Abstract
Little is known about the three-dimensional (3-D) intramural activation sequences during long-duration ventricular fibrillation (VF), including the role of the subendocardium and its Purkinje fibers (PFs) in long-duration VF maintenance. Our aim was to explore the mechanism of long-duration VF maintenance with 3-D electrical mapping. We recorded 10 min of electrically induced VF in the left ventricular anterior free wall of six 10-kg, open-chest dogs using a 3-D transmural unipolar electrode matrix (9 × 9 × 6, 2-mm spacing) that allowed us to map intramural activation sequences. At 2.5 ± 1.8 min of VF, although the body surface ECG continued to exhibit a disorganized VF pattern, intramurally a more organized, synchronous activation pattern was first observed [locally synchronized VF (LSVF)]. This pattern occurred one or more times in all dogs and was present 33.4 ± 31.4% of the time during 5–10 min of VF. As opposed to the preceding changing complex activation sequences of VF, during LSVF, wavefronts were large and highly repeatable near the endocardium, first exciting the endocardium almost simultaneously and then rapidly spreading toward the epicardium with different levels of conduction block en route. During LSVF, PF activations always preceded working myocardium activations near the endocardium. In conclusion, long-duration VF in dogs frequently becomes highly organized in the subendocardium, with activation fronts arising in this region and passing intramurally toward the epicardium, even though the surface ECG continues to exhibit a disorganized pattern. PFs appear to play an important role during this stage of VF.
Keywords: intramural ventricular fibrillation, locally synchronized ventricular fibrillation, Purkinje fibers
sudden cardiac arrest is a leading cause of death in the industrialized world (25). Many lethal cardiac arrests are caused by ventricular fibrillation (VF) (25). The mean time from collapse until defibrillation in the prehospital setting, where most cardiac arrests occur, ranges from 4 to 10 min (21, 22). Yet, relatively few studies have dealt with activation sequences during VF lasting >1 min [long-duration VF (20)], and little is known about the mechanisms of long-duration VF maintenance.
Some studies have suggested an important role of Purkinje fibers (PFs) during long-duration VF maintenance (1, 4, 20, 23). As in humans, PFs in canines are primarily located in the subendocardium (19). In canines, an endocardial-epicardial gradient of the activation rate develops during long-duration VF (1, 4, 23), with discrete, regular, rapid deflections persisting in the subendocardium (23), possibly caused by PFs. After endocardial ablation with Lugol's solution to eliminate PF activations, subendocardial PF signals were no longer recorded, and the transmural activation rate gradient disappeared (4, 7). In an endocardial mapping study (20) of canine hearts, frequent PF activations and their interactions with working myocardium (WM) activations were observed during long-duration VF. However, determining the role of the subendocardium and its PFs in long-duration VF maintenance requires three-dimensional (3-D) transmural mapping.
We (14) recently developed a 3-D electrical mapping technique that, combined with 3-D wavefront analysis programs, allows quantitative wavefront analysis. The aims of the present study were to use these techniques in canines to 1) test the hypothesis that the subendocardium is the source of the intramural wavefronts during long-duration VF, 2) determine if there is more than one type of activation pattern during long-duration VF, and 3) further define the relationship between PF and WM activations during long-duration VF.
MATERIALS AND METHODS
Animal preparation.
All experiments were performed in accordance with the American Physiology Society's guiding principles in the care and use of animals. The protocol was approved by the Institution Animal Care and Use Committee of the University of Alabama at Birmingham.
Six beagles (either sex, 9.7 ± 0.7 kg) were fasted overnight, intramuscularly injected with atropine (0.04 mg/kg), anesthetized with pentothal (∼20mg/kg iv), intubated, and mechanically ventilated with isoflurane in 100% O2. Femoral arterial blood pressure, blood gases, pH, and electrolytes were monitored every 30 min and maintained within acceptable physiological ranges. Throughout the experiment, the animal was in a restrained, dorsally recumbent position, with lactated Ringer solution administrated (2–5 ml·kg−1·h−1). The chest was opened by a median sternotomy, and the pericardial sac was opened to expose the heart. The heart was supported in a pericardial sling.
Data acquisition.
A transparent plaque (14) containing 9 × 9 holes 2 mm apart was sutured to the anterior left ventricular (LV) epicardium ∼5 mm from the left anterior descending artery and ∼10 mm from the apex. Up to 81 fiberglass-reinforced epoxy needles containing 6 unipolar electrodes with 2-mm interelectrode spacing (16) were inserted through the holes in the plaque. To minimize ischemia, no needles were inserted through any holes with visible vessels beneath them. The size of the mapped region was 16 × 16 × 10 mm3. The thickness of the LV free wall in the mapped region was ∼8.5 mm.
After needle insertion, we waited 30 min to allow injury currents to subside. Activations and the ECG lead II signal during sinus rhythm and a 10-min VF episode induced by a 9-V battery touching the right ventricle were recorded with a 528-channel, 2-kHz sampling rate mapping system and bandpass filtered between 0.5 Hz and 1 kHz. After the experiment, the locations of the electrodes were determined as previously described (3, 5).
Definition of cavity, endocardial, nonendocardial, and epicardial electrodes.
The six electrodes along each needle were divided into three types from the endocardium to epicardium as follows: 1) cavity electrodes, located in the LV cavity; 2) the endocardial electrode, the most endocardial electrode not in the cavity; and 3) nonendocardial electrodes, all other electrodes not in the cavity. Cavity and endocardial electrodes were identified based on the following algorithm. The voltage complex of the cavity electrodes during a sinus activation cycle 1) exhibited a QS pattern, 2) was more than twice as wide as the endocardial electrode complex, and 3) had a negative peak of the first temporal derivative (dV/dt) less than half that of the endocardial electrode. The most epicardial electrode on each plunge needle was defined as the epicardial electrode.
Locally synchronized VF identification.
During long-duration VF, an activation pattern was frequently observed in which activation occurred in discrete cycles separated by large temporal gaps and in which the subendocardial WM activated almost simultaneously, and then the activation propagated toward the epicardium, sometimes with block en route, which we called locally synchronized VF (LSVF). LSVF was automatically detected by the following algorithm. WM activation was identified whenever the five-point dV/dt of the recordings reaching a most negative value less than or equal to −0.5 mV/ms. A refractory period of ≥50 ms was assumed between successive activations. See the Supplement Material for the details of WM activation detection. All nonexcited gaps were identified, during which no WM activations were detected at any electrode for >55 ms. Whenever the interval between two nonexcited gaps was <250 ms, the activation sequence during this interval was assessed. This activation sequence was considered a LSVF beat if 1) none of the electrodes recorded more than one WM activation and 2) WM activations were recorded at >5% of the channels. A LSVF beat with the nonexcited gap after it was defined as a LSVF cycle. The time of the earliest LSVF cycle in each animal was identified, and the percentage of the time that LSVF cycles were present during 0–5 and 5–10 min of VF was calculated. The 10 min of VF were divided into 300 2-s segments. If LSVF cycles were present for 1 s or more, the 2-s segment was called a LSVF segment; otherwise, it was called a segment of VF without locally synchronized activation (non-LSVF).
Evaluation of the transmural activation sequence during LSVF.
In each six-electrode needle, there are five pairs of transmurally neighboring electrodes. During each LSVF beat, the endocardium-to-epicardium ratio was defined as the number of pairs of transmurally neighboring electrodes on all needles with activation propagating from the endocardium to the epicardium divided by the number of pairs of transmurally neighboring electrodes with activations recorded on both electrodes. The pairs of neighboring electrodes containing at least one cavity electrode were excluded from the above analysis. To quantify block en route when activation propagated from the endocardium to the epicardium during LSVF, we defined a block at epicardium ratio as follows. During a LSVF beat, the needles on which the endocardial electrode recorded activation before the epicardial electrode (propagation) and the needles on which the endocardial electrode recorded activation while the epicardial electrode did not (block at epicardium) were counted. The block at epicardium ratio was defined as the number of needles with block at epicardium divided by the number of needles with propagation or block at epicardium.
Evaluation of the organized pattern during LSVF.
The 10 min of VF were divided into 600 1-s segments. For each segment, individual wavefronts were isolated by grouping WM activations that were adjacent in space and time (14). The following wavefront descriptors were calculated for each 1-s segment using algorithms similar to those previously described (14, 17, 18): 1) maximum wavefront extent, the maximum number of electrodes recording activation from a single wavefront; 2) activation rate, the mean number of activations per second detected at each electrode; 3) multiplicity, a descriptor that measures the number of distinct wavefront pathways, which describes the spatiotemporal complexity of the activation patterns; and 4) repeatability, a descriptor that expresses, in an average sense, the number of wavefronts that propagate along each of the distinct pathways indicated by the multiplicity descriptor.
Based on the results of LSVF identification, eight 20-s VF recordings from six animals were identified in which the first 10-s of recording was in non-LSVF and the second 10-s recording was in LSVF. In each recording, the maximum wavefront extent, multiplicity, and repeatability were calculated for the 10 1-s non-LSVF segments and 10 1-s LSVF segments.
Evaluation of the LSVF beat-to-beat difference.
To quantify the origination of LSVF beats, the location of the earliest activated endocardial electrode was recorded for each LSVF beat. For each animal, we defined the earliest activation incidence for each endocardial electrode as the number of LSVF beats with the earliest activation recorded by that electrode divided by the total number of LSVF beats during 10 min of VF. Similar analysis was conducted for sinus beats for each animal for comparison.
To evaluate the cycle length difference, during each 2-s LSVF segment, the coefficient of variation (COV) of the cycle lengths was calculated for each endocardial electrode. Mean and SD values of the COV from all the endocardial electrodes were calculated for the segment and averaged for all LSVF segments and all animals.
LSVF onset analysis.
In each animal, an episode was found in which 10 successive VF cycles did not exhibit LSVF but the next 11 cycles did. Only the electrodes recording activations during all 21 cycles were analyzed. The mean minimum dV/dt and mean activation time of the recordings from these electrodes were calculated for each cycle. The mean cycle length was defined as the mean activation time difference for all electrodes between the current cycle and the next cycle.
PF signal analysis.
PF activations were identified and distinguished from WM activations with an algorithm similar to that previously described (20). The two-point dV/dt was calculated as follows: [V(n + 1) − V(n)]/t. PF activation was identified whenever the two-point dV/dt of the recordings was negative for ≤2 ms and the most negative value was less than or equal to −0.3 mV/ms. See the Supplemental Material for the details of the algorithm. Those electrodes with PF activations detected during sinus rhythm (a mean of 4.7 electrodes/animal) were used for PF analysis throughout long-duration VF. The 10 min of VF were divided into 300 2-s segments. For each segment, the numbers of PF and WM activations per second at each electrode in which PFs could be detected during sinus rhythm were counted, and means of the minimum dV/dt of the PF and WM activations were averaged. The PF-WM interval at an electrode was defined as the time of a PF activation subtracted from the time of its nearest WM activation (at most 50 ms apart). The positive PF-to-WM ratio was defined as the ratio of the number of positive PF-WM intervals to the number of all PF-WM intervals for all of the electrodes analyzed for the segment. The above parameters were compared between LSVF and non-LSVF segments. For each animal, a 2-s segment was also chosen during sinus rhythm, and all of the above parameters were calculated.
Statistical analysis.
Group data are expressed as mean ± SD. Paired Student's t-tests were used to compare the maximum wavefront extent, multiplicity, and repeatability averaged for 10 s before and 10 s after the onset of LSVF (n = 8 recordings). A paired Student's t-test was also used to compare the mean minimum dV/dt and mean cycle lengths before and after the onset of LSVF as well as the number of PF activations per second, mean minimum dV/dt of PF activations, PF-WM intervals, and positive PF-to-WM ratios for sinus rhythm versus LSVF and sinus rhythm versus non-LSVF. These variables were first averaged for each animal and were compared with n = 6 animals. ANOVA with repeated measures was used to compare the above four PF-related variables during LSVF and non-LSVF, with animal as subject effect and time as a repeated measure. P values of <0.05 were considered statistically significant.
RESULTS
Typical LSVF pattern.
All VF episodes began with typical irregular, complex activation patterns. As VF continued, however, a highly organized LSVF pattern was observed in all animals, with wavefronts activating the endocardium almost simultaneously and then propagating toward the epicardium, sometimes with block en route.
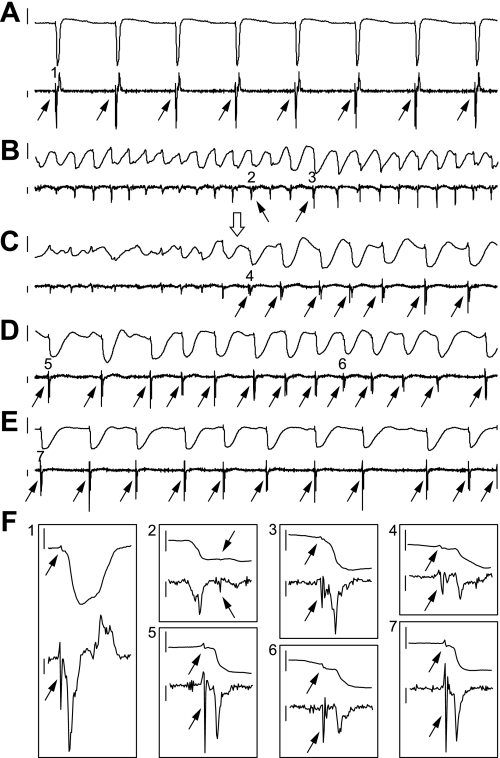
Figure 1 shows typical recordings from an endocardial electrode during sinus rhythm, non-LSVF, and LSVF. Figure 1B shows a typical VF activation pattern during which PF activations were infrequently observed and might follow WM activation. At 4.6 min of VF (Fig. 1C), the activation pattern suddenly changed to LSVF, in which PF activations occurred immediately before WM activations, as during sinus rhythm (Fig. 1A). PF activations appeared as small, sharp complexes with narrow spikes in the dV/dt trace (Fig. 1F).
Fig. 1.
Recordings from an endocardial electrode in animal 2 during long-duration ventricullar fibrillation (VF). A–E: a 3-s electrical recording (top trace) and its dV/dt (bottom trace) during sinus rhythm (A) and at 1.7 min (B), 4.6 min (C), 5 min (D), and 10 min (E) of VF. The onset of locally synchronized VF (LSVF) is shown by the open arrow in C. F: 50-ms enlargements of the electrical recording and its dV/dt from areas 1–7 in A–E. For all images, the height of the vertical bars to the left of the electrical recording is 10 mV. The height of the vertical bars to the left of dV/dt is 0.5 mV/ms, with the top at 0 mV/ms. Purkinje fiber (PF) activations are indicated by the solid arrows.
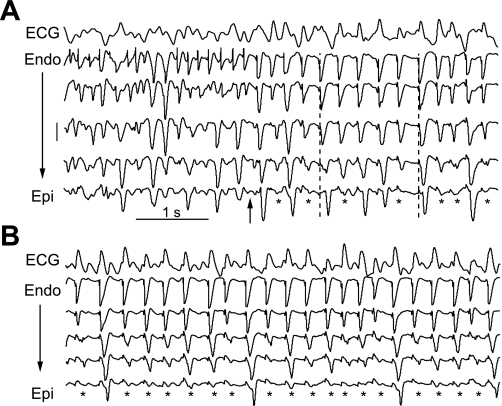
Figure 2 shows representative recordings from a transmural plunge needle during LSVF. After the onset of LSVF, the ECG lead II continued to exhibit a typical VF-like or polymorphic ventricular tachycardia-like pattern (Fig. 2A). Activation propagated from the endocardium toward the epicardium along the needle with about half the wavefronts appearing to block en route. A higher incidence of conduction block was observed at 10 min of VF (Fig. 2B). The peaks of the ECG complexes were approximately aligned with the endocardial activations.
Fig. 2.
Example of LSVF wavefront block near the epicardium in animal 6. In A, the surface ECG lead II (row 1) and recordings [rows 2–6, from the endocardium (Endo) to epicardium (Epi)] from one needle at 5.9 min of VF are shown. The most endocardial electrode recording was in the cavity and is not shown. The arrow indicates the onset of LSVF. The height of the vertical scale to the left is 10 mV and applies to all needle recordings. *Activations propagating from the endocardium that blocked before reaching the epicardium. The two dashed lines are aligned with the endocardial activations in row 2 to indicate the endocardial to epicardial spread of activation. B: the same ECG and electrical recordings at 10 min of VF.
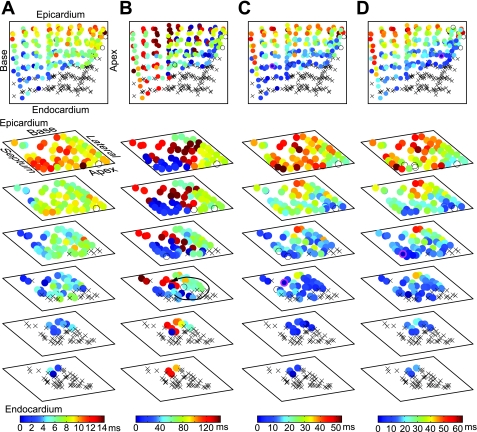
Figure 3 shows typical activation sequences during LSVF and non-LSVF. The propagation sequences of LSVF beats (Fig. 3, C and D) were similar to that during sinus rhythm (Fig. 3A), first activating the endocardium almost simultaneously and then spreading toward the epicardium, but with a slower transmural conduction velocity than during sinus rhythm. LSVF beats had different propagation sequences along the endocardium. As shown in Fig. 3C, the wavefront propagated into the mapped endocardium from the base of the heart. As shown in Fig. 3D, however, the wavefront first entered the mapped region from the apical-septal side. Figure 3B shows activations during non-LSVF containing an intramural reentrant cycle (arrow). The entire mapped volume was excited in 145 ms (Fig. 3B), whereas during LSVF (Fig. 3, C and D), the entire volume was excited in 52 and 62 ms.
Fig. 3.
Isochronal maps in animal 1 during sinus rhythm (A) and at 1 min of VF during non-LSVF (B) and at 4.6 (C) and 4.9 min (D) of VF during LSVF. Row 1 shows the three-dimensional isochronal maps. Rows 2–7 show the same activation sequence as in row 1, with the electrodes displayed in six planes 2-mm apart, composed of the first (epicardium) to sixth (endocardium) electrodes on all needles. xCavity electrodes. Circles represent electrodes located in the myocardium with color indicating the activation time according to the scale at the bottom. Open circles represent electrodes not recording activation. Circles with magenta boundaries in C and D indicate the earliest activation sites.
LSVF incidence.
In two animals, one transition from non-LSVF to LSVF was observed. The other four animals alternated more than once between the two patterns during 10 min of VF. The earliest occurrence of LSVF cycles was 2.5 ± 1.8 min after VF initiation. During 0–5 and 5–10 min of VF, LSVF cycles were present 5.2 ± 4.7% and 33.4 ± 31.4% of the time.
Transmural activation sequence during LSVF.
The endocardium-to-epicardium ratio of all LSVF beats from the six animals was 88 ± 4%. Of all the LSVF beats, 96% had an endocardium-to-epicardium ratio of >0.8. Both findings indicate a dominant propagation pattern from the endocardium to the epicardium.
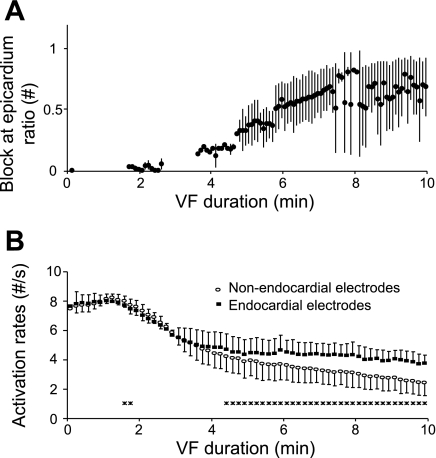
In all animals, the incidence of activations blocking before reaching the epicardium during LSVF beats increased as VF progressed (Fig. 4A). At 5 and 10 min of VF, the block at epicardium ratios were 33% and 68% separately. The increasing incidence of conduction block contributed to the transmural activation rate gradient during long-duration VF. During early VF, activation rates at the endocardial and nonendocardial electrodes were not significantly different (Fig. 4B). After 4.3 min of VF, however, activation rates at endocardial electrodes were significantly higher than at nonendocardial electrodes and remained so until the end of the recording period.
Fig. 4.
Endocardium-epicardium activation rate difference during long-duration VF. A: means and SDs of the block at epicardium ratio of LSVF beats every 5 s during 10 min of VF for all animals. There is no data point when no LSVF was present in any animal. B: means and SDs of activation rates every 10 s for all animals. *Activation rate of the endocardial electrodes differed significantly from that of the nonendocardial electrodes during this 10-s interval.
Organized LSVF activation sequence.
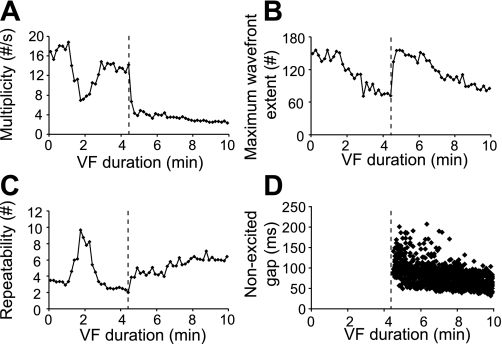
Sudden changes were observed in wavefront descriptors during the transition from non-LSVF to LSVF, indicating significant changes of activation patterns. As shown in Fig. 5, at the onset of LSVF at 4.6 min of VF (dashed lines), multiplicity decreased and repeatability increased, both of which indicated simpler and more repeatable activation sequences during LSVF. Maximum wavefront extent increased to 158 electrodes, indicating that large wavefronts swept across most of the mapped volume. The occurrence of nonexcited gaps (Fig. 5D), an indicator of LSVF, coincided with the sudden changes in the wavefront descriptors. In eight recordings from six animals during the transition to LSVF, multiplicity decreased significantly (7.3 ± 4.3 vs. 4.3 ± 1.9 /s, P = 0.023), repeatability increased significantly (2.4 ± 1.2 vs. 4.0 ± 1.0, P = 0.011), and maximum wavefront extent increased significantly (76.1 ± 41.2 vs. 133.7 ± 44.6, P = 0.00019).
Fig. 5.
Wavefront descriptors in animal 2 indicating sudden changes at 4.6 min of VF. A–C: multiplicity (A), maximum wavefront extent (B), and repeatability (C) averaged every 10 s. D: distributions of nonexcited gaps. Vertical dashed lines in A–D indicate the moment of the transition from non-LSVF to LSVF.
Beat-to-beat differences during LSVF.
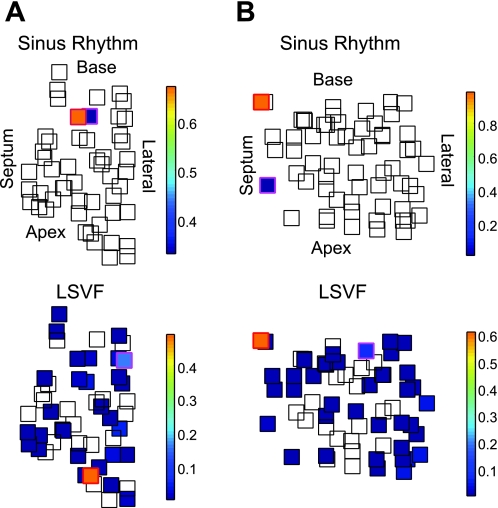
Although activation always propagated from the endocardium to the epicardium during LSVF, the earliest activation sites at the endocardium varied from animal to animal and from beat to beat. The endocardial electrode recording the largest number of earliest activations recorded earliest activations for 25.5–65.5% of all LSVF cycles in the six animals. The endocardial electrode recording the second largest number of earliest activations recorded earliest activations for 7.3–33.6% of all LSVF cycles. Thus, in most animals, LSVF has multiple activation sequences during 10 min of VF. In two animals, the endocardial electrode usually recording earliest activations during sinus rhythm was the same electrode recording the largest (animal 6) or second largest (animal 4) number of earliest activations during LSVF. Figure 6 shows the distributions of the earliest activation sites during sinus rhythm and LSVF in two animals. In animal 1 (Fig. 6A), the electrode recording the largest number of earliest activations during sinus rhythm was neither the electrode recording the largest nor the second largest number of earliest activations during LSVF. In animal 6 (Fig. 6B), during 61% of LSVF beats, the earliest activation site was the same as during sinus rhythm. As shown in Fig. 6, in most animals, the electrodes frequently recording earliest activations during LSVF/sinus rhythm were located on the endocardial boundary of the mapped region, suggesting that the origination sites of the LSVF and sinus cycles were outside the mapped volume.
Fig. 6.
Endocardial spatial distributions of the earliest activation sites of LSVF beats during 10 min of VF and of sinus beats. A and B: distributions in the two-dimensional projection plane composed of the endocardial electrodes from animal 1 (A) and animal 6 (B). Squares represent endocardial electrodes. The color of the square indicates the earliest activation incidence during sinus rhythm and LSVF in that electrode, according to the color bar shown at the right. Empty squares represent electrodes in which no earliest activation was observed. The electrodes recording the largest and second largest number of earliest activations have red and magenta outside boundaries.
Cycle lengths were highly variable during LSVF, with a mean COV of the endocardial cycle lengths of 0.19 ± 0.09. Typically, a 40% difference could be observed during each 2-s LSVF segment between the longest and shortest cycle lengths. However, the SD of the COV of the endocardial cycle lengths during LSVF was only 0.02 ± 0.02, indicating that the cycle length variations were almost the same in each of the endocardial recordings because they were highly synchronized.
Tissue excitability during LSVF onset.
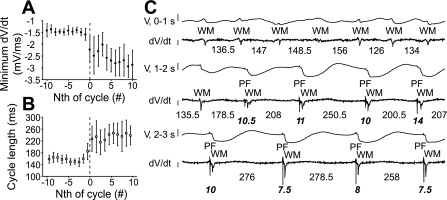
Minimum dV/dt decreased (Fig. 7A) and cycle length increased (Fig. 7B) when LSVF began (cycles −10 to −1 vs. cycles 1 to 10, minimum dV/dt: −1.46 ± 0.08 vs. −2.60 ± 0.52 mV/ms, P = 0.003, cycle length: 159 ± 11.2 vs. 235 ± 32.4 ms, P = 0.0007). The decrease of minimum dV/dt was likely caused by the increased recovery time allowed by prolongation of the cycle length at LSVF initiation, both of which implied an increase of tissue excitability. Figure 7C shows an endocardial electrode recording during LSVF initiation. The second WM activation in the 1- to 2-s segment (Fig. 7C, rows 3 and 4) was the first LSVF activation and was the first activation in which a preceding PF activation could be observed. The cycle length before this activation was 178.5 ms, which was longer than the previous cycle and shorter than the next cycle. The minimum dV/dt of LSVF WM activations stabilized after approximately five to six cycles and became visibly more negative than before LSVF initiation. This transition zone exhibited varying PF-WM intervals and WM dV/dt morphology.
Fig. 7.
Transition from non-LSVF to LSVF. In A and B, means and SDs of minimum dV/dt and cycle lengths during 21 cycles averaged for all animals and all electrodes are shown with the first LSVF cycle labeled cycle 0 (dashed line). C: recordings from an endocardial electrode during LSVF initiation in animal 2. Rows 1, 3, and 5 show continuous 1-s recordings (V). Rows 2, 4, and 6 show the corresponding dV/dt traces. The vertical bar at the left of the V recordings corresponds to 10 mV. The vertical bar at the left of the dV/dt traces corresponds to 0.5 mV/ms with the top at 0 mV/ms. Working myocardium (WM) and PF activations are indicated. Bold italic numbers indicate PF-WM intervals (in ms); regular numbers indicate cycle lengths (in ms) between WM activations. A few WM activations did not reach the −0.5-mV/ms threshold and were manually selected.
Role of PF.
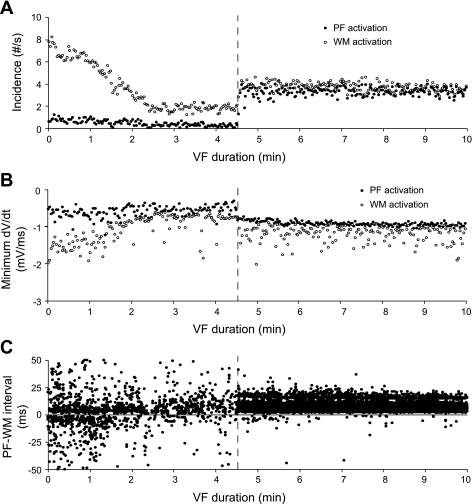
Although the incidence of PF activations detected by our algorithm during non-LSVF was low, during LSVF, PF activations were frequently observed and were almost equal to the incidence of WM activations (Fig. 8A). After the onset of LSVF, a smaller SD of the minimum PF dV/dt was observed (Fig. 8B). The PF-WM intervals during early VF were randomly distributed with both positive and negative values and a large SD (Fig. 8C). After LSVF developed, almost all PF-WM intervals were positive, indicating that PF activations usually preceded WM activations (Fig. 8C).
Fig. 8.
PF and WM activation properties in animal 2. A and B: incidence (A) and minimum dV/dt (B) of PF and WM activations. C:PF-WM intervals. Dashed lines indicate the transition to LSVF at 4.6 min of VF. The horizontal line in C corresponds to a PF-WM interval of 0 ms.
The number per second of PF activations, minimum dV/dt, PF-WM intervals, and positive PF-to-WM ratio were significantly different for LSVF versus non-LSVF (Table 1) and changed significantly with VF duration (P < 0.0001 by repeated-measures ANOVA). The PF activation dynamics during sinus rhythm (Table 1) were more similar to LSVF than to non-LSVF.
Table 1.
PF activation properties during sinus rhythm, LSVF, and non-LSVF
| PF incidence, number•s−1•electrode−1 | Minimum dV/dt of PF, mV/ms | PF-WM Interval, ms | Positive PF-to-WM Ratio | |
|---|---|---|---|---|
| Sinus rhythm | 2.43 ± 0.37 | −1.30 ± 0.29 | 5.28 ± 1.11 | 1.00 ± 0.00 |
| LSVF | 3.06 ± 0.73 | −0.82 ± 0.22 | 7.02 ± 3.52 | 0.97 ± 0.14 |
| Non-LSVF | 0.77 ± 0.60 | −0.79 ± 0.50 | 1.66 ± 9.27 | 0.61 ± 0.38 |
| P values | ||||
| LSVF vs. nonLSVF | <0.0001 | 0.03 | <0.0001 | <0.0001 |
| Sinus rhythm vs. LSVF | 0.30 | 0.11 | 0.33 | 0.15 |
| Sinus rhythm vs. non-LSVF | 0.001 | 0.02 | 0.007 | 0.0001 |
Means ± SD during sinus rhythm were calculated for six animals. Means ± SD during locally synchronized ventricullar fibrillation (LSVF) and non-LSVF were calculated for all 2-s LSVF episodes and all 2-s non-LSVF episodes from six animals. PF, Purkinje fibers; WM, working myocardium.
DISCUSSION
Mechanisms of long-duration VF maintenance.
Our study revealed that periods of organization in which large wavefronts spread across almost the entire mapped region occur during long-duration VF in canines. The transition from the disorganized non-LSVF pattern to the organized LSVF pattern was rapid and distinct. Sudden changes in the quantitative wavefront descriptors were observed at the transition points (Fig. 5). The transitions could also be identified from changes in PF and WM activation descriptors (Figs. 7 and 8). The activation patterns during LSVF and non-LSVF are markedly different. During LSVF, the activation wavefronts are large and organized. They arise at the endocardium and propagate toward the epicardium with large temporal gaps between successive wavefronts. Furthermore, during LSVF, PF signals robust enough to be detected by our algorithm were observed at the endocardial electrodes. These PF activations preceded and maintained a relatively consistent relationship with the WM activations, as indicated by the PF-WM interval and the positive PF-to-WM ratio (Fig. 8 and Table 1). In comparison, during non-LSVF, the wavefronts were small; the wavefront paths were complex, indicated by significantly greater multiplicity and smaller repeatability, and PF signals were not coupled with WM signals (Fig. 8 and Table 1). These findings strongly suggest that the mechanism of VF maintenance during LSVF is different from that during non-LSVF.
One candidate for the mechanism causing LSVF is a mother rotor (24). If the region exhibiting LSVF were a small part of a large mother rotor, the large temporal gaps in activation could occur during the time period when the rotor was sweeping out the remainder of its path. However, neither the activation sequence nor the cycle length was constant during LSVF. Both of these findings are inconsistent with the mapped region being part of a stable mother rotor (24). It is possible that a mother rotor was present elsewhere in the heart that gave rise to daughter wavelets that followed variable pathways into the mapped region.
The following findings suggest that LSVF is maintained by activation spreading from the PF network to the WM: 1) the endocardium, the location of the PF-WM junctions, had the highest activation rate in our canine study, as opposed to previously reported findings that the activation rate during VF is high transmurally in porcine hearts (1, 15), in which the PF-WM junctions are distributed intramurally almost to the epicardium (10, 11); 2) PF activation was coupled to and preceded WM activation during LSVF; 3) the activation sequences from the endocardium to the epicardium and the PF activation properties in LSVF were similar to sinus rhythm; and 4) the canine PF network is more resistant to the global ischemia caused by VF than is WM (2, 8, 9, 13), so that during long-duration VF, it was possible for activation to initiate in the PF network and propagate through the PF-WM junctions and then spread in the WM. During LSVF, the WM activations have multiple initiation sites on the endocardium (Figs. 3 and 6). Some of these sites were similar to those during sinus rhythm; however, the majority were different, suggesting that activation was not proceeding downward from the His bundle or fascicles. Although the PF activation may have arisen from retrograde propagation through the PF-WM junctions, as has been reported previously (20), it may have arisen focally within PFs, as has also been reported previously (20). It is not known if this focal activation is caused by early afterdepolarization, delayed afterdepolarization, or abnormal automaticity. Whatever the mechanism, the fact that the cycle length is increased during LSVF suggests that this mechanism was overdrive suppressed by the shorter cycle lengths when LSVF was not present.
Relation of LSVF to previous findings.
Although previous studies have not identified the existence of LSVF, they have made observations consistent with LSVF. An endocardial-epicardial activation rate gradient has been observed in canines after 2–5 min of VF, with the highest activation rate region at the endocardium (1, 4, 7, 15, 23). Discrete, regular, rapid deflections have been reported in the most subendocardial electrograms after this transmural activation rate gradient developed (23). Activations have been reported to propagate more frequently from the endocardium toward the epicardium than in the opposite direction (1, 7). PF-WM interactions, including the passage of activation from PFs into WM, have been observed at the endocardium in canine hearts during long-duration VF (20).
Janse et al. (12) reported that endocardial activity preceded epicardial activity during spontaneous premature beats induced after 2–10 min of coronary artery occlusion, Purkinje spikes preceding the myocardial deflections were observed in these beats, and sometimes these beats initiated VF. It is possible that these premature beats were generated through a similar mechanism as for LSVF due to the similar propagation patterns. Interestingly, the application of phenol to destroy the endocardium in that study did not abolish premature activity caused by acute ischemia. However, the nature of the premature activity after the application of phenol was quite different from that before phenol, and VF was never induced. Our findings after the acute ischemia caused by the lack of perfusion during the first 10 min of VF may be related to these results about the importance of PFs during acute ischemia caused by coronary occlusion (12).
ECG signal during LSVF.
It is interesting that the ECG continued to exhibit a disorganized VF-like or a polymorphic ventricular tachycardia-like pattern while LSVF was present (Fig. 2). The ECG pattern during LSVF may have occurred because there were beat-to-beat changes in the activation sequence and cycle length during LSVF. During each LSVF cycle, wavefronts typically propagated into the mapped endocardium from a different direction, as shown in Figs. 3 and 6. Also, many wavefronts blocked as they propagated intramurally toward the epicardium, and the site of block was different for different wavefronts. In addition, other portions of the heart might not have been in a synchronized pattern when LSVF was observed in the mapped region. However, we observed that the peaks of the ECG complexes were approximately aligned with the endocardial activations in the mapped region, especially later during long-duration VF. Therefore, some LSVF beats might have been present throughout much of the ventricles.
Clinical implications.
Since LSVF beats may initiate from a focal source, the administration of drugs that prevent focal activation might stop LSVF beats. However, the choice of the specific antiarrhythmic drug(s) depends on further understanding of the mechanism of focal activity, such as abnormal automaticity, early afterdepolarization, or delayed afterdepolarization. It is also possible that the mechanism of defibrillation differs during LSVF and non-LSVF. Although a shock may need to halt reentry within the WM when non-LSVF is present, it may need to halt focal activity within PFs when LSVF is present. It is possible that the waveform parameters, such as waveshape and duration, that best defibrillate may be different during LSVF and non-LSVF. It is even possible that a series of pacing stimuli could defibrillate during LSVF. These ideas are speculative and require experimental testing.
Limitations.
The limitations of this study are as follows. First, due to the 2-mm resolution, the entire heart could not be mapped. It is not known if LSVF extends to unmapped regions. Second, with 2-mm spacing along the needles, the endocardial electrodes were not precisely at the endocardium. Therefore, the quality of PF signals was not as good as in other studies where a two-dimensional plaque was sutured directly to the endocardium (7, 20). The PF signal quality and limited endocardial area mapped made it difficult to analyze the PF activation pattern in detail (20). Third, during non-LSVF, few PF activations were detected. Criteria for PF activation in this study may have missed PF activation during this stage. Finally, during long-duration VF, the exposed epicardium, without the perfusion of warm blood, may experience mild hypothermia, which may lead to decrease of the activation rates near the epicardium (6). Although the decrease of the activation rates in our study may not be as significant as that induced by the acute reduction of temperature in the isolated hearts (6), the possibility that mild hypothermia contributes to the activation rate gradient in our study cannot be excluded.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-28429, HL-66256, HL-85370, and 5K99-HL-091138.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
Present address of Q. Jin: Shanghai Ruijin Hospital, Jiaotong University, School of Medicine, Shanghai, China.
REFERENCES
- 1.Allison JS, Qin H, Dosdall DJ, Huang J, Newton JC, Allred JD, Smith WM, Ideker RE. The transmural activation sequence in porcine and canine left ventricle is markedly different during long-duration ventricular fibrillation. J Cardiovasc Electrophysiol 18: 1306–1312, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Bagdonas AA, Stuckey JH, Piera J, Amer NS, Hoffman BF. Effects of ischemia and hypoxia on the specialized conducting system of the canine heart. Am Heart J 61: 206–218, 1961 [DOI] [PubMed] [Google Scholar]
- 3.Barnette AR, Bayly PV, Zhang S, Walcott GP, Ideker RE, Smith WM. Estimation of 3-D conduction velocity vector fields from cardiac mapping data. IEEE Trans Biomed Eng 47: 1027–1035, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Cha YM, Uchida T, Wolf PL, Peters BB, Fishbein MC, Karagueuzian HS, Chen PS. Effects of chemical subendocardial ablation on activation rate gradient during ventricular fibrillation. Am J Physiol Heart Circ Physiol 269: H1998–H2009, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Chattipakorn N, Fotuhi PC, Chattipakorn SC, Ideker RE. Three-dimensional mapping of earliest activation after near-threshold ventricular defibrillation shocks. J Cardiovasc Electrophysiol 14: 65–69, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chorro FJ, Guerrero J, Ferrero A, Tormos A, Mainar L, Millet J, Canoves J, Porres JC, Sanchis J, Lôpez-Merino V, Such L. Effects of acute reduction of temperature on ventricular fibrillation activation patterns. Am J Physiol Heart Circ Physiol 283: H2331–H2340, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Dosdall DJ, Tabereaux PB, Kim JJ, Walcott GP, Rogers JM, Killingsworth CR, Huang J, Robertson PG, Smith WM, Ideker RE. Chemical ablation of the Purkinje system causes early termination and activation rate slowing of long-duration ventricular fibrillation in dogs. Am J Physiol Heart Circ Physiol 295: H883–H889, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman PL, Stewart JR, Fenoglio JJ, Jr, Wit AL. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ Res 33: 597–611, 1973 [DOI] [PubMed] [Google Scholar]
- 9.Gilmour RF, Jr, Zipes DP. Different electrophysiological responses of canine endocardium and epicardium to combined hyperkalemia, hypoxia, and acidosis. Circ Res 46: 814–825, 1980 [DOI] [PubMed] [Google Scholar]
- 10.Hamlin RL, Burton RR, Leverett SD, Burns JW. Ventricular activation process in minipigs. J Electrocardiol 8: 113–116, 1975 [DOI] [PubMed] [Google Scholar]
- 11.Holland RP, Brooks H. The QRS complex during myocardial ischemia. An experimental analysis in the porcine heart. J Clin Invest 57: 541–550, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janse MJ, Kleber AG, Capucci A, Coronel R, Wilms-Schopman F. Electrophysiological basis for arrhythmias caused by acute ischemia. Role of the subendocardium. J Mol Cell Cardiol 18: 339–355, 1986 [DOI] [PubMed] [Google Scholar]
- 13.Lazzara R, el-Sherif N, Scherlag BJ. Electrophysiological properties of canine Purkinje cells in one-day-old myocardial infarction. Circ Res 33: 722–734, 1973 [DOI] [PubMed] [Google Scholar]
- 14.Li L, Jin Q, Huang J, Cheng KA, Ideker RE. Intramural foci during long duration fibrillation in the pig ventricle. Circ Res 102: 1256–1264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newton JC, Johnson PL, Justice RK, Smith WM, Ideker RE. Estimated global epicardial distribution of activation rate and conduction block during porcine ventricular fibrillation. J Cardiovasc Electrophysiol 13: 1035–1041, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Rogers JM, Melnick SB, Huang J. Fiberglass needle electrodes for transmural cardiac mapping. IEEE Trans Biomed Eng 49: 1639–1641, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. A quantitative framework for analyzing epicardial activation patterns during ventricular fibrillation. Ann Biomed Eng 25: 749–760, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Rogers JM, Usui M, KenKnight BH, Ideker RE, Smith WM. Recurrent wavefront morphologies: a method for quantifying the complexity of epicardial activation patterns. Ann Biomed Eng 25: 761–768, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Schnabel PA, Richter J, Schmiedl A, Bach F, Bartels U, Ramsauer B, Gebhard MM, Bretschneider HJ. Patterns of structural deterioration due to ischemia in Purkinje fibres and different layers of the working myocardium. Thorac Cardiovasc Surg 39: 174–182, 1991 [DOI] [PubMed] [Google Scholar]
- 20.Tabereaux PB, Walcott GP, Rogers JM, Kim J, Dosdall DJ, Robertson PG, Killingsworth CR, Smith WM, Ideker RE. Activation patterns of Purkinje fibers during long-duration ventricular fibrillation in an isolated canine heart model. Circulation 116: 1113–1119, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela TD, Roe DJ, Cretin S, Spaite DW, Larsen MP. Estimating effectiveness of cardiac arrest interventions: a logistic regression survival model. Circulation 96: 3308–3313, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med 343: 1206–1209, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Worley SJ, Swain JL, Colavita PG, Smith WM, Ideker RE. Development of an endocardial-epicardial gradient of activation rate during electrically induced, sustained ventricular fibrillation in dogs. Am J Cardiol 55: 813–820, 1985 [DOI] [PubMed] [Google Scholar]
- 24.Zaitsev AV, Berenfeld O, Mironov SF, Jalife J, Pertsov AM. Distribution of excitation frequencies on the epicardial and endocardial surfaces of fibrillating ventricular wall of the sheep heart. Circ Res 86: 408–417, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Zipes DP, Jalife J. Cardiac Electrophysiology: From Cell to Bedside (4th ed.). Philadelphia, PA: Saunders, 2004 [Google Scholar]