Abstract
Nuclear factors of activated T cells (NFATs) are Ca2+-sensitive transcription factors that have been implicated in hypertrophy, heart failure (HF), and arrhythmias. Cytosolic NFAT is activated by dephosphorylation by the Ca2+-sensitive phosphatase calcineurin, resulting in translocation to the nucleus, which is opposed by kinase activity, rephosphorylation, and nuclear export. Four different NFAT isoforms are expressed in the heart. The activation and regulation of NFAT in adult cardiac myocytes, which may depend on the NFAT isoform and cell type, are not fully understood. This study compared basal localization, import, and export of NFATc1 and NFATc3 in adult atrial and ventricular myocytes to identify isoform- and tissue-specific regulatory mechanisms of NFAT activation under physiological conditions and in HF. NFAT-green fluorescent protein fusion proteins and NFAT immunocytochemistry were used to analyze NFAT regulation in adult cat and rabbit myocytes. NFATc1 displayed basal nuclear localization in atrial and ventricular myocytes, an effect that was attenuated by reducing intracellular Ca2+ concentration and inhibiting calcineurin, and enhanced by the inhibition of nuclear export. In contrast, NFATc3 was localized to the cytoplasm but could be driven to the nucleus by angiotensin II and endothelin-1 stimulation in atrial, but not ventricular, cells. Inhibition of nuclear export (by leptomycin B) facilitated nuclear localization in both cell types. Ventricular myocytes from HF rabbits showed increased basal nuclear localization of endogenous NFATc3 and reduced responsiveness of NFAT translocation to phenylephrine stimulation. In control myocytes, Ca2+ overload, leading to spontaneous Ca2+ waves, induced substantial translocation of NFATc3 to the nucleus. We conclude that the activation of NFAT in adult cardiomyocytes is isoform and tissue specific and is tightly controlled by nuclear export. NFAT is activated in myocytes from HF animals and may be secondary to Ca2+ overload.
Keywords: nuclear factor of activated T cells, intracellular Ca2+ concentration, calcineurin, nuclear translocation
during pathological remodeling associated with hypertension, hypertrophy, heart failure (HF), and arrhythmias, the cardiovascular system experiences changes in gene transcription and protein expression. Nuclear factor of activated T cells (NFAT) transcription factors play a key role in cellular remodeling, integrating intracellular Ca2+ signals and gene transcription. NFAT is controlled by phosphorylation [e.g., by the kinases glycogen synthase kinase (GSK)3β, p38, and JNK], and its phosphorylation status regulates its translocation into the nucleus. Intracellular Ca2+ signals stimulate the Ca2+/calmodulin-dependent phosphatase calcineurin (CaN), which dephosphorylates NFAT, causing translocation to the nucleus, where it regulates gene transcription (4, 43, 59). Rephosphorylation of NFAT causes NFAT nuclear export and relieves its transcriptional effect. Cardiac myocytes display large rhythmic changes in intracellular Ca2+ concentration ([Ca2+]i) with every heart beat (5). This raises the question of how NFAT is activated in a Ca2+-dependent fashion either normally or during pathological situations (5, 35, 57). Several mechanisms have been hypothesized to selectively activate CaN and NFAT dephosphorylation [e.g., Ca2+ fluxes through voltage-gated L-type Ca channels and Ca2+-induced Ca2+ release (CICR) from ryanodine receptor (RyR) sarcoplasmic reticulum (SR) Ca2+-release channels, Ca2+ entry via T-type Ca2+ channels, store-operated Ca2+ entry, and inositol 1,4,5-trisphosphate (IP3)-dependent Ca2+ release; for a review, see Ref. 58]. However, none of these pathways has been demonstrated experimentally in conclusive and unequivocal ways. Nonetheless, several recent reports have shed new light on two Ca2+ signaling mechanisms that appear to be involved in the activation of NFAT in diseased cardiomyocytes: first, under conditions of tachycardia, the high frequency of action potentials can cause a net intracellular Ca2+ gain that increases [Ca2+]i and can activate the CaN/NFAT pathway (30). Two more recent studies (42, 61) have revealed a potential link between NFAT activation and remodeling of ion channels [L-type Ca2+ channels and voltage-gated K+ channels (transient outward K+ current)] in canine myocytes in response to high-frequency pacing. Second, chronic activation of Gq protein-coupled receptors by neurohumoral agonists [e.g., angiotensin (ANG) II and endothelin (ET)-1] may represent an alternative mechanism for NFAT activation observed during hypertrophy (32, 36). Gq proteins stimulate phospholipase C, which generates the second messengers IP3 and diacylglycerol. IP3 promotes Ca2+ release from intracellular stores by activating specific Ca2+-release channels of the SR (IP3 receptors). IP3-mediated Ca2+ release could either act as a Ca2+ source independent from RyR-mediated Ca2+ release or modulate RyR-dependent CICR, resulting in arrhythmogenic Ca2+ release and elevated systolic [Ca2+]i (63). The application of ET-1 has been shown to generate cytoplasmic Ca2+ signals that affect the nucleus and activate NFAT, thereby inducing hypertrophy in ventricular myocytes (23). In addition, IP3-dependent Ca2+ signaling also occurs locally at the nuclear envelope and affects the nuclear Ca2+ concentration, which, in turn, controls many cellular functions in cardiac cells (22, 27), including the regulation of transcription factors (60).
The mammalian heart expresses four different NFAT isoforms (NFATc1, NFATc2, NFATc3, and NFATc4), which are regulated by [Ca2+]i via CaN (54). Open questions regarding our understanding of NFAT regulation in adult cardiac myocytes are: Is there a common mechanism that activates all NFAT isoforms in cardiac cells, or are there isoform-specific or even tissue-specific (atrium vs. ventricle) differences? Is NFAT solely regulated via the balance of cytosolic phosphatase (CaN) and kinase activity, or are regulated nuclear export pathways and nuclear kinases involved in NFAT activation/deactivation?
The present study analyzed basal and agonist-induced (ET-1 and ANG II) activation of NFATc1 and NFATc3 in quiescent myocytes (measured as nuclear localization of NFAT) using NFAT-green fluorescent protein (GFP) fusion proteins in conjunction with confocal microscopy. We found isoform- and tissue-specific differences in the activation of NFATc1 and NFATc3 in adult cardiac myocytes as well as increased basal activity of NFATc3 in myocytes from failing hearts.
Part of this work has been previously published in abstract form (45, 47).
MATERIALS AND METHODS
Isolation, Cell Culture, and Viral Transduction of Cardiac Myocytes
Atrial and ventricular myocytes were isolated from cat or rabbit hearts as previously described (26, 50). The procedure for cell isolation was approved by the Institutional Animal Care and Use Committees. Briefly, atrial and ventricular myocytes were isolated from cat or rabbit hearts using animals of either sex. Animals were anesthetized with thiopental sodium (35 mg/kg ip). After a thoracotomy, the hearts were quickly excised, mounted on a Langendorff apparatus, and retrogradely perfused via the aorta. After an initial washing step with an oxygenated Ca2+-free solution [which contained (in mM) 137 NaCl, 5.4 KCl, 1.0 MgCl2, 12 NaHCO3, 0.6 NaH2PO4, and 11 glucose], the heart was perfused with oxygenated HEPES-buffered saline solution (HBSS) containing 36 μM Ca2+ and collagenase at 37°C (0.06% collagenase type II, Worthington Biochemical, Freehold, NJ) to obtain single myocytes. Isolated cells were adapted to the final Ca2+ concentration of the medium (1.8 mM) over a period of 2 h and plated on sterile, laminin-coated glass coverslips. Myocytes were cultured using serum-free medium 199, which was supplemented with 25 μg/ml gentamycin and 25 μg/ml kanamycin (all from Mediatech, Hernon, VA). One day after isolation, cells were infected with adenoviruses encoding for NFATc1-GFP and NFATc3-GFP, and experiments were performed 24 h (ventricular myocytes) or 48 h (atrial myocytes) after infections. In addition, ventricular myocytes were isolated from rabbits with nonischemic HF induced by combined aortic insufficiency and stenosis (38, 39). This HF model is characterized by the combination of the gradual development of hypertrophy and HF (over 4–9 mo, as monitored by serial echocardiography), depressed systolic function, and arrhythmogenesis in an animal that has human-like Ca2+ handling and cellular electrophysiological properties. The echocardiographic index of severe left ventricular dysfunction is a left ventricular end-systolic dimension of >1.4 cm (>40% increase). This model has been well characterized on structural, biochemical, molecular, Ca2+ handling, and electrophysiological levels (14, 15, 38, 40, 41, 49).
Solutions and Chemicals
During all experiments, cells were bathed in an extracellular solution (HBSS), which contained (in mmol/l) 135 NaCl, 4 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES (pH 7.3 with NaOH). Agonists or antagonists were prepared in HBSS for acute application or added to the medium for longer incubations. Unless otherwise stated, all chemicals, agonists, or inhibitors were purchased from Sigma (St. Louis, MO) or Tocris (Ellisville, MO).
Fluorescence Measurements
Measurements of NFAT-GFP.
To analyze the subcellular localization of NFAT-GFP with confocal microscopy (Biorad Radiance 2000/MP) in virally transduced myocytes, we measured the mean fluorescence of a region of interest (ROI) covering the nucleus (NFATnuc) and a cytoplasmic ROI (NFATcyt) of the same size. The subcellular distribution of NFAT was then quantified as the ratio of NFATnuc to NFATcyt. In some experiments, where agonists or antagonists were applied acutely, this ratio was normalized to the ratio measured from the same cell before the stimulus application. GFP was excited with an argon ion laser (wavelength: 488 nm), and emitted fluorescence was collected at 500–520 nm.
Intracellular Ca2+ measurements.
Intracellular Ca2+ transients from single myocytes evoked by electrical field stimulation (0.5 Hz) or [Ca2+]i signals evoked by Ca2+ overload were measured with rhod-2 and confocal microscopy in the line-scan mode (3 ms/line, pixel size: 1.5 μm). Briefly, cells were loaded for 20 min at room temperature with 5 μM rhod-2 AM and 5 μM Pluronic F-127 in HBSS (Pluronic stock solution: 0.2 g/ml DMSO). After the removal of excessive dye, rhod-2 was excited at 543 nm (green He-Ne laser), and emitted fluorescence was recorded at a wavelength of ≥570 nm. Changes in rhod-2 fluorescence were normalized to the level of fluorescence before stimulus application. Rhod-2 was chosen as the Ca2+-sensitive dye because it allows for measurements of [Ca2+]i in the presence of GFP (46).
Immunocytochemistry.
The preparation and immunofluorescence staining of rabbit ventricular myocytes were carried out as previously described (46). Myocytes were plated on laminin-coated coverslips and washed with solution A (450 mM NaCl, 20 mM phosphate buffer, pH 7.2). Cells were fixed for 30 min using 4% (wt/vol) paraformaldehyde in 200 mM phosphate buffer (pH 7.4). Excessive paraformaldehyde was washed out using solution A, and cells were permeabilized using solution B (0.3% Triton X-100, 450 mM NaCl, and 20 mM phosphate buffer; pH 7.2) supplemented with 1% goat serum. Cells were then incubated with goat polyclonal antibody C-20 against NFATc3 (sc-1152, Santa Cruz Biotechnology, Santa Cruz, CA) using a 1:300 dilution. For visualization, an Alexa fluor488 donkey anti-goat secondary antibody (A11055, Invitrogen, Carlsbad, CA) was used at a 1:500 dilution. Fluoromount G was used as a mounting medium.
Data Analysis and Presentation
Data are presented as individual observations or as means ± SE and were analyzed using a Student's t-test. n represents the number of individual cells, and differences were considered significant at P < 0.05.
RESULTS
Localization of NFAT in Resting Myocytes Is Isoform Specific
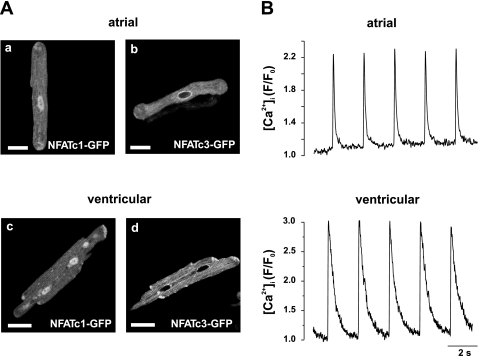
The subcellular distribution of NFAT-GFP in resting (extracellular Ca2+ concentration: 2 mmol/l) adult cat myocytes was analyzed with confocal microscopy 24–48 h after infections. NFATc1-GFP was predominantly localized to the nucleus of atrial and ventricular cells (Fig. 1, A,a and A,c). Average NFATc1nuc-to-NFATc1cyt ratios were 1.71 ± 0.31 (n = 31) for atrial myocytes and 2.50 ± 0.12 (n = 22) for ventricular myocytes. In contrast, NFATc3-GFP displayed cytoplasmic localization under basal conditions, as indicated by lower average NFATc3nuc-to-NFATc3cyt ratios of 0.54 ± 0.05 (n = 17) and 0.51 ± 0.04 (n = 34) for atrial myocytes (Fig. 1A,b) and ventricular (Fig. 1A,d) myocytes, respectively.
Fig. 1.
Isoform-specific subcellular localization of nuclear factors of activated T cells (NFATs) in adult cardiomyocytes. A: representative confocal images showing the basal nuclear localization of NFATc1-green fluorescent protein (GFP) in atrial (a) and ventricular (c) myocytes. In contrast, NFATc3-GFP was localized to the cytoplasm in cardiac myocytes [atrial cells (A,b) and ventricular cells (A,d)]. Images were taken 24 h (ventricular cells) or 48 h (atrial cells) after adenoviral transduction. B: representative intracellular Ca2+ transients from adult cultured myocytes expressing NFATc1-GFP in response to electrical stimulation (0.5 Hz). Changes in intracellular Ca2+ concentration ([Ca2+]i) were measured with confocal laser scanning microscopy in the line-scan mode using the Ca2+-sensitive dye rhod-2. Changes in rhod-2 fluorescence (F) were normalized to the level of fluorescence before stimulus application (F0). Scale bars = 30 μm.
Adult myocytes in culture may be subject to morphological and functional changes that are characterized by the loss of t-tubules and insensitivity to electrical excitability (20, 33, 34, 55) and may affect Ca2+ handling. To test for functional changes of excitation-contraction coupling and CICR, we measured action potential-evoked intracellular Ca2+ transients from myocytes expressing NFATc1-GFP using the Ca2+-sensitive dye rhod-2. Cultured myocytes responded to electrical field stimulation (0.5 Hz) with Ca2+ transients that were typical for atrial cells (Fig. 1B, top) or ventricular cells (Fig. 1B, bottom), indicating no significant functional changes due to time in culture.
Pharmacological Manipulation of NFATc1 in Cardiac Myocytes
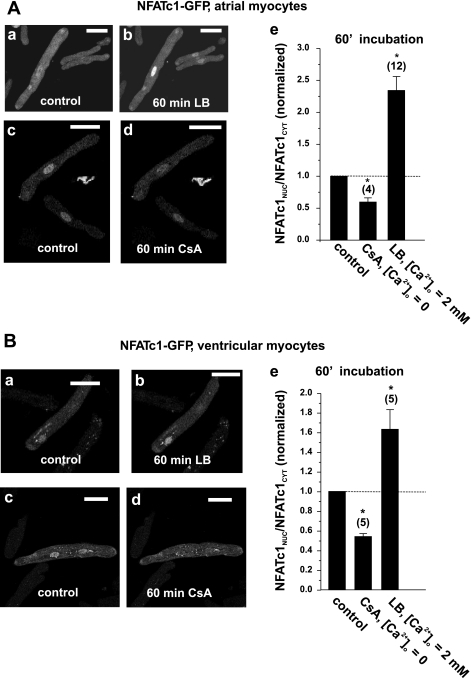
If the nuclear localization of NFATc1 is due to basal Ca2+-dependent CaN activity, then reduction of [Ca2+]i or inhibition of CaN with cyclosporin A (CsA) would be expected to shift NFATc1 toward the cytoplasm (i.e., decrease the NFATnuc-to-NFATcyt ratio). Indeed, the acute application of CsA (1 μmol/l) in Ca2+-free HBSS for 60 min resulted in the redistribution of NFATc1 to the cytoplasm in atrial cells (Fig. 2, A,c and A,d), which was quantified as a decrease in the NFATnuc-to-NFATcyt ratio by up to 50% of the initial level (summary data shown in Fig. 2A,e); however, NFATc1 retained the preferential nuclear localization.
Fig. 2.
Pharmacological manipulation of NFATc1 basal activity. A: nuclear localization of NFATc1 in atrial cells was sensitive to [Ca2+]i, calcineurin (CaN) activity, and the nuclear export processes. Short-term incubation (60 min) of atrial cells with 40 nM leptomycin B (LB) caused further accumulation of NFATc1 in the nucleus (A,a and A,b), whereas the inhibition of CaN, induced by an incubation with nominally Ca2+-free extracellular solution containing cyclosporin A (CsA; 1 μmol/l), resulted in the export of NFATc1 back to the cytoplasm (A,c and A,d). A,e: summary data from A,a–A,d. B: as in atrial cells, basal nuclear localization in ventricular cells was also sensitive to CaN activity (B,a, B,b, and B,e) and enhanced when nuclear export was inhibited with LB (B,c–B,e). Nuclear-to-cytoplasmic NFAT ratios (NFATnuc/NFATcyt ratios) were normalized to the control ratio at an extracellular Ca2+ concentration ([Ca2+]o) of 2 mmol/l before incubation. Numbers in parentheses indicate the number of individual cells tested. Scale bars = 30 μm. *Significantly different from control at P < 0.05.
The observation that nuclear localization of NFAT is reversible indicates the involvement of a nuclear export process for NFAT. It has been suggested that the nuclear export of NFAT involves the transport protein Crm1 (exportin 1), which can be inhibited by leptomycin B (LB) (28). The application of LB for 60 min resulted in an even higher NFATnuc-to-NFATcyt ratio for NFATc1 (Fig. 2, A,a, A,b, and A,e). This indicates that in the basal state there is some dynamic balance of NFATc1 import (driven by CaN activity) and export via a Crm1-dependent pathway (as opposed to a maximal nuclear concentration).
These observations were not restricted to atrial tissue, since LB also increased the steady state ratio by a factor of ∼1.8 (Fig. 2, B,a and B,b), and CaN inhibition (and reduction of [Ca]i) caused nearly a 50% reduction in the ratio (Fig. 2, B,c and B,d) in cat ventricular myocytes.
Kinase activity and rephosphorylation of NFAT can also influence NFAT localization in ventricular myocytes (4, 9, 53). When we inhibited the cellular kinases GSK3β with 1 μmol/l alsterpaullone or JNK2 using 1 μmol/l SP-600125 in ventricular cells by incubations overnight, there was a substantial nuclear accumulation of NFATc1, as indicated by an increase in the NFATnuc-to-NFATcyt ratio from 2.10 ± 0.08 (control, n = 34) to 4.17 ± 0.32 (SP-600125, n = 20) and 5.28 ± 0.36 (alsterpaullone, n = 24).
These data suggest that the CaN/NFATc1 pathway has a high basal activity in resting atrial myocytes and even more so in ventricular myocytes. Nonetheless, nuclear kinase activity and export processes can shift the nuclear/cytosolic localization of NFATc1 in adult cardiac myocytes.
Activation of NFATc3 by Neurohumoral Stimuli
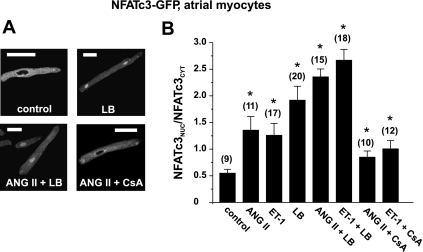
The NFATc3 isoform has been shown to play an important role in hypertrophy and HF-related remodeling in the cardiovascular system (11, 19, 48, 61). In contrast to NFATc1, we found NFATc3 to be localized to the cytoplasm of resting atrial and ventricular myocytes (cf. Fig. 1A) under basal conditions. We tested whether the hypertrophy-related extracellular agonists ANG II or ET-1 were capable of inducing the translocation of NFATc3 to the nucleus. These agonists activate the Gq protein/IP3 pathway and liberate Ca2+ from the SR via IP3 receptor Ca2+-release channels (44, 63), a pathway that has been linked to the activation of transcription factors in myocytes (22, 60). Atrial myocytes expressing NFATc3-GFP were incubated overnight in medium containing 2 μmol/l ANG II or 100 nmol/l ET-1. Both agonists induced the nuclear translocation of NFATc3, which was quantified as a two- to threefold increase in the NFATnuc-to-NFATcyt ratio (representative images are shown in Fig. 3A; summary data are shown in Fig. 3B). The degree of agonist-induced translocation was comparable with nuclear accumulation induced by LB (Fig. 3B). The combination of agonist stimulation and block of nuclear export further enhanced the nuclear accumulation of NFAT (4- to 5-fold increase of the NFATnuc-to-NFATcyt ratio compared with control). Shorter incubation times (up to 4 h) did not result in detectable changes in the subcellular distribution of NFATc3 (data not shown).
Fig. 3.
Nuclear translocation of NFATc3 is induced by angiotensin (ANG) II and endothelin (ET)-1 in adult atrial myocytes. A and B: overnight incubation of atrial myocytes with ANG II (2 μmol/l), ET-1 (100 nmol/l), or LB (40 nmol/L) resulted in the nuclear translocation of NFATc3-GFP, which was sensitive to CsA (1 μmol/l). This effect could be enhanced when agonist stimulation was combined with LB incubation. Numbers in parentheses indicate the number of individual cells tested. Scale bars = 30 μm. *Significantly different from control at P < 0.05.
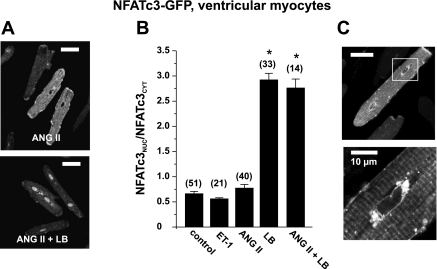
In striking contrast to atrial myocytes, stimulation with ANG II and ET-1 failed to induce the nuclear accumulation of NFATc3 in ventricular myocyes (Fig. 4, A and B). However, the inhibition of nuclear export (40 nmol/l LB) resulted in the substantial nuclear accumulation of NFATc3-GFP in time-matched parallel cultures (Fig. 4B). Combined incubation with ANG II and LB did not induce further nuclear accumulation of ventricular NFATc3, in contrast to observations in atrial cells (compare Fig. 3B).
Fig. 4.
Regulation of NFATc3 is tissue specific. A and B: agonists ANG II and ET-1 failed to induce the nuclear translocation of NFATc3 in ventricular myocytes. In contrast, inhibition of nuclear export with LB caused substantial nuclear accumulation of NFATc3, which was not further enhanced by ANG II (compare with Fig. 3B). C: NFATc3-GFP accumulated in a region surrounding the nucleus in resting myocytes. Numbers in parentheses indicate the number of individual cells tested. Scale bars = 30 μm except for C, bottom, where the scale bar = 10 μm. *Significantly different from control at P < 0.05.
Furthermore, the inhibition of cellular kinases (with alsterpaullone or SP-600125), an experimental intervention that induced the nuclear accumulation of NFATc1 in ventricular cells (see above), did not induce nuclear localization of NFATc3 in ventricular myocytes [NFATnuc-to-NFATcyt ratios: control, 0.66 ± 0.04 (n = 51); alsterpaullone, 0.65 ± 0.06 (n = 12); and SP-600125, 0.63 ± 0.07 (n = 13)].
Another notable observation was a strong fluorescence signal of NFATc3-GFP around the nucleus (Fig. 4C; see also Fig. 1A,a for atrial cells). As shown for ventricular cells at two different magnifications, NFATc3 did accumulate around the nucleus in nonstimulated cells (Fig. 4C). This finding might support the hypothesis that NFATc3 is regulated locally by IP3-dependent nuclear Ca2+ signals (see the discussion) (27).
Our data suggest that the activation and regulation of NFATc3 are different in atrial cells compared with ventricular cells. The activation of atrial (but not ventricular) IP3 pathways by hypertrophic, neurohumoral agonists appears to be capable of inducing the nuclear translocation of NFATc3. Comparable with the NFATc1 isoform above, nuclear NFATc3 accumulation is also limited by dynamic nuclear export mechanisms under basal conditions.
HF and Ca2+ Overload Induce Nuclear Localization of NFATc3
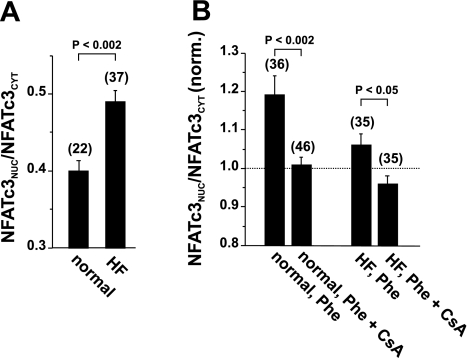
HF is characterized by profound changes in Ca2+ handling (38) and gene transcription (7). Since the activation of CaN depends on the sustained elevation of [Ca2+]i (59), a mechanism that results in Ca2+ overload would favor the activation of NFAT transcription factors under pathological conditions. NFATc3, in particular, is active during pathological situations, including atrial and ventricular fibrillation or the presence of hypertrophic extracellular agonists (23, 42, 61). We analyzed the nuclear localization of endogenous NFATc3 in ventricular cells from a chronic rabbit HF model (38) using immunocytochemistry (NFATc3-specific antibody). In ventricular myocytes from HF rabbits, nuclear localization of NFATc3 was enhanced compared with myocytes from normal rabbits [NFATnuc-to-NFATcyt ratios: 0.49 ± 0.014 (n = 37) vs. 0.40 ± 0.013 (n = 22), P < 0.002; Fig. 5A]. The application of phenylephrine (100 μmol/l) resulted in further nuclear accumulation of NFATc3 in ventricular cells from control rabbits but failed to facilitate nuclear localization in myocytes from HF animals (Fig. 5B). Similar to cat ventricular myocytes, ET-1 and ANG II, however, failed to induce translocation in rabbit ventricular cells (data not shown). Phenylephrine application in the presence of CsA prevented the agonist-induced nuclear translocation in control cells and reduced the NFATnuc-to-NFATcyt ratio below control levels in HF myocytes. These data suggest that the Ca2+/CaN pathway may be basally activated in ventricular myocytes from HF animals.
Fig. 5.
NFATc3 is activated in ventricular myocytes from rabbits with heart failure (HF). A: basal nuclear localization of endogenous NFATc3 (NFATc3 antibody) was enhanced in ventricular myocytes from HF rabbits compared with control rabbit myocytes. B: stimulation with phenylephrine (Phe; 100 μmol/l) induced further nuclear accumulation of NFATc3 in control myocytes but not in HF myocytes. NFATnuc/NFATcyt ratios were normalized to the respective level of unstimulated cells as shown in A (dashed line). In A and B, endogenous NFAT was detected by immunocytochemistry using a specific antibody against NFATc3. Numbers in parentheses indicate the number of individual cells tested.
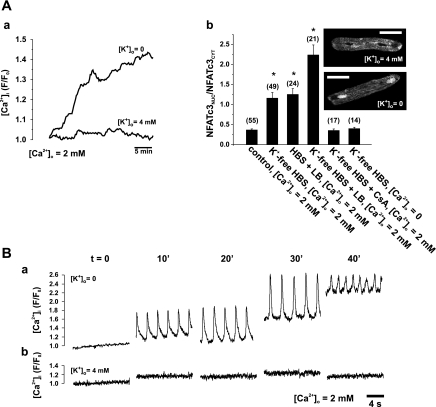
Cardiac myocytes from failing hearts develop irreversible changes in Ca2+ homeostasis due to altered function and/or the expression of several Ca2+-handling proteins, among them, the Na+/Ca2+ exchanger (NCX). In HF rabbits, alterations in [Ca2+]i contribute to systolic dysfunction and arrhythmogenesis (39, 40) and may also contribute to altered transcriptional regulation (7). To test whether diastolic [Ca2+]i elevation (e.g., at a high heart rate in HF) activates NFATc3 in ventricular myocytes from normal rabbits, we induced Ca2+ overload in myocytes expressing NFATc3-GFP by Na+,K+-ATPase inhibition (K+-free solution), which elevates the intracellular Na+ concentration and, in turn, [Ca2+]i [via NCX, leading to SR Ca2+ overload (18)]. Figure 6A,a shows the sustained elevation of [Ca2+]i in K+-free solution, which caused the translocation of NFATc3 to the nucleus (over 2 h) to a similar extent as LB in normal HBSS (Fig. 6A,b). This NFATc3 translocation was prevented by either blocking CaN with CsA or removing extracellular Ca2+ (Fig. 6A,b) and further enhanced by 40 nM LB. The further analysis of [Ca2+]i signals under Ca2+ overload conditions using higher temporal resolution (confocal line scan mode) indicated that these cells developed spontaneous Ca2+ release in the form of spontaneous Ca2+ waves and a net increase in diastolic [Ca2+]i (see Fig. 6B,a). In contrast, control cells did not show comparable changes in [Ca2+]i (control myocytes; Fig. 6B,b).
Fig. 6.
Ca2+ overload caused the nuclear translocation of NFATc3 in ventricular myocytes from normal rabbits. A,a: removal of external K+ caused an increase of [Ca2+]i. A,b: removal of external K+ caused the nuclear translocation of NFATc3-GFP in control myocytes (inset). The summary data show that nuclear translocation after incubation in K+-free HEPES-buffered saline (HBS) solution for 2 h was sensitive to CsA (1 μmol/l) and dependent on [Ca2+]o. Inhibition of nuclear export with LB (40 nmol/l, 2-h incubation) further facilitated the nuclear accumulation of NFATc3. B: [Ca2+]i profiles derived from confocal line-scan images indicating that Ca2+ overload resulted in spontaneous Ca2+ release and Ca2+ waves in an extracellular K+ concentration ([K+]o) of 0 mmol/l (a) but not of 4 mmol/l (b). All experiments were performed in the presence of 10 mmol/l 2,3-butanedione monoxime to prevent contractions. Numbers in parentheses indicate the number of individual cells tested. Scale bars = 30 μm. *Significantly different from control at P < 0.05.
These data indicate that the nuclear localization of NFATc3 is regulated by a dynamic balance between import and export rates. Under normal conditions, a net nuclear export rate prevents the nuclear localization of NFATc3. A pathological Ca2+ signal (e.g., Ca2+ waves and elevated diastolic [Ca2+]i, as in Fig. 6) changes this balance to a net nuclear import, thereby stabilizing the nuclear localization of NFATc3 in cardiomyocytes.
DISCUSSION
Transcription factors of the NFAT family are involved in the pathological remodeling of cardiac myocytes (42, 61). The mammalian heart expresses four different Ca2+-sensitive isoforms of NFAT (NFATc1, NFATc2, NFATc3, and NFATc4) (54). Although Ca2+-dependent activation and regulation of NFAT have been investigated in detail in many cell types (10, 21, 59), it is not fully understood how NFAT is regulated in adult cardiac myocytes, which experience large changes in [Ca2+]i during every heart beat (5, 35, 57). Several Ca2+-dependent signals for the activation of NFAT have been proposed for neonatal and adult cardiac tissue, including contributions of extracellular Ca2+ (i.e., Ca2+ influx through L-type voltage-operated Ca2+ channels) (24, 42, 56) or local spatially restricted Ca2+ signals, such as nuclear Ca2+ and IP3-mediated Ca2+ release (27). The activation of NFAT in adult myocytes has been observed during high-frequency pacing (42, 61) or the application of neurohumoral stimuli (ET-1) that activate the IP3 pathway (23). In addition, recent evidence has suggested an upstream regulatory function of Ca2+/calmodulin-dependent kinase II (CaMKII) during NFAT activation by means of CaN phosphorylation (31, 61); however, the details of this type of regulation remain to be substantiated. The studies mentioned above have also indicated that the activation of NFAT is tissue specific and may also be regulated through pathways other than the Ca2+-dependent activation of CaN alone.
The present study focused on the analysis of basal and agonist-induced activation of NFAT (measured as nuclear translocation of NFAT) in adult myocytes. To visualize and analyze the subcellular localization of NFAT, we used NFAT-GFP fusion proteins (NFATc1 and NFATc3 isoforms) and quantified the subcellular localization of NFAT as the NFATnuc-to-NFATcyt ratio. NFAT-GFP fusion proteins are widely used to study NFAT in living cells because they behave similarly to endogenous proteins (13, 17, 25, 31, 46, 51).
We found that the NFATc1 isoform, but not the NFATc3 isoform, displayed nuclear localization in resting myocytes. To our best knowledge, this is the first study to compare these isoforms in adult cardiac myocytes. Consistent with the notion of the high activity of NFATc1 compared with other isoforms, a recent study on skeletal muscle cells has described the robust nuclear translocation of NFATc1 but only the transient nuclear localization of NFATc3 in response to electrical stimulation. In those cells, as well as in endothelial cells, it was found that NFATc3, but not NFATc1, was tightly controlled by nuclear export processes (46, 51).
The molecular mechanism for the basal nuclear localization of NFATc1 is not well understood. Transcriptional activity and the localization of NFAT in the nucleus are determined by binding DNA and other accessory transcription factors in the nucleus (52, 59). This mechanism would provide another layer of (isoform-specific) regulation of NFAT in the nucleus. Cardiac NFATc1 controls valve formation and morphogenesis of the heart, and disruption of this isoform results in lethal defects (12). Thus, the basal activity of NFATc1 in cardiac myocytes is likely to be required to maintain the differentiated phenotype of adult myocytes. This idea is supported by a more recent study that analyzed the role of different NFAT isoforms for the differentiation of skeletal muscle fibers. The study (8) demonstrated that the activation of a single NFAT isoform or the concerted activation of up to four NFAT isoforms controls the differentiation of skeletal muscle fibers into slow or fast fibers. However, it remains to be determined how exactly specific patterns of NFAT isoforms are activated differentially. It is conceivable that NFAT isoforms reside in distinct cytosolic domains and restricted Ca2+ signals activate CaN locally, that the spatiotemporal organization of the Ca2+ signal (e.g., steady-state elevation vs. oscillatory changes) is responsible for the activation of a specific isoform, or that different NFAT isoforms have different levels of sensitivity for dephosphorylation by CaN.
Another striking result of the present study is the specific activation of NFATc3 by the neurohumoral agonists ANG II and ET-1 in atrial, but not ventricular, myocytes from the cat. Our laboratory has previously demonstrated that the density of IP3 receptors is higher in atrial than ventricular myocytes (16) and that IP3 signaling strongly influences Ca2+ handling, excitation-contraction coupling, and arrhythmogenesis in atrial myocytes (29, 62, 63). While the more pronounced IP3-dependent Ca2+ release in atrial cells would support the hypothesis that this specific source of Ca2+ preferentially activates NFAT, further investigation will be required to clarify whether this difference in IP3 receptor signaling indeed represents the molecular mechanism responsible for the cellular differences in NFAT regulation or if there are other mechanisms involved, such as different expression levels of membrane surface receptors for these agonists.
We also observed a concentrated localization of NFATc3 around the nucleus. This observation supports the notion that cardiac NFAT and other Ca2+-dependent transcription factors are regulated by local, nuclear Ca2+ signals, independent from normal “beat-to-beat” Ca2+ (22, 27, 60). In support of this idea are the observations that the nuclear envelope is well equipped with IP3 receptors (3) and that IP3 can release Ca2+ from the cytosolic side of the nuclear envelope as well as into the nucleoplasm via IP3 receptors located in the inner membrane of the nuclear envelope (62). Thus, IP3-mediated Ca2+ release can act locally around the nucleus and affect nuclear envelope [Ca2+] and the nuclear Ca2+ concentration (62, 63), which could represent the basic elements of a mechanism for NFAT activation independent from global cytoplasmic Ca2+ signals. Thus, the perinuclear region could reflect a local reserve of NFAT that is poised for shuttling in and out of the nucleus when local [Ca2+]i is elevated.
In ventricular myocytes from HF rabbits, basal nuclear localization of NFATc3 was increased compared with control cells (Fig. 5A). HF myocytes are characterized by increased expression of IP3 receptors and increased diastolic Ca2+ release (1) as well as calmodulin/CaMKII-dependent nuclear export of HDAC5 (another transcriptional regulator) (7).
The higher circulating levels of neurohumoral factors in HF, which trigger IP3 signaling (e.g., ET-1 and ANG II), may contribute to the enhanced IP3-dependent NFAT nuclear import seen here and may reinforce the HF phenotype. In HF, the intracellular Na+ concentration is increased (2, 6, 37) and can increase [Ca2+]i and spontaneous SR Ca2+ release (especially at higher heart rates). This could also contribute to enhanced CaN activity and nuclear translocation of NFATc3-GFP in HF, as seen here in Ca2+-overloaded control ventricular myocytes (Fig. 6).
The fact that there are substantial basal levels of nuclear NFATc3 (and especially NFATc1) that are acutely increased by block of nuclear export implies that there is a significant rate of basal CaN-dependent import and export of NFAT that results in the steady-state distribution. Moreover, this may poise this system for dynamic manipulation by cytosolic or perinuclear Ca2+ signals.
We conclude that the regulation of NFAT in adult cardiac myocytes is isoform specific and differs among atrial and ventricular tissue. Nuclear localization of NFAT is regulated by both the Ca2+ signal, which activates CaN, but also by nuclear NFAT kinases and the nuclear export machinery.
GRANTS
This work was supported by grants National Heart, Lung, and Blood Institute Grants HL-62231 and HL-80101 (to L. A. Blatter), HL-089617 (to K. Banach), HL-30077 and HL-80101 (to D. M. Bers), HL-62927 (to J. D. Molkentin), and HL-46929 and HL-73966 (to S. M. Pogwizd) and by American Heart Association Grant 0820080Z (to A. Rinne).
DISCLOSURES
No conflicts of interest are declared by the author(s).
REFERENCES
- 1.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Baartscheer A, van Borren MM. Sodium ion transporters as new therapeutic targets in heart failure. Cardiovasc Hematol Agents Med Chem 6: 229–236, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J Biol Chem 280: 15912–15920, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1934, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol 70: 23–49, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bers DM, Pogwizd SM, Schlotthauer K. Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97, Suppl 1: I36–I42, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Bossuyt J, Helmstadter K, Wu X, Clements-Jewery H, Haworth RS, Avkiran M, Martin JL, Pogwizd SM, Bers DM. Ca2+/calmodulin-dependent protein kinase IIδ and protein kinase D overexpression reinforce the histone deacetylase 5 redistribution in heart failure. Circ Res 102: 695–702, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, Pallafacchina G, Tothova J, Schiaffino S, Murgia M. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci USA 106: 13335–13340, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow W, Hou G, Bendeck MP. Glycogen synthase kinase 3beta regulation of nuclear factor of activated T-cells isoform c1 in the vascular smooth muscle cell response to injury. Exp Cell Res 314: 2919–2929, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell 109, Suppl: S67–S79, 2002 [DOI] [PubMed] [Google Scholar]
- 11.de Frutos S, Spangler R, Alo D, Bosc LVG. NFATc3 mediates chronic hypoxia-induced pulmonary arterial remodeling with α-actin up-regulation. J Biol Chem 282: 15081–15089, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Pompa JL, Timmerman LA, Takimoto H, Yoshida H, Elia AJ, Samper E, Potter J, Wakeham A, Marengere L, Langille BL, Crabtree GR, Mak TW. Role of the NF-ATc transcription factor in morphogenesis of cardiac valves and septum. Nature 392: 182–186, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Dean DA, Urban G, Aragon IV, Swingle M, Miller B, Rusconi S, Bueno M, Dean NM, Honkanen RE. Serine/threonine protein phosphatase 5 (PP5) participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling. BMC Cell Biol 2: 6, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despa S, Islam MA, Weber CR, Pogwizd SM, Bers DM. Intracellular Na+ concentration is elevated in heart failure but Na/K pump function is unchanged. Circulation 105: 2543–2548, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol 587: 5197–5209, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol 294: H596–H604, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald DJ, Burgoyne RD, Haynes LP. Neuronal calcium sensor proteins are unable to modulate NFAT activation in mammalian cells. Biochim Biophys Acta 1780: 240–248, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glitsch HG. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol Rev 81: 1791–1826, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez Bosc LV, Wilkerson MK, Bradley KN, Eckman DM, Hill-Eubanks DC, Nelson MT. Intraluminal pressure is a stimulus for NFATc3 nuclear accumulation: role of calcium, endothelium-derived nitric oxide, and cgmp-dependent protein kinase. J Biol Chem 279: 10702–10709, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gorelik J, Yang LQ, Zhang Y, Lab M, Korchev Y, Harding SE. A novel Z-groove index characterizing myocardial surface structure. Cardiovasc Res 72: 422–429, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Graef IA, Chen F, Crabtree GR. NFAT signaling in vertebrate development. Curr Opin Genet Dev 11: 505–512, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Guatimosim S, Amaya MJ, Guerra MT, Aguiar CJ, Goes AM, Gomez-Viquez NL, Rodrigues MA, Gomes DA, Martins-Cruz J, Lederer WJ, Leite MF. Nuclear Ca2+ regulates cardiomyocyte function. Cell Calcium 44: 230–242, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Higazi DR, Fearnley CJ, Drawnel FM, Talasila A, Corps EM, Ritter O, McDonald F, Mikoshiba K, Bootman MD, Roderick HL. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol Cell 33: 472–482, 2009 [DOI] [PubMed] [Google Scholar]
- 24.Houser SR, Molkentin JD. Does contractile Ca2+ control calcineurin-NFAT signaling and pathological hypertrophy in cardiac myocytes? Sci Signal 1: pe31, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kehlenbach RH, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT in vitro. J Cell Biol 141: 863–874, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kockskamper J, Blatter LA. Subcellular Ca2+ alternans represents a novel mechanism for the generation of arrhythmogenic Ca2+ waves in cat atrial myocytes. J Physiol 545: 65–79, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc Natl Acad Sci USA 96: 9112–9117, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin-1-induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5-trisphosphate (IP3)-receptor type 2-deficient mice. Circ Res 96: 1274–1281, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Lin CC, Lin JL, Lin CS, Tsai MC, Su MJ, Lai LP, Huang SK. Activation of the calcineurin-nuclear factor of activated T-cell signal transduction pathway in atrial fibrillation. Chest 126: 1926–1932, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Macdonnell SM, Weisser-Thomas J, Kubo H, Hanscome M, Liu Q, Jaleel N, Berretta R, Chen X, Brown JH, Sabri AK, Molkentin JD, Houser SR. CaMKII negatively regulates calcineurin-NFAT signaling in cardiac myocytes. Circ Res, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mende U, Kagen A, Cohen A, Aramburu J, Schoen FJ, Neer EJ. Transient cardiac expression of constitutively active Gαq leads to hypertrophy and dilated cardiomyopathy by calcineurin-dependent and independent pathways. Proc Natl Acad Sci USA 95: 13893–13898, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitcheson JS, Hancox JC, Levi AJ. Action potentials, ion channel currents and transverse tubule density in adult rabbit ventricular myocytes maintained for 6 days in cell culture. Pflügers Arch 431: 814–827, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res 39: 280–300, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Molkentin JD. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J Clin Invest 116: 623–626, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93: 215–228, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res 57: 874–886, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Pogwizd SM. Nonreentrant mechanisms underlying spontaneous ventricular arrhythmias in a model of nonischemic heart failure in rabbits. Circulation 92: 1034–1048, 1995 [DOI] [PubMed] [Google Scholar]
- 39.Pogwizd SM, Bers DM. Na/Ca exchange in heart failure: contractile dysfunction and arrhythmogenesis. Ann NY Acad Sci 976: 454–465, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Pogwizd SM, Qi M, Yuan W, Samarel AM, Bers DM. Upregulation of Na+/Ca2+ exchanger expression and function in an arrhythmogenic rabbit model of heart failure. Circ Res 85: 1009–1019, 1999 [DOI] [PubMed] [Google Scholar]
- 41.Pogwizd SM, Schlotthauer K, Li L, Yuan W, Bers DM. Arrhythmogenesis and contractile dysfunction in heart failure: roles of sodium-calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ Res 88: 1159–1167, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Qi XY, Yeh YH, Xiao L, Burstein B, Maguy A, Chartier D, Villeneuve LR, Brundel BJJM, Dobrev D, Nattel S. Cellular signaling underlying atrial tachycardia remodeling of L-type calcium current. Circ Res 103: 845–854, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Rao A. Signaling to gene expression: calcium, calcineurin and NFAT. Nat Immunol 10: 3–5, 2009 [DOI] [PubMed] [Google Scholar]
- 44.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J Biol Chem 281: 608–616, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Rinne A, Banach K, Bers DM, Blatter LA. Isoform-specific regulation of the Ca-sensitive transcription factor NFAT in the cardiovascular system (Abstract). Biophys J 96: 559a, 2009 [Google Scholar]
- 46.Rinne A, Banach K, Blatter LA. Regulation of nuclear factor of activated T cells (NFAT) in vascular endothelial cells. J Mol Cell Cardiol 47: 400–410, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rinne A, Kapur N, Bossuyt J, Bers DM, Blatter LA, Banach K. Pharmacological characterization of nuclear NFAT translocation in cardiac myocytes (Abstract). Biophys J 94: 977, 2008. 17890388 [Google Scholar]
- 48.Rossow CF, Dilly KW, Yuan C, Nieves-Cintron M, Cabarrus JL, Santana LF. NFATc3-dependent loss of Ito gradient across the left ventricular wall during chronic beta adrenergic stimulation. J Mol Cell Cardiol 46: 249–256, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon TR, Pogwizd SM, Bers DM. Elevated sarcoplasmic reticulum Ca2+ leak in intact ventricular myocytes from rabbits in heart failure. Circ Res 93: 592–594, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Sheehan KA, Blatter LA. Regulation of junctional and non-junctional sarcoplasmic reticulum calcium release in excitation-contraction coupling in cat atrial myocytes. J Physiol 546: 119–135, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen T, Liu Y, Cseresnyes Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell 17: 1570–1582, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shyu YJ, Suarez CD, Hu CD. Visualization of AP-1 NF-κB ternary complexes in living cells by using a BiFC-based FRET. Proc Natl Acad Sci USA 105: 151–156, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Velden JL, Schols AM, Willems J, Kelders MC, Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem 283: 358–366, 2008 [DOI] [PubMed] [Google Scholar]
- 54.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, de Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem 277: 48617–48626, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Veldkamp MW, de Jonge B, van Ginneken AC. Decreased inward rectifier current in adult rabbit ventricular myocytes maintained in primary culture: a single-channel study. Cardiovasc Res 42: 424–433, 1999 [DOI] [PubMed] [Google Scholar]
- 56.Voigt N, Maguy A, Yeh YH, Qi X, Ravens U, Dobrev D, Nattel S. Changes in IK,ACh single-channel activity with atrial tachycardia remodelling in canine atrial cardiomyocytes. Cardiovasc Res 77: 35–43, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541: 1–8, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun 322: 1178–1191, 2004 [DOI] [PubMed] [Google Scholar]
- 59.Wu H, Peisley A, Graef IA, Crabtree GR. NFAT signaling and the invention of vertebrates. Trends Cell Biol 17: 251–260, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest 116: 675–682, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le Bouter S, Allen BG, Nattel S. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res 103: 733–742, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J Physiol 584: 601–611, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J Physiol 555: 607–615, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]