Abstract
Studies from animal models suggest that myocardial mitochondrial damage contributes to cardiac dysfunction after burn injury. In this report, we used an ex vivo model of primary cardiomyocyte culture to investigate the mechanisms of burn-induced mitochondrial impairment. Briefly, blood serum was collected from Sprague-Dawley (SD) rats subjected to 40% total body surface area burn and added (10% vol/vol) to primary cardiomyocytes prepared from SD rats. The effect of the burn serum on mitochondrial function and membrane integrity in the myocytes was analyzed. Exposure of myocytes to burn serum doubled the mitochondrial membrane damage measured by two independent assays. This treatment also significantly elevated mitochondrial oxidative stress, indicated by a more than 30% increase in lipid oxidation. Downregulation of mitochondrial antioxidant defense was also evident since the activities of the antioxidant enzymes superoxide dismutase and glutathione peroxidase were reduced by about 30% and 50%, respectively. Burn serum also induced deficiency of mitochondrial metabolism, indicated by a 30% decrease in the activity of cytochrome c oxidase. These mitochondrial dysfunctions appear to be generated by oxidative stress because burn serum induced a significant increase of mitochondrial oxygen species (mtROS) in cardiomyocytes, and pretreatment of cardiomyocytes with the antioxidant N-acetyl-cysteine prevented the mitochondrial damages induced by burn serum. Remarkably, the increase in mtROS was abolished by an antibody-mediated blockade of CD14. Furthermore, burn injury-induced mitochondrial damage in cardiomyocytes was prevented in CD14 knockout mice. Taken together, these data suggested that burn injury produces CD14-dependent mitochondrial damage via oxidative stress in myocardium.
Keywords: oxidative stress, mitochondrial metabolism, burn injury
burn injury has been shown to induce cardiac dysfunction previously in animal models as well as in clinical studies (23, 24, 26, 35, 47). This pathological condition is associated with systemic and myocardial inflammation (3, 30), abnormal iron homeostasis (2, 31, 34, 42, 46), and disordered metabolisms (48, 52). To date, intensive research has been focused on deducing the relevant molecular mechanisms and the development of novel therapeutic interventions.
Abnormalities in mitochondrial structure and function have been found to occur in numerous myocardial injury and disease states (32). Mitochondria are a major source of intracellular oxidative stress because reactive oxygen species (ROS) are generated as by-products of mitochondrial metabolism. Increased accumulation of ROS can alter the function of proteins, lipids, and DNA through structural modifications in various pathological conditions (6, 17). In burn injury, oxidative stress has been well recognized as a contributor to the development of distant organ injury/failure (20, 22, 39). However, most studies to date have focused on the effects of total intracellular ROS. We hypothesize that burn injury produces functional defects of myocardial mitochondria and causes overproduction of mitochondrial ROS (mtROS), leading to a cascade of molecular events that generate cardiac contractile dysfunction.
CD14, a member of the toll-like receptor 4 (TLR4) signalosome, is involved in the regulation of postburn pathogenic responses in distant organs such as the liver (11, 13, 14), lung (14), skin (43), and adrenal gland (12). Consistently, recent clinical research has correlated genetic variations within the TLR4 gene with the risk of post-trauma organ failure (4, 41). In the heart, we have previously detected burn-induced overexpression of CD14 in cardiomyocytes and provided evidence showing that CD14 promoted myocardial inflammation and dysfunction (4, 5). However, the role of CD14 in the regulation of mitochondria in the heart has not been directly examined. Studies in the present report allowed us to understand CD14 signaling from extracellular stimuli to the induction of cardiac dysfunction in burn injury.
Previously, we demonstrated in an ex vivo primary cardiomyocte cell culture model that challenging the myocytes with 10% serum (vol/vol) collected from burn-injured adult rats stimulated mitochondrial calcium accumulation (31), activated inflammatory responses (30, 31), and inhibited Na-K-ATPase activation (44). These studies helped us to understand the molecular mechanisms underlying burn-related cardiac dysfunction. The objective of the present report was to resolve more completely burn-induced myocardial mitochondrial alteration using this primary cell culture model. We examined mitochondrial function in primary cardiomyocytes following treatment with serum collected from adult male Sprague-Dawley (SD) rats 24 h after receiving a 40% total body surface area (TBSA) burn. Furthermore, cardiomyocytes isolated from CD14 knockout (KO) mice were included in the analysis to determine the functional role of CD14 in burn-induced myocardial mitochondrial damage.
MATERIALS AND METHODS
Experimental Animals
Adult SD male rats weighing 325–360 g were obtained from Harlan Laboratories (Houston, TX). CD14-deficient mice as well as wild-type (WT) mice (C57BL/6) were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were allowed 5–10 days to acclimate to their surroundings before experimentation, and commercial food and tap water were available throughout the experiment. All work described herein was approved by The University of Texas Southwestern Medical Center Institutional Animal Care and Research Advisory Committee and was performed according to the guidelines outlined in the Guide for the Care and Use of Laboratory Animals published by the American Physiological Society.
Burn Procedure and Burn Serum Isolation
Protocols producing full-thickness cutaneous burns covering 40% TBSA in rats and in mice were previously described (2, 21). Briefly, animals were deeply anesthetized (2–2.5% isoflurane) and shaved before injury. For the rats subjected to burn, animals were secured in a custom template device, with the skin on the back and upper sides of the body exposed through the template. Rats were inverted with the dorsal side down and immersed in 100°C water for 12 s. For mice, the same percent TBSA burn injury was generated by applying brass probes (2 × 3 cm with 3 mm thickness) that were preheated to 100°C in boiling water to the animal's sides and back for 5 s. The TBSA was calculated using rodent or murine-specific data (19), and this calculation was verified by removing the animal pelt and measuring the actual burned area. Sham animals were subjected to the same procedures except that a room-temperature water bath was used for rats and room-temperature brass probes were used for mice. All animals were given lactated Ringer solution (4 ml/kg per percent burn) intraperitoneally after burn, according to the Parkland burn formula. One-half of the calculated volume was given immediately postburn, and the remaining volume was given 8 h postburn. All animals received analgesia (buprenorphine, 0.05 mg/kg im) every 8 h after burn. Animals were monitored closely for the first 8-h postburn period to ensure adequate recovery from the anesthesia, responsiveness to external stimuli, absence of pain, and the ability to consume food and water. Serum was collected from burn and sham animals 24 h following the burn procedure.
Cardiomyocyte Isolation and Culture
Isolation of cardiomyocytes from rats and mice was performed as previously described (25, 51). Briefly, animals were given an intraperitoneal injection of heparin (2,000 units/rat; 500 units/mouse) 20–30 min before they were killed by decapitation. Hearts were harvested and placed in a petri dish containing room-temperature heart medium that contained (in mM) 113 NaCl, 4.7 KCl, 0.6 KH2PO4, 0.6 Na2HPO4, 1.2 MgSO4, 12 NaHCO3, 10 KHCO3, 20 d-glucose, 10 HEPES, 30 taurine, 2 carnitine, 2 creatine, and plus 0.5× MEM amino acids (Invitrogen, Carlsbad, CA), which was bubbled constantly with 95% O2-5% CO2. Hearts were cannulated via the aorta and perfused with heart medium at a rate of 1 ml/min for 5 min in a nonrecirculating mode. Enzymatic digestion was initiated by perfusing the heart with digestion solution prepared by adding 50 mg of collagenase II (Worthington Biochemical, Lakewood, NJ) and 50 mg bovine serum albumin (BSA), Fraction V (Invitrogen) to 34.5 ml of heart medium, plus 1× trypsin (Invitrogen), 15 μM CaCl2, and 40 mM 2,3-butanedione monoxime (BDM). Enzymatic digestion was accomplished by recirculating this solution through the heart at a flow rate of 1 ml/min for 20 min. All solutions perfusing the heart were maintained at a constant 37°C. At the end of the enzymatic digestion, the ventricles were removed and mechanically disassociated in 6 ml of enzymatic digestion solution containing a 6-ml aliquot of 2× BDM/BSA solution (3 mg BSA, Fraction V to 150 ml of BDM stock, 40 mM). After mechanical disassociation with fine forceps, the tissue homogenate was filtered through a mesh filter into a conical tube. The cells adhering to the filter were collected by washing with an additional 10-ml aliquot of 1× BDM/BSA solution, prepared by combining 100 ml of BDM stock, 40 mM; 100 ml of heart medium; and 2 g of BSA, Fraction V. Cells were then allowed to pellet in the conical tube for 10 min. The supernatant liquid was removed, and the pellet was resuspended in 10 ml of 1× BDM/BSA. The cells were washed and pelleted further in BDM/BSA buffer with increasing concentrations of calcium (100, 200, 500, and 1,000 μM). After the final pelleting step, the supernatant liquid was removed, and the pellet was resuspended in MEM containing 1× MEM (Sigma-Aldrich), 11.9 mM NaHCO3, 10 mM HEPES, and 1× penicillin/streptomycin (Invitrogen). At the time of MEM preparation, the medium was bubbled with 95% O2-5% CO2 for 15 min, and the pH was adjusted to 7.1 with 1 M NaOH. The solution was then filter sterilized and stored at 4°C until use. At the final concentration of calcium, the cell viability was measured, and only cell suspensions with greater than 85% viability were used for subsequent studies.
Cardiomyocytes were plated at a density of 106cells/plate on 100-mm petri dishes and incubated with MEM medium alone (control) or in the presence of burn serum or sham serum (10% vol/vol) in CO2 incubator at 37°C. In some experiments, cells were treated with N-acetyl-l-cysteine (NAC; 5 mM in MEM; Sigma-Aldrich) or CD14 antibody (10 μg/ml in MEM; Santa Cruz Biotechnology, Santa Cruz, CA) for 30 min before burn serum or sham serum was added in the culture. Cells were harvested 18 h after incubation.
Isolation of Cytosolic and Mitochondrial Fractions from Heart Tissues
Cytosolic and mitochondrial fractions were prepared by differential centrifugation according to previously described procedures (49, 50). Cardiomyocyte cell pellets were homogenized in three volume of homogenizing buffer containing (in Mm) 20 HEPES-KOH (pH 7.5), 250 sucrose, 10 KOH, 1.5 MgCl2, 1 EDTA, 1 EGTA, 1 DTT, and 0.1 PMSF, followed by centrifugation at 600 g for 5 min to remove the unbroken tissue debris, cells, and cell nuclei. The supernatants were collected and centrifuged at 10,000 g for 30 min to pellet mitochondria, which were then resuspended in resuspension buffer containing (in mM) 200 mannitol, 10 HEPES-KOH (pH 7.5), 70 sucrose, and 1 EGTA. The resulting supernatants were further centrifuged at 100,000 g for 30 min to eliminate contamination by the membrane fraction. All procedures were performed at 4°C. Concentrations of cytosolic and mitochondrial protein fractions were quantified using Bio-Rad DC RC protein assay kit (Bio-Rad, Hercules, CA).
Measurement of Cytochrome c Oxidase Activity and Assessment of Outer Mitochondrial Membrane Damage
Both mitochondrial cytochrome c oxidase (COX) activity and outer membrane integrity were evaluated using a COX assay (Sigma-Aldrich). Experimental procedures were performed according to the manufacturer's protocol; 20 μg freshly isolated mitochondrial fraction were used for each reaction, and duplicate reactions were conducted for each assay. For measurement of total mitochondrial COX activity, the mitochondria fraction was diluted in enzyme dilution buffer of 10 mM Tris·HCL (pH 7.0) containing 250 mM sucrose with 1 mM N-dodecyl β-d-maltoside and incubated on ice for 30 min. The reaction was initiated by adding freshly prepared ferrocytochrome c substrate solution (0.22 mM) to the sample. The decrease in absorbance at 550 nm, which is related to oxidation of ferrocytochrome c by COX, was recorded using a kinetic program (5-s delay; 10-s interval; 6 repeated readings). COX activity was calculated and normalized for the amount of protein per reaction and results were expressed as units per milligram mitochondrial protein. Mitochondrial outer membrane integrity was assessed by measuring COX activity of mitochondria in the presence or absence of the detergent, N-dodecyl β-d-maltoside. Mitochondrial outer membrane damage was assessed from the ratio between COX activity without and with detergent.
Measurement of Superoxide Dismutase Activity in Mitochondria
Superoxide dismutase (SOD) activity was measured using a commercial assay kit (Calbiochem, San Diego, CA). Mitochondrial protein (∼100 μg) was used for each reaction, and all assays were performed in duplicate. According to the manufacturer's protocol, samples were first incubated with 1-methyl-2-vinylpyridinium in assay buffer at 37°C for 1 min to eliminate interference. Immediately after substrate (5,6,6α,11β-tetrahydro-3,9,10-trihydroxybenzo[c]fluorine, the oxidation of which is regulated by SOD) was added, and absorbance at 525 nm was recorded using a kinetic program: 10-s interval and six readings. SOD activity was determined from the ratio of the autoxidation rates measured in the presence and in the absence of SOD. Results were normalized by protein amount per reaction and expressed as units per milligram mitochondrial protein.
Measurement of Glutathione Peroxidase Activity in Mitochondria
Glutathione peroxidase (GPx) activity was measured using a commercial assay kit (Calbiochem). Mitochondrial extracts were at first diluted with assay buffer, and ∼50 μg protein was used per reaction; all assays were performed in duplicate. Samples were added to a solution containing 1 mM glutathione (GSH), ≥0.4 unit/ml glutathione reductase, and 0.2 mM NADPH. The reaction was initiated by adding substrate tert-butyl hydroperoxide (final concentration, 0.22 mM), and reduction was recorded at A340 nm using a kinetic program: 30-s interval and six readings. GPx activity was determined by the rate of decrease in absorbance at A340 nm [1 mU/ml GPx = (A340/min)/0.0062]. Results were normalized by protein amount per reaction and expressed as micro units per milligram mitochondrial protein.
Measurement of Lipid Peroxidation in Mitochondria
Lipid peroxidation was determined by levels of malondialdehyde (MDA), a marker of lipid oxidation using a lipid peroxidation assay kit (Calbiochem). To prevent sample oxidation, concentrations of all mitochondrial extracts were adjusted to 1–1.5 mg/ml in a resuspension buffer containing 5 mM butylated hydroxyl toluene. Standards were prepared, and experimental procedures were performed according to manufacturer's protocol. For each reaction, 200 μl of sample or standard was added to 650 μl chromogenic reagent (provided by the manufacturer) and 150 μl 12 N HCL. After incubation at 45°C for 60 min, the samples were cooled at 4°C and centrifuged at 10,000 g for 5 min. The supernatants were collected, and absorbance at 586 nm was recorded. MDA concentration was calculated using a standard curve. All measurements were performed in duplicate.
Detection of Mitochondrial ROS
MtROS were detected by florescent labeling with MitoSox Red (Invitrogen). According to manufacturer's protocol, cultured cardiomyocytes were loaded with 5-μM MitoSox Red (in HEPES-buffered medium) for 20 min at 37°C. After washed three times, the cells were resuspended in PBS and analyzed by flow cytometry.
Mitochondrial Aconitase Assay
The colometric aconitase assay (BioVision, Mountain View, CA) was used to determine aconitase activities in the mitochondrial fractions isolated from cardiomyocytes. Mitochondrial protein (100 μg per reaction) was applied, and aconitase activity was determined via converting citrate to isocitrate according to manufacturer's protocol. The final product was measured at λ = 450 nm.
Western Blots
Protein samples were mixed with Laemmli's loading buffer, boiled for 5 min, subjected to SDS-PAGE gels, and then transferred to polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat milk-PBS at room temperature for 1 h and subsequently probed with anti-cytochrome c, anti-GAPDH (both from Chemicon, Temecula, CA), mitochondrial total OXPHOS antibody cocktail (MitoSciences, Eugene, OR), or anti-Cu/Zn-SOD (Assay Designs, Ann Arbor, MI). The membranes were then rinsed and incubated with corresponding horseradish peroxidase conjugated secondary antibodies. Antibody dilutions and incubation time were according to manufacturer's instructions. Membranes were then rinsed, and bound antibodies were detected by using enhanced chemiluminescence (ECL; Amersham, Piscataway, NJ).
Statistical Analysis
All values are expressed as means ± SE. ANOVA was used to assess an overall difference among the groups for each of the variables. Levene's test for equality of variance was used to suggest the proper multiple comparison procedure to be used; once equality of variance among the four groups was assessed, the appropriate multiple comparison procedures were performed. Student-Neuman-Keuls was used for examining differences in myocardial performance. One-way ANOVA and a two-sided post hoc Dunnett t-test were used to compare the control group with each of the other groups for mitochondrial outer membrane damage, lipid peroxidation, GPx, cytochrome c, and MDA levels. Probability values less than 0.05 were considered statistically significant (analyses were performed using SPSS for Windows, Version 7.5.1).
RESULTS
Burn Serum Challenge Produces Mitochondrial Damage in Cardiomyocytes
Membrane integrity.
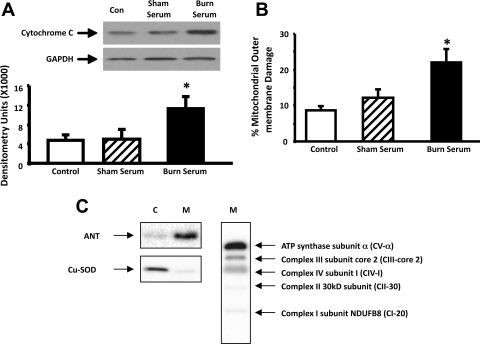
Leaking of mitochondrial protein cytochrome c to the cytosolic compartment is a well-recognized measure of impaired mitochondrial membrane integrity (1, 18, 40). To determine whether challenge with burn serum produced mitochondrial damage in cardiomyocytes, primary myocytes isolated from SD rats were exposed for 18 h to 10% (vol/vol in culture medium) burn serum or sham serum obtained from SD rats 24 h after delivery of a 40% TBSA burn or sham-burn injury. Cytosolic cytochrome c levels were examined by Western blots in control cells and cells challenged by burn serum or sham serum. A greater than twofold increase of cytosolic cytochrome c was detected in cells treated with burn serum, compared with either control cells or cells treated with sham serum (Fig. 1A). As expected, expression of the marker protein GAPDH was unaltered. These data suggest that burn serum challenge damages mitochondrial membrane integrity in cardiomyocytes.
Fig. 1.
Impairment of mitochondrial membrane integrity by burn serum challenge in primary cardiomyocytes. Cytosol and mitochondrial fractions were extracted from control myocytes (Con) and myocytes challenged with 18-h exposure to 10% (vol/vol) sham serum or burn serum. A: burn serum challenge induced cytosolic accumulation of mitochondrial protein cytochrome c. Cytochrome c and cytosolic marker GAPDH were detected by Western blots and analyzed by densitometry. B: burn serum challenge increased mitochondrial outer damage. Mitochondrial outer membrane damage was measured in the mitochondrial preparations. C: confirmation of subcellular fractionation. Equal amount of cytosolic (C) and mitochondrial (M) fractions were analyzed by Western blot using antibodies against marker proteins ANT or Cu-SOD. Mitochondrial respiratory complexes (I-V) were detected in the mitochondrial fraction (M) by OXPHOS antibody cocktail. All values are means ± SE. *Significant difference when comparing the burn serum-challenged samples with control or sham serum-treated counterparts (P < 0.05; n ≥ 3).
We also examined mitochondrial membrane integrity in the mitochondrial fractions isolated from control cells and cells challenged by either burn or sham serum. In this measurement, the ratio of COX activity in the presence/absence of detergent N-dodecyl β-d-maltoside reports the percentage of mitochondrial outer membrane damage. Consistent with the result from the cytosolic cytochrome c assay, cardiac mitochondrial outer membrane damage was nearly doubled in the cardiomyocytes challenged by burn serum (Fig. 1B).
In the experiments of this report, the quality of subcellular fractionation was confirmed by Western blots using antibodies against cytosol and mitochondria marker proteins. As shown in Fig. 1C, cytosolic isoform of superoxide dismutase CuSOD and mitochondrial adenine nucleotide translocase (ANT) were exclusively present in the cytosolic and mitochondrial fractions, respectively. Mitochondrial respiratory complex I-V was also verified in the mitochondrial fractions.
Oxidative stress.
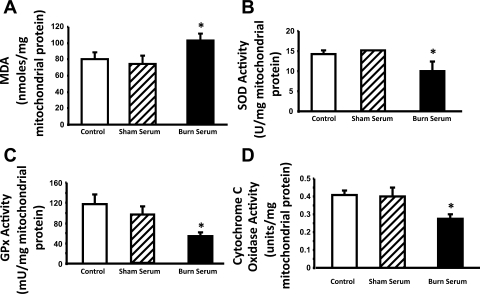
Excessive oxidative stress causes mitochondrial damage and dysfunction through oxidation of mitochondrial molecules (7, 15, 36). To determine the effects of burn serum on mitochondrial oxidative stress in cardiomyocytes, we measured the levels of lipid peroxidation in the mitochondrial fractions isolated from control myocytes and myocytes challenged with either burn serum or sham serum. As shown in Fig. 2A, MDA content, which is a marker of lipid peroxidation, was 100 nmol per mg of mitochondrial protein in the cells treated with burn serum, compared with 75 nmol per mg of mitochondrial protein in controls or sham serum-treated cells. This represented more than a 30% increase of oxidative stress within the mitochondria of cardiomyocytes following challenge with burn serum.
Fig. 2.
Burn serum challenge increased oxidative stress, downregulated antioxidant defense, and decreased respiration activity in the mitochondria of primary cardiomyocytes. Mitochondrial fractions were extracted from control myocytes and myocytes challenged with 18-h exposure to 10% sham serum or burn serum. Malondialdehyde (MDA) levels (A), activities of SOD (B), glutathione peroxidase (GPx; C), and cytochrome c oxidase (COX; D) were measured and normalized by the amount of protein per reaction. All values are means ± SE. *Significant difference when comparing the burn serum-challenged samples with control or sham serum-treated counterparts (P < 0.05; n ≥ 3).
Antioxidant defense.
A major component of mitochondrial antioxidant defense mechanism is the expression of mitochondrial antioxidant enzymes, including manganese-dependent (Mn)-SOD and GPx (27, 50). We compared the enzymatic activities of SOD and GPx in mitochondrial fractions isolated from control, burn serum-treated, and sham serum-treated cells. We found that 18 h of exposure to burn serum reduced SOD activity by ∼30% (Fig. 2B) and GPx activity by more than 50% (Fig. 2C), indicating that burn serum challenge impairs the mitochondrial antioxidant capacity of cardiomyocytes.
Mitochondrial respiratory function.
COX is one of the most important enzymes of mitochondrial respiratory electron transport chain that controls ATP production (28). We determined COX activity in the mitochondrial fractions isolated from control cardiomyocytes and myocytes treated with either burn serum or sham serum. A significant decrease (∼30%) of COX activity was detected in burn serum-challenged cells, relative to that in the control or sham serum-treated cells, suggesting that burn serum challenge impairs mitochondrial energy production in cardiomyocytes (Fig. 2D).
Increasing Antioxidant Defense Protects Mitochondria from Burn Serum-mediated Injury in Cardiomyocytes
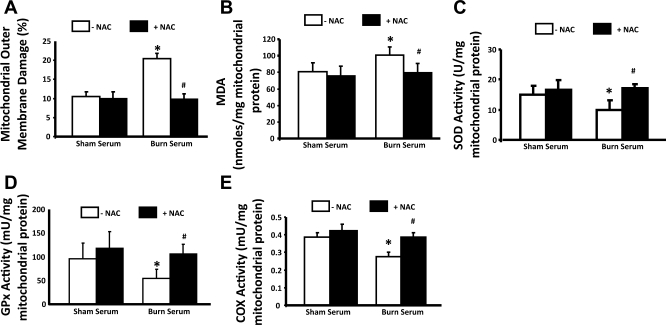
To confirm that burn-related oxidative stress damages myocardial mitochondria, the effects of NAC, currently the most clinically utilized pharmacological antioxidant (9, 10, 38), was evaluated in the primary cardiomyocytes. Myocytes were pretreated with 5 mM NAC for 30 min before exposure to 10% burn serum or sham serum. Mitochondrial metabolism (COX activity), outer membrane damage, oxidative stress levels (lipid oxidation), and antioxidant defense (SOD and GPx activities) were then compared. As shown in Fig. 3, A–E, NAC prevented mitochondrial alterations caused by burn serum challenge in myocytes.
Fig. 3.
Antioxidant N-acetyl-cysteine (NAC) protected cardiomyocyte mitochondrial damage from burn serum challenge. Myocytes were pretreated with 5 mM NAC for 30 min before challenge with 18-h exposure to 10% sham serum or burn serum. Mitochondrial fractions were prepared and applied to the measurements of mitochondrial outer membrane damage (A), MDA levels (B), activities of SOD (C), GPx (D), and COX (E). All values are means ± SE. *Significant difference between burn serum- and sham serum-treated cells; #significant difference between with and without NAC (P < 0.05; n ≥ 3).
Burn Serum Challenge Causes CD14-dependent mtROS Production in Cardiomyocytes
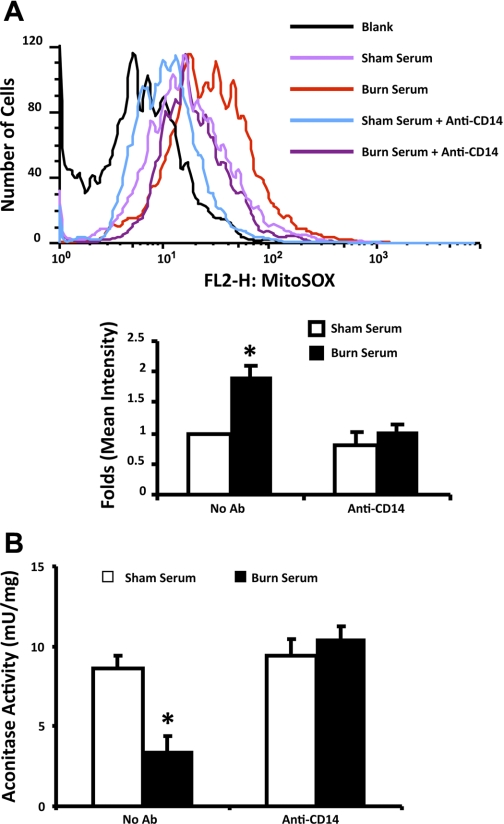
To determine whether burn serum challenge produces an increase of ROS in mitochondria, cardiomyocytes were prechallenged by sham or burn serum and labeled with MitoSox Red, a fluorescent probe that preferentially reacts with superoxide in mitochondria (33). Cells were then analyzed by flow cytometry to qualify mitochondrial superoxide levels. As shown in Fig. 4A, a nearly 100% increase of mitochondrial superoxide was detected in burn serum-challenged myocytes. Furthermore, this increase was completely abolished by pretreatment of the cells with a CD14 neutralizing antibody.
Fig. 4.
Burn serum challenge produced a CD14-dependent mitochondrial reactive oxygen species (mtROS) increase in primary cardiomyocytes. Myocytes were challenged with 18-h exposure to 10% (vol/vol) sham serum or burn serum. In a parallel experiment, myocytes were in addition treated with 10 μg/ml anti-CD14. A: cells were then labeled with 5 μM MitoSox Red and analyzed by flow cytometry. Top: example of flow cytometry analysis. Bottom: summary of multiple independent experiments. B: aconitase activities were measured in the mitochondrial fractions isolated from all cardiomyocytes. Enzyme activities were normalized by the amount of mitochondrial proteins. All values are means ± SE. *Significant difference when comparing the burn serum-challenged samples with control or sham serum-treated counterparts (P < 0.05; n ≥ 3). Ab, antibody.
Aconitase exists in tissue as mitochondria- and cytoplasmic-specific isoforms that catalyze the stereospecific isomerization of citrate to isocitrate. Because aconitase is sensitive to ROS oxidation, loss of aconitase activity has been interpreted as an indicator of ROS elevation (16, 29, 45). We measured aconitase activities in the mitochondrial fractions as an alternative approach to compare mtROS levels in sham serum- and burn serum-challenged myocytes. As shown in Fig. 4B, burn serum challenge suppressed mitochondrial aconitase activity from 8.6 to 3.4 micro units per mg mitochondrial protein, indicating a more than 50% reduction. However, with the presence of CD14 neutralizing antibody, no difference was detected when comparing burn serum-challenged myocytes with sham serum-challenged cells. These data suggest that burn serum causes a CD14-dependent elevation of mtROS production in primary cardiomyocytes.
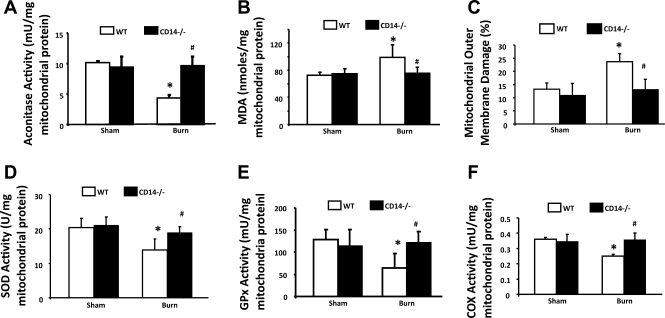
Burn Injury-induced Mitochondrial Damage in Cardiomyocytes is Protected by CD14 KO
To determine whether burn-induced mitochondrial damage is mediated via the CD14 signaling pathway, we determined whether burn serum-produced mitochondrial alterations were present in the primary cardiomyocytes of CD14 KO mice. In the experiments summarized in Fig. 5, cardiomyocytes were isolated from WT and CD KO mice 24 h postburn or sham injury. As expected, in cardiomyocytes from WT animals, burn injury caused a significant increase in mitochondrial oxidative stress, signified by the inactivation of aconitase (Fig. 5A), elevation of lipid oxidation (Fig. 5B), and increase of mitochondrial membrane damage (Fig. 5C). Consistent with the results obtained from burn serum-challenged rat myocytes, burn injury impaired mitochondrial antioxidant defense, as shown by the downregulation of the enzymatic activities of SOD (Fig. 5D) and GPx (Fig. 5E), and caused mitochondrial functional deficiency, as indicated by the decrease of COX activity (Fig. 5F). In CD14 KO mice, however, no statistically significant difference was observed when cells from burn-injured subjects were compared with sham controls. These data suggest that CD14 plays an important role in mitochondrial dysfunction in the heart after burn injury.
Fig. 5.
CD14 knockout prevented burn-induced mitochondrial impairments in primary cardiomyocytes. Wild-type (WT) and CD14 knockout mice were subjected to burn or sham injury followed by cardiomyocytes isolation. Mitochondrial fractions were prepared and applied to the measurements of aconitase activities (A), MDA levels (B), mitochondrial outer membrane damage (C), activities of SOD (D), GPx (E), and COX (F). All values are means ± SE. *Significant difference between burn and sham samples; #significant difference between WT and CD14 knockout (P < 0.05; n ≥ 3).
DISCUSSION
We utilized a previous established ex vivo primary cardiomyocte cell culture model to investigate burn injury-related myocardial mitochondrial damage. Our results confirmed that challenge of rat myocytes with 10% (vol/vol) serum collected from animals given a 40% TBSA burn impaired mitochondrial integrity, decreased mitochondrial metabolism, increased oxidative stress levels, and downregulated antioxidant defense (Figs. 1 and 2). Pretreatment of myocytes with antioxidant NAC prevented these burn serum-induced mitochondrial alterations (Fig. 3), suggesting that burn serum-induced damage to cardiomyocyte mitochondria is mediated by oxidative stress. Interestingly, a CD14 antibody was able to inhibit burn serum-induced mtROS production and consequent aconitase inactivation in cardiomyocytes, suggesting that CD14 is an important signaling molecule to initiate mitochondrial oxidative damage in response to burn injury (Fig. 4). This conclusion was further supported by the observation that burn injury produced mitochondrial dysfunction in cardiomyocytes from WT but not CD14 KO mice (Fig. 5). To date, our results are the first to suggest that the CD14 pathway mediates alterations of mitochondrial function in cardiomyocytes following burn injury.
Burn injury increases oxidative stress via imbalanced generation of ROS and subsequently induces pathophysiological conditions in various organs including the heart (37). Because mitochondria contribute to major intracellular ROS production, we hypothesized that cardiac dysfunction related to burn injury is at least in part mediated via mitochondrial oxidative stress in the myocardium. Previously, using a rat burn model, we obtained evidence showing that burn injury altered mitochondrial function in the heart by producing abnormal calcium accumulation, increase of oxidative stress, and downregulation of antioxidant defense (31, 49, 50). In the present study, we confirmed burn injury-mediated mitochondrial oxidative damage and directly detected burn serum-induced mtROS production in a primary cardiomyocyte cell culture model.
Although CD14 has been recognized as an important inflammation mediator in burn-associated pathological responses in multiple organs including the liver (11, 13, 14), lung (14), skin (43), and adrenal gland (12), its role in myocardium and myocardial mitochondrial function has not been elucidated. A recent study from our laboratory suggests that burn injury significantly elevated CD14 expression in the heart tissue and in cardiomyocytes (5). Correspondingly, both pharmacological inhibition of CD14 and CD14 KO prevented myocardial inflammation and dysfunction in burn animals (5). These results suggest that CD14 mediates myocardial inflammation after burn. In the current study, preexposure of cardiomyocytes to a CD14 antibody blocked burn serum induction of mtROS (Fig. 4). Comparison of myocytes from WT and CD14 KO mice subjected to burn or sham injury showed that CD14 KO attenuated burn injury-induced mitochondrial damage and dysfunction (Fig. 5). These data suggest that burn-associated alteration of mitochondrial function in the heart is regulated through the CD14 pathway. Future investigations to elucidate the underlying mechanism will require the analysis of other intracellular components of the CD14 signaling pathway, such as IRAK, TRAF6, and NF-κB (8), in the changes of cardiac mitochondrial function after burn injury.
In the current study, a statistically significant decrease in COX activity was detected in cardiomyocytes challenged by burn serum (Fig. 2D). However, previous investigations in the heart tissue collected from burned rats failed to detect any change of COX activity following injury (50). The discrepancy of data obtained from heart tissue versus primary cardiomyocytes could have been caused by different experimental sensitivities. In animal experiments, a certain degree of mitochondrial damage is expected from the procedure of tissue harvesting and homogenization. Consistent with this notion, myocardial mitochondrial membrane damage was measured in nearly 30% of the heart tissue from sham control rats (50), but in fewer than 10% of the control cardiomyocytes (Fig. 1B). Therefore, in animal models, mitochondrial damage caused by experimental procedures may mask differences between control and burn animals. In addition, primary cardiomyocyte culture provided a homogeneous cell population, which also enhanced the sensitivity of biochemical measurements. However, the possibility that COX activity was altered by artificial conditions associated with the culture of primary myocytes cannot be completely excluded.
In summary, the results presented in this report showed that challenging primary cardiomyocytes with serum collected from burn-injured animals elevated mtROS production and produced mitochondrial damage including impaired membrane integrity, increased oxidative stress, decreased antioxidant defense, and downregulated mitochondrial metabolism. A CD14 antibody was able to inhibit burn serum-mediated mtROS generation. Furthermore, CD14 KO prevented these burn-related mitochondrial alterations in cardiomyocytes. These results suggest that the CD14 pathway plays a pivotal role in the regulation of myocardial mitochondrial function after burn.
Our future focus is to understand the molecular pathway(s) from CD14 to cardiac mitochondrial responses in burn trauma. A cardiomyocytes cell culture model will offer the advantage of taking measurements in live cells and manipulating mitochondrial signaling by viable genetic and/or pharmacological approaches. Targeting the CD14 signaling pathway, mtROS, and/or specific aspects of mitochondrial function are expected to lead to more efficient and more sensitive therapeutic strategies to improve cardiac function in trauma injuries.
GRANTS
This project was supported by National Institute of General Medical Sciences R01-GM57054-05 (to J. W. Horton) and American Heart Association south central affiliate Beginning-in-Aid Grant 09BGIA2220114 (to Q. S. Zang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Dr. Hui Zou for critical comments of this manuscript and Drs. Youxue Wang and Zijuan Liu for helpful suggestions on cell labeling with MitoSox Red.
REFERENCES
- 1.Akao M, O′Rourke B, Teshima Y, Seharaseyon J, Marban E. Mechanistically distinct steps in the mitochondrial death pathway triggered by oxidative stress in cardiac myocytes. Circ Res 92: 186–194, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Ballard-Croft C, Carlson D, Maass DL, Horton JW. Burn trauma alters calcium transporter protein expression in the heart. J Appl Physiol 97: 1470–1476, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ballard-Croft C, White DJ, Maass DL, Hybki DP, Horton JW. Role of p38 mitogen-activated protein kinase in cardiac myocyte secretion of the inflammatory cytokine TNF-α. Am J Physiol Heart Circ Physiol 280: H1970–H1981, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Barber RC, Aragaki CC, Chang LY, Purdue GF, Hunt JL, Arnoldo BD, Horton JW. CD14–159 C allele is associated with increased risk of mortality after burn injury. Shock 27: 232–237, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barber RC, Maass DL, White DJ, Chang LY, Horton JW. Molecular or pharmacologic inhibition of the CD14 signaling pathway protects against burn-related myocardial inflammation and dysfunction. Shock 30: 705–713, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med 32: 797–803, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Benderdour M, Charron G, Comte B, Ayoub R, Beaudry D, Foisy S, Deblois D, Des Rosiers C. Decreased cardiac mitochondrial NADP+-isocitrate dehydrogenase activity and expression: a marker of oxidative stress in hypertrophy development. Am J Physiol Heart Circ Physiol 287: H2122–H2131, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Beutler B. Tlr4: central component of the sole mammalian LPS sensor. Curr Opin Immunol 12: 20–26, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Bourraindeloup M, Adamy C, Candiani G, Cailleret M, Bourin MC, Badoual T, Su JB, Adubeiro S, Roudot-Thoraval F, Dubois-Rande JL, Hittinger L, Pecker F. N-acetylcysteine treatment normalizes serum tumor necrosis factor-alpha level and hinders the progression of cardiac injury in hypertensive rats. Circulation 110: 2003–2009, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Bulger EM, Maier RV. Antioxidants in critical illness. Arch Surg 136: 1201–1207, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Cho K, Adamson LK, Jeong J, Crivello SD, Vanhook TG, Palmieri T, Greenhalgh DG. CD14-dependent alterations in c-Jun expression in the liver after burn injury. J Surg Res 122: 36–42, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Cho K, Crivello SD, Vanhook TG, Greenhalgh DG. CD14- and toll-like receptor 4-dependent regulation of c-Fos, c-Jun and c-Jun phosphorylation in the adrenal gland after burn injury. Pathobiology 71: 302–307, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Cho K, Pham TN, Crivello SD, Jeong J, Green TL, Greenhalgh DG. Involvement of CD14 and toll-like receptor 4 in the acute phase response of serum amyloid A proteins and serum amyloid P component in the liver after burn injury. Shock 21: 144–150, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Cho K, Thomas RL, Greenhalgh DG. CD14-dependent regulation of Grp78 in the liver and lungs of mice after burn injury. Exp Mol Pathol 75: 148–153, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Choksi KB, Boylston WH, Rabek JP, Widger WR, Papaconstantinou J. Oxidatively damaged proteins of heart mitochondrial electron transport complexes. Biochim Biophys Acta 1688: 95–101, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Correa F, Garcia N, Robles C, Martinez-Abundis E, Zazueta C. Relationship between oxidative stress and mitochondrial function in the post-conditioned heart. J Bioenerg Biomembr 40: 599–606, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Croteau DL, Bohr VA. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J Biol Chem 272: 25409–25412, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res 77: 334–343, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Harkness JE, Wagner JE. Biology and Medicine of Rabbits and Rodents Williams & Wilkins, 1995 [Google Scholar]
- 20.Haycock JW, Ralston DR, Morris B, Freedlander E, MacNeil S. Oxidative damage to protein and alterations to antioxidant levels in human cutaneous thermal injury. Burns 23: 533–540, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Horton J, Maass D, White J, Sanders B. Effect of aspiration pneumonia-induced sepsis on post-burn cardiac inflammation and function in mice. Surg Infect (Larchmt) 7: 123–135, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology 189: 75–88, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Horton JW. Left ventricular contractile dysfunction as a complication of thermal injury. Shock 22: 495–507, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Horton JW, Baxter CR, White DJ. Differences in cardiac responses to resuscitation from burn shock. Surg Gynecol Obstet 168: 201–213, 1989 [PubMed] [Google Scholar]
- 25.Horton JW, Maass DL, White J, Sanders B. Hypertonic saline-dextran suppresses burn-related cytokine secretion by cardiomyocytes. Am J Physiol Heart Circ Physiol 280: H1591–H1601, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Horton JW, White DJ. Cardiac responses to burn injury in young and adult guinea pigs. Shock 12: 280–287, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Imai H, Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34: 145–169, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Kadenbach B, Huttemann M, Arnold S, Lee I, Bender E. Mitochondrial energy metabolism is regulated via nuclear-coded subunits of cytochrome c oxidase. Free Radic Biol Med 29: 211–221, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Korge P, Ping P, Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res 103: 873–880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maass DL, Hybki DP, White J, Horton JW. The time course of cardiac NF-kappaB activation and TNF-alpha secretion by cardiac myocytes after burn injury: contribution to burn-related cardiac contractile dysfunction. Shock 17: 293–299, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Maass DL, White DJ, Sanders B, Horton JW. Cardiac myocyte accumulation of calcium in burn injury: cause or effect of myocardial contractile dysfunction. J Burn Care Rehabil 26: 252–259, 2005 [PubMed] [Google Scholar]
- 32.Marin-Garcia J, Goldenthal MJ. Understanding the impact of mitochondrial defects in cardiovascular disease: a review. J Card Fail 8: 347–361, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay P, Rajesh M, Hasko G, Hawkins BJ, Madesh M, Pacher P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat Protoc 2: 2295–2301, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murphy JT, Giroir B, Horton JW. Thermal injury alters myocardial sarcoplasmic reticulum calcium channel function. J Surg Res 82: 244–252, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Murphy JT, Horton JW, Purdue GF, Hunt JL. Cardiovascular effect of 7.5% sodium chloride-dextran infusion after thermal injury. Arch Surg 134: 1091–1097, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med 27: 42–50, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns 34: 6–17, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Paterson RL, Galley HF, Webster NR. The effect of N-acetylcysteine on nuclear factor-kappa B activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit Care Med 31: 2574–2578, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Ritter C, Andrades M, Guerreiro M, Zavaschi L, Gelain DP, Souza LF, Ribeiro CA, Clausell N, Menna-Barreto S, Moreira JC, Dal-Pizzol F. Plasma oxidative parameters and mortality in patients with severe burn injury. Intensive Care Med 29: 1380–1383, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Saelens X, Festjens N, Vande Walle L, van Gurp M, van Loo G, Vandenabeele P. Toxic proteins released from mitochondria in cell death. Oncogene 23: 2861–2874, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Shalhub S, Junker CE, Imahara SD, Mindrinos MN, Dissanaike S, O′Keefe GE. Variation in the TLR4 gene influences the risk of organ failure and shock posttrauma: a cohort study. J Trauma 66: 115–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikes PJ, Zhao P, Maass DL, Horton JW. Time course of myocardial sodium accumulation after burn trauma: a 31P- and 23Na-NMR study. J Appl Physiol 91: 2695–2702, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Steinstraesser L, Alarcon W, Fan MH, Klein RD, Aminlari A, Zuccaro C, Su GL, Wang SC. Thermal injury induces expression of CD14 in human skin. Burns 28: 223–230, 2002 [DOI] [PubMed] [Google Scholar]
- 44.Tan J, Maass DL, White DJ, Horton JW. Effects of burn injury on myocardial signaling and cytokine secretion: possible role of PKC. Am J Physiol Regul Integr Comp Physiol 292: R887–R896, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Tong JJ, Schriner SE, McCleary D, Day BJ, Wallace DC. Life extension through neurofibromin mitochondrial regulation and antioxidant therapy for neurofibromatosis-1 in Drosophila melanogaster. Nat Genet 39: 476–485, 2007 [DOI] [PubMed] [Google Scholar]
- 46.White DJ, Maass DL, Sanders B, Horton JW. Cardiomyocyte intracellular calcium and cardiac dysfunction after burn trauma. Crit Care Med 30: 14–22, 2002 [DOI] [PubMed] [Google Scholar]
- 47.White J, Maass DL, Giroir B, Horton JW. Development of an acute burn model in adult mice for studies of cardiac function and cardiomyocyte cellular function. Shock 16: 122–129, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Xia ZF, Horton JW, Zhao P, Lin C, Sherry AD, Malloy CR. Relationship between energetic, ionic, and functional status in the perfused rat heart following thermal injury: a 31P and 23Na NMR study. J Surg Res 69: 212–219, 1997 [DOI] [PubMed] [Google Scholar]
- 49.Zang Q, Maass DL, Tsai SJ, Horton JW. Cardiac mitochondrial damage and inflammation responses in sepsis. Surg Infect (Larchmt) 8: 41–54, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zang Q, Maass DL, White J, Horton JW. Cardiac mitochondrial damage and loss of ROS defense after burn injury: the beneficial effects of antioxidant therapy. J Appl Physiol 102: 103–112, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol 292: H2408–H2416, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Zhaofan X, Jianguang T, Guangyi W, Hongtai T, Shengde G, Horton JW. Effect of thermal injury on relative anaplerosis and gluconeogenesis in the rat during infusion of [U-13C] propionate. Burns 28: 625–630, 2002 [DOI] [PubMed] [Google Scholar]