Abstract
Alterations to cell-to-cell electrical conductance and to the structural arrangement of the collagen network in cardiac tissue are recognized contributors to arrhythmia development, yet no present method allows direct in vivo measurements of these conductances at their true microscopic scale. The present report documents such a plan, which involves interstitial multisite stimulation at a subcellular to cellular size scale, and verifies the performance of the method through biophysical modeling. Although elements of the plan have been analyzed previously, their performance as a whole is considered here in a comprehensive way. Our analyses take advantage of a three-dimensional structural framework in which interstitial, intracellular, and membrane components are coupled to one another on the fine size scale, and electrodes are separated from one another as in arrays we fabricate routinely. With this arrangement, determination of passive tissue resistances can be made from measurements taken on top of the currents flowing in active tissue. In particular, our results show that measurements taken at multiple frequencies and electrode separations provide powerful predictions of the underlying tissue resistances in all geometric dimensions. Because of the small electrode size, separation of interstitial from intracellular compartment contributions is readily achieved.
Keywords: gap junctions, anisotropy, microfabrication, collagen network, arrhythmia
alterations to cell-to-cell electrical conductance and to the structural arrangement of the collagen network in cardiac tissue are recognized contributors to arrhythmia development (12, 15, 24). The passive electrical properties of both the intracellular and interstitial compartments influence local circuit currents that control action potential propagation (4, 8, 23). Although altered intercellular coupling is accepted as an arrhythmia mechanism, the actual magnitudes for the intrinsic electrical impedances on the cellular size scale, i.e., the microimpedances, remain largely unknown. This lack of data is primarily a consequence of the lack of a sufficiently robust measurement strategy that can interpret voltage recordings made during current injection within a structural framework that distinguishes microimpedances along myocyte axes from those across myocyte axes and transmural to myocyte layers. Delineation between interstitial (Rox,Roy,Roz) and intracellular (Rix,Riy,Riz) contributions is even more problematic, since the historic challenge in cardiac tissue preparations has been an inability to account, adequately, for the extent to which current supplied to one compartment redistributes to the other compartment. That redistribution depends on the effective membrane resistance, which is difficult to assess in practice, as well as on the microimpedances. This model-based report documents the potential measurement accuracy of a new approach (1, 18, 20) in which extensions address these challenges with a three-dimensional structural framework that incorporates very small and closely spaced electrodes for stimulation and recording over a range of frequencies to modulate effective membrane impedance. Recorded voltages are formally analyzed by considering many alternative (Rox,Roy,Roz) and (Rix,Riy,Riz) combinations as part of the measurement process.
We believe the new approach builds favorably on the highly successful cell pair method [see Metzger and Weingart (14)], which is the only approach presently available for electrical impedance measurement on a cellular size scale. With cell pairs, the structural framework is simple and takes advantage of the high membrane resistance for an isolated myocyte. This preparation allows changes in transmembrane potentials (Vm) recorded in adjacent cells during intracellular stimulation to be considered in terms of a junctional resistance (or conductance) that accounts for myoplasm and gap junction contributions. Cell pairs with myocyte-myocyte connections that are predominantly end to end are assumed to reflect in vivo intracellular coupling along myocyte axes (3). Pairs with more side-to-side connections provide descriptions across myocyte axes and transmural to myocyte layers. Although powerful, implementation requires dissolution of the extracellular matrix. Therefore, interstitial microimpedance measurements cannot be obtained. Fragile glass microelectrodes must be attached to adjacent cells for intracellular stimulation and recording. Such intracellular access is technically challenging and complicates data acquisition. Moreover, the focus on pairs limits identification of possible differences between intracellular microimpedances across myocyte axes and transmural to myocyte layers. Locations from which pairs are isolated cannot be controlled precisely enough.
The alternative we propose builds on our recent reports in which we assessed the feasibility of multisite interstitial stimulation for microimpedance measurements. Multisite interstitial stimulation requires no intracellular access or cell isolation, so many of the practical difficulties with the cell pair method are avoided. The method assumes a linear array of small electrodes is positioned interstitially, with outer stimulating electrodes bounding a central pair from which a microscopic potential difference (uPD) is recorded. In tests using fiber (1, 20) and sheet (18) models for cardiac tissue, DC current supplied between finely spaced stimulating electrodes established uPDs that primarily reflected interstitial current flow because the high myocyte membrane resistance limited intracellular redistribution of supplied current over the short distance between electrodes. Magnitudes for uPDs then decreased when current was supplied between more widely spaced stimulating electrodes because the separation lowered effective membrane resistance. For analyses, we assembled large tables in which every line associated a reciprocal case (ARC) of a microimpedance combination to the set of uPDs simulated with that combination. Including wide ranges of possible microimpedances allowed a search in which the minimum difference between the recorded and tabulated uPDs was found on an ARC table line to identify the underlying microimpedances.
To employ interstitial stimulation for (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements under conditions directly applicable to in vitro and in vivo preparations used experimentally, we incorporated a number of extensions that we view as essential for practical implementation. The structural framework was extended to include a three-dimensional network of subcellular building blocks representing myocytes from small tissue volumes and the necessary interstitial electrodes. Electrode components for a specific linear array that is practical to assemble with microelectrical mechanical systems (MEMS) technology (19) are included. To obtain test data for ARC table analyses, the network was used in simulations that included 1) a limited number of electrode combinations consistent with the MEMS arrays, 2) sarcolemmal current through channels described by a subset of the Puglisi and Bers (PB) membrane equations (22), 3) a wide frequency range to incorporate different capacitive contributions to effective membrane impedance, and 4) nominal values prescribed for (Rox,Roy,Roz) and (Rix,Riy,Riz). ARC table analyses with the simulated records were refined to provide accuracy under conditions that are practical to implement as a routine part of cardiac electrophysiology studies. That accuracy was maintained during sensitivity analyses in the presence of different noise levels.
MATERIALS AND METHODS
Structural Framework
Myocyte network building blocks.
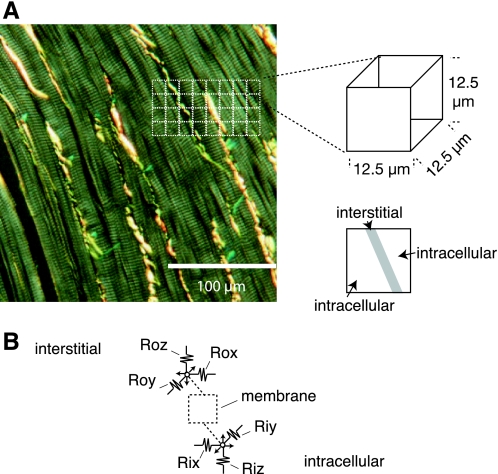
The framework we designed included a network of building blocks that measured only 12.5 × 12.5 × 12.5 μm3. This small size ensured microimpedances introduced into the network resolved cellular to subcellular currents. To demonstrate the block size, Fig. 1A shows 12.5 × 12.5 μm2 regions (dashed white boxes) drawn on a micrograph acquired from a 5 μm-thick section of rabbit ventricular subepicardium. The section was stained with picrosirius red, which is specific for collagen types I, II, and III and enhances visualization of collagen (yellow bands) during acquisition with polarized light. Collagen bands are located between individual myocytes in the image. Within each building block, we assumed a portion of the volume was interstitial, whereas the rest of the volume was intracellular. Intracellular and interstitial compartments in adjacent blocks were then coupled as shown schematically in Fig. 1B, with interstitial coupling via (Rox,Roy,Roz) and intracellular coupling via (Rix,Riy,Riz).
Fig. 1.
A: micrograph of rabbit ventricular subepicardium stained with picrosirius red and imaged using polarized light. Squares mark 12.5 × 12.5 μm2 regions in the micrograph. B: circuit representation for a building block. (Rox,Roy,Roz) make interstitial connections to neighboring blocks, (Rix,Riy,Riz) make intracellular connections to neighboring blocks, and the interstitial and intracellular compartments are coupled by membrane.
Stimulation and recording.
Our framework also insured sufficiently fine spatial resolution to represent interstitial electrode separations from linear MEMS arrays we are able to fabricate (19). That fabrication strategy allows precise location and dimensions for platinum black electrodes patterned into silicon-backed arrays. Figure 2A shows a schematic diagram of the electrode arrangement analyzed in this report. Central uPDs were recorded from blocks separated by 25 μm, which is the spacing available in our MEMS arrays. Multisite stimulation was then performed by supplying current between blocks separated by either 75, 125, or 175 μm. Only eight total electrodes were considered. That number will be advantageous in practice since limiting the electrodes simplifies stimulation and recording under experimental conditions. In the structural framework, arrays were located on the surface of the tissue block shown in Fig. 2B, which measured 625 × 312.5 × 250 μm3. Electrodes were aligned with the longest axis of the model, an alignment that limited boundary effects on the flow of supplied current during simulations.
Fig. 2.
A: arrangement of stimulation (■) and recording (☐) electrodes assumed for all simulations. B: schematic diagram of the 3-dimensional myocyte network used for active and passive membrane simulations, including the surface location for the electrode array.
Literature-based microimpedances.
Once the dimensions for the building blocks were specified, we identified nominal values for (Rox,Roy,Roz) and (Rix,Riy,Riz) from the limited macroscopic conductivity data available for rabbit ventricular myocardium. Myocytes in the network were assumed to align with the x-axis. We used Kléber and Rieger's (11) specific intracellular conductivity for perfused rabbit papillary muscles (6.02 mS/cm), assumed that 80% (17) of tissue volume in each building block was intracellular, and derived a Rix of 166 kΩ. Similarly, we used Kléber and Rieger's (11) specific extracellular conductivity (15.85 mS/cm), assumed 20% of each block was interstitial, and derived a Rox of 255 kΩ. Since data for macroscopic conductivities across myocyte axes was unavailable for rabbit ventricle, we relied on ratios provided by Clerc (6) to prescribe Riy and Roy. We assumed interstitial and intracellular conductivities across myocyte axes were 2.7- and 9.4-fold lower, respectively, than conductivities along myocytes axes and prescribed a Roy of 680 kΩ and a Riy of 1,560 kΩ. We also prescribed Riy = Riz and Roy = Roz.
Active Membrane Simulations
Governing equations.
To complete simulations in which the flow of supplied current through building blocks in the myocyte network was carefully reconstructed across a wide range of stimulation frequencies, we considered the intracellular and interstitial contributions to total membrane current (Im) as is commonly done in modeling local circuit currents. Within each building block, the intracellular contribution to Im was determined from spatial differences in intracellular potentials
| (1) |
where Vm is the transmembrane potential, ϕo is the interstitial potential, and superscripts + and − denote directional differences and j denotes x, y, and z directions. The interstitial contribution to Im was determined from
| (2) |
These contributions were balanced by membrane capacitive charging (and discharging) and by sarcolemmal current flow
| (3) |
In Eq. 3, Cm was the membrane capacitance, which we set to 8.85 pF based on the Giles and Imaizumi (9) data for isolated rabbit ventricular myocytes. Because those investigators reported total cell capacitance of 72.5 pF and an average cell volume of 16,021 μm3, our capacitance was reduced 8.2-fold to represent the smaller size of our building blocks. Iion was determined using the PB (22) membrane equations for the inward rectifier potassium current (IK1) in adult rabbit ventricular myocytes. Only IK1 was considered because it is dominant for resting myocytes and our simulations never increased or decreased Vm by more than 2 mV.
Time-dependent solutions.
The computational sequence for the active membrane simulations involved an implicit solution of Eqs. 1–3 with timestepping that advanced Vm, ϕo, and Iion. Values for (Rox,Roy,Roz), (Rix,Riy,Riz), Vm, ϕo, Cm, and the terms necessary to evaluate Iion were embedded in the linear system
| (4) |
In Eq. 4, [G] was a coefficient matrix that accounted for resistive coupling via the microimpedances from Eqs. 1 and 2 and a capacitive contribution from Eq. 3. As such, v̄ was a vector of unknown potentials expressed at time t, with the lower half of v̄ including Vm for all building blocks and the upper half of v̄ including ϕo for all building blocks. The b̄ term was a source vector in which the lower half depended upon Iion and a capacitive contribution from Eq. 3 determined for time t − Δt and in which the upper half included currents (Istim) of equal and opposite magnitudes at building blocks selected for stimulation. Initial conditions for all integrated parameters used to establish b̄ at t = 0 ms were prescribed according to the PB membrane equations. Resting Vm was set to −85.6 mV. Iterative solution of Eq. 4 at t = Δt with a conjugate gradient method established v̄, in which values were then used to advance simulations in time and resolve Vm and ϕo over the selected duration for a given simulation (18, 20).
Stimulation at multiple frequencies.
The frequency response to stimulation with the different electrode combinations was identified by scanning the 1 Hz to 63.1 kHz range at five frequencies per decade. This range minimized the capacitive contribution to effective membrane impedance (Zm) at lower frequencies and ensured a capacitive contribution at higher frequencies. In each simulation, alternating current of peak amplitude Istim was supplied over a single cycle of electrical activity at the specified frequency. For high frequency simulations, the cycle was divided into 500 total time steps. At lower frequencies, larger numbers of time steps were used to ensure no step exceeded 10 μs in any simulation. To replicate likely experimental conditions where changes in Istim are expected to occur during different uPD recordings, each response was expressed as a microscopic composite impedance (uCI = uPD/Istim).
Microimpedance Measurements with ARC Table Analyses
Steady-state solution for tabulated uCIs.
To interpret the uCIs recorded in the active membrane simulations within our new structural framework, we used ARC tables to compare the recorded uCIs with uCIs tabulated from simulations in which membrane was considered passive. For passive membrane, Im was described by
| (5) |
with
| (6) |
In Eq. 6, Rm was a membrane resistance, f was a stimulation frequency, and τm was a membrane time constant. For each simulation, unique values for (Rox,Roy,Roz), (Rix,Riy,Riz), and (Rm,τm) were specified with a single f. The linear system (Eq. 4) that resulted from combination of Eqs. 1, 2, and 5 was assembled, with adjustment to b̄ to supply current between blocks separated by either 75, 125, or 175 μm. Steady-state solutions for the Vm and ϕo distributions were then obtained rapidly, since the computational costs for these simulations were considerably lower than the costs for the active membrane simulations. One tabulated uCI was determined from the uPD/Istim in each simulation.
ARC table assembly.
ARC tables were built using systematic adjustments to selected combinations of (Rox,Roy,Roz), (Rix,Riy,Riz), and (Rm,τm) parameters in different simulations. For each table, we defined a parameter set Rr, as a combination of N parameters located on table row r. Following the structure shown in Table 1, lower bound values (L1 to LN) that were well below nominal literature-based values were assigned to the set R1. R1 was used to build the linear system, a simulation with a given stimulating electrode combination and frequency was completed to tabulate a uCI that we denoted A[1,1], and then stimuli were adjusted over S different stimuli for A[1,2] through A[1,S]. L1 was then multiplied by a step between levels (γ) to combine new parameters for set R2. Stimulus adjustments as with R1 were used for A[2,1] through A[2,S] and repeated multiplications of L1 by γ were performed over M levels. When k reached M + 1, L2 was multiplied by γ to tabulate uCIs for lines M + 1 to 2M. Analogous adjustments were made over MN total ARC table rows.
Table 1.
ARC table structure
| 1 | | | L1 | L2 | — | LN | | | A[1,1] | A[1,2] | — | A[1,s] | — | A[1,S] |
| 2 | | | γL1 | L2 | — | LN | | | A[2,1] | A[2,2] | — | A[2,s] | — | A[2,S] |
| · | | | · | · | — | · | | | · | · | — | · | — | · |
| m | | | γmL1 | L2 | — | LN | | | A[m,1] | A[m,2] | — | A[m,s] | — | A[m,S] |
| · | | | · | · | — | · | | | · | · | — | · | — | · |
| M | | | γML1 | L2 | — | LN | | | A[M,1] | A[M,2] | — | A[M,s] | — | A[M,S] |
| M+1 | | | L1 | γL2 | — | LN | | | A[M+1,1] | A[M+1,2] | — | A[M+1,s] | — | A[M+1,S] |
| M+2 | | | γL1 | γL2 | — | LN | | | A[M+2,1] | A[M+2,2] | — | A[M+2,s] | — | A[M+2,S] |
| · | | | · | · | — | · | | | · | · | — | · | — | · |
| 2M | | | γML1 | γL2 | — | LN | | | A[2M,1] | A[2M,2] | — | A[2M,s] | — | A[2M,S] |
| · | | | · | · | — | · | | | · | · | — | · | — | · |
| MN | | | γML1 | γML2 | — | γMLN | | | A[MN,1] | A[MN,2] | — | A[MN,s] | — | A[MN,S] |
ARC table analyses.
Microimpedances were then measured using different ARC tables to obtain root mean square (RMS) estimates for the individual parameters in a given parameter set (1). This was achieved with a Bayesian approach in which a component probability p[r, s] was computed for every A[r, s]
| (7) |
In Eq. 7, the associated uCI recorded from the active membrane simulations was denoted Z[s]. The parameter σ was an estimate for the standard deviation of the measurement noise, which was necessary for the Bayesian analysis. In considering estimates, we recognized that traditional sources of noise such as those resulting from experimental recordings of low amplitude potentials with small electrodes would not be pertinent for our records because they were obtained from simulations. Noise in this setting was more likely to depend upon differences between the active and passive membrane responses to stimulation with the different electrode combinations and frequencies. The effect of using a relatively small σ on individual p[r, s] values was to exclude ARC table rows from consideration in which collective parameter values might represent a reasonable approximation to the underlying parameters we were attempting to measure. Alternatively, the effect of using a relatively large σ was to include rows with parameters that were less representative. We set σ to 5 kΩ because this value was 10–15% of the largest uCI we identified in any of the simulations with active membrane. After each p[r, s] value was computed, we calculated an a priori probability [p(Rr)] for each ARC table row
| (8) |
Individual p(Rr) values were then scaled by the sum of p(Rr) over all MN rows in a table and used as a weighting function for every parameter in Rr. RMS estimates were computed as weighted sums for the N parameters. Each RMS estimate was then recomputed using σ at 2.5 and 10 kΩ to assess the influence of the selected σ the microimpedance measurements. All RMS estimates were compared with the prescribed values and expressed as percent errors [100%(1+ measured-prescribed /prescribed)].
RESULTS
Current-Voltage Relationships in the Three-dimensional Network
As a preliminary step before selected uCI values were used in ARC table analyses to assess (Rox,Roy,Roz) and (Rix,Riy,Riz) measurement accuracy, we completed a set of simulations with active and passive membrane to examine relationships between supplied current amplitudes and ϕo distributions in the three-dimensional network. One goal in completing these simulations was to identify likely stimulus current amplitudes to be used in experiments with arrays fabricated on the fine size scale documented in our recent report (19). Istim values that produced uPDs of ≈1 mV amplitude were identified. Such stimuli establish signals that are large enough to record with high fidelity yet are small enough to have little or no impact on the intrinsic electrical activity of myocytes adjacent to the electrodes. The signal amplitude was only an issue of interest in the active membrane simulations, since uCI values obtained from the passive membrane simulations were independent of stimulus amplitude. Another goal in completing these simulations was to provide an initial test of our assumption that interstitial current supplied between identical building blocks in active and passive membrane simulations with the same prescribed (Rox,Roy,Roz) and (Rix,Riy,Riz) established comparable voltage distributions.
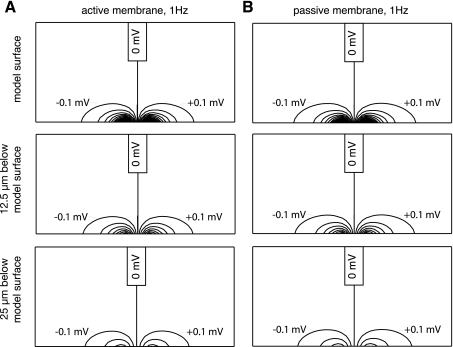
Figure 3A shows contour maps of ϕo assembled using 0.1-mV steps between contour lines for the surface myocyte layer (top) where the electrode array was positioned, the layer 12.5 μm below that surface (middle) and the layer 25 μm below that surface (bottom), from an active membrane simulation with 1 Hz stimulation and 75 μm electrode separation. A 14-nA current established a 1.04-mV uPD. In most building blocks away from the neighborhood of the electrode array, interstitial potential differences resulting from stimulation were very low. For example, the potential difference between blocks located 137.5 and 150 μm from each stimulating electrode along the myocyte axis, which was also aligned with the electrodes, were below 10 μV. Comparable reductions were observed between blocks located only 37.5 and 50 μm from the stimulating electrodes across myocyte axes and transmural to myocyte layers.
Fig. 3.
A: isopotential contour maps of ϕo assembled from values obtained in an active membrane simulation with 1 Hz stimulation and 75 μm separation between stimulating electrodes. Maps were built using ϕo distributions from the model surface where the electrodes were positioned (top), from the layer of building blocks located 12.5 μm below the model surface (middle), and from the layer of blocks located 25 μm below the model surface (bottom). B: isopotential contour maps of ϕo assembled from values obtained a passive membrane simulation that represented the conditions shown in A.
Figure 3B shows maps from these same layers assembled from the ϕo distribution in a passive membrane simulation in which literature-based values for (Rox,Roy,Roz) and (Rix,Riy,Riz) were prescribed with (Rm,τm) of (276 MΩ, 10 ms). The selected Rm was based on Giles and Imaizumi (9), who reported a cell input resistance for rabbit ventricular myocytes of 33.7 MΩ, which we scaled to account for the smaller size of our network building blocks. A 14-nA current established a 1.06-mV uPD. Qualitatively, the ϕo distributions established in the two simulations were highly similar. Quantitatively, the maximum difference between a ϕo from any building block in the active and passive membrane simulations was only 0.02 mV. Larger amplitudes for Istim were required to establish ≈1-mV amplitude uPDs when the stimulating electrodes were separated more widely, since 31 and 58 nA currents led to 0.99 and 1.00 mV uPDs with 125 and 175 μm electrode separations, respectively. Istim increases accompanying the wider stimulating electrode separations did establish potential differences between adjacent building blocks that were larger than with 75 μm separation. However, those difference were lower than 10 μV between blocks located 200 and 212.5 μm from stimulating electrodes along myocyte axes, 75 and 87.5 μm across myocyte axes, and 75 and 87.5 μm transmural to myocyte layers. These results were favorable in that they supported using passive tissue as a representation for active tissue, insofar as the tissue microimpedances dictated local responses to stimulation.
With 10 kHz stimulation, increased Istim was also needed to establish ≈1 mV uPDs, since 25, 53, and 91 nA currents led to 1.02, 1.01, and 1.00 mV uPDs in active membrane simulations with 75, 125, and 175 μm electrode separations, respectively. For passive membrane simulations that represented 10 kHz stimulation, Zm was lowered to 439 kΩ based on Eq. 6. The uPD amplitudes from the passive membrane simulations at these same Istim values differed from those with the active membrane simulations by less than 0.1 mV. Here, we note that we made no attempt to identify the (Rm,τm) parameters intrinsic to the PB membrane equations, which we recognized as parameters that would initially need to be treated as unknowns in ARC table analyses in which frequency adjustments were considered. Overall, these observations also supported use of passive membrane simulations to obtain uCI entries in different ARC tables.
Frequency Response
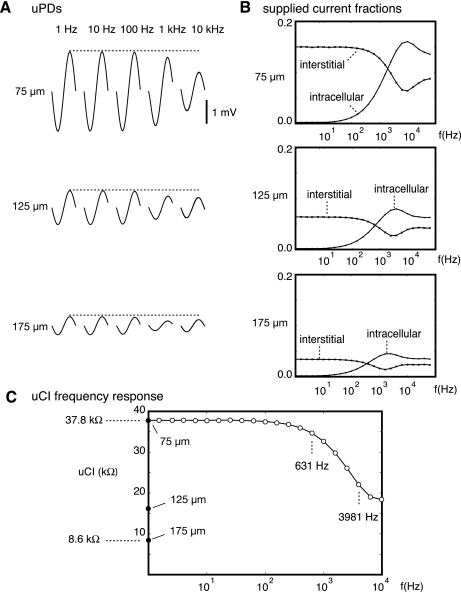
A second key assumption of our measurement strategy was that the fine adjustments in electrode separation available with the subcellular resolution of our building blocks would establish voltage distributions during low frequency stimulation that primarily reflected the interstitial flow of supplied current, whereas voltage distributions established during higher frequency stimulation would reflect redistribution of supplied current to the intracellular compartment. Figure 4A shows uPDs recorded from active membrane simulations in which frequencies were adjusted between 1 Hz (left) and 10 kHz (right) with Istim fixed at 14 nA. Waveforms at all stimulating electrode separations are included. At 1 Hz, the uPD amplitude fell as the electrode separations increased from 75 to 125 μm and 175 μm separation. Amplitudes at all three electrode separations were unchanged when the frequency was increased to 10 Hz. Above 100 Hz, however, uPD amplitudes fell. With the use of the most closely spaced electrode arrangement, the uPD amplitude at 10 kHz was only 52% of that at 1 Hz.
Fig. 4.
A: microscopic potential differences (uPDs) recorded during active simulations in which Istim supplied between electrodes separated by 75 (top), 125 (middle), and 175 μm (bottom) and frequencies adjusted from 1 Hz (left) to 10 kHz (right). Each dashed line marks peak uPD at 1 Hz. All uPDs were aligned at 0 mV. B: fraction of supplied current measured between building blocks used for the uPD recordings expressed as a function of frequency for the stimulating electrode separations considered in A. C: UcIts using different stimulating electrode combinations (●) and frequencies (○).
To assess the extent to which these observations depended upon interstitial current flow across myocyte axes and transmural to myocyte layers as opposed to being related to intracellular redistribution of supplied current, we measured the interstitial and intracellular currents between the building blocks selected for the uPD recordings in all active membrane simulations. Those currents were expressed as fractions of the supplied current. Figure 4B shows those fractions plotted as a function of frequency for the 75, 125, and 175 μm stimulating electrode separations. At frequencies below 10 Hz, intracellular redistribution was negligible, whereas at frequencies between 1 and 10 kHz, supplied current flowed readily to the intracellular compartment. Changes in the supplied current fractions were primarily due to the enhanced capacitive contribution to Zm at higher frequencies.
Figure 4C summarizes all uCIs. At 1 Hz, the uCIs ranged from 37.8 kΩ with a 75 μm stimulating electrode separation to 8.6 kΩ with a 175 μm stimulating electrode separation. Note that all of the uCIs were considerably lower than the prescribed microimpedances, highlighting the importance of interpreting the uCIs within the structural framework to obtain (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements. With a 75 μm stimulating electrode separation, uCIs were effectively unchanged at frequencies below 100 Hz and fell most dramatically at frequencies between 631 Hz and 3.98 kHz.
ARC Table Analyses
Two types of ARC tables were then analyzed to assess microimpedance measurement accuracy. In the first type (termed interstitial ARC tables), only (Rox,Roy,Roz) were adjusted in the parameter sets, whereas (Rix,Riy,Riz) and (Rm,τm) were held constant at (100, 100, and 100 kΩ) and (276 MΩ, 10 ms), respectively. The A[r, s] values were tabulated assuming a frequency of 1 Hz and stimulating electrode separations of 75, 125, and 175 μm. Our rationale for limiting parameter adjustments to (Rox,Roy,Roz) was based on the frequency response, which suggested supplied current remained primarily interstitial at frequencies below 10 Hz. We found RMS estimates for (Rox,Roy,Roz) of (278, 742, and 743 kΩ) with an interstitial ARC table built using seven levels, steps between levels of 1.25, and lower bound values of (129, 348, and 348 kΩ). All three RMS estimates therefore differed from the prescribed microimpedances by 9.0%. With an interstitial ARC table built using 11 levels, steps between levels of 1.05, and lower bound values of (197, 533, and 533 kΩ), the RMS estimates improved to (254, 685, and 685 kΩ). All three RMS estimates therefore differed from the prescribed values by less than 1%. Adjustments to the estimate for measurement noise necessary for the Bayesian analysis had minimal impact, since percent errors remained below 1%.
We then built an intracellular ARC table using (Rox,Roy,Roz) of (254, 685, and 685 kΩ) with (Rix,Riy,Riz) and (Rm,τm) adjusted in the parameter sets. Because of the improvement in the accuracy of the RMS estimates we found with the interstitial ARC table analysis, we chose to build one large table that included 11 levels with steps between levels of 1.05 (161,051 table rows). The A[r, s] values were tabulated for a 75 μm stimulating electrode separation with five frequencies from 631 Hz to 3.98 kHz. These frequencies were selected as ones at which intracellular redistribution of supplied current was identified from the frequency response. RMS estimates for (Rix,Riy,Riz) were (166, 1,575, and 1,575 kΩ) and therefore differed from the prescribed (Rix,Riy,Riz) by less than 1%. As with the interstitial ARC table analyses, these percent error levels were maintained during adjustments to the estimate for measurement noise.
To assess the extent to which these findings depended on the accuracy with which the uCI amplitudes were estimated, we then randomly adjusted component uCIs by maxima of either 0.1%, 1.0%, or 10% for 200 separate interstitial ARC table analyses and 200 separate intracellular ARC table analyses. In experiments, we approximate uPDs recorded with controlled Istim as sinusoidal waveforms at the specified frequencies (19). The 0.1% adjustments were therefore intended to reflect conditions with very low levels of experimental noise, whereas the 10% adjustments were intended to represent conditions with experimental noise exceeding levels we anticipate. As expected, the percent errors in (Rox,Roy,Roz) and (Rix,Riy,Riz) measurement with the 0.1% uCI amplitude adjustments changed little, since no RMS estimate from the ARC table analyses exceeded 1%. Figure 5A shows percent error in Rox, Roy, and Roz from the interstitial ARC table analyses. Even with 10% adjustments in uCI amplitudes, all three interstitial microimpedances matched the prescribed values to within 5%. Perhaps most strikingly, percent errors in Rix, Riy, and Riz shown in Fig. 5B remained low.
Fig. 5.
A: percent error between prescribed and measured Rox (top), Roy (middle), and Roz (bottom) from 200 different trials in which overestimation and underestimation of uCI amplitude was considered in interstitial associated ARC table analyses. B: Rix (top), Riy (middle), and Riz (bottom) percent errors during different trials with in intracellular ARC table analyses.
DISCUSSION
This model-based study documents a set of steps that we believe can be implemented rapidly in cardiac electrophysiology experiments for routine (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements. The work builds substantively on our earlier reports assessing theoretical feasibility in fiber and sheet models because a three-dimensional structural framework that incorporated very small and closely spaced electrodes for stimulation and recording over a range of frequencies was used. The new approach is likely to provide advantages over the state-of-the-art cell pair method in that it requires no dissolution of the extracellular matrix, does not involve intracellular penetration of myocytes with fragile glass microelectodes, and is achievable with interstitial electrode arrays that we build using MEMS fabrication. The importance of obtaining (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements is underscored by the recent report of Cabo and Boyden (2), who showed discontinuous microscopic conduction associated with gap junction remodeling in the epicardial border zone was modulated by the surrounding extracellular volume conductor. Their observations suggest quantitative microimpedance measurements for both the intracellular and interstitial compartments will ultimately be needed to improve understanding of microstructural contributions to propagagation failure and arrhythmia development. Three steps provided accurate (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements in this report. First, resolution of the uCI frequency dependence allowed clear definition of ranges over which supplied current remained primarily interstitial and/or redistributed to the intracellular compartment. Second, low frequency uCIs used in interstitial ARC table analyses allowed (Rox,Roy,Roz) measurements when sufficiently fine table resolution was employed. Finally, comparison of high frequency uCIs in intracellular ARC table analyses using the identified (Rox,Roy,Roz) allowed accurate (Rix,Riy,Riz) measurements.
Structural Framework
Demonstration that these steps provided accurate microimpedance measurements depended substantively on the specific network of building blocks used to obtain the uCIs with both active and passive membrane. The subcellular block size (12.5 × 12.5 × 12.5 μm3) was important in this regard. This size allowed electrode separations that were consistent with separations in cell pair experiments and therefore ensured electrical impedance measurements were on a subcellular to cellular scale. It also allowed careful representation of stimulating and recording electrode arrangement features available in our linear MEMS arrays, which are built with platinum black electrodes patterned onto silicon substrates at 25 μm center-to-center separations (19). Combination of the blocks into volumes that measured 625 × 312.5 × 250 μm3 established models that were sufficiently small to allow routine uCI identification for the many ARC table entries needed to complete our analyses. These volumes were sufficiently large, however, to limit influences of the model boundaries on uCI amplitudes, since our preliminary simulations showed stimulation with 10–50 nA currents established ≈1 mV uPDs and potential differences between adjacent blocks throughout models that were generally below 10 μV. Although the block size and overall volume considered here were sufficient to demonstrate the ability of the presented steps to provide accurate (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements, we emphasize that the computational demands for implementing all the steps were manageable. Assembly of our finest interstitial ARC table, which included three parameters adjusted over 11 levels to generate 1,331 table lines, only required hours of computational time. Assembly of our finest intracellular ARC table, which included 161,051 table lines, was much more computationally intensive, although distribution of the different table sections over different processors allowed that table to be built in a few days. Analyses with assembled tables were completed in seconds of computational time. The modest overall computational requirements suggest the use of smaller building blocks and/or larger total volumes will be practical to implement in going forward, should such refinements prove necessary in adapting the approach for use with animal models of acute and chronic heart disease.
Our decision to make the microimpedance measurements with Bayesian mathematical methods as opposed to developing an approach in which the underlying equations were solved for the individual unknown parameters based on uCI measurements was influenced by a number of factors. First, our earlier report (1) supported Bayesian interpretation of the measured data in a noise-free environment. In that report, uPD recordings were obtained from solutions of the core-conductor equations for a fiber in which nominal and extreme impedances were considered. Steps analogous to those implemented here consistently identified the underlying impedance state from eight possible combinations, suggesting such identification could potentially be achieved here under conditions where either 1,331 or 161,051 possible combinations were considered. Second, that report demonstrated superior performance in the presence of experimental noise. We recognize noise will be a significant issue as we adapt the strategy for use with our MEMS arrays. Although we documented steps that lowered electrode-electrolyte impedances that contribute substantively to the noise typically associated with potential recordings using very small electrodes in electrophysiological experiments in Pollard et al. (19), we appreciate that the interpretation of uCIs in the presence of noise will be important in going forward. Our findings here suggest that up to 10% fluctuations in the uPD amplitude estimations contribute less than 5% error to the (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements. Weighted contributions from the many possible impedance states embedded in the ARC tables provided this protection. Third, the ARC table analyses are completed rapidly. This suggests (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements during experiments will be practical as a component of the overall strategy. Analyses that take place on a time scale comparable with the uPD and Istim acquisition, i.e., seconds as opposed to minutes or hours, represent an attractive aspect of the procedure. Finally, the approach will be straightforward to refine further as control (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements are obtained. Bayesian search allows integration of a priori probabilities for different ARC table entries that reflect the likely range of intrinsic values. In Barr et al. (1), we found that integration allowed focused solutions in the presence of even higher noise levels than the levels considered here.
Frequency Dependence
The electrode arrangement we assumed based on our MEMS arrays allowed identification of a uCI frequency response that was fundamentally different from responses presented in previous experimental reports. The flat response we observed at frequencies below 100 Hz was not seen in reports where more macroscopic separations between electrodes (1–3 mm) were included in traditional arrays. Instead, composite impedances measured below 100 Hz in those reports declined with increasing frequency, consistent with the response we observed at frequencies closer to the 1 to 10 kHz range. One way to appreciate this important difference is to consider the relationship between the electrode span and the space constant. According to Plonsey and Barr (16), only a tiny fraction of the current supplied over an extent shorter than a space constant crosses membrane. Although cardiac space constant measurements vary with species, tissue type, and experimental conditions, Chapman and Fry (5) reported a 328 μm space constant for frog ventricular trabeculae. Therefore, all eight electrodes in our arrangement were positioned inside a space constant. Electrodes in traditional arrays used in previous studies were not. With stimulation below 100 Hz in the present study, the small capacitive contribution to effective membrane impedance established a space constant that likely reflected that at rest. At higher frequencies, however, the capacitive contribution lowered effective membrane impedance and redistribution of supplied current comparable with that with traditional arrays was observed. The inability of traditional arrays to resolve the low frequency response we observed is a significant impediment to their use in distinguishing intracellular from interstitial electrical impedances in cardiac tissue preparations.
Interstitial Microimpedance Measurements
Our finding that interstitial ARC table analyses using low frequency uCIs identified with 75, 125, and 175 μm stimulating electrode separations led to accurate (Rox,Roy,Roz) measurements is significant because it suggests practical resolution in vitro and in vivo. Quantitative interstitial microimpedance data are unavailable, which represents a significant gap in understanding of the link between microstructural alterations and arrhythmia development. Impedances in this part of the local circuit current loop are undoubtedly important, since interstitial potentials recorded during propagation have amplitudes ≈½ of the amplitude of the action potential upstroke (23). The quantitative macroscopic interstitial impedance data that are available are primarily for the longitudinal fiber axis, since the voltage ratio method used with papillary muscle preparations provides core-conductor interstitial impedance estimates (8, 11). The method has been essential for documenting the time course of impedance changes during exposure to ischemic and ischemia-like conditions, where collapse of the interstitial compartment accompanies loss of flow and causes a rapid impedance rise. Consistent with this response, Fleischauer et al. (8) showed sensitivity of core-conductor interstitial impedance to compartment size changes induced by altering perfusate dextran. Quantitative macroscopic interstitial impedance data across fibers remain limited to Clerc (6), who identified core-conductor impedances along and across fibers by moving superfused calf trabeculae between different experimental chambers to supply current in orthogonal directions. The special preparations and apparatus required have led most investigators to simply use the 30+-year-old Clerc measurements as if they remained valid for all other species and disease states despite the fact that virtually no present day investigators use the calf preparation that Clerc studied.
Intracellular Microimpedance Measurements
Our finding that intracellular ARC table analyses using higher frequency uCIs led to accurate (Rix,Riy,Riz) measurements is also significant, and we emphasize that this accuracy was obtained without any need for Vm recordings with fine glass microelectrodes or Vm estimation based on fluorescence collected from dye-stained tissue. As with our interstitial microimpedance measurements, we note that our strategy will likely be effective in distinguishing intracellular microimpedances along myocyte axes from those across myocyte axes and transmural to myocyte layers. More broadly, however, the strategy to resolve (Rix,Riy,Riz) here provides an opportunity to describe intracellular electrical properties in vivo or in vitro, suggesting new measurements that will improve understanding of mechanisms for conduction failure associated with heterogeneities in gap junction distributions and myocyte arrangements. The size scale of our building blocks was approximately that of the diameter of a ventricular myocyte and was much lower than that of the length of a myocyte. As a consequence, we believe intracellular microimpedance measurements will ultimately refine understanding of source-load relationships during discontinuous conduction hypothesized to contribute to arrhythmogenesis. For example, one-dimensional modeling with explicitly characterized gap junction and cytoplasmic contributions showed propagation with normal gap junction coupling that differed little from propagation in the continuous core-conductor model (26). However, with moderate uncoupling the gap junctions caused discontinuous propagation in which large transmembrane potential differences and long conduction latencies were maintained between adjacent myocytes. More severe uncoupling caused propagation failure, but only at extremely low propagation velocities. In ventricular myocardium, Cx43 hemichannels form most gap junctions. Cx43 turnover is rapid with a channel half-life of ≈1 to 2 h (25). Assembly requires combination of individual connexons into hemichannels that are transported to the membrane where they locate preferentially at myocyte ends and dock to hemichannels from neighboring myocytes to form gap junctions under normal conditions (25). Although these issues have been considered, to a limited extent, along myocyte axes, no quantitative details are available for directions across myocyte axes or intramurally. Identifying such differences in the intracellular microimpedances is therefore crucial to improved understanding of conditions under which discontinuous propagation that accompanies gap junction uncoupling causes unidirectional block and initiation of anisotropic reenty (12, 15, 24, 27, 28).
Refinements Based on Circuit Analyses
Although the building blocks used for the present study were effective in allowing accurate (Rox,Roy,Roz) and (Rix,Riy,Riz) measurements in settings that were advantageous in terms of recording lead noise and other possible sources for uCI estimation error, we note that refinement of our analyses to account for the frequency dependence of uCI phase with amplitude will be straightforward to adopt. Such refinement may prove beneficial, since previous impedance measurement strategies using circuit analyses have been effective. For example, Cooklin et al. (7) placed guinea pig trabeculae isolated from the left ventricles of sham and hypertrophied hearts in a three-compartment chamber in which the central compartment was filled with oil to insure current supplied between electrodes positioned in the outer compartments flowed along myocyte axes. System resistance and capacitance measured with a balanced Wien bridge during stimulation over the 20 Hz to 300 kHz range were then interpreted in terms of a circuit response that accounted for electrode polarization, cytoplasmic and junctional resistance elements in the intracellular compartment, a restricted extracellular shunt, and membrane impedance contributions as identified in separate cell suspension experiments. The collective steps were crucial to identification of a 144% increase in junctional resistance accompanying hypertrophy. Le Guyader et al. (13) stimulated two-dimensional bidomain models with orthogonal four-electrode arrangements over a 10 Hz to 10 kHz range and used a fast Fourier transform approach to evaluate interstitial potential distributions. Numerical optimization through successive computations of those distributions with different bidomain parameter combinations allowed accurate parameter estimations at frequencies below 2.4 kHz. Above 2.4 kHz, however, refinements to account for possible contributions of junctional resistance and capacitance in the intracellular compartment were needed to represent simulated phase changes. Incorporating uCI phase changes with frequency in ARC table analyses is likely to expand identification of microscale electrical parameters beyond those considered here.
Limitations
In assessing our findings, it is important to recognize certain limitations. First, our simulations assumed a continuous and homogeneous bidomain representation for ventricular myocardial tissue structure. Although this served as an effective starting point to validate the steps we propose for routine and rapid measurements in cardiac electrophysiology experiments, our approach did not account for naturally occurring architectural discontinuities that are believed to establish spatially heterogeneous microimpedance distributions. For (Rox,Roy,Roz), refinements to account for locations where the 12.5 × 12.5 μm2 building blocks contained more and less collagen were not considered. For (Rix,Riy,Riz), variabilities in cell size and gap junction density that contribute to discontinuous microscopic conduction were not analyzed. These important issues warrant further study, and we note that including such microscopic heterogeneities in the three-dimensional structural framework will be straightforward. That inclusion is likely to allow identification of spatial differences in (Rox,Roy,Roz) and (Rix,Riy,Riz) because adjustments in the component microimpedances cause changes in the uCI frequency response. It is that response that guides the microimpedance measurements.
Second, we only considered microimpedance measurements for the case where the linear MEMS array and the myocyte axes were aligned and did not attempt to identify the impact of this assumption on measurement sensitivity. Under experimental conditions, we believe there are regions of the ventricle where such alignment will be straightforward to achieve. For example, epicardial fibers emerge from the margin where the left anterior descending (LAD) coronary artery runs apically in the left ventricle of rabbit hearts. In this region, careful registration of the MEMS array location in combination with histological measurement of the angle between the electrode and myocyte axes can be used to confirm alignment. The precision with which the electrodes are fabricated will be advantageous in this regard, since guides for fiducial markings of the array boundaries can be integrated into the measurement apparatus. In other ventricular regions, we appreciate that alignment will be more difficult to achieve, although histological assessment of the angle between the electrode and myocyte axes should be routine. Transformation of the (Rox, Roy, Rix, Riy) measurements our method provides to microimpedance measurements along and across myocyte axes will depend upon this angle.
Third, we did not assess measurement accuracy under conditions Roy≠Roz or Riy≠Riz. Although quantitative macroscopic interstitial and intracellular impedance data transmural to myocyte layers is unavailable, a recent report by Hooks et al. (10) showed unique total tissue impedances associated with this direction from total tissue impedances impedances along and across fibers in pig ventricle. Those investigators hypothesized the total tissue impedance differences were primarily a consequence of reduced gap junction availability, which they attributed to the presence of laminar sheets embedded between myocyte layers. Although likely, the demonstration by Pope et al. (21) that perimysial collagen surrounding myolaminae formed a mesh with a wide range of sizes and orientations that were typically oblique to myocyte axes in rat ventricle suggests improved understanding of any interstitial contribution to the Hooks et al. (10) observation is also important. The extent to which our method, as presented, will be able resolve influences of cleavage planes between the myolaminae that form at separations of ∼4 myocytes, on Roz and Riz, may be limited. As shown, the depth of interrogation was only 25–50 μm from the surface where the electrodes were positioned. Although we anticipate this will provide an advantage in the sense that the impact of any myocyte axis rotation with depth will be limited, we recognize the flow of supplied current through cleavage planes in this setting may be restricted.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Award HL-092049 and National Science Foundation Award CBET-0756078.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1.Barr RC, Nolte LW, Pollard AE. Bayesian analysis of fiber impedance measurements. Conf Proc IEEE Eng Med Biol Soc 1: 423–429, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Cabo C, Boyden PA. Extracellular space attenuates the effect of gap junctional remodeling on wave propagation: a computational study. Biophys J 96: 3092–3101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabo C, Yao JA, Boyden PA, Chen W, Hussain W, Duffy HS, Ciaccio EJ, Peters NS, Wit AL. Heterogeneous gap junction remodeling in reentrant circuits in the epicardial border zone of the healing canine infarct. Cardiovasc Res 72: 241–249, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Cascio WE, Yan GX, Kléber AG. Passive electrical properties, mechanical activity and extracellular potassium in arterially perfused and ischemic rabbit ventricular muscle. Effects of calcium entry blockade or hypocalcemia. Circ Res 66: 1461–1473, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Chapman RA, Fry CH. An analysis of the cable properties of frog ventricular myocardium. J Physiol 283: 263–282, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerc L. Directional differences of impulse spread in trabecular muscle from mammalian heart. J Physiol 255: 335–346, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooklin M, Wallis WRJ, Sheridan DJ, Fry CH. Changes in cell-to-cell electrical coupling associated with left ventricular hypertrophy. Circ Res 80: 765–771, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Fleischauer J, Lehmann L, Kléber AG. Electrical resistances of interstitial and microvascular space as determinants of the extracellular electrical field and velocity of propagation in ventricular myocardium. Circulation 92: 587–594, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Giles WR, Imaizumi Y. Comparison of potassium currents in rabbit atrial and ventricular cells. J Physiol 405: 123–145, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooks DA, Trew ML, Caldwell BJ, Sands GB, LeGrice IJ, Smaill BH. Laminar arrangement of ventricular myocytes influences electrical behavior of the heart. Circ Res 101: e103–e112, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Kléber AG, Rieger CB. Electrical constants of arterially perfused rabbit papillary muscle. J Physiol 385: 307–324, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev 84: 431–488, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Le Guyader P, Trelles F, Savard P. Extracellular measurement of anisotropic bidomain myocardial conductivities. I. Theoretical analysis. Ann Biomed Eng 29: 862–877, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Metzger P, Weingart R. Electrical current flow in cell pairs isolated from adult rat hearts. J Physiol 366: 177–195, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters NS, Wit AL. Myocardial architecture and ventricular arrhythmogenesis. Circulation 97: 1746–1754, 1998 [DOI] [PubMed] [Google Scholar]
- 16.Plonsey R, Barr RC. The four electrode resistivity technique as applied to cardiac muscle. IEEE Trans Biomed Eng 29: 541–546, 1982 [DOI] [PubMed] [Google Scholar]
- 17.Polimeni PI, Williams S, Weisman H. Application of automatic electrotonic image analyzer to the measurement of myocardial extracellular space. Comp Biomed Res 16: 522–530, 1983 [DOI] [PubMed] [Google Scholar]
- 18.Pollard AE, Barr RC. Cardiac microimpedance measurement in two-dimensional models using multisite interstitial stimulation. Am J Physiol Heart Circ Physiol 290: H1976–H1987, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Pollard AE, Ellis CD, Smith WM. Linear electrode arrays for stimulation and recording within cardiac tissue space constants. IEEE Trans Biomed Eng 55: 1408–1414, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Pollard AE, Smith WM, Barr RC. Feasibility of cardiac microimpedance measurement using multisite interstitial stimulation. Am J Physiol Heart Circ Physiol 287: H2402–H2411, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Pope AJ, Sands GB, Smaill BH, LeGrice IJ. Three-dimensional transmural organization of perimysial collagen in the heart. Am J Physiol Heart Circ Physiol 295: H1243–H1252, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puglisi JL, Bers DM. LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol 281: C2049–C2060, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Roberts DE, Hersh LT, Scher AM. Influence of cardiac fiber orientation on wavefront voltage conduction velocity and tissue resistivity in the dog. Circ Res 44: 701–712, 1979 [DOI] [PubMed] [Google Scholar]
- 24.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62: 309–322, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Saffitz JE, Laing JG, Yamada KA. Connexin expression and turnover. Implications for cardiac excitability. Circ Res 86: 723–728, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Shaw RM, Rudy Y. Ionic mechanisms of propagation in cardiac tissue. Roles of sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81: 727–741, 1997 [DOI] [PubMed] [Google Scholar]
- 27.Spach MS. Anisotropic structural complexities in the genesis of reentrant arrhythmias. Circulation 84: 1447–1450, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Spach MS, Dobler PC, Heidlage JF. Influence of the passive anisotropic properties on directional differences in propagation following modification of the sodium current. Circ Res 62: 811–832, 1988 [DOI] [PubMed] [Google Scholar]