Abstract
Integrins link the extracellular matrix (ECM) with the intracellular cytoskeleton and other cell adhesion-associated signaling proteins to function as mechanotransducers. However, direct quantitative measurements of the cardiomyocyte mechanical state and its relationship to the interactions between specific ECM proteins and integrins are lacking. The purpose of this study was to characterize the interactions between the ECM protein fibronectin (FN) and integrins in cardiomyocytes and to test the hypothesis that these interactions would vary during contraction and relaxation states in cardiomyocytes. Using atomic force microscopy, we quantified the unbinding force (adhesion force) and adhesion probability between integrins and FN and correlated these measurements with the contractile state as indexed by cell stiffness on freshly isolated mouse cardiomyocytes. Experiments were performed in normal physiological (control), high-K+ (tonically contracted), or low-Ca2+ (fully relaxed) solutions. Under control conditions, the initial peak of adhesion force between FN and myocyte α3β1- and/or α5β1-integrins was 39.6 ± 1.3 pN. The binding specificity between FN and α3β1- and α5β1-integrins was verified by using monoclonal antibodies against α3-, α5-, α3 + α5-, or β1-integrin subunits, which inhibited binding by 48%, 65%, 70%, or 75%, respectively. Cytochalasin D or 2,3-butanedione monoxime (BDM), to disrupt the actin cytoskeleton or block myofilament function, respectively, significantly decreased the cell stiffness; however, the adhesion force and binding probability were not altered. Tonic contraction with high-K+ solution increased total cell adhesion (1.2-fold) and cell stiffness (27.5-fold) compared with fully relaxed cells with low-Ca2+ solution. However, it could be partially prevented by high-K+ bath solution containing BDM, which suppresses contraction by inhibiting the actin-myosin interactions. Thus, our results demonstrate that integrin binding to FN is modulated by the contractile state of cardiac myocytes.
Keywords: integrins, extracellular matrix protein, mechanobiology, atomic force microscopy, cell mechanics
in cardiac muscle, a salient example of the link between mechanical strain and physiological function is the regulation of cardiac output in response to increasing ventricular pressure. This interaction defines the cardiac pressure-volume relationship (i.e., the Frank-Starling law of the heart). The mechanism for this relationship is believed to involve stretching of the myocytes within the cardiac wall, which impacts the myofilament overlap in such a way that filament interaction and, hence, contractile performance are affected (44). In addition to the acute effects of mechanical load, prolonged mechanical loading of the myocardium leads to hypertrophy as well as changes in cardiac wall and myocyte stiffness (54, 56, 69, 71). These changes are accompanied by significant alterations in integrin and extracellular matrix (ECM) protein expression (4, 8, 38, 58, 75, 78, 80, 81). Fibronectin (FN) is one of the major ECM proteins that promotes the adhesion of cardiomyocytes. FN is normally expressed in the heart and undergoes increased expression in the hypertrophic and injured myocardium (1, 7, 30, 38, 74). Moreover, an accumulation of immunoreactive FN is seen in the ischemic injured myocardium during the early stages of acute myocardial infarction and may have functions related to the repair process and fibrotic remodeling of the ventricular wall (6, 32). An imbalance in the production and degradation of ECM proteins may lead to structural alterations such as basement membrane thickening and ECM protein deposition in tissues during the development of cardiovascular diseases (2, 5, 8, 17, 20, 31, 38, 40, 52, 58, 63, 64, 75, 78, 80, 81). These studies have implied that FN is potentially of major importance to the functioning of the myocardium. However, the roles of FN in the myocardium with respect to its interaction with integrins and other downstream signaling events have not been addressed. In this study, our aim was to characterize the relationship between integrin adhesion and FN in normal myocytes as an essential starting point in understanding its functional significance.
α3β1-Integrin and α5β1-integrin are two important adhesion receptors for FN, and both bind to the arginine-glycine-aspartic acid (RGD) motif in FN domain III (11, 16, 18, 37, 61). Integrins, by virtue of their cytoskeletal and signaling protein linkages, are thus believed to form an important mechano-sensing and -transducing axis capable of inside-out and outside-in signaling (12, 28, 55, 79). In cardiomyocytes, integrins have been found localized in costameres, the sites where Z bands connect to the basement membrane. The costamere is structurally integrated with cytoskeletal components and signaling complexes, further supporting the proposition that integrins are involved in mechanical signaling (14, 19, 30, 57). It has been reported that an application of mechanical stress to integrin adhesion sites causes increased cytoskeletal stiffening, second messenger signals, and phosphorylation of proteins anchored to the cytoskeleton (41, 60, 79). Thus, there is strong evidence suggesting that integrins act as conduits for the transmission of mechanical force across the cell membrane and the initiation of intracellular signaling (12, 28, 55).
Cardiomyocytes have been shown to express at least four dominant integrin subtypes, which include α1β1, α3β1, α5β1, and α7β1 (50). Both α3β1- and α5β1-integrins recognize ECM proteins containing the RGD sequence, such as found in FN (57, 59). In addition to β1-integrin expression, expression of β3- and β5-integrins has been reported in cardiomyocytes (33, 46, 66). In this study, our focus was to determine whether the adhesion properties of two major β1-integrin subtypes, α3β1 and α5β1, which interact with FN, change during the contracted and relaxed states in cardiomyocytes. We hypothesized that the regulation of the interaction between integrins and ECM could form a basis for the physiological modulation of myocyte function as well as for the pathological changes in myocyte function that are correlated with the altered expression of ECM proteins and integrins. If integrin interactions with ECM are capable of rapid modifications, then it is also possible that they may ultimately be shown operative on a beat-to-beat basis.
Currently, little is known about the mechanical characteristics of integrin-ECM interactions in myocytes, and there is nothing known about how these interactions are affected by the contractile state. Technical limitations have somewhat slowed progress toward understanding adhesive interactions at the cellular and molecular levels, particularly in mechanically active cells such as cardiac myocytes. In this study, we used atomic force microscopy (AFM) to measure forces at the pico- and nano-Newton scales to evaluate the adhesion between FN and integrins and to measure cell stiffness as an index of activation during the contracted and relaxed states of cardiomyocytes.
MATERIALS AND METHODS
Adult cardiomyocyte preparation.
Adult male mouse cardiomyocytes (FVB/N strain, 2–4 mo) were prepared as previously described (82). Briefly, the heart was harvested under anesthetic conditions and put into ice-cold Ca2+-free physiological saline solution (PSS) containing (in mM) 133.5 NaCl, 4 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 10 HEPES, and 11 glucose (pH 7.4). 2,3-Butanedione monoxime (BDM; 10 mM) was present during the dissecting procedure. The aorta was cannulated, and the heart was mounted in a Langendorff perfusion system with Ca2+-free control solution containing 1 mg/ml BSA (Amersham Life Science, Arlington Heights, IL) at 37°C. After 5 min, perfusion was continued with the same solution containing 25 μM Ca2+ together with collagenase type I (62.4 U/ml, Worthington) and type II (73.7 U/ml, Worthington). After ∼15–20 min, the heart was removed and transferred to a petri dish containing PSS with 100 μM Ca2+. The ventricles were cut into small pieces, which were then gently triturated using a fire-polished Pasteur pipette to release single cells. The cell suspension was filtered through a 250-μm mesh collector, and cells were allowed to settle. Collected cells were then resuspended in PSS containing 200 μM Ca2+. After this procedure, cells were stored at room temperature and used within 6 h. A suspension of freshly dispersed cells was plated onto a laminin type I-coated (10 μg/ml) dish for at least 30 min in PSS with 1.8 mM Ca2+ before experiments. All experiments were carried out at 22–23°C. All procedures using mouse hearts were approved by the Texas A&M University Animal Care Committee.
AFM force mode operation for force and stiffness measurements.
The force contact mode of operation was used for measurements of unbinding force (adhesion force) and was detected and quantified in the AFM probe retraction curve. Relative cortical membrane stiffness (elastic modulus) was measured from the approach curve as previously described (68, 73). AFM experiments were performed using a Bioscope system (model 3A, Digital Instruments, Santa Barbara, CA), which was mounted on an Axiovert 100 TV inverted microscope (Carl Zeiss). The Bioscope system is equipped with Nanoscope IIIa controller and Nanoscope III software (version 5.12). The AFM probes used were silicon nitride microlevers with conical tips (model MLCT-AUHW). Tip diameters were <40 nm, and the mean spring constant was ∼14.4 ± 0.6 pN/nm. For each experiment, the position of the protein (e.g., FN)-labeled probe was controlled to repeatedly touch and retract (z-axis) from the cell surface. Force curves were recorded for these repeated cycles of probe approach and retraction at 0.5-Hz scan frequency and a z-axis movement of 800 nm. The AFM probe tip was selectively positioned between the nucleus and the edge of the cell.
To measure cell cortical stiffness (i.e., elastic modulus or cell resistance to shape deformation; see Fig. 2), approach force curves were used for analysis (see dark line in Fig. 2). Approach force curves were fitted with the Hertz model between points 2 and 3 (see Fig. 2) using MATLAB software (Mathwork) and NForceR software to calculate the cortical stiffness based on tip displacement and membrane indentation (73). The retraction curve (see dotted line in Fig. 2) was used to analyze the specific adhesion forces related to bonding between the AFM tip and cell surface. During retraction, if a specific adhesion event occurred, it was detected as small sharp shift (bond rupture) in the deflection curve obtained during probe retraction from the cell surface. No adhesions were apparent as a smooth retraction curve similar in appearance to the approach curve. These deflection shifts, referred to as “snap-offs,” were recorded in the force curve and represent the force required to cause bond failure between FN on the AFM probe and the cell surface (68, 73). With the application of the spring constant of the AFM probe cantilevers, all deflection shifts (snap-offs) in a retraction curve were detected, quantified, and used to compute the rupture force with NForceR software. Deflection shifts represent the rupture force between the probe and cell surface. With each group of experiments, 500 force curves were sampled from 10 randomly selected cells (obtained from 3 to 5 hearts, 50 curves/cell) for each treatment. Collectively, the adhesion force measurements obtained from all retraction curves were analyzed as force-density (normalized events that with adhesion) relationships. Smooth plots of relative density versus force or stiffness were obtained with standard kernel density estimation methods (48). The algorithm centers a smoothing window on the distribution of force values, and the predicted density [f(x)] is calculated as a weighted average of the density values for nearby points. The weight given to each point in the smoothing window is controlled by a Gaussian kernel (K) based on the distance between each force (or stiffness) value (xi) and the center of the interval (x) in which xi falls (29) as follows:
| 1 |
where d is dimension. The bandwidth (h) determines the degree of smoothing of the density curve. For these analyses, h was based on the normal reference distribution (77).
Fig. 2.
Raw force curves generated using fibronectin (FN)-coated atomic force microscopy (AFM) probes on cardiomyocytes. FN-coated probes (1 mg/ml) were controlled to repeatedly (800 nm/s z-axis movement at 0.5-Hz frequency) approach (solid trace) and retract (dotted line trace) from freshly isolated cardiomyocytes. Points 1–6 represent the stages of approach and retraction (explained in detail in the results). The insets show the AFM probe approaching the myocyte (top left inset, point 1 in the curve). Note that the image of the AFM cantilever is blurred as it is above the plane of focus. The bottom right inset shows the AFM probe in contact with the myocyte (point 2 in the curve). Note that the AFM cantilever is in sharp focus. CVM, cardiomyocyte.
From the density distributions, we recorded peak force/stiffness as well as integrated estimates of force and stiffness. The peak value (mode) represents the most frequent or maximum likelihood value obtained over 500 approach and retraction curves in each experiment. However, the peak values do not convey information about the spread of the force or stiffness measures. We therefore measured integrated values of force and stiffness as well. The integrated values correspond to the areas under the force/stiffness density distribution curves; departures from symmetry in the density distribution (e.g., rightward skew) will thus cause the integrated measures to be greater than the peak values. In probability theory, the integral of the density corresponds to the mean. Although force and stiffness are continuous variables, their density curves can only be estimated at discrete points. We measured the center and spread of the force/stiffness distributions using standard algorithms for the mean and variance of random variables, allowing summations to approximate integration over the discrete points. X represents the set of plotted values for either force or stiffness having values x1, x2, x3, . . . xk. For each of these values, there is a corresponding set of probabilities (p1, p2, p3, . . . pk) that can be obtained from the density curve (i.e., plotted relative densities divided by n, the total area under the curve). The mean (i.e., integrated value) of X (μX) is as follows:
| 2 |
SEs of the peak or integrated values of force and stiffness were computed from each density curve by resampling (i.e., sampling with replacement). A bootstrap distribution of the peak or integrated value of a density curve was obtained by constructing density curves from 5,000 random resamples of the data and estimating the peak and integral for each curve. The bootstrap SE for each measure is the SD of the bootstrap distributions (13, 25).
Labeling of AFM probes.
AFM probes were labeled with the ECM protein (i.e., FN) or control proteins using methods we have previously described, which were adopted from Lehenkari and Horton (34, 68). Polyethylene glycol (PEG; Sigma) was used to cross-link proteins onto silicon nitride probes at room temperature. The probe was first incubated with 10 mg/ml PEG for 5 min, washed with PBS four times, and then incubated with FN (1 mg/ml, Invitrogen, Grand Island, NY) for 1 min. The tip was again washed with PBS for four times. The spring constants were assumed to be unchanged after the protein labeling because only the very end of the cantilever was coated. In nonintegrin antibody, control adhesion experiments, anti-rat major histocompatibility complex class I (MHC) monoclonal antibody (U.S. Biological, Swampscott, MA, 1 mg/ml)-coated AFM probes were used. In nonspecific protein experiments, BSA-coated AFM probes were used.
Ligand application.
To determine the specificity of FN-integrin bond, experiments were performed on cells pretreated with either mouse anti-α3-integrin monoclonal antibody (60 nM) or anti-α5-integrin monoclonal antibody (HMα5–1, 60 nM, BD Bioscience, San Jose, CA). α3- and α5-integrin subunits are known to associate only with β1-integrin (but not the reverse) (mouse anti-β1-integrin monoclonal antibody, 50 μM, BD Bioscience), making these antibodies specific for the α3β1- and α5β1-heterodimers. Anti-mouse α11-integrin monoclonal antibody (60 nM, R&D Systems) was used as a control for non-FN-recognized integrin.
Fluorescence confocal microscopy.
Overnight cultured cardiac myocytes were fixed with 2% paraformaldehyde for 1 h followed by several glycine-PBS washes. Cells were permeabilized with ice-cold methanol for 3 min at 4°C followed again by several rinses with PBS. Cells were then incubated with blocking solution containing 1% BSA (Vector Laboratories), 2.5% normal goat serum, and 0.1% Triton X-100 for 1 h. After the blockade, cells were incubated together with primary mouse anti-α3-integrin and rat anti-mouse α5-integrin (5H10–27, 1:100, BD Bioscience) or primary mouse anti-vinculin (1:100, Chemicon) and rat anti-mouse α5-integrin (1:100, BD Bioscience) for 1 h. Samples were rinsed and then incubated with secondary antibodies of goat anti-mouse Oregon green 488 IgG and goat anti-rat red Cy5 IgG (1:200, Molecular Probes, Invitrogen) for 1 h in the dark, washed extensively, and treated with ProLong AntiFade (Molecular Probes, Invitrogen). Serial image sections through focus with a step size of 0.1–0.3 μm thickness were collected using a Leica AOBS SP2 confocal microscope (Leica Microsystems, Wetzlar, Germany).
Solutions.
For normal physiological conditions, PSS with 1.8 mM Ca2+ was used. For tonic contractions, cells were incubated with high-K+ Ringer solution (high-K+ solution) containing (in mM) 150 KCl, 2 CaCl2, 1.17 MgSO4, 5 glucose, 1.22 NaH2PO4, 0.02 EDTA, 2 sodium pyruvate, and 3 MOPS (pH 7.4). High-K+ solution was used to depolarize the cell membrane and activate Ca2+ entry. For relaxing conditions, cells were incubated with low-Ca2+ solution containing (in mM) 53.3 KCl, 6.8 MgCl2, 0.025 CaCl2, 10 EGTA, 20 MOPS, 5.35 ATP, 12 creatine phosphate, and 10 BDM [Sigma; pH 7.2, Ca2+ concentration ([Ca2+]) of 50 nM]. Low-Ca2+ solution served to keep the sarcomeres in a relaxed state through the combination of BDM, low [Ca2+], and high [ATP] (72). All chemicals, except as specifically stated, were obtained from Sigma-Aldrich.
General data analysis.
For adhesion force and stiffness measurements, the NForceR program, Matlab software (MathWorks, Natick, MA), Origin (OriginLab), StatView, and SAS were used. Adhesion force between FN and integrins on myocytes were plotted as a function of the frequency (events) of occurrence. Single-rupture forces (F) were determined using Hooke's law as follows: F = kd, where d is the height of the step change in the retraction curve representing bond rupture (see point 5 in Fig. 2) and k is the spring constant. Differences between means for the effect of a given treatment were determined using ANOVA or with an independent two-tail t-test as appropriate. Averaged values are expressed as mean ± SE. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Distribution of integrins on cardiomyocytes.
To confirm the presence of α3β1- and α5β1-integrins in cardiomyocytes, the distribution of these integrins was determined by fluorescent immunocytochemistry using monoclonal antibodies against α3- and α5-integrin subunits. A single cardiac myocyte dual immunolabeled with α3-integrin (Fig. 1A, green) and α5-integrin (Fig. 1A, red) monoclonal antibodies showed that these two integrins are heavily concentrated in the costameric structures of myocytes. The overlay of the α3- and α5-integrin images with “zoom-in” revealed that the two integrins are distributed in costameric structures of the myocyte (Fig. 1A, α3 + α5), which is consistent with previous studies (21, 57) describing integrin distribution in cardiomyocytes. To further determine the proximity of the integrins to the cytoskeleton structure in cardiomyocytes, we examined the distribution of α5β1-integrin and vinculin as a cytoskeletal protein. Cardiac myocytes dual immunolabeled with vinculin (Fig. 1B, green) and α5β1-integrin (Fig. 1B, red) monoclonal antibodies showed that vinculin and α5β1-integrin are concentrated in costameres and the intercalated disk of myocytes. The overlay of α5-integrin and vinculin with zoom-in magnifications revealed that these two structures are closely localized in the vicinity of the costamere structure (Fig. 1B).
Fig. 1.
Immunofluorescence localization of integrins and the cytoskeleton protein vinculin in adult mouse cardiomyocytes under high confocal magnification (×63). A: immunofluorescence labeling was performed on isolated cardiomyocytes using anti-α3-integrin with an Oregon green-labeled secondary monoclonal antibody and anti-α5-integrin with a Cy5-labeled secondary monoclonal antibody. The enlarged inset (right image) shows the overlay of anti-α3 and anti-α5-integrin micrographs, where the closest or colocalized areas are indicated as yellow-orange. Regions of costameric (arrows), striated (arrowheads), and nuclear (red asterisks) structures are shown. B: immunofluorescence staining of a single cardiomyocyte using anti-vinculin with an Oregon green-labeled secondary monoclonal antibody and anti-α5-integrin with a Cy5-labeled secondary monoclonal antibody. The enlarged inset (right image) shows the overlay of anti-vinculin and anti-α5-integrin micrographs, where the closest or colocalized areas are shown as yellow-orange. Integrin and vinculin exhibited costameric and intercalated disk (blue arrow) localization. Bar = 10 μm.
Analyses of the adhesion force between the FN-coated probe and a single cardiomyocyte.
To measure the cell membrane stiffness and adhesion force (unbinding force) between FN and the cardiomyocyte surface, AFM probes coated with FN were applied to the membrane surface of noncontracting cardiomyocytes. A typical force curve is shown in Fig. 2. When the FN-coated probe moved to approach the cell surface (from point 1 to point 2), force remained at zero level. The cantilever bent, encountered a resistance, and changed the deflection signal after contact with the surface (dark “approach” line, from point 2 to 3). Point 2 represents the “reflection” point or “contact” point. Data in the region of points 2–3 were used to fit the Hertz model to calculate cell cortical stiffness. The stiffer the cell, the less the indentation and the steeper the upslope of the force curve. As probe retraction started (dotted “retraction” line), resistance force decreased (from point 3 to 4). The snap-off that represents bond rupture (i.e., adhesion force) between FN and the cardiomyocyte is shown in the retraction line (dotted line point 5). As shown in Fig. 2, the example trace shows three adhesion events (bond rupture) that occurred when the FN-coated probe retracted. When all adhesions between the FN-coated probe and cardiomyocyte have been broken, the retraction curve again overlies the initial approach curve level (point 6) because net forces acting on the cantilever are zero (i.e., equivalent to force acting on the probe during the approach).
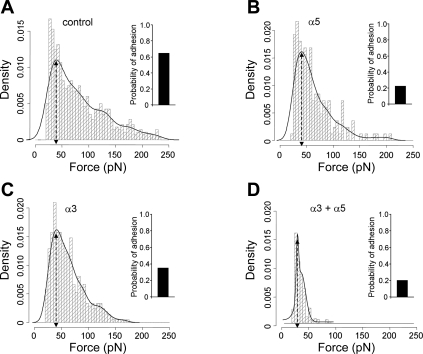
To analyze the distribution of adhesion events, the observed adhesion events plotted as histograms were fitted with Gaussian distributions to resolve integrin-FN bond adhesion force. Data analyses showed a good agreement between the raw data (histogram) and the fitted line (Fig. 3A, solid line). The peak bond rupture force (initial peak of the adhesion force in Fig. 3A) of FN-integrin was ∼39.6 ± 1.3 pN (Fig. 3A, control, n = 10), which likely represents a FN-integrin single bond unbinding force (67, 68, 73). The bar at the right of Fig. 3A shows the probability of FN-integrin adhesion events defined as the percentage of the curves with adhesion divided by total recorded curves. Under conditions defined for these experiments, the probability of adhesion events between the FN-coated probe and cardiomyocyte was 65% (Fig. 3). We also performed control experiments in which myocytes were plated directly on the glass plate without laminin and measured adhesive force and probability. Our results showed that the adhesion (force and probability of binding) between FN and integrins was not significantly different from the cells plated on laminin-coated plates (adhesion force: 38.6 ± 0.9 vs. 39.6 ± 1.3 and probability: 61% vs. 65%), indicating that there was no significant influence of the substrate laminin on the measured interactions with FN. We also examined the adhesion force and probability of adhesion using FN-coated microbeads (5 μm, n = 6) on the tip of the AFM probe. While the adhesion force between FN-integrin was not significantly different from the adhesion force measured with the conically tipped AFM probe (38.1 ± 1.0 vs. 39.6 ± 1.3 pN), the probability of adhesion was significantly greater with the microbead-tipped AFM probe due to the larger contact area of the microbead with the cardiomyocyte cell membrane (84% vs. 65%).
Fig. 3.
Summary of adhesion force results with the FN-coated AFM probe in cardiomyocytes. A: analyses of force-density plots of adhesion events during FN-coated probe retraction in cardiomyocytes. The observed adhesion force and corresponding number of events in the experiments (50 curves/cell for a total of 500 curves) were plotted as histograms. Solid lines represent the results that fitted with multiple Gaussian distributions. Insets: integrin-FN binding probabilities (solid bars). B–D: force-density plots of adhesion events and integrin-FN binding probabilities (solid bars) in the presence of function-blocking antibodies against α5-integrin (B; 60 nM), α3-integrin (C; 60 nM), or the combination of α3- and α5-integrins. n = 10 for each group.
To determine whether adhesion or mechanical characteristics would vary in different regions of the cell body, force curve measurements were performed on three regions of the cell body. Measurement sites were selected 25% from either end of the myocyte and in the lengthwise center of the cell (n = 4). Results demonstrated that there were no differences in cell stiffness or adhesion characteristics at either of the two cell ends (data not shown). However, the adhesion probability and cell stiffness from the central region of the cell were 14% and 47%, respectively, lower than similar measurements made at the end of the cells (data not shown). A comparison of the FN adhesion force indicated that there were no differences among the three different regions. For all experiments reported below, data presented were collected with the probe positioned 25% from the end of the myocyte.
FN exhibits binding specificity for α3β1- and α5β1-integrins on cardiomyocytes.
α3β1-Integrin and α5β1-integrin have been documented to bind FN (50). To verify their roles in adhesion events between FN and cardiomyocytes, cells were pretreated with integrin monoclonal antibodies. We used anti-α3- or anti-α5-integrin antibody (60 nM) to block α3- or α5-integrin subunits. Since α3- and α5-integrin subunits are known to associate only with the β1-integrin subunit, we assumed that these antibodies provide information regarding binding specificity for α3β1- and α5β1-integrins (27). There were no significant changes in the initial peak adhesion force in the presence of either α3-integrin (41.1 ± 3.2 pN, 60 nM, n = 10; Figs. 3C and 4A) or α5-integrin (41.2 ± 2.2 pN, 60 nM, n = 10; Figs. 3B and 4A) monoclonal antibodies. However, the probability of attachment to FN was inhibited by 48% or 65% in the presence of anti-α3- or anti-α5-integrin monoclonal antibodies, respectively (Fig. 3, B and C). Pretreatment with antibodies also reduced the area under the adhesion force density distribution curve (i.e., displayed less spread than with FN alone), indicating fewer total adhesion events (Fig. 3, A–C). The integrated force value, determined as average force from all adhesion events, was also calculated as described in materials and methods. The integrated adhesion force between FN and the myocyte was 81.1 ± 2.3 pN. The integrated force was inhibited by 21% in the presence of α3-integrin monoclonal antibody and by 23% in the presence of α5-integrin monoclonal antibody (Fig. 4B). Treatment with the combination of anti-α3- and α5-integrin monoclonal antibodies significantly reduced theadhesion force (to 28.1 ± 1.2 pN), probability of attachment, and integrated force by 29%, 70%, and 59%, respectively, compared with FN binding alone (Figs. 3D and 4). In addition, treatment with β1-integrin monoclonal antibody, to block all available αβ-integrin combinations, including α3β1- and α5β1-integrins, significantly reduced the adhesion force (to 25.2 ± 0.1 pN), probability of attachment, and integrated force by 36%, 75%, and 61%, respectively (n = 10; Fig. 4). To further determine the specificity of the adhesion force and probability between α3β1-integrin-FN and α5β1-integrin-FN, a non-FN α11-integrin subunit monoclonal antibody was used. The results showed no significant difference in adhesion force, probability of attachment, and integrated force compared with FN alone in the presence of α11-integrin monoclonal antibody (n = 10; Fig. 4).
Fig. 4.
Summary data of adhesion force and integrated force with the FN-coated AFM probe in cardiomyocytes. The adhesion force (A) represents the first peak force, and the integrated force (B) represents the total area under the force-density distribution curves. Calculations are presented in materials and methods. Adhesion force was not changed in the presence of α3- or α5-integrin monoclonal antibodies alone, whereas the combination of α3- and α5-integrin monoclonal antibodies and β1-integrin monoclonal antibody decreased adhesion force. Integrated force, which provides a metric reflecting the average overall adhesiveness, was decreased by α3- or α5-integrin monoclonal antibodies and further decreased by the combination of α3- and α5-integrin monoclonal antibodies and β1-integrin monoclonal antibody (50 μM). Non-FN α11-integrin (60 nM) showed no significant effects on adhesion force and integrated force. *P < 0.05 vs. control (FN-coated AFM probe alone). n = 10 for each group.
Furthermore, as an antibody control, nonintegrin binding antibody, anti-rat MHC monoclonal antibody (1 mg/ml)-coated AFM tips were tested (n = 10). MHC class I receptor molecules were detected on almost every cell, but the probability of binding and adhesion force were significantly lower compared with FN (n = 10)-coated AFM probes (−85% and −29%, respectively). As a nonspecific protein control, AFM probes were coated with BSA. BSA-coated probes (n = 10) also exhibited a significantly lower probability of binding and adhesion force compared with FN-coated probes (−75% and −24%, respectively). Finally, uncoated AFM probes alone exhibited 65% lower probability of binding and 45% lower adhesion force than FN-coated probes.
Adhesion force and membrane stiffness of cardiomyocytes are dependent on the actin-myosin cytoskeleton.
The membrane cytoskeleton is a critical junction for mechanotransduction in cardiomyocytes. Complexed with integrins at focal adhesions, mechanical forces are transmitted bidirectionally across the cell membrane. Forces transmit from the ECM to the cytoskeleton when extracellular forces act on the myocyte and from the cytoskeleton to the extracellular environment when myocytes generate contractile force. Thus, the cytoskeleton has been proposed to provide an intracellular structure for transmitting contractile forces out of the cell to the matrix as well as a pathway for transmitting external forces into the cell and nucleus (27, 42, 57). To investigate the changes in the actin-myosin cytoskeleton in cardiomyocytes, force curve measurements were performed in the presence of cytochalasin D, an F-actin-disrupting agent, or BDM, an agent that suppresses contraction by inhibiting actin-myosin interactions (23, 72). Treatment of myocytes (n = 10) with cytochalasin D or BDM had no effect on adhesion force (Fig. 5A) or adhesion probability (Fig. 5B). However, integrated adhesion force was significantly reduced (Fig. 5D) by both cytochalasin D (−32%) and BDM (−24%). Cell stiffness in control myocytes was 31.0 ± 0.5 kPa; the decrease in myocyte cell stiffness after cytochalasin D (−85%) or BDM (−70%) treatment supported the cytoskeletal effects of these agents (Fig. 5D).
Fig. 5.
Summary of results of adhesion characteristics of cardiomyocytes in the absence or presence of cytochalasin D or 2,3-butanedione monoxime (BDM). The adhesion force, probability, and cell stiffness were calculated as described in detail in materials and methods from the force curves obtained from cells under normal condition or cells incubated in the presence of cytochalasin D or BDM. Adhesion force (A) and adhesive probability (B) were not changed in the presence of cytochalasin D or BDM. Integrated force (C) and cell stiffness (D) were significantly decreased in the presence of cytochalasin D or BDM. *P < 0.05 vs. control. n = 10 for each group.
Adhesion force and membrane stiffness of cardiomyocytes change under contracted and relaxed states.
To quantify the changes in FN adhesion during contraction and relaxation of the myocyte, adhesion measurements were compared under conditions where high-K+ solution was used to induce a tonically contracted state and low-Ca2+ solution with EGTA and BDM was to induce a fully relaxed state. Images of typical cardiomyocytes in these two solutions are shown as insets in Fig. 6, A and B. All cardiac cells that were studied were visibly observed to contract in high-K+ solution (Fig. 6A, inset). During tonic contraction, peak adhesion force was significantly increased (1.2-fold, P < 0.05) from 38.4 ± 2.5 pN in relaxed cells (n = 10, Fig. 6B with inset cell image) compared with 47.9 ± 1.5 pN in contracted cells (n = 10; Fig. 6, A and B). Contraction was also associated with a 2.8-fold increase (P < 0.05) in adhesion probability compared with myocytes under relaxed conditions (53% in contracted vs. 19% in relaxed, n = 10; Fig. 6, A and B). In addition, the adhesion force density distribution curve observed in cells under the contracted state displayed significantly more adhesion events (i.e., more area under probability distribution) than occurred in cells under the relaxed state (Fig. 6, A and B). The increase in myocyte adhesiveness during contraction was also apparent as a 1.6-fold higher integrated force in contracted cardiomyocytes compared with cells in the relaxed state (Fig. 7B).
Fig. 6.
Summary of results of adhesion force in contracted (high-K+ solution), relaxed (low-Ca2+ solution), and high-K+ solution + BDM-treated cardiomyocytes. A–C: analyses of force-density plots of adhesion events during FN-coated probe retraction in cardiomyocytes. Adhesion to FN was enhanced during contraction or contraction with BDM compared with the relaxed state. The observed adhesion force and corresponding number of events in the experiments (50 curves/cell for a total of 500 curves) were plotted as histograms in contracted cells (A), relaxed cells (B), and high-K+ solution + BDM-treated cells (C). Solid lines represent the results that fitted with multiple Gaussian distributions. Insets: integrin-FN binding probabilities (solid bars) in contracted (A) and relaxed (B) cardiomyocytes and high-K+ solution + BDM treated cells (C). Top: AFM images of cardiomyocytes under each condition. n = 10 for each group.
Fig. 7.
Summary of results of cell adhesive and mechanical properties of cardiomyocytes in the contracted and relaxed states. The adhesion force (A), integrated force (B), and stiffness (C) of relaxed cells and high K+ solution + BDM-treated cells were significantly smaller than in contracted cells. In addition, the integrated force (B) and stiffness (C) of high-K+ solution + BDM-treated cells were significantly greater than in relaxed cells. *P < 0.05 vs. contracted cells; #P < 0.05 vs. relaxed cells. n = 10 for each group.
Consistent with a state of cell contraction and relaxation, the cell stiffness or elasticity was 137.5 ± 5.3 kPa in the contracted state, which was 27.5-fold greater than in the relaxed condition (Fig. 7C). To control for the effect of cell shortening and force generation by actin-myosin cross-bridge cycling, myocytes were treated with a combination of high-K+ solution plus BDM (Fig. 6C). With BDM (10 mM) in high-K+ bath solution, the adhesion force and integrated force decreased by 17% and 25%, respectively (Fig. 7, A and B) compared with high-K+ solution alone. There were no significant differences in adhesive probability without or with BDM in high-K+ state, and the probability of binding remained significantly higher than in the relaxed state. The presence of BDM in high-K+ solution prevented visible cell shortening (Fig. 6C, inset) and the increase in cell stiffness observed with high-K+ solution alone. BDM decreased cell stiffness by 82% compared with high-K+ solution alone (Fig. 7C). In addition, the probability, integrated force, and stiffness of high-K+ solution + BDM-treated cells were 2.7-, 1.2-, and 5.1-fold greater than in relaxed cells, respectively (Fig. 7, B and C).
DISCUSSION
The results of this study demonstrate that FN-cardiomyocyte adhesion through α3- and α5-integrins varies with the contractile status of the myocyte, indicative of dynamic regulation of integrin adhesion during cell contraction.
Transmembrane integrins that link the ECM and intracellular cytoskeleton are important for mechanosensation and mechanotransduction. Integrins can act as conduits to mechanically transfer forces and initiate biochemical signals from the outside of the cell to the inside and from the inside of the cell to the outside (12, 28, 55, 79). In cardiomyocytes, costameres in general are considered as the subsarcolemmal protein assemblies that circumferentially align in register with the Z disk of peripheral myofibrils. Thus, costameres physically couple force-generating sarcomeres with the sarcolemma in cardiac muscle (9, 15, 47). The costamere structure, including integrin linkage to the Z disk of the sarcomeres via the cytoskeleton proteins talin, vinculin, desmin, FAK, and α-actinin, plays an important role in mechanotransduction (57) and is analogous to the focal adhesions found in other cell types. Cardiac myocytes normally express α1-, α3-, α5-, α6-, α7-, α9-, α10-, and β1-integrins; of these, the four most prevalent subtypes are α1β1-, α3β1-, α5β1-, and α7β1-integrins (49, 50). We (59) have previously shown the expression of α5β1-integrin in the intact mouse myocardium. The data in the present study show that the presence of α5β1-integrin in isolated cardiomyocytes qualitatively matches the expression of integrins in the intact heart tissue. The expression of α3β1- and α5β1-integrins and the cytoskeleton protein vinculin in the vicinity of the costameres and intercalated disk (Fig. 1) in cardiomyocytes is consistent with the importance of the ECM-integrin-cytoskeleton axis in mechanotransduction.
The ECM of heart tissue is largely composed of collagen, FN, and laminin (26, 39). FN is deposited as an insoluble complex of either a cellular form that is synthesized and secreted locally by nonmuscle cells or a circulating form that is synthesized and secreted by hepatocytes (30, 58). The presence of FN in normal rat and mouse cardiac tissues has been previously reported (10, 76), and an increase in the amount of FN deposition in the hypertrophied or injured myocardium has also been documented (6, 38, 58, 75). However, in isolated myocytes using the collagenase digestion method as we used in this study, the basement membrane is completely disrupted and collagen and pericelluar matrix proteins, like FN, are mostly removed from the cell surface (10, 36). In this study, we sought to explore the relationship between FN adhesion and mechanosensation in normal myocytes. Our hypothesis was that transmission of an increased level of force during cell contraction and a decreased level of force during relaxation would correlate with a corresponding increase in integrin adhesion to FN during contraction and a decrease in adhesion during relaxation (Fig. 8). It was speculated that this would allow the myocyte to be mechanically and energetically more efficient at transferring force. AFM has been proven to be an unparalleled tool for the study of adhesion kinetics in cells (24, 45). It has both the spatial and temporal resolution to permit the study of adhesion at the scale of single molecules and is a proven technique for the study of adhesion in cells. In our experiments, we used AFM to measure adhesion force and adhesion probability as indicators of FN-integrin interactions and to measure cell stiffness as an indicator of the cell contractile state.
Fig. 8.
Simplified schematic representation of the FN-integrin-costamere axis. A: during relaxation, decreased adhesion force, probability of adhesion, and cell stiffness could be related to the decreased availability of activated integrin receptors within the costamere complex. B: in comparison, during contraction, increased adhesion force, probability of adhesion, and stiffness could be related to the increased availability of active integrin receptors and fortification of the costamere complex. The activation of integrins could hypothetically be related to inside-out ligand-activated signaling mechanisms (e.g., Ca2+) that can act to coordinate the contractile state with adhesion. Myofilaments: actin, myosin, tropomyosin, and troponin. ECM, extracellular matrix; +, activation; −, inhibition.
To quantify the adhesive events between FN and myocyte integrin proteins, the force that caused the bond between integrin and FN to rupture (unbinding force) was quantified. Previous work in our laboratory and by others (35, 68, 73) has provided evidence to show that the force required to disrupt adhesion between FN and α5β1-integrin is between 35 and 80 pN. In the present study, we measured the comparable unbinding force for the interaction between FN and integrins as 39.6 ± 1.3 pN using a conically tipped FN-coated AFM probe and as 38.1 ± 1.0 pN using a microbead-tipped AFM probe. A number of factors have been shown to contribute to the measured unbinding force and probability of an adhesion event occurring, such as the sampling location on the cell surface, ligand concentration, probe spring constant, and loading rate (3, 68). To minimize contributions from these sources of variation, we held each of these variables constant for our experiments.
The data from the present study demonstrated that the probability of adhesion to FN-integrins significantly decreased but not the bond rupture force in the presence of α3- or α5-integrin monoclonal antibodies, whereas in the presence of both α3- and α5-integrin antibodies or β1-integrin monoclonal antibody, the adhesion force was significantly reduced (Figs. 3 and 4). Non-FN α11-integrin monoclonal antibody had no effects on adhesion force and adhesion probability, demonstrating the specificity of FN binding to α3β1- and α5β1-integrins. Presumably, α3- or α5-integrin monoclonal antibodies act by preventing FN on the AFM probe from interacting with these integrins on the cell surface in the manner of a competitive inhibitor; thus, the antibodies bind to the integrin and reduce the availability of these integrins to the probe, which would decrease the binding probability and binding force between FN-integrin. These results support the α3- and α5-integrin specificity of the binding to FN. The residual adhesion force observed in the presence of combined antibodies to α3- and α5-integrins (28.1 ± 1.2 pN) or to β1-integrin (25.2 ± 0.1 pN) may be due to cell membrane force and/or membrane tether formation and/or the nonspecific binding we observed with control BSA protein on the AFM tip or tip alone. In particular, these residual forces are similar in magnitude to the membrane tether forces reported by Sun et al. (65) of 28.6 ± 10, 29.6 ± 9, and 29.6 ± 10 pN in Chinese hamster ovary cells, a malignant human brain tumor cell line, and human endothelial cells, respectively.
The data shown in Fig. 6 clearly demonstrate that the FN-integrin adhesion force and adhesion probability in contracted cells are greater than in cells under relaxed conditions. An interpretation of the increase in bond rupture force and adhesion probability observed in cardiomyocytes under the contracted state compared with cells in the relaxed state raises several possibilities. First, the increase in the probability of adhesion could have resulted from an increased number of integrins available for binding at the cell surface during contraction. Whether the increased probability is mediated by an increase in the number of receptors being expressed/inserted in the cell membrane or increased activation of receptors already present on the surface has not yet been determined. Second, the increase in measured force could result from integrin activation, leading to a more tightly bound state. Both of these types of inside-out integrin regulation have been reported to occur (27, 42). Finally, another possibility of enhanced adhesion force during contraction is that the cytoskeletal attachments to integrins at the cell surface are enhanced (43). It has been shown that the stability of the ECM-integrin-cytoskeleton axis is crucial to and required for cell contraction (70).
Furthermore, our data demonstrate that the cell stiffness or elasticity of cardiac myocytes during tonic contraction was higher than that of cells under the relaxed state (Fig. 7). This is consistent with the view that membrane depolarization caused by high extracellular K+ would lead to Ca2+ entry and subsequent myofilament activation and cytoskeleton stiffening (Fig. 8). Similar to our observations, an increase in cell stiffness during contraction has been reported in rat atrial myocytes using AFM. This increase in stiffness was associated with actin-myosin formation and force generation (62). It has also been shown that changes in [Ca2+]i levels and activation of cytoskeletal filaments are major components responsible for the cellular indentation stiffness (51). Thus, in addition to providing valuable insights into changes in cell adhesion, the stiffness measurements obtained from the AFM force curves can provide important temporal information related to the cell activation status.
To our knowledge, this is the first report to advance and provide support for the hypothesis that integrin adhesive properties are modulated differently in a contracted condition versus a relaxed condition in the myocyte. The mechanism we propose is one whereby the cell signaling that activates contraction or altered contractility will also activate integrins through an inside-to-out signaling pathway. The activated integrins would be evident as the altered adhesion that we examined and demonstrated in these investigations with AFM. A simplified schematic representation showing the adhesive events, probability, and cell stiffness at the contracted and relaxed states of the cardiac cell is shown in Fig. 8. In the context of our hypothesis, the subsequent alteration in integrin adhesion to the ECM could conceivably initiate a secondary round of outside-in signaling changes including the changes/activation in the costamere complex and activation of Ca2+ channels, as shown in Fig. 8. As an example of possible feedback from altered ECM-integrin interactions, we recently observed that FN induces a Ca2+ increase and an increase in force development in mouse papillary muscle fibers (unpublished observations). Rueckschloss et al. (53) demonstrated that contraction could induce an enhancement of Ca2+ currents by FN and RGD peptides in guinea pig cardiomyocytes. We (22, 83–85) have also previously reported that FN modulates Ca2+ and K+ channels through α5β1-integrin in vascular smooth muscle and human embryonic kidney cells heterologously expressing neuronal and smooth muscle Ca2+ channel isoforms. Sorting out the integrin signaling pathways and cytoskeletal changes involved in feedforward and feedback processes during cell contraction and relaxation will be an important challenge for the future.
In conclusion, our data indicate that FN interactions on cardiomyocytes involve α3β1- and α5β1-integrins and that adhesive interactions with these integrins are modulated by the contractile state in cardiomyocytes. We speculate that these changes in adhesion would act to match the adhesive state of the cell with the generation of contractile force thereby enhancing mechanical efficiency. Further studies will be required to clarify the mechanisms by which these adhesive changes occur and to determine if such changes in adhesion can be modulated on a beat-to-beat basis in cardiomyocytes.
GRANTS
This work was supported by National Institutes of Health Grants R21-EB-003888-01A1, and KO2-HL-86650 and by Texas A&M Health Science Center Research Development and Enhancement Award Program 244441-20702.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGEMENTS
The authors thank Dr. Wei Wang (Texas A&M Health Science Center) and Zhaohui Li (University of Missouri) for the skillful technical assistance.
REFERENCES
- 1.Ahumada GG, Saffitz JE. Fibronectin in rat heart: a link between cardiac myocytes and collagen. J Histochem Cytochem 32: 383–388, 1984 [DOI] [PubMed] [Google Scholar]
- 2.Belke DD, Dillmann WH. Altered cardiac calcium handling in diabetes. Curr Hypertens Rep 6: 424–429, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Benoit M, Gabriel D, Gerisch G, Gaub HE. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat Cell Biol 2: 313–317, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res 70: 422–433, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Casscells W, Ferrans VJ. Growth factors in the heart. In: The Development and Regenerative Potential of Cardiac Muscle, edited by Oberpriller JO, Oberpriller JS, Mauro A. New York: Harvard, 1990, p. 8–21 [Google Scholar]
- 7.Chen H, Huang XN, Stewart AF, Sepulveda JL. Gene expression changes associated with fibronectin-induced cardiac myocyte hypertrophy. Physiol Genomics 18: 273–283, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Contard F, Koteliansky V, Marotte F, Dubus I, Rappaport L, Samuel JL. Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest 64: 65–75, 1991 [PubMed] [Google Scholar]
- 9.Craig SW, Pardo JV. Gamma actin, spectrin, and intermediate filament proteins colocalize with vinculin at costameres, myofibril-to-sarcolemma attachment sites. Cell Motil 3: 449–462, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Dalen H, Saetersdal T, Roli J, Larsen TH. Effect of collagenase on surface expression of immunoreactive fibronectin and laminin in freshly isolated cardiac myocytes. J Mol Cell Cardiol 30: 947–955, 1998 [DOI] [PubMed] [Google Scholar]
- 11.Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol 189: 1–13, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Davis MJ, Wu X, Nurkiewicz TR, Kawasaki J, Davis GE, Hill MA, Meininger GA. Integrins and mechanotransduction of the vascular myogenic response. Am J Physiol Heart Circ Physiol 280: H1427–H1433, 2001 [DOI] [PubMed] [Google Scholar]
- 13.Efron B, Tibshirani R. An Introduction to the Bootstrap London: Chapman and Hall, 1993 [Google Scholar]
- 14.Epstein ND, Davis JS. Sensing stretch is fundamental. Cell 112: 147–150, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Ervasti JM. Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Farhadian F, Contard F, Sabri A, Samuel JL, Rappaport L. Fibronectin and basement membrane in cardiovascular organogenesis and disease pathogenesis. Cardiovasc Res 32: 433–442, 1996 [PubMed] [Google Scholar]
- 17.Fredersdorf S, Thumann C, Ulucan C, Griese DP, Luchner A, Riegger GA, Kromer EP, Weil J. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol 13: 11–19, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane crosstalk between the extracellular matrix and the cytoskeleton. Nat Rev Mol Cell Biol 2: 793–805, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Glukhova M, Koteliansky V. Integrins, cytoskeletal and extracellular matrix proteins in developing smooth muscle cells of human aorta. In: The Vascular Smooth Muscle Cell: Molecular and Biological Responses to the Extracellular Matrix, edited by Schwartz S, Mecham R. San Diego, CA: Academic, 1995, p. 37–79 [Google Scholar]
- 20.Grimm D, Huber M, Jabusch HC, Shakibaei M, Fredersdorf S, Paul M, Riegger GA, Kromer EP. Extracellular matrix proteins in cardiac fibroblasts derived from rat hearts with chronic pressure overload: effects of β-receptor blockade. J Mol Cell Cardiol 33: 487–501, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Guan K, Czyz J, Furst DO, Wobus AM. Expression and cellular distribution of αvintegrins in β1integrin-deficient embryonic stem cell-derived cardiac cells. J Mol Cell Cardiol 33: 521–532, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Gui P, Wu X, Ling S, Stotz SC, Winkfein RJ, Wilson E, Davis GE, Braun AP, Zamponi GW, Davis MJ. Integrin receptor activation triggers converging regulation of Cav1.2 calcium channels by c-Src and protein kinase A pathways. J Biol Chem 281: 14015–14025, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Gwathmey JK, Hajjar RJ, Solaro RJ. Contractile deactivation and uncoupling of crossbridges. Effects of 2,3-butanedione monoxime on mammalian myocardium. Circ Res 69: 1280–1292, 1991 [DOI] [PubMed] [Google Scholar]
- 24.Helenius J, Heisenberg CP, Gaub HE, Muller DJ. Single-cell force spectroscopy. J Cell Sci 121: 1785–1791, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Hesterberg T, Moore DS, Monaghan S, Clipson A, Epstein R. Bootstrap methods and permutation tests. In: Introduction to the Practice of Statistics, edited by Moore DS, McCabe GP. New York: Freeman, 2005 [Google Scholar]
- 26.Hynes RO. Fibronectins. Sci Am 254: 42–51, 1986 [DOI] [PubMed] [Google Scholar]
- 27.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25, 1992 [DOI] [PubMed] [Google Scholar]
- 28.Ingber D. Integrins as mechanochemical transducers. Curr Opin Cell Biol 3: 841–848, 1991 [DOI] [PubMed] [Google Scholar]
- 29.Izenman AJ. Recent developments in nonparametric density estimation. J Am Stat Assoc 86: 205–224, 1991 [Google Scholar]
- 30.Jane-Lise S, Corda S, Chassagne C, Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail Rev 5: 239–250, 2000 [DOI] [PubMed] [Google Scholar]
- 31.Kaur H, Chen S, Xin X, Chiu J, Khan ZA, Chakrabarti S. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes 55: 3104–3111, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Knowlton AA, Connelly CM, Romo GM, Mamuya W, Apstein CS, Brecher P. Rapid expression of fibronectin in the rabbit heart after myocardial infarction with and without reperfusion. J Clin Invest 89: 1060–1068, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuppuswamy D, Kerr C, Narishige T, Kasi VS, Menick DR, Cooper GT. Association of tyrosine-phosphorylated c-Src with the cytoskeleton of hypertrophying myocardium. J Biol Chem 272: 4500–4508, 1997 [DOI] [PubMed] [Google Scholar]
- 34.Lehenkari PP, Horton MA. Single integrin molecule adhesion forces in intact cells measured by atomic force microscopy. Biochem Biophys Res Commun 259: 645–650, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Li F, Redick SD, Erickson HP, Moy VT. Force measurements of the α5β1 integrin-fibronectin interaction. Biophys J 84: 1252–1262, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundgren E, Gullberg D, Rubin K, Borg TK, Terracio MJ, Terracio L. In vitro studies on adult cardiac myocytes: attachment and biosynthesis of collagen type IV and laminin. J Cell Physiol 136: 43–53, 1988 [DOI] [PubMed] [Google Scholar]
- 37.Magnusson M, Mosher D. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol 18: 1363–1370, 1998 [DOI] [PubMed] [Google Scholar]
- 38.Mamuya WS, Brecher P. Fibronectin expression in the normal and hypertrophic rat heart. J Clin Invest 89: 392–401, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin GR, Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol 3: 57–85, 1987 [DOI] [PubMed] [Google Scholar]
- 40.McCrossan ZA, Billeter R, White E. Transmural changes in size, contractile and electrical properties of SHR left ventricular myocytes during compensated hypertrophy. Cardiovasc Res 63: 283–292, 2004 [DOI] [PubMed] [Google Scholar]
- 41.McNamee HP, Ingber DE, Schwartz MA. Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J Cell Biol 121: 673–678, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267: 883–885, 1995 [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol 131: 791–805, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss RL, Fitzsimons DP. Frank-Starling relationship: long on importance, short on mechanism. Circ Res 90: 11–13, 2002 [PubMed] [Google Scholar]
- 45.Muller DJ, Helenius J, Alsteens D, Dufrene YF. Force probing surfaces of living cells to molecular resolution. Nat Chem Biol 5: 383–390, 2009 [DOI] [PubMed] [Google Scholar]
- 46.Nagai T, Laser M, Baicu CF, Zile MR, Cooper Gt, Kuppuswamy D. β3-Integrin-mediated focal adhesion complex formation: adult cardiocytes embedded in three-dimensional polymer matrices. Am J Cardiol 83: 38H–43H, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Pardo JV, Siliciano JD, Craig SW. A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80: 1008–1012, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parzen E. On the estimation of a probability density and mode. Ann Math Stat 33: 1065–1076, 1962 [Google Scholar]
- 49.Ross RS. Molecular and mechanical synergy: cross-talk between integrins and growth factor receptors. Cardiovasc Res 63: 381–390, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Ross RS, Borg TK. Integrins and the myocardium. Circ Res 88: 1112–1119, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Rotsch C, Braet F, Wisse E, Radmacher M. AFM imaging and elasticity measurements on living rat liver macrophages. Cell Biol Int 21: 685–696, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci USA 87: 404–408, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rueckschloss U, Isenberg G. Contraction augments L-type Ca2+ currents in adherent guinea-pig cardiomyocytes. J Physiol 560: 403–411, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47: 23–37, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Sachs F. Mechanical transduction by ion channels: how forces reach the channel. Soc Gen Physiol Series 52: 209–218, 1997 [PubMed] [Google Scholar]
- 56.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 59: 551–571, 1997 [DOI] [PubMed] [Google Scholar]
- 57.Samarel AM. Costameres, focal adhesions, and cardiomyocyte mechanotransduction. Am J Physiol Heart Circ Physiol 289: H2291–H2301, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Samuel JL, Barrieux A, Dufour S, Dubus I, Contard F, Koteliansky V, Farhadian F, Marotte F, Thiery JP, Rappaport L. Accumulation of fetal fibronectin mRNAs during the development of rat cardiac hypertrophy induced by pressure overload. J Clin Invest 88: 1737–1746, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sarin V, Gaffin RD, Meininger GA, Muthuchamy M. Arginine-glycine-aspartic acid (RGD)-containing peptides inhibit the force production of mouse papillary muscle bundles via α5β1 integrin. J Physiol 564: 603–617, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt C, Pommerenke H, Durr F, Nebe B, Rychly J. Mechanical stressing of integrin receptors induces enhanced tyrosine phosphorylation of cytoskeletally anchored protein. J Biol Chem 273: 5081–5085, 1998 [DOI] [PubMed] [Google Scholar]
- 61.Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol 3: 786–791, 1991 [DOI] [PubMed] [Google Scholar]
- 62.Shroff SG, Saner DR, Lal R. Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol Cell Physiol 269: C286–C292, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Siperstein MD, Unger RH, Madison LL. Studies of muscle capillary basement membranes in normal subjects, diabetic, and prediabetic patients. J Clin Invest 47: 1973–1999, 1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smoak IW. Hyperglycemia-induced TGFβ and fibronectin expression in embryonic mouse heart. Dev Dyn 231: 179–189, 2004 [DOI] [PubMed] [Google Scholar]
- 65.Sun M, Graham JS, Hegedus B, Marga F, Zhang Y, Forgacs G, Grandbois M. Multiple membrane tethers probed by atomic force microscopy. Biophys J 89: 4320–4329, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun M, Opavsky MA, Stewart DJ, Rabinovitch M, Dawood F, Wen WH, Liu PP. Temporal response and localization of integrins beta1 and beta3 in the heart after myocardial infarction: regulation by cytokines. Circulation 107: 1046–1052, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Sun Z, Martinez-Lemus LA, Hill MA, Meininger GA. Extracellular matrix-specific focal adhesions in vascular smooth muscle produce mechanically active adhesion sites. Am J Physiol Cell Physiol 295: C268–C278, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Z, Martinez-Lemus LA, Trache A, Trzeciakowski JP, Davis GE, Pohl U, Meininger GA. Mechanical properties of the interaction between fibronectin and α5β1-integrin on vascular smooth muscle cells studied using atomic force microscopy. Am J Physiol Heart Circ Physiol 289: H2526–H2535, 2005 [DOI] [PubMed] [Google Scholar]
- 69.Tagawa H, Wang N, Narishige T, Ingber DE, Zile MR, Cooper GT. Cytoskeletal mechanics in pressure-overload cardiac hypertrophy. Circ Res 80: 281–289, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Takagi J, Erickson HP, Springer TA. C-terminal opening mimics “inside-out” activation of integrin α5β1. Nat Struct Biol 8: 412–416, 2001 [DOI] [PubMed] [Google Scholar]
- 71.Tavi P, Laine M, Weckstrom M, Ruskoaho H. Cardiac mechanotransduction: from sensing to disease and treatment. Trends Pharmacol Sci 22: 254–260, 2001 [DOI] [PubMed] [Google Scholar]
- 72.Tong CW, Kolomenskii A, Lioubimov VA, Schuessler HA, Trache A, Granger HJ, Muthuchamy M. Measurements of the cross-bridge attachment/detachment process within intact sarcomeres by surface plasmon resonance. Biochemistry 40: 13915–13924, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Trache A, Trzeciakowski JP, Gardiner L, Sun Z, Muthuchamy M, Guo M, Yuan SY, Meininger GA. Histamine effects on endothelial cell fibronectin interaction studied by atomic force microscopy. Biophys J 89: 2888–2898, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Trial J, Rossen RD, Rubio J, Knowlton AA. Inflammation and ischemia: macrophages activated by fibronectin fragments enhance the survival of injured cardiac myocytes. Exp Biol Med (Maywood) 229: 538–545, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ulrich MM, Janssen AM, Daemen MJ, Rappaport L, Samuel JL, Contard F, Smits JF, Cleutjens JP. Increased expression of fibronectin isoforms after myocardial infarction in rats. J Mol Cell Cardiol 29: 2533–2543, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Valencik ML, Zhang D, Punske B, Hu P, McDonald JA, Litwin SE. Integrin activation in the heart: a link between electrical and contractile dysfunction? Circ Res 99: 1403–1410, 2006 [DOI] [PubMed] [Google Scholar]
- 77.Venables WN, Ripley BD. Modern Applied Statistics with S New York: Springer, 2002 [Google Scholar]
- 78.Villarreal FJ, Dillmann WH. Cardiac hypertrophy-induced changes in mRNA levels for TGF-β1, fibronectin, and collagen. Am J Physiol Heart Circ Physiol 262: H1861–H1866, 1992 [DOI] [PubMed] [Google Scholar]
- 79.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993 [DOI] [PubMed] [Google Scholar]
- 80.Weber K. Extracellular matrix remodeling in heart failure: a role for de novo angiotensin II generation. Circulation 96: 4065–4082, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol 179: 321–325, 1996 [DOI] [PubMed] [Google Scholar]
- 82.Wolska BM, Solaro RJ. Methood for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am J Physiol Heart Circ Physiol 271: H1250–H1255, 1996 [DOI] [PubMed] [Google Scholar]
- 83.Wu X, Davis GE, Meininger GA, Wilson E, Davis MJ. Regulation of the L-type calcium channel by α5β1 integrin requires signaling between focal adhesion proteins. J Biol Chem 276: 30285–30292, 2001 [DOI] [PubMed] [Google Scholar]
- 84.Wu X, Mogford JE, Platts SH, Davis GE, Meininger GA, Davis MJ. Modulation of calcium current in arteriolar smooth muscle by αvβ3 and α5β1 integrin ligands. J Cell Biol 143: 241–252, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu X, Yang Y, Gui P, Sohma Y, Meininger GA, Davis GE, Braun AP, Davis MJ. Potentiation of large conductance, Ca2+-activated K+ (BK) channels by α5β1 integrin activation in arteriolar smooth muscle. J Physiol 586: 1699–1713, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]