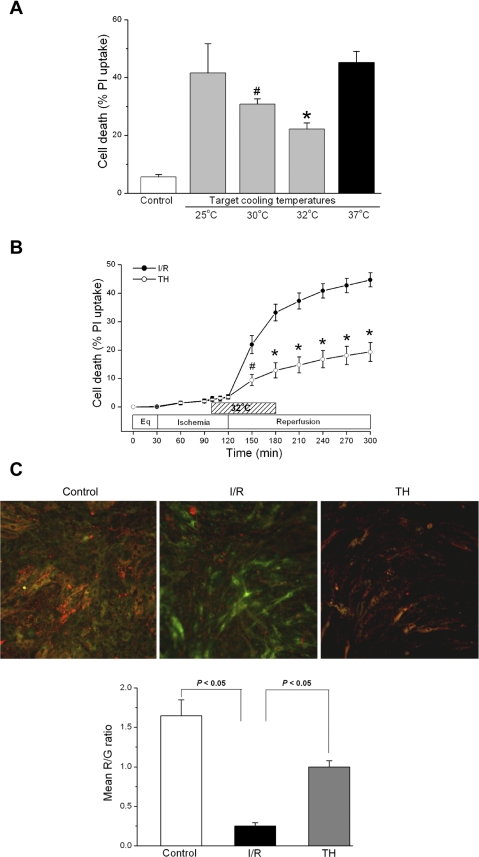
We next determined the optimal cooling temperature for cardioprotection. Cells were subjected to simulated ischemia for 70 min at 37°C and an additional 20 min of cold ischemia (total ischemia time = 90 min) at 25°, 30°, or 32°C; cooling was continued for the first 1 h of reperfusion. Cells were then rewarmed to 37°C (TH protocol, see Fig. 1). Since 32°C induced the greatest protection (Fig. 3A), this target temperature was used for further study. As shown in Fig. 3B, TH significantly reduced cell death compared with I/R control cells [19.3 ± 3.3% (n = 11) vs. 44.7 ± 2.7% (n = 10), P < 0.001]. Synchronous cell contractions, a physiological measurement of cell function, also returned in 9 of 11 TH experiments compared with 0 of 10 normothermic I/R experiments.
Fig. 3.
Protective effect of TH on I/R-induced cell death. A: cells were cooled to different target temperatures (25°, 30°, or 32°C) during the last 20 min of ischemia; this temperature was maintained during the first 1 h of reperfusion after which cells were rewarmed to 37°C for the remaining 2 h reperfusion. The target temperature of 32°C induced the greatest protection against I/R cell death. *P < 0.001, cooling at 32°C (n = 3) vs. I/R (n = 3). #P < 0.01, cooling at 30°C (n = 3) vs. I/R (n = 3). P = not significant (NS), cooling at 25°C (n = 3) vs. I/R (n = 3). B: protection against I/R-induced cell death by TH. TH was induced to the target temperature of 32°C as in A, resulting in decreased cell death during reperfusion. *P < 0.001, TH (n = 11) vs. I/R (n = 10). C: assessment of mitochondrial membrane potential (ΔΨm) by 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazoly-carbocyanine iodide (JC-1). Healthy mitochondria are characterized by a punctate red fluorescence; depolarized mitochondria are characterized by a diffuse green fluorescence. Individual color channels were acquired with the same exposure settings for quantitative measurement of ΔΨm. As quantified by red-to-green (R/G) image ratio, ΔΨm decreased during normothermic I/R but was maintained during TH. Image representative of 3 individual experiments (5 fields/experiment). P < 0.05, I/R vs. control. P < 0.05, TH vs. I/R. Data are presented as means ± SE.
Effect of TH on ΔΨm.
The induction of cell death is generally associated with a perturbation in mitochondrial function. The dissipation of ΔΨm is one indication of failing mitochondria. We used the membrane potential sensitive dye JC-1 to assess the impact of I/R and TH on cardiomyocyte ΔΨm. Figure 3C shows representative images of JC-1 uptake in cardiomyocyte mitochondria. Ischemia followed by 30 min reperfusion resulted in the prominent dissipation of ΔΨm compared with control cells. As seen in the representative images and quantified by red-to-green image ratios (n = 3 experiments), TH ameliorated the ΔΨm disruption caused by I/R (P < 0.05).
Role of NO in TH.
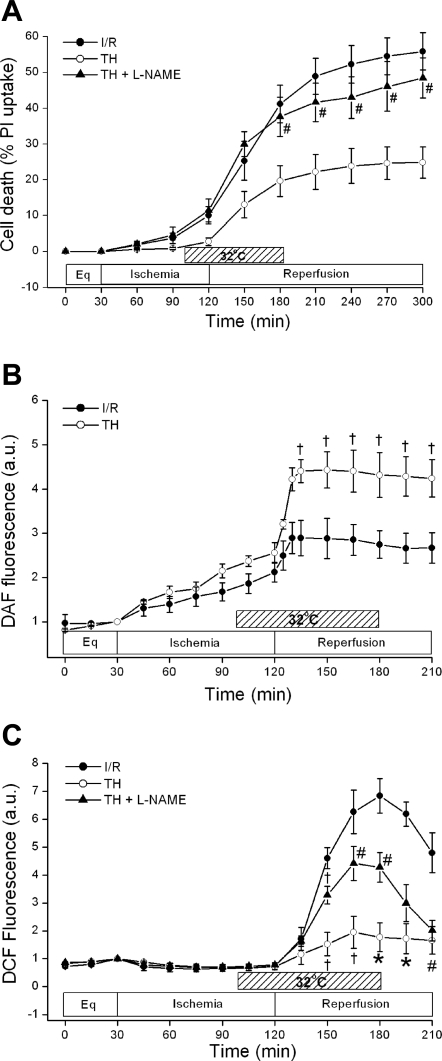
NO plays a critical role in adaptive cardioprotection against I/R injury and increased, sustained levels of NO correlate with improved avian cardiomyocyte survival (23, 29). To assess whether NO plays a role in hypothermic protection of murine cardiomyocytes, we examined the effect of the NOS inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 200 μM, Sigma) on TH cell death. l-NAME was preincubated for 2 h and used continuously throughout the study. As shown in Fig. 4A, l-NAME reversed TH protection [24.8 ± 4.3% TH (n = 7) vs. 48.5 ± 5.6% TH + l-NAME (n = 4), P < 0.01]. l-NAME alone did not increase cell death compared with control I/R [52.8 ± 6.1% l-NAME + I/R (n = 3) vs. I/R 55.9 ± 5.2% (n = 7), P = not significant (NS)].
Fig. 4.
Role of nitric oxide (NO) during TH. A: effect of blocking NO generation during TH on cell death. The NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME, 200 μM) was used to assess the effect of blocking NO generation on TH cell survival. l-NAME reversed TH-induced cardioprotection. #P < 0.01, TH + l-NAME (n = 4) vs. TH (n = 4). P = NS, I/R + l-NAME (n = 3) vs. I/R (n = 3). B: effect of TH on NO generation as measured by 4,5-diaminofluorescein (DAF-2) fluorescence. TH induced an elevated level of NO that was sustained throughout reperfusion compared with I/R. †P < 0.05, TH (n = 5) vs. I/R (n = 5). AU, arbitrary units. C: effect of TH and blocking NO generation on reactive oxygen species (ROS) generation as measured by 6-carboxy-2′,7′-dichloro-dihydrofluorescein diacetate (DCF). TH blunted DCF fluorescence during reperfusion. †P < 0.05, #P < 0.01, and *P < 0.001, TH (n = 5) vs. I/R (n = 5). Blocking NO generation with l-NAME partially reversed the DCF signal during reperfusion. †P < 0.05 and #P < 0.01, TH + l-NAME (n = 5) vs. TH (n = 5). The results are shown as means ± SE.
Additionally, we used the NO-sensitive dye DAF-2 to further test the effect of TH on endogenous NO production. Figure 4B demonstrates that DAF-2 fluorescence gradually increased during both normothermic and hypothermic ischemia. When compared with normothermic reperfusion, TH resulted in a significantly higher level of NO production throughout reperfusion [2.8 ± 0.3 (n = 5) vs. 4.3 ± 0.5 AU (n = 5), P < 0.05].
Effect of I/R and TH on ROS.
We next sought to determine the relationship between I/R and ROS generation in murine cardiomyocytes in the presence and absence of TH. As seen in Fig. 4C, I/R led to a burst of increased DCF fluorescence at reperfusion, suggesting significant ROS generation. TH significantly attenuated this burst [peak value at 1 h: 6.8 ± 0.1 (n = 4) vs. 1.8 ± 0.5 AU (n = 5), P < 0.01]. ROS originate from many intracellular sources, and mitochondrial ROS can be generated at complex I as well as complex III (33, 34). To further study the role of mitochondrial ROS during reperfusion, we used stigmatellin (50 nM, Sigma), a mitochondrial complex III inhibitor. Stigmatellin attenuated the reperfusion ROS burst, decreasing peak DCF fluorescence from 1.8 ± 0.1 (n = 4) to 0.7 ± 0.1 AU (n = 4, P < 0.01). This is consistent with our prior work suggesting that significant reperfusion ROS in cardiomyocytes are mitochondrial in origin (2, 23).
NO is cardioprotective and has been demonstrated to decrease ROS generation. Given our above findings, we next investigated whether TH-associated NO could be blocked with l-NAME and how this might affect ROS. l-NAME partially reversed the attenuation in reperfusion ROS during TH [peak value at 1 h: 4.3 ± 0.5 AU (n = 5), P < 0.01], suggesting that NOS-mediated NO may be protecting cardiomyocytes by decreasing ROS (23). NO modulation of ROS may be cGMP dependent or due to direct nitrosylation of mitochondrial proteins (8). To determine whether TH cardioprotection was independent of cGMP, we used the cGMP inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) (20). ODQ (10 μM, EMD Biosciences, San Diego, CA) was preincubated for 2 h and then given all course. Although there was a trend toward increased cell death, ODQ at this dose or higher doses ranging up to 50 μM did not significantly inhibit TH cardioprotection [22.5 ± 4.3% TH (n = 5) vs. 31.7 ± 4.2% TH + ODQ (n = 5), P = 0.20].
Role of Akt in TH protection.
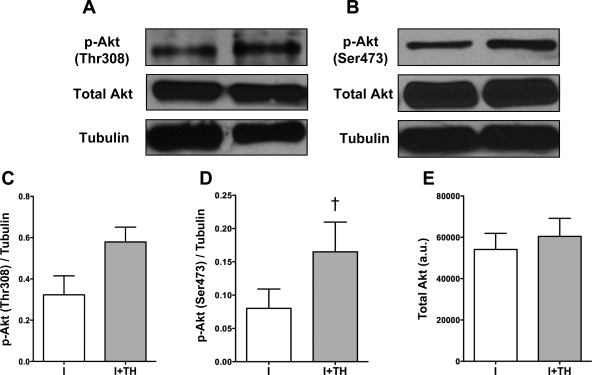
Akt is an important survival kinase that, when overexpressed in cardiomyocytes, results in increased I/R tolerance. It is also a key regulator of NO through the activation of NOS3. Our prior work has shown that TH is associated with increased NO generation, but whether Akt was involved in TH protection was not determined (29). Therefore, we sought to elucidate the relationship between TH and Akt phosphorylation. When compared with normothermia, TH increased Akt phosphorylation by the end of ischemia at the Thr308 and Ser473 sites (Fig. 5, A and B). Densitometric analysis showed a significant increase in phosphorylation at the Ser473 site (Fig. 5D, n = 5, P < 0.05) with a trend toward increased Thr308 phosphorylation (Fig. 5C, n = 5, P = NS). TH was not associated with changes in total Akt (Fig. 5 E). In addition we found that TH increased Akt during reperfusion but was not significantly different compared with normothermic reperfusion.
Fig. 5.
Effect of TH on Akt phosphorylation. A and B: Western blot analysis showed that TH induced an increase in Akt phosphorylation (Thr308 and Ser473) following 90 min ischemia (I). C: densitometric analysis showed a trend toward an increase in phosphorylation of Akt (Thr308). P = NS. I + TH (n = 5) vs. I (n = 5). D: densitometric analysis showed that TH induced a significant elevation in phospho (p)-Akt (Ser473) by the end of ischemia. †P < 0.05, I + TH (n = 5) vs. I (n = 5). E: total Akt was unchanged with I + TH compared with I (P = NS). Tubulin was used as a loading control. Data are presented as means ± SE.
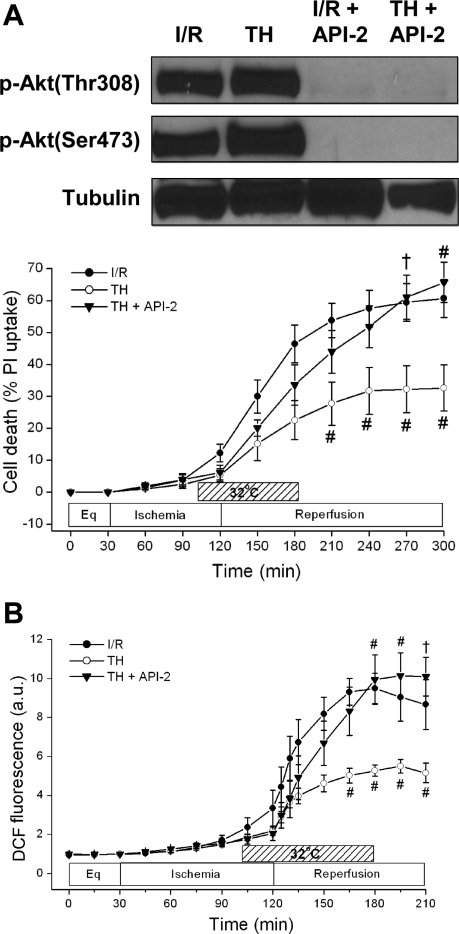
To determine whether the TH-induced increase in Akt phosphorylation was associated with cardioprotection, we tested the effect of the Akt inhibitor Akt/PKB-signaling inhibitor-2 (API-2) on cell death. API-2 is a specific inhibitor of Akt and is known to block its phosphorylation at both the Thr308 and Ser473 sites (39). As shown in Fig. 6A, API-2 (10 μM) preincubated for 2 h and present all-course reversed TH cardioprotection from 32.6 ± 7.2% (n = 4) to 65.7 ± 6.3% (n = 4) (P < 0.01). API-2 given during I/R resulted in 53.6 ± 7.9% cell death (n = 3) vs. 60.7 ± 6.0% (n = 4) during I/R without API-2 (P = NS). Western blot analysis confirmed that API-2 blocked the phosphorylation of Akt during both normothermic and hypothermic reperfusion (Fig. 6A, top).
Fig. 6.
Effects of Akt inhibition on cell death and ROS generation. A: effect of Akt/PKB-signaling inhibitor-2 (API-2) on phosphorylation of Akt and cell death. API-2 completely blocked the phosphorylation of Akt at both sites (Thr308 and Ser473). API-2 (10 μM) also abrogated TH-induced cardioprotection. †P < 0.05 and #P < 0.01, TH + API-2 (n = 4) vs. TH (n = 4). P = NS, I/R + API-2 (n = 4) vs. I/R (n = 3). B: effect of API-2 on ROS generation as measured by DCF fluorescence. API-2 reversed the attenuated DCF fluorescence seen during TH, resulting in an ROS burst similar to I/R. †P < 0.05 and #P < 0.01, TH + API-2 (n = 4) vs. TH (n = 4). P = NS, I/R + API-2 (n = 3) vs. I/R (n = 3).
Given that TH was associated with increased Akt-NO signaling while decreasing ROS, we next sought to determine whether inhibiting Akt would reverse TH effects on ROS. As shown in Fig. 6B, the Akt inhibitor API-2 (10 μM) reversed the effects of TH, resulting in an increased ROS burst at reperfusion [I/R + TH + API-2, 9.9 ± 1.2 (n = 4) vs. I/R + TH, 5.3 ± 0.3 AU (n = 4), P < 0.01]. Consistent with the results that API-2 did not increase normothermic I/R cell death, API-2 + I/R also did not increase ROS generation above that of I/R alone [7.9 ± 0.4 AU (n = 4), P = NS].
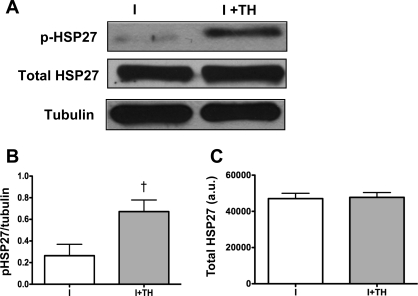
HSP27 is a heat shock protein affected by temperature change and is a known regulator of Akt and ROS (24, 36). Western blot analysis demonstrated that TH increased HSP27 (Ser82) phosphorylation compared with normothermia following 90 min ischemia but did not alter total HSP27 levels (Fig. 7A). Densitometric analysis showed significant increases in phosphorylation of HSP27 (ischemia, 0.3 ± 0.1 vs. TH, 0.7 ± 0.1 AU, n = 6, P < 0.05, Fig. 7B). No changes in total HSP27 were seen [ischemia, 47,049 ± 2,949 vs. TH, 47,742 ± 2,649 AU, n = 4, P = NS, Fig. 7C].
Fig. 7.
Effect of TH on heat shock protein 27 (HSP27) phosphorylation. A: Western blot analysis demonstrated that TH induced increased HSP27 (Ser82) phosphorylation following 90 min ischemia but did not alter total HSP27 levels. B: densitometric analysis showed significant increase in phosphorylation of HSP27 (Ser82). †P < 0.05, I + TH (n = 6) vs. I (n = 6). C: densitometric analysis showed no changes in total HSP27. P =NS, I + TH (n = 4) vs. I (n = 4). Tubulin was used as a loading control. Data are presented as means ± SE.
DISCUSSION
In this study, we developed a murine cardiomyocyte model of I/R to investigate the mechanisms of TH cardioprotection. We found that murine cardiomyocytes exhibit an ROS burst evident by 30 min reperfusion following 90 min ischemia. ROS generation peaks within 1 h of reperfusion, is attenuated by the mitochondrial electron transport chain inhibitor stigmatellin, and is associated with an accelerated cell death of 40–50% at 3 h reperfusion. Similar to previous studies in avian cardiomyocytes (35), prolonged ischemia (4.5 h) without reperfusion did not lead to accelerated cell death. An intra-ischemic induction of cardiomyocyte cooling to 32°C maintained through the first hour of reperfusion significantly decreased reperfusion ROS and cell death, preserved ΔΨm, improved recovery of spontaneous contractile function, and enhanced NO generation that was sustained throughout 90 min reperfusion. TH increased Akt phosphorylation by end ischemia. Both the inhibition of enzymatic NO generation using the NOS inhibitor l-NAME and Akt inhibition using API-2 abrogated TH protection against cell death. API-2 completely blocked Akt phosphorylation and reversed the TH attenuation of reperfusion ROS. Along with Akt, HSP27 demonstrated a simultaneous increase in phosphorylation with cooling by end ischemia. Collectively, these results suggest that TH protection requires an enhanced Akt phosphorylation as early as end ischemia before reperfusion. Increased Akt activation and associated enhanced NO generation improve cell survival and contractile function by attenuating mitochondrial ROS during reperfusion.
TH and I/R.
There are many studies in the literature examining the effects of cooling before the onset of ischemia, i.e., surgical or accidental hypothermia, but there are few studies of cooling induced after potentially lethal warm ischemia has already occurred, i.e., TH. Hale and Kloner (16) have previously demonstrated the efficacy of TH in a rabbit model of I/R in limiting reperfusion injury following prolonged ischemia. The optimal target temperature in our study is similar to the range used in our mouse model of cardiac arrest (1) and with human post-cardiac arrest cooling guidelines (27). Of note, the optimal target temperature for TH benefit in our previous study in avian cardiomyocytes was 25°C (29), a difference likely related to species (murine vs. avian) and developmental stage (neonatal vs. embryonic). The timing of TH induction before reperfusion may be critical, with our past work and work by others suggesting that delaying reperfusion to reach target temperature significantly improves TH cardioprotection (15, 29). As highlighted in the current work, ROS generation and injury are evident within minutes of reperfusion. Therefore, the induction of cooling after reperfusion that has already occurred may be too late to affect the outcome. The increased phospho-Akt by end-ischemia is consistent with the notion that TH induced during ischemia may better prepare ischemic heart tissue for reperfusion. This timing of protection is clinically relevant since some cardiac arrest survivors with evidence of AMI are cooled to 32–34°C before reperfusion with percutaneous coronary intervention (38).
TH and ROS.
Given the role of mitochondrial ROS generation in cardiomyocyte reperfusion injury, TH could work by directly decreasing ROS generation. Alternatively, TH may affect stress pathways that act as endogenous antioxidant responses to attenuate ROS generation. The cardioprotective mechanism of TH remains unknown and has largely been ascribed to decreased cellular metabolism and oxygen uptake (27). The current results showing that cooling decreases cardiomyocyte ROS generation at reperfusion are consistent with prior studies of surgical cooling. Reiss et al. (28) found evidence that hypothermia can suppress ROS generation at reperfusion following ischemia in whole hearts. Our findings here extend this work in two important ways. First, it extends findings in surgical hypothermia models to a model of TH, showing that reperfusion ROS can be similarly decreased even when cooling is induced later in ischemia and targets higher temperatures (i.e., 32°C) than used in surgical hypothermia. Second, the current results suggest that mitochondrial ROS generation from complex III (i.e., the stigmatellin binding site) is attenuated by cooling via Akt related stress responses.
TH and NO.
NO is a known regulator of mitochondrial respiration, ROS generation, antioxidant gene induction, and cardioprotection, making it a logical target for TH (17, 21, 37). Consistent with this, past work in our avian cardiomyocyte I/R model has shown that two different cardioprotective strategies at reperfusion, controlling CO2 or temperature, each result in enhanced cardioprotective NO generation at reperfusion (23, 29). Recently, an NO donor given during CPR has been found to attenuate mitochondrial ROS generation and improve survival in a mouse model of cardiac arrest (11). However, there are few studies investigating the relationship between NO and hypothermia. TH protection in a model of lung I/R injury was associated with enhanced NO and was blocked in NOS3−/− mice (41). TH is also associated with enhanced cardiac function and survival in a murine model of cardiac arrest but is significantly decreased in NOS3−/− mice (26). The current study builds on this work to directly demonstrate that TH enhances NO generation and attenuates reperfusion ROS in mammalian cardiomyocytes. In the cardiovascular system, the major target of NO is soluble guanylyl cyclase that results in the conversion of guanosine triphosphate to the second messenger cGMP. However, NO is known to have cardioprotective effects including the modulation of ROS generation independent of cGMP due to direct effects on mitochondrial protein nitrosylation (8, 17). Here we find that TH cardioprotection was not blocked by cGMP inhibition, suggesting a more direct effect on mitochondria.
TH and Akt/HSP27.
The overexpression of Akt results in I/R cardioprotection (13), and the activation of Akt via growth factors has been found to be cardioprotective via a NOS-mediated generation of NO (14). The activation of Akt by TH has also been described in the brain and is proposed to be neuroprotective (42) and has been associated with TH cardioprotection in a rat model of cardiac arrest after ROSC (18). Our study is the first to examine the changes in cardiomyocyte Akt phosphorylation during I/R and its relationship to ROS generation and TH. Here we demonstrate that TH increases phospho-Akt during ischemia before reperfusion and that this was associated with decreased ROS generation and increased cell survival. The inhibition of Akt activity before reperfusion with API-2 abrogated the effects of TH on cell survival and ROS generation, further supporting the hypothesis that Akt activation before or immediately after reperfusion is necessary for TH cardioprotection. Interestingly, Akt phosphorylation was enhanced following I/R with or without TH, implying that Akt activation following reperfusion may be less important than activation before reperfusion. This is further supported by our data demonstrating that API-2 in the absence of TH did not increase I/R cell death or ROS generation in normothermic I/R controls. These results, taken together, indicate that Akt activation before reperfusion may have protective effects, whereas its activation following reperfusion is less protective. This would be consistent with the narrow therapeutic window for TH, which must be implemented before or immediately after reperfusion to have cardioprotective effects (29).
One potential regulator of Akt and ROS is HSP-27, a known stress-activated protein that is cardioprotective (22, 24, 36). Here we find that TH during ischemia increased phospho-HSP27/total HSP27 without significant increases in total HSP27 protein levels. These findings were similar to those of Akt during ischemia. While there are many studies examining the cellular response to heat shock, there are few examining the response to hypothermia. To the best of our knowledge, this the first study to examine the effects of hypothermia on HSP27 protein phosphorylation and expression.
Cardioprotective mechanism of TH.
Temperature alterations have long been known to affect the rate of chemical and biochemical reactions. The current work suggests that cardioprotective hypothermia with a 5°C drop in temperature does more than simply slow reperfusion ROS generation. The cooling-related increase in Akt phosphorylation could result from relative inhibition of regulatory phosphatases. In addition, mild cooling can alter the strength of weak bonds that determine the conformation and association of individual proteins/enzymes that increase protein function (31). For example, temperature-mediated alterations in mRNA translational efficiency have also been recognized (7). It is thus likely that mild alterations in temperature such as those used in TH result in significant alterations in the cellular activity of proteins like Akt and HSP27.
Cardioprotection is associated with several kinase signaling pathways including the G-coupled receptors, the reperfusion injury salvage kinase, and the JAK-STAT pathway (17). In our study, enhanced Akt-NO signaling with an attenuation of ROS independent of cGMP suggests that TH is associated with reperfusion injury salvage kinase pathway signaling. These results are encouraging because they suggest that the beneficial effects of TH may be replaced or enhanced by pharmacological agents.
Limitations.
A limitation of our study was the use of cultured neonatal cardiomyocytes that may not entirely reflect the response of adult cardiomyocytes in the intact heart following I/R. Neonatal cardiomyocytes were chosen over adult cardiomyocytes in this study because of their inherent spontaneous contractile activity, low rates of baseline apoptotic activity, longevity of cell culture for a number of days, and the availability of sufficient protein to assess cellular signaling events. The use of neonatal cells to study molecular cardiac events is well established in the literature, and Bahi et al. (3) have demonstrated similarities between neonatal and adult cardiomyocyte response to I/R injury. Recent studies by our laboratory also demonstrate that TH cardioprotection in a murine model of cardiac arrest is similarly dependent on Akt (5).
Conclusion.
To our knowledge, this study is the first to suggest a role for TH in modulating ROS via Akt-NO signaling. It also implicates a cardiomyocyte-specific cell signaling pathway independent of fibroblasts and endothelial cells, which confound whole heart preparations and studies. Many questions remain unanswered regarding the mechanistic details of the link between hypothermia and Akt-NO activation. Although there is unlikely a “hypothermia receptor,” future studies are needed to determine the signaling pathways manipulated by TH.
GRANTS
This work was supported in part by National Institutes of Health Grants HL-68951 (to T. L. Vanden Hoek), HL-84643 and HL-79641 (to K. J. Hamann), and AT-003441 (to Z.-H. Shao).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Abella B, Zhao D, Alvarado J, Hamann K, Vanden Hoek T, Becker L. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation 109: 2786–2791, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Anderson T, Li C, Shao Z, Hoang T, Chan K, Hamann K, Becker L, Vanden Hoek T. Transient and partial mitochondrial inhibition for the treatment of postresuscitation injury: getting it just right. Crit Care Med 34: S474–S482, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Bahi N, Zhang J, Llovera M, Ballester M, Comella JX, Sanchis D. Switch from caspase-dependent to caspase-independent death during heart development: essential role of endonuclease G in ischemia-induced DNA processing of differentiated cardiomyocytes. J Biol Chem 281: 22943–22952, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Baines CP. The mitochondrial permeability transition pore as a target of cardioprotective signaling. Am J Physiol Heart Circ Physiol 293: H903–H904, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Beiser DG, Wojcik K, Danhong Z, Orbelyan GA, Hamann K, Vanden Hoek TL. Akt1 genetic deficiency limits hypothermia cardioprotection following murine cardiac arrest. Am J Physiol Heart Circ Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernard SA, Rosalion A. Therapeutic hypothermia induced during cardiopulmonary resuscitation using large-volume, ice-cold intravenous fluid. Resuscitation 76: 311–313, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Breaker RR. RNA switches out in the cold. Mol Cell 37: 1–2, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burwell LS, Brookes PS. Mitochondria as a target for the cardioprotective effects of nitric oxide in ischemia-reperfusion injury. Antioxid Redox Signal 10: 579–599, 2008 [DOI] [PubMed] [Google Scholar]
- 9.DeAtley SM, Aksenov MY, Aksenova MV, Harris B, Hadley R, Cole Harper P, Carney JM, Butterfield DA. Antioxidants protect against reactive oxygen species associated with adriamycin-treated cardiomyocytes. Cancer Lett 136: 41–46, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Deng XF, Rokosh DG, Simpson PC. Autonomous and growth factor-induced hypertrophy in cultured neonatal mouse cardiac myocytes comparison with rat. Circ Res 87: 781–788, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation 120: 897–905, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg MS, Mengert TJ. Cardiac resuscitation. N Engl J Med 344: 1304–1313, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao F, Gao E, Yue TL, Ohlstein EH, Lopez B, Christopher T, Ma XL. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-Kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation 105: 1497–1502, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Hale SL, Dae MW, Kloner RA. Hypothermia during reperfusion limits ‘no-reflow’ injury in a rabbit model of acute myocardial infarction. Cardiovasc Res 59: 715–722, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Hale SL, Kloner RA. Myocardial temperature reduction attenuates necrosis after prolonged ischemia in rabbits. Cardiovasc Res 40: 502–507, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation 118: 1915–1919, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Hsu C, Huang C, Chang WT, Chen H, Cheng H, Tsai M, Wang T, Yen Z, Lee C, Chen S, Chen WJ. Cardioprotective effect of therapeutic hypothermia for postresuscitation myocardial dysfunction. Shock 32: 210–216, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Idris A, Roberts L, Caruso L, Showstark M, Layon A, Becker L, Vanden Hoek T, Gabrielli A. Oxidant injury occurs rapidly after cardiac arrest, cardiopulmonary resuscitation, and reperfusion. Crit Care Med 33: 2043–2048, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Iwase H, Robin E, Guzy R, Mungai P, Vanden Hoek T, Chandel N, Levraut J, Schumacker P. Nitric oxide during ischemia attenuates oxidant stress and cell death during ischemia and reperfusion in cardiomyocytes. Free Radic Biol Med 43: 590–599, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol 40: 16–23, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett 410: 493–498, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lavani R, Chang W, Anderson T, Shao Z, Wojcik K, Li C, Pietrowski R, Beiser D, Idris A, Hamann K, Becker L, Vanden Hoek T. Altering CO2 during reperfusion of ischemic cardiomyocytes modifies mitochondrial oxidant injury. Crit Care Med 35: 1709–1716, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Liu L, Zhang XJ, Jiang SR, Ding ZN, Ding GX, Huang J, Cheng YL. Heat shock protein 27 regulates oxidative stress-induced apoptosis in cardiomyocytes: mechanisms via reactive oxygen species generation and Akt activation. Chin Med J 120: 2271–2277, 2007 [PubMed] [Google Scholar]
- 25.Manning B, Cantley L. AKT/PKB signaling: navigating downstream. Cell 129: 1261–1274, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishida T, Yu JD, Minamishima S, Sips PY, Searles RJ, Buys ES, Janssens S, Brouckaert P, Bloch KD, Ichinose F. Protective effects of nitric oxide synthase 3 and soluble guanylate cyclase on the outcome of cardiac arrest and cardiopulmonary resuscitation in mice. Crit Care Med 37: 256–262, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nolan JP, Morley PT, Hoek TL, Hickey RW; Advancement Life support Task Force of the International Liaison committee on Resuscitation Therapeutic hypothermia after cardiac arrest. An advisory statement by the Advancement Life support Task Force of the International Liaison committee on Resuscitation. Resuscitation 57: 231–235, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17°C ischemia in intact hearts. Cardiovasc Res 61: 580–590, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Shao Z, Chang W, Chan K, Wojcik K, Hsu C, Li C, Li J, Anderson T, Qin Y, Becker L, Hamann K, Vanden Hoek T. Hypothermia-induced cardioprotection using extended ischemia and early reperfusion cooling. Am J Physiol Heart Circ Physiol 292: H1995–H2003, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Shao ZH, Wojcik KR, Dossumbekova A, Hsu C, Mehendale SR, Li CQ, Qin Y, Sharp WW, Chang WT, Hamann KJ, Yuan CS, Hoek TL. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J Cell Biochem 107: 697–705, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Storey KB. Functional Metabolism Hoboken: John Wiley and Sons; 2004 [Google Scholar]
- 32.Tissier R, Couvreur N, Ghaleh B, Bruneval P, Lidouren F, Morin D, Zini R, Bize A, Chenoune M, Belair MF, Mandet C, Douheret M, Dubois-Rande JL, Parker JC, Cohen MV, Downey JM, Berdeaux A. Rapid cooling preserves the ischaemic myocardium against mitochondrial damage and left ventricular dysfunction. Cardiovasc Res 83: 345–353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turrens J. Mitochondrial formation of reactive oxygen species. J Physiol 552: 335–344, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J 191: 421–427, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanden Hoek TL, Shao ZH, Li C, Zak R, Schumacker PT, Becker LB. Reperfusion injury on cardiac myocytes after simulated ischemia. Am J Physiol Heart Circ Physiol 270: H1334–H1341, 1996 [DOI] [PubMed] [Google Scholar]
- 36.Wei H, Vander Heide RS. Ischemic preconditioning and heat shock activate Akt via a focal adhesion kinase-mediated pathway in Langendorff-perfused adult rat hearts. Am J Physiol Heart Circ Physiol 298: H152–H157, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, Keith RJ, Schrader J, Guo Y, Conklin DJ, Prabhu SD, Zweier JL, Bolli R, Bhatnagar A. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation 118: 1970–1978, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolfrum S, Pierau C, Radke PW, Schunkert H, Kurowski V. Mild therapeutic hypothermia in patients after out-of-hospital cardiac arrest due to acute ST-segment elevation myocardial infarction undergoing immediate percutaneous coronary intervention. Crit Care Med 36: 1780–1786, 2008 [DOI] [PubMed] [Google Scholar]
- 39.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, Hamilton AD, Polokoff M, Nicosia SV, Herlyn M, Sebti SM, Cheng JQ. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res 64: 4394–4399, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Yellon D, Hausenloy D. Myocardial reperfusion injury. N Engl J Med 357: 1121–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Zhang L, Kumar S, Kaminski A, Kasch C, Sponholz C, Stamm C, Ladilov Y, Steinhoff G. Importance of endothelial nitric oxide synthase for the hypothermic protection of lungs against ischemia-reperfusion injury. J Thorac Cardiovasc Surg 131: 969–974, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Zhao H, Shimohata T, Wang J, Sun G, Schaal D, Sapolsky R, Steinberg G. Akt contributes to neuroprotection by hypothermia against cerebral ischemia in rats. J Neurosci 25: 9794–9806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]