Abstract
Sympathetic nerves stimulate cardiac function through the release of norepinephrine and the activation of cardiac β1-adrenergic receptors. The sympathetic innervation of the heart is sculpted during development by chemoattractive factors including nerve growth factor (NGF) and the chemorepulsive factor semaphorin 3a. NGF acts through the TrkA receptor and the p75 neurotrophin receptor (p75NTR) in sympathetic neurons. NGF stimulates sympathetic axon extension into the heart through TrkA, but p75NTR modulates multiple coreceptors that can either stimulate or inhibit axon outgrowth. In mice lacking p75NTR, the sympathetic innervation density in target tissues ranges from denervation to hyperinnervation. Recent studies have revealed significant changes in the sympathetic innervation density of p75NTR-deficient (p75NTR−/−) atria between early postnatal development and adulthood. We examined the innervation of adult p75NTR−/− ventricles and discovered that the subendocardium of the p75NTR−/− left ventricle was essentially devoid of sympathetic nerve fibers, whereas the innervation density of the subepicardium was normal. This phenotype is similar to that seen in mice overexpressing semaphorin 3a, and we found that sympathetic axons lacking p75NTR are more sensitive to semaphorin 3a in vitro than control neurons. The lack of subendocardial innervation was associated with decreased dP/dt, altered cardiac β1-adrenergic receptor expression and sensitivity, and a significant increase in spontaneous ventricular arrhythmias. The lack of p75NTR also resulted in increased tyrosine hydroxylase content in cardiac sympathetic neurons and elevated norepinephrine in the right ventricle, where innervation density was normal.
Keywords: sympathetic innervation, nerve growth factor, semaphorin 3a, ventricular arrhythmias
the sympathetic innervation of the heart regulates cardiac function by stimulating heart rate, contractility, and conduction velocity through the release of norepinephrine (NE) and the activation of cardiac β1-adrenergic receptors (β1ARs). Sympathetic innervation of the heart is sculpted during development by chemoattractive and chemorepulsive factors (17, 24). For example, nerve growth factor (NGF) supports sympathetic neuron survival and promotes cardiac axon outgrowth during development (14, 17), whereas the chemorepulsive factor semaphorin 3a (Sema3a) attenuates sympathetic axon extension in the heart (24) and in the peripheral vasculature (38).
Neurotrophins such as NGF act through two distinct types of receptors: tropomyosin-related tyrosine kinase (Trk) receptors and the lower-affinity p75 neurotrophin receptor (p75NTR) (16, 60). The actions of these receptors have been most thoroughly characterized in the context of development. NGF acts through TrkA to promote the extension of sympathetic axons into the heart (17, 34), whereas the role of p75NTR is more complicated because p75NTR modulates signaling by coreceptors that can either stimulate or inhibit axon outgrowth (2, 28). Thus, in mice lacking p75NTR, the sympathetic innervation density in target tissues ranges from denervation to hyperinnervation, with many tissues exhibiting normal innervation (25, 31, 34, 35).
Developmental studies have examined cardiac innervation in p75NTR-deficient (p75NTR−/−) mice, but neurotrophins also maintain neurotransmitter production, and little is known about the role of p75NTR in adult cardiac innervation. The lack of p75NTR delays sympathetic innervation of the heart (34), but despite this delay, sympathetic innervation in the ventricle is qualitatively normal soon after birth (25, 35). It is unknown if the lack of p75NTR impacts sympathetic cardiac innervation of the adult ventricles, and recent studies have raised the possibility that cardiac innervation might be altered in the adult ventricle. First, sympathetic innervation density in p75NTR−/− atria is elevated 4 wk after birth but is decreased in adults (18), whereas parasympathetic innervation is normal at both ages despite the parasympathetic expression of p75NTR (22). This suggests that p75 plays a role in both the establishment and maintenance of sympathetic, but not parasympathetic, neurons. Second, Sema3a expression in the left ventricular (LV) subendocardium retards the growth of sympathetic fibers, generating a gradient of sympathetic innervation across the ventricle with more fibers in the subepicardium than in the subendocardium (24, 47). p75NTR attenuates Sema3a signaling in sensory neurons (3), and, therefore, we hypothesized that the lack of p75NTR would result in decreased sympathetic innervation in the LV subendocardium due to an enhanced susceptibility to Sema3a repulsion. To test this hypothesis, we used tyrosine hydroxylase (TH) immunohistochemistry to determine the sympathetic innervation density and pattern in the p75NTR−/− adult LV and used ganglia explants to determine the susceptibility of sympathetic axons to Sema3a. Furthermore, we used ECG telemetry to determine if potential sympathetic heterogeneity would be associated with increased ventricular arrhythmias as seen in mice overexpressing Sema3a (24).
MATERIALS AND METHODS
Materials
(d,l)-Dobutamine hydrochloride was from Hospira, mouse β-NGF was from Austral Biologicals, and the Sema3a/Fc chimera was from R&D Systems. TH-specific antibody was from Chemicon; protein gene product 9.5 (PGP9.5)-specific antibody was from Accurate Chemicals; β1AR-specific antibody was from Affinity Bioreagents; horseradish peroxidase-conjugated rabbit IgG-specific antibody was from Pierce; and Alexa fluor488-conjugated rabbit IgG-specific antibody was from Molecular Probes. DMEM-F-12 media was obtained from Life Technologies.
Animals
Wild-type (WT) C57BL/6J and p75NTR−/− mice (B6.129S4-Ngfrtm1Jae/J) were obtained from Jackson Laboratories. B6.129S4-Ngfrtm1Jae/J mice contain two mutated exon III alleles, which removes the coding region for the last three of four cysteine-rich repeats in the extracellular domain and prevents the expression of functional p75NTR (36, 57, 58). p75NTR−/− mice were genotyped upon arrival (15), and a colony was maintained using homozygous breeder pairs, with additional genotyping every 6 mo. All mice were kept on a 12:12-h light-dark cycle with ad libitum access to food and water. Age- and gender-matched male and female mice between 12 and 18 wk old were used for all experiments. All procedures were approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
Axon Outgrowth
To test the role of p75NTR in modulating Sema3a inhibition of axon outgrowth, we used explants of stellate ganglia, which contain most of the sympathetic neurons that project to the heart. Ganglia were desheathed to facilitate axon outgrowth, embedded in reduced growth factor Matrigel, and covered with serum-free DMEM-F-12 with penicillin-streptomycin (1:10,000) and 10 ng/ml NGF. Explants were maintained at 37°C with 5% CO2. Twenty hours after being plated (time 0), explants were photographed, treated with vehicle or Sema3a/Fc, and then photographed again 6 h later (time = 6 h). Axon length was measured at each time point using Nikon Elements AR 3.0, and the rate of axon growth per hour was calculated. For each treatment group, 6–10 axon measurements were obtained and averaged from a minimum of 3 different sites. The experiment was repeated three times, and data from a single representative experiment are shown.
Immunohistochemistry
Hearts were fixed for 1 h in 4% paraformaldehyde, rinsed in PBS, and cryoprotected in 30% sucrose, and 10-μm transverse sections were thaw mounted onto charged slides. To reduce fixative-induced autofluorescence, sections were rinsed in 10 mg/ml sodium borohydride three times for 10 min and then rinsed in PBS three time for 10 min. Sections were then blocked in 2% BSA-0.3% Triton X-100 in PBS for 1 h, incubated with TH-specific antibody (1:300) overnight, rinsed three times for 10 min in PBS, and incubated for 1.5 h with Alexa fluor488-conjugated rabbit IgG-specific antibody (1:300). Sections were rinsed three times for 10 min in PBS, coverslipped, and visualized by fluorescence microscopy. Sections from WT and p75−/− hearts were always stained and photographed side by side to minimize variations between the groups due to the immunohistochemistry procedures.
Imaging and Analysis of the Ventricles
Composite analysis.
Immunohistochemical staining was visualized on a Zeiss (Axiophot II) fluorescent microscope with a ×10 objective. Composite images were used to generate a representative heart section from each mouse that included the LV and right ventricle (RV). The composite was divided into the RV and the subepicardium or subendocardium (including the interventricular septum) of the LV and quantified using ImageJ software (NIH). In order for two observers to analyze the endocardium versus epicardium consistently, we measured the ventricular wall and identified half as the endocardium and half as the epicardium rather than trying to follow the border between the two layers of muscle. Innervation density was determined by threshold discrimination using ImageJ (Fig. 1C). All photographs were treated in an identical manner. Black and white photos were opened in ImageJ, and the brightness/contrast tool was used to adjust each image so that the minimum was set at the left side of the histogram. The threshold tool was then used to identify nerve fibers. The automated function set a beginning threshold level, but this did not reliably include all sympathetic fibers while excluding all non-neuronal tissue. The threshold was then manually adjusted to ensure that only specific TH staining was identified. The ventricle has two layers of muscle set at different angles, so each section included some nerve fibers cut longitudinally along the plane of the section and other fibers cut in cross section and appearing as small round dots. Thus, specific criteria for size and shape were not used in our analysis. Innervation density was expressed as the percent area that was above the threshold (TH-positive fibers). Each image was quantified by two independent observers. The coefficient of variance between the two independent analyses averaged 10 ± 8.3% (means ± SD).
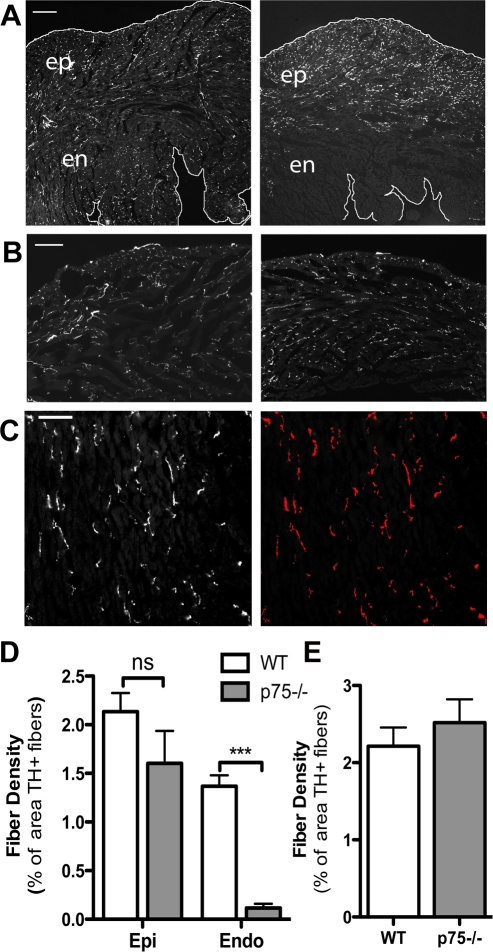
Fig. 1.
Fiber distribution is altered in the p75 neurotrophin receptor-deficient (p75NTR−/−) left ventricle (LV). Sympathetic fiber distribution was identified by tyrosine hydroxylase (TH) immunoreactivity. A: representative pictures taken at ×5 magnification showing the entire width of the LV free wall revealing the altered pattern of sympathetic innervation in p75NTR−/− mice (right) compared with wild-type (WT) mice (left). This low magnification was not used for quantitative analysis. The edges of the tissue have been outlined for clarity. Scale bar = 0.25 mm. B: sympathetic fiber distribution in the right ventricle (RV) of WT mice (left) and p75NTR−/− mice (right). Scale bar = 0.1 mm. C: example of the threshold discrimination used to determine innervation density in which the TH-positive nerves above the threshold are highlighted. Innervation density was then expressed as the percent area that was above the determined threshold (TH+ fibers). Scale bar = 0.05 mm. D: quantification of sympathetic fiber density in the subendocardium (en and Endo) and subepicardium (ep and Epi) of WT and p75NTR−/− mice. ns, Not significant. Values are means ± SE; n = 4. ***P < 0.001. E: quantification of sympathetic fiber density of the RV in WT and p75NTR−/− mice. Values are means ± SE; n = 4.
Analysis of specific regions.
TH staining was visualized using a ×20 objective, and pictures were obtained for areas A–E as shown in Fig. 2C. Innervation density was determined by threshold discrimination (ImageJ), and each image was quantified by two independent observers. Five sections at least 150 μm apart were analyzed from each heart and averaged together. Data shown are the average of the two independent determinations. The coefficient of variance between the two independent analyses averaged 9.8 ± 7.8% (means ± SD).
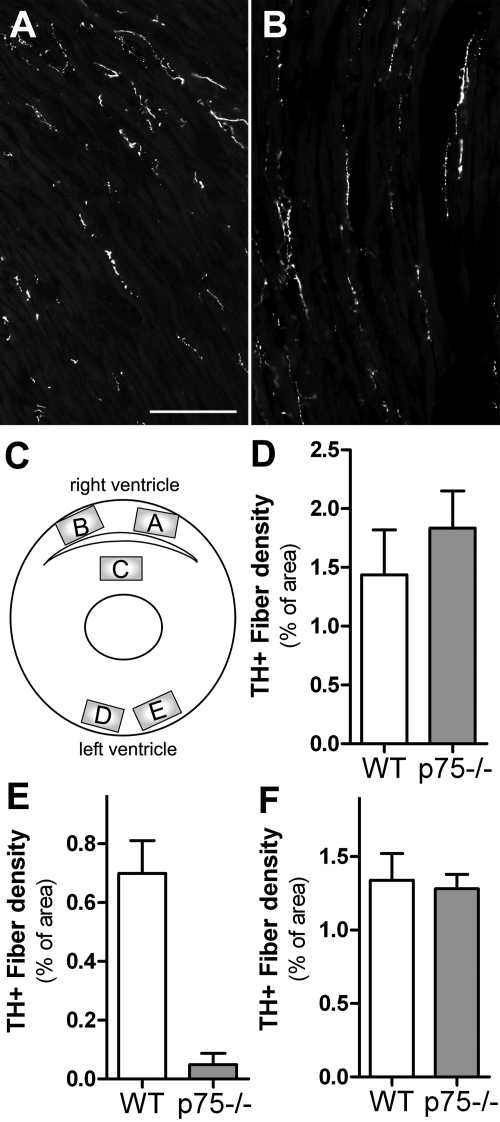
Fig. 2.
Innervation density is normal in the p75NTR−/− subepicardium. A and B: representative pictures at ×20 magnification of TH+ fibers in the WT (A) and p75NTR−/− (B) subepicardium that were used for quantitative analysis. Scale bar = 0.1 mm. C: diagram showing the regions (areas A–E) analyzed to further characterize the innervation density of WT and p75NTR−/− ventricles. D–F: quantification of sympathetic fiber density in the WT and p75NTR−/− RV (D), interventricular septum (E), and LV (F). Values are means ± SE; n = 7.
HPLC
NE was measured by HPLC with electrochemical detection as previously described (37, 46). Detection limits were ∼0.05 pmol with recoveries from the alumina extraction >60%.
Immunoblot Analysis
TH and β1ARs were quantified via Western blot analysis as previously described (37, 46). For the biochemical analysis of the subendocardium versus the subepicardium, the inner vertical loop was dissected from the outer transverse loop. The pan-neuronal marker PGP9.5 (1:1,000) was used to normalize TH content to the total amount of neuronal protein in each sample. Actin (1:1,000) was quantified as a loading control for β1AR expression in cardiac myocytes. Data were analyzed using LabWorks software (UVP, Upland, CA).
Real-Time PCR
Hearts and ganglia were stored in RNAlater. RNA was isolated, and real-time PCR was performed as previously described (46). Samples were assayed using prevalidated Taqman gene expression assays for mouse β1AR, neuropilin (NP)-1, plexin 4A, actin, and GAPDH. β1AR expression was normalized to actin, and NP-1 and plexin A4 expression were normalized to GAPDH.
Hemodynamics and Ventricular Function
Ventricular function was measured in isoflurane-anesthetized mice using both transthoracic echocardiography and a micromanometer-tipped pressure transducer (SPR1000, Millar Instruments) inserted retrograde into the ventricle. LV dimensions and function were evaluated using transthoracic echocardiography at a transmission frequency of 40 MHz (Vevo 770, VisualSonics) and a cycle length of 1. Parasternal imaging was performed in the midpapillary short-axis and parasternal long-axis imaging planes. Image sequences were acquired with ECG gating of sequential M-mode sweeps (EKV mode) for an effective two-dimensional frame rate of 1,000 Hz. LV anteroseptal and posterolateral wall thickness in the short axis were measured at end systole and end diastole and were used to calculate fractional thickening. LV cavity dimensions in the anterior-posterior dimension at end systole (LVIDs) and end diastole (LVIDd) were measured and used to calculate fractional shortening as follows: 100(LVIDd − LVIDs)/LVIDd and LV volumes as follows: [7.0/(2.4 + LVID)] × LVID3 as well as LV ejection fraction. Stroke volume was measured by the product of the proximal aortic area and time-velocity integral derived from pulsed-wave Doppler at the same level. The product of stroke volume and heart rate was used to calculate cardiac output. Tissue Doppler imaging was performed to determine peak radial endocardial velocities in systole (S′) and early diastole (E′) in the anterior wall. (d,l)-Dobutamine hydrochloride (32 μg/kg) was injected intraperitoneally to assess β1AR responsiveness.
Ventricular pressure and arterial pressure were monitored with a microtipped pressure transducer (1.0-Fr, Millar). Anesthesia was induced with 4% inhaled isoflurane. Mice were intubated and ventilated, and anesthesia was maintained with 2–3% inhaled isoflurane. Body temperature was maintained at 37 ± 0.2°C. The microtipped pressure transducer was inserted into the right carotid artery for arterial pressure measurements and then advanced into the LV for measurement of LV pressure using a PowerLab data-acquisition system. LV peak systolic pressure (LVPSP), dP/dtmax, and dP/dtmin were analyzed using ChartPro software.
NE Uptake
The uptake of [3H]NE into sympathetic nerve terminals was assayed as previously described (45). Mouse ventricles were separated into sections of 1 mm thick. Each section contained the RV, LV, and septum. Two sections from each heart used for total uptake and two for background uptake (defined by the addition of 1 mM desipramine). Ten minutes after the addition of 50 nmol/l [3H]NE, reactions were terminated by adding ice-cold Krebs-Ringer-HEPES buffer.
ECG Telemetry Recordings and Analysis
ECGs were obtained from conscious adult mice using telemetry implants and were analyzed with Dataquest ART software (Data Sciences). Mice were anaesthetized with 4% inhaled isofluorane and maintained on 2% inhaled isoflurane. The transmitter was implanted into the abdominal cavity in a lead II configuration, with the negative lead placed in the right pectoral muscle and the positive lead to the left of the xyphoid process. A subcutaneous injection of 0.1 mg/kg buprenorphine was administered postoperatively for analgesia. Mice recovered for at least 72 h before data acquisition. ECG recordings were collected for 24 h and were analyzed at four time points within the circadian cycle (6–7 PM, 12–1 AM, 6–7 AM, and 12–1 PM) to determine heart rates and the presence of spontaneous premature ventricular complexes (PVCs). PVCs were defined as a single premature QRS complex in relation to the P-wave.
Statistics
Student's t-test was used for a single comparison between two groups (WT vs. p75NTR mice). Two-way ANOVA with a Bonferroni posttest was used to compare across genotypes and treatment groups.
RESULTS
We used TH immunohistochemistry to determine the sympathetic innervation density in the RVs and LVs of WT and p75NTR−/− mice. Surprisingly, we discovered a consistent and profound alteration in the pattern of sympathetic nerve fibers in p75NTR−/− LVs (Fig. 1A). Few sympathetic nerve fibers projected into the subendocardium of the p75NTR−/− LV, but many fibers were present in the subepicardium, and RV innervation appeared normal (Fig. 1B). We generated composite images of the ventricular sections and quantified the innervation density in low-magnification images using threshold discrimination (Fig. 1C). Density in the subendocardium and RV were not significantly different than the control, but there was a significant deficit in sympathetic fiber density in the LV subendocardium and septum (Fig. 1, D and E). To confirm this striking result, we carried out additional experiments quantifying pictures taken at a higher magnification that sampled a smaller number of specific sites throughout the RV, LV, and septum (Fig. 2). This confirmed the initial assessment that innervation density in the p75NTR−/− RV and LV subepicardium were normal, whereas the septum was essentially devoid of sympathetic fibers (Fig. 2).
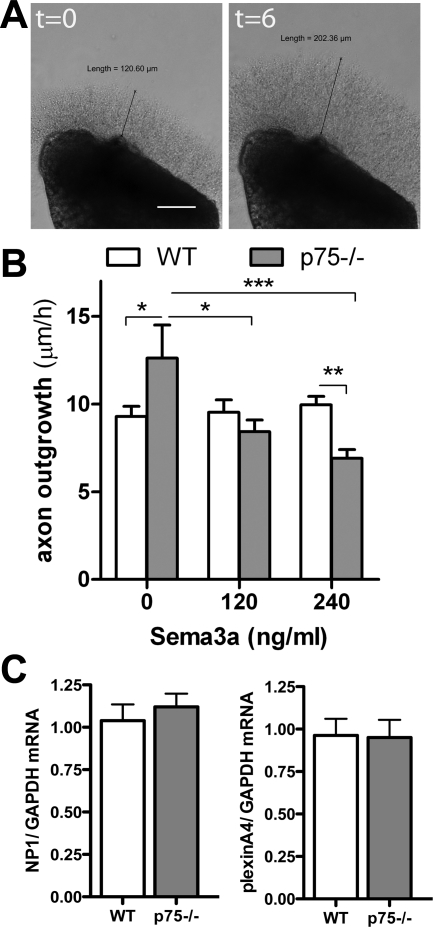
The altered innervation pattern in the adult p75NTR−/− LV raised the question of why sympathetic fibers are excluded from the subendocardium. Sema3a expression in the subendocardium decreases sympathetic innervation density in that part of the heart (24), and p75NTR disrupts a NP-1/plexin Sem3a receptor complex in sensory neurons and attenuates Sema3a signaling (3). We evaluated the growth of cardiac sympathetic axons lacking p75NTR using stellate ganglion explants (Fig. 3A). The addition of Sema3a into solution, rather than from a point source, causes axon collapse and essentially inhibits axon outgrowth (21, 32). We found that Sema3a (at 120 and 240 ng/ml) did not inhibit axon outgrowth from WT ganglia but dose dependently inhibited outgrowth from p75NTR−/− ganglia (Fig. 3B). NP-1 and plexin mRNA were identical in both genotypes (Fig. 3C), suggesting that Sema3a receptor expression is normal in p75NTR−/− mice, and the lack of p75NTR increased the sensitivity to Sema3a in cardiac sympathetic neurons.
Fig. 3.
p75NTR−/− sympathetic axons are more sensitive to semaphorin 3a (Sema3a) growth cone collapse/inhibition. A: Representative stellate ganglia explants at time 0 (left) and 6 h later (right). Scale bar = 0.1 mm. B: quantification of axon growth rate from WT and p75NTR−/− sympathetic ganglia in the presence of 0, 120, or 240 ng/ml Sema3a. Values are means ± SE; n = 3. *P < 0.05; **P < 0.01; ***P < 0.001. C: neuropilin (NP)-1 (left) and plexin A4 (right) mRNA normalized to GAPDH in WT and p75NTR−/− stellate ganglia. Values are means ± SE; n = 5.
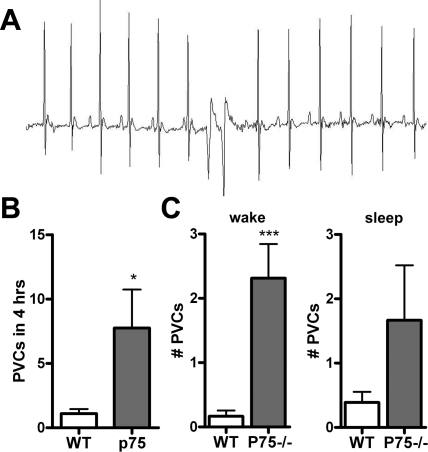
Experimental manipulations and pathologies that cause heterogeneous sympathetic innervation in the LV increase the occurrence of ventricular arrhythmias (7, 8, 24, 48). To determine if the aberrant development of sympathetic innervation was sufficient to trigger arrhythmias, we examined heart rhythm in conscious mice. ECGs were monitored for 24 h, and rhythm abnormalities were quantified during four 1-h periods throughout the circadian cycle. p75NTR−/− mice had significantly more spontaneous PVCs than WT mice during the 4 h that were analyzed in detail (Fig. 4, A and B). However, there was significant circadian variability in the presence of PVCs so that the difference between genotypes was significant during the wake phase but not significant during the sleep phase (Fig. 4C). The analysis of heart rates in conscious mice confirmed the previous observation in anesthetized mice (18) that heart rates are lower in p75NTR−/− mice compared with WT mice (heart rate at 12–1 AM: 618 ± 52 beats/min in WT mice vs. 551 ± 27 beats/min in p75NTR−/− mice, means ± SD, P < 0.01, n = 7–8).
Fig. 4.
Mice lacking p75NTR have increased spontaneous ventricular arrhythmias. A: representative ECG showing spontaneous premature ventricular complexes (PVCs) in a p75NTR−/− mouse. B: p75NTR−/− mice experienced more spontaneous PVCs than WT mice. Values are means ± SE; n = 8–9. *P < 0.05. C: number of PVCs in WT and p75NTR−/− mice during 2 h of the awake phase and sleep phase. Values are means ± SE; n = 8–9. ***P < 0.01.
To determine if the absence of sympathetic innervation in the subendocardium altered cardiac function, we measured ventricular dimensions and function by transthoracic echocardiography. Ventricle size, fractional shortening, ejection fraction, stroke volume, and cardiac output at rest were not significantly different in p75NTR−/− mice (Table 1), suggesting that the absence of sympathetic innervation in the subendocardium of the LV had no effect on ventricular function. This was surprising, and we asked if the lack of p75NTR had other effects on sympathetic nerves that might contribute to normal stroke volume and cardiac output.
Table 1.
LV size and function
| WT Mice | p75−/− Mice | |
|---|---|---|
| Heart dimensions | ||
| IVSd, mm | 0.68 ± 0.1 | 0.68 ± 0.05 |
| LVIDd, mm | 3.3 ± 0.27 | 3.1 ± 0.12 |
| LVPWd, mm | 0.64 ± 1.12 | 0.64 ± 0.06 |
| IVSs, mm | 1.16 ± 0.1 | 1.18 ± 0.04 |
| LVIDs, mm | 2.06 ± 0.24 | 1.87 ± 0.07 |
| LVPWs, mm | 1.11 ± 0.09 | 1.15 ± 0.03 |
| Ventricular function | ||
| Heart rate, beats/min | 432 ± 22 | 463 ± 30 |
| Fractional shortening, % | 38.2 ± 10.8 | 39.7 ± 3.7 |
| LV ejection fraction, % | 68.4 ± 13.8 | 71.6 ± 4.3 |
| Stroke volume, ml | 38.8 ± 8.7 | 42.9 ± 6.5 |
| Cardiac output, ml/min | 16.9 ± 2.8 | 20.5 ± 4.8 |
| Arterial pressure | ||
| Mean, mmHg | 76.0 ± 4.1 | 60.1 ± 4.5* |
| Systolic, mmHg | 88.6 ± 4.4 | 74.5 ± 4.3* |
| Diastolic, mmHg | 64.0 ± 3.9 | 47.7 ± 4.3* |
Values are means ± SD; for heart dimensions and ventricular function, n = 4 mice/group and for arterial pressure, 7 mice/group in the wild-type (WT) group and 6 mice/group in the p75 neurotrophin receptor (p75NTR)-deficient (p75NTR−/−) group. IVSd and IVSs, interventricular septum at diastole and systole, respectively; LVIDd and LVIDs, left ventricular (LV) internal dimension at diastole and systole, respectively; LVPWd and LVPWs, LV posterior wall at diastole and systole, respectively.
P < 0.05 vs. WT mice.
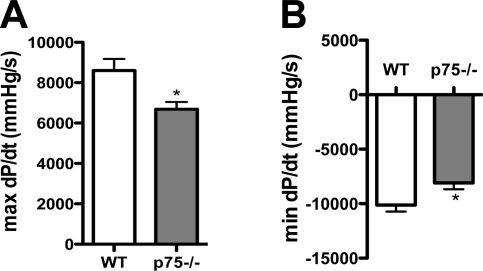
Afterload affects stroke volume, and a recent study (38) has revealed that Sema3a impacts the sympathetic innervation density of vascular beds. Thus, vessels with high Sema3a expression have a lower innervation density, resulting in decreased arterial pressure. Since p75NTR−/− sympathetic fibers are more sensitive to Sema3a, we asked if arterial pressure was low in p75NTR−/− mice. Mean arterial pressure was normal in C57Bl6/J control mice under isoflurane anesthesia (63) but was decreased significantly in p75NTR−/− mice compared with the controls (Table 1). LVPSP tended to be lower in p75NTR−/− mice (WT mice: 93 ± 2.1 mmHg vs. p75NTR−/− mice: 83 ± 5.4 mmHg, means ± SE, n = 6–7, P = 0.1), and both dP/dtmax and dP/dtmin were significantly decreased in p75NTR−/− mice (Fig. 5, A and B).
Fig. 5.
dP/dtmax and dP/dtmin are low in p75NTR−/− mice. A: baseline dP/dtmax in WT and p75NTR−/− mice. B: dP/dtmin in WT and p75NTR−/− mice. Values are means ± SE; n = 6–7. *P < 0.05.
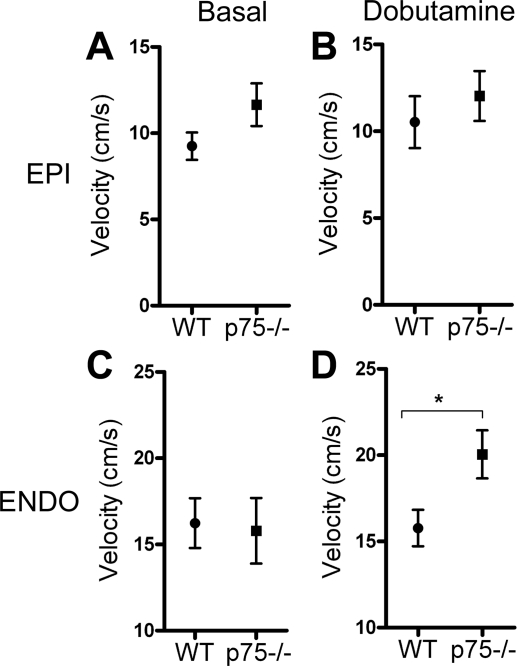
One drawback of measuring stroke volume or LVPSP is that these values reflect the function of the entire LV and do not allow specific analysis of the innervated p75NTR−/− subepicardium separate from the denervated subendocardium. To specifically examine the function of each layer of the heart, spectral tissue Doppler was used to compare subendocardial and subepicardial radial thickening velocities under basal conditions and after the stimulation of β1ARs with the agonist dobutamine. No differences were observed between the genotypes in basal or stimulated systolic thickening velocities (basal S′ in the endocardium: 12.8 ± 1.7 cm/s in WT mice vs. 11.8 ± 0.9 cm/s in p75NTR−/−, basal S′ in the epicardium: 8.0 ± 1.5 cm/s in WT mice vs. 7.8 ± 0.4 cm/s in p75NTR−/− mice, dobutamine-stimulated S′ in the endocardium: 13.3 ± 1.4 cm/s in WT mice vs. 15.7 ± 0.5 cm/s in p75NTR−/− mice, and dobutamine-stimulated S′ in the epicardium: 9.8 ± 0.6 cm/s in WT mice vs. 8.9 ± 0.4 cm/s in p75NTR−/− mice, means ± SE, n = 4). Under basal conditions, there were no differences in E′ between the genotypes in either layer of cardiac muscle (Fig. 6, A and C). However, the denervated subendocardium of p75NTR−/− mice exhibited a significantly greater E′ after stimulation with dobutamine than the WT subendocardium (Fig. 6D). In contrast, the normally innervated subepicardium of p75NTR−/− mice did not respond differently than the WT subepicardium to β1AR stimulation (Fig. 6B).
Fig. 6.
p75NTR−/− mice have an enhanced response to β1-adrenergic receptor (β1AR) stimulation in the denervated subendocardium. A and B: peak radial thickening velocity of the subepicardium at early diastole in WT and p75NTR−/− mice as determined by tissue Doppler analysis before (A) and after (B) an intraperitoneal injection of dobutamine (32 μg/kg). C and D: peak radial thickening velocity of the subendocardium in WT and p75NTR−/− mice determined at early diastole before (C) or after (D) an intraperitoneal injection of dobutamine (32 μg/kg). Values are means ± SE; n = 4. *P < 0.05.
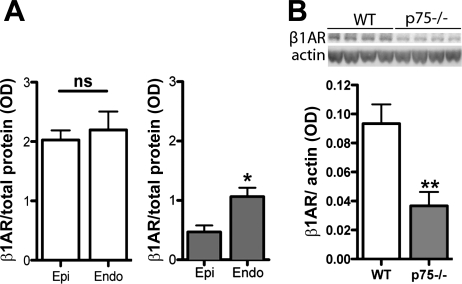
Given the enhanced responsiveness of the denervated p75NTR−/− subendocardium to β-agonists, we suspected that β1AR levels were increased in that layer of the p75NTR−/− LV. We examined β1AR levels by Western blot analysis and found that β1ARs were significantly higher in the denervated subendocardium than in the subepicardium of the p75NTR−/− LV but were evenly distributed across the WT LV (Fig. 7A). However, overall, β1AR levels in the p75NTR−/− LV were only 50% of WT controls (Fig. 7B). β1AR mRNA was also lower in the p75NTR−/− LV compared with WT controls (data not shown). Therefore, β-receptor responsiveness was high in the subendocardium and normal in the subepicardium despite significantly fewer receptors.
Fig. 7.
β1AR expression is low, and its distribution is altered in the p75NTR−/− LV. A: β1AR protein in the subendocardium and subepicardium of WT (left; open bars) and p75NTR−/− mice (right; shaded bars) normalized to total protein. Values are means ± SE; n = 4. *P < 0.05. B: representative Western blot (top) and quantification (bottom) of β1ARs and actin in WT and p75NTR−/− LVs. Values are means ± SE; n = 6. *P < 0.05; **P < 0.01.
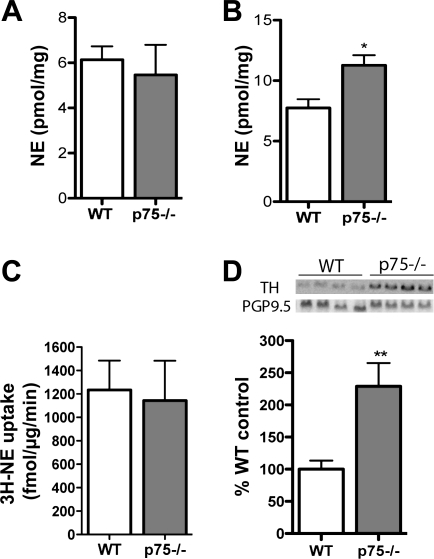
The significant lack of cardiac β-receptors in p75NTR−/− mice raised additional questions about normal ventricular function observed in those mice. NE can regulate β1AR expression, and NGF signaling through TrkA and p75NTR can modulate NE production in addition to axon outgrowth (4, 12). Therefore, we measured cardiac NE content in the LV and found that NE levels in p75NTR−/− mice were identical to WT mice despite the decreased sympathetic innervation density (Fig. 8A). NE was elevated in the p75NTR−/− RV, where innervation density was normal (Fig. 8B). These data suggest that sympathetic neurons lacking p75 produce more NE and are consistent with elevated NE, as previously reported in p75NTR−/− atria (18). [3H]NE uptake was similar in WT and p75NTR−/− ventricles (Fig. 8C), but TH, the rate-limiting enzyme in NE synthesis, was elevated in p75NTR−/− LVs (Fig. 8D). Thus, increased TH may lead to higher NE synthesis in sympathetic neurons lacking p75NTR.
Fig. 8.
Norephinephrine (NE) content, NE uptake, and TH levels in the LV. A: NE content in the p75NTR−/− LV was not significantly different than that in the WT LV. Values are means ± SE; n = 5–6. B: NE is elevated in the RV of p75NTR−/− mice. Values are means ± SE; n = 5–6. *P < 0.05. C: NE uptake in p75−/− mouse ventricles is identical to uptake in WT ventricles. Values are means ± SE; n = 6. D: TH protein, normalized to the pan-neuronal marker protein gene product 9.5 (PGP9.5), is elevated in the LV of p75NTR−/− mice compared with WT mice. Values are means ± SE; n = 5–6. **P < 0.01.
DISCUSSION
The sympathetic innervation of target tissues is critically dependent on NGF activation of TrkA (14, 17, 50), but the precise role of p75NTR has been less clear (25, 31, 35). p75NTR interacts with and modulates several signaling pathways involved in cell survival, axon outgrowth, and axon degeneration and repulsion (3, 11, 49). For example, in the pineal gland, the activation of p75NTR inhibits NGF-induced axon outgrowth, and the absence of p75NTR results in sympathetic hyperinnervation (31). Likewise, p75NTR comprises part of a Nogo-R/Lingo/p75 complex that mediates Nogo inhibition of axon regeneration after injury (44), and Nogo is present in the heart (6, 23), where it might interact with p75NTR on sympathetic neurons. p75NTR blunts axon outgrowth in both of these scenarios, although the receptors and signaling involved are quite distinct, and the absence of p75NTR would be expected to enhance axon outgrowth. Indeed, the absence of p75NTR enhances NGF-stimulated sympathetic axon outgrowth in vivo (15, 31) and in vitro (19). Sema3a inhibits sympathetic axon extension into the LV subendocardium (24), and p75NTR disrupts Sema3a signaling in sensory neurons by breaking up its NP-1/plexin receptor complex. Thus, the absence of p75NTR results in decreased sensory innervation to the skin, which expresses Sema3a (3). We found that the absence of p75NTR renders adult sympathetic axons more susceptible to inhibition by Sema3a. The doses of Sema3a that we used did not inhibit axon outgrowth in WT neurons, but we expect that using higher doses of Sema3a would inhibit WT outgrowth since Sema3a is clearly able to cause growth cone collapse and axon repulsion in embryonic sympathetic neurons (59). Given the clear role identified for Sema3a in controlling sympathetic innervation density in the subendocardium (24), we think it likely that the lack of subendocardial innervation in p75NTR−/− mice is due to an enhanced sensitivity to Sema3a. Thus, the ability of p75NTR to modulate multiple different signaling pathways can explain the variable degree of sympathetic organ innervation when p75NTR is absent (25, 31, 35).
Sympathetic heterogeneity in the heart contributes to the generation of ventricular arrhythmias in pathological conditions (30, 48, 51), and our data support the model that sympathetic heterogeneity in the ventricle is arrhythmogenic even in the absence of pathology. p75NTR−/− mice exhibited significantly more PVCs than age-matched control mice, and the circadian variation in the number of PVCs implicated the sympathetic nervous system as a contributor (1, 27, 42) despite other alterations in autonomic transmission that normally prevent arrhythmias. First, the atria of adult p75NTR−/− hearts exhibit a relative increase in cholinergic parasympathetic nerve fibers compared with sympathetic fibers, which are decreased in number (18). Enhanced parasympathetic transmission in the atria typically protects against arrhythmias (26, 54). Second, there is a lower overall density of sympathetic nerve fibers in the p75NTR−/− ventricle and decreased expression of β1ARs. Inhibition of noradrenergic transmission by β-blockers normally prevents arrhythmias (20, 39). Although total cardiac β1ARs were low in the p75NTR−/− LV, the distribution was altered compared with WT hearts, with significantly higher β1ARs in the denervated p75NTR−/− subendocardium. Transmural gradients of ion channel expression and myocyte repolarization are tightly regulated across the LV (5, 13, 33, 43, 56). Disruption of these gradients can destabilize cardiac rhythm (13, 33), and aberrant distribution of sympathetic nerve fibers and β1ARs may alter the regulation of repolarization across the LV (53), resulting in an enhanced susceptibility to ventricular arrhythmias despite the overall lower number of β1ARs and sympathetic fibers. These findings have significant clinical implications since neurotrophins are critical regulators of neuronal remodeling after cardiac injury (7, 62).
The uneven distribution of sympathetic nerves in the p75NTR−/− ventricle may contribute to the gradient in β1AR expression. The lack of p75NTR enhances NGF-induced TrkA signaling in sympathetic neurons (19, 31), and NGF stimulates TH expression and NE synthesis in the superior cervical ganglion (41, 52). Our data confirmed that cardiac p75NTR−/− sympathetic neurons produced more TH in vivo, and the RV exhibited increased NE content. NE content in the LV was normal in p75NTR−/− mice, but we expect that if we dissected the subendocardium and subepicardium separately, we would detect little NE in the denervated endocardium and elevated NE in the epicardium. β1AR expression is regulated by NE release (40, 55), and heterogeneity of NE release in the heart may contribute to the corresponding gradient in β1ARs.
While alterations in sympathetic innervation pattern and density seem to affect arrhythmogenic potential, basal LV function determined by echocardiography was normal in p75NTR−/− mice. The impact of aberrant sympathetic innervation on cardiovascular function only becomes evident upon direct activation of β1ARs, which is consistent with other animal models that have altered ventricular sympathetic innervation (24, 29). Although basal stroke volume and cardiac output were normal in p75NTR−/− mice, catheterization experiments revealed some differences not detected via echocardiography, including decreased mean arterial pressure and decreased dP/dtmax. Sema3a is a critical regulator of sympathetic innervation density in the vasculature (38), so low arterial pressure might result from decreased sympathetic innervation of the vasculature due to enhanced Sema3a signaling. Arterial pressure can affect dP/dtmax, so the low dP/dtmax may result from decreased afterload rather than the denervation of endocardial muscle. It is not possible to do these experiments in conscious mice, but the need for anesthesia is a limitation of our study as it likely impacts our results. For example, the heart rate in p75NTR−/− mice in the echocardiography experiment tended to be higher than that in control mice, even though the heart rate in conscious p75NTR−/− mice was significantly lower than in WT mice. This discrepancy may be a combination of isoflurane anesthesia and handling during the echocardiography procedure, since p75NTR−/− mice anesthetized with isoflurane but maintained in the dark without disruption have a significantly lower heart rate than WT mice (18), similar to conscious animals.
Tissue Doppler analysis revealed additional functional consequences in the heart, even though stroke volume and cardiac output were normal. Tissue Doppler allowed us to separately examine the tissue velocities of the normally innervated subepicardium and denervated subendocardium. Basal tissue velocities in the p75NTR−/− heart were normal, but treatment with the β1AR agonist dobutamine stimulated a significantly greater E′ in the p75NTR−/− subendocardium compared with the WT subendocardium, despite lower β1AR levels. Thus, significant differences in muscle function only become apparent using techniques that allowed for the separate analysis of the subendocardium and subepicardium. These mice provided a unique opportunity to examine the responsiveness of cardiac muscle that developed in the absence of sympathetic innervation, and we completed in vivo experiments to examine cardiac function in the context of the other hemodynamic changes in these animals. However, a limitation of this approach is that it does not allow us to examine the fractional shortening of isolated myocytes in vitro and to identify molecular differences in cardiac cells that develop in the presence of sympathetic nerves versus those that develop without innervation.
We focused on the role of p75NTR in controlling sympathetic innervation of the LV, but p75NTR is expressed in cardiac interstitial cells as well, and its expression in those cells is increased after injury (61). There is no evidence that p75NTR is expressed in cardiac myocytes in vivo (61) or in vitro (9, 10), but we cannot rule out the possibility that some of the changes observed in p75NTR−/− mice are due in part to an effect of non-neuronal cells within the heart. Nevertheless, given the lack of p75NTR in cardiac myocytes and the profound changes in the distribution and NE content of p75NTR−/− sympathetic nerves, it seems reasonable to attribute many of the changes observed in p75NTR−/− hearts to altered innervation.
Our results identify p75NTR as a key player in the establishment of ventricular sympathetic innervation and are consistent with the model that heterogeneous sympathetic innervation enhances the susceptibility to ventricular arrhythmias. We found that p75NTR has a greater functional impact on the cardiovascular system and cardiac rhythm stability than previously understood. Our findings are particularly relevant to myocardial infarction, heart failure, and diabetic autonomic neuropathy, where heterogeneous sympathetic innervation is correlated with altered neurotrophin expression and the development of ventricular arrhythmias (30, 48, 51).
GRANTS
This work was supported by American Heart Association Grants 0715669Z and 09PRE2110052 (to C. U. Lorentz) and 0555553Z (to B. A. Habecker) and National Heart, Lung, and Blood Institute Grant HL-093056 (to B. A. Habecker).
DISCLOSURES
No conflicts of interest are declared by the author(s).
ACKNOWLEDGMENTS
The authors thank Dr. William Woodward and Laura Pahlmeyer for technical assistance and Dr. Susan Birren for helpful discussions.
REFERENCES
- 1.Aronow WS, Ahn C, Mercando AD, Epstein S. Circadian variation of sudden cardiac death or fatal myocardial infarction is abolished by propranolol in patients with heart disease and complex ventricular arrhythmias. Am J Cardiol 74: 819–821, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Bandtlow C, Dechant G. From cell death to neuronal regeneration, effects of the p75 neurotrophin receptor depend on interactions with partner subunits. Sci STKE 2004: pe24, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Ben-Zvi A, Ben-Gigi L, Klein H, Behar O. Modulation of semaphorin3A activity by p75 neurotrophin receptor influences peripheral axon patterning. J Neurosci 27: 13000–13011, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bjerre B, Bjorklund A, Mobley W, Rosengren E. Short- and long-term effects of nerve growth factor on the sympathetic nervous system in the adult mouse. Brain Res 94: 263–277, 1975 [DOI] [PubMed] [Google Scholar]
- 5.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bullard TA, Protack TL, Aguilar F, Bagwe S, Massey HT, Blaxall BC. Identification of Nogo as a novel indicator of heart failure. Physiol Genomics 32: 182–189, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Cao JM, Chen LS, KenKnight BH, Ohara T, Lee MH, Tsai J, Lai WW, Karagueuzian HS, Wolf PL, Fishbein MC, Chen PS. Nerve sprouting and sudden cardiac death. Circ Res 86: 816–821, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Cao JM, Fishbein MC, Han JB, Lai WW, Lai AC, Wu TJ, Czer L, Wolf PL, Denton TA, Shintaku IP, Chen PS, Chen LS. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 101: 1960–1969, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Caporali A, Emanueli C. Cardiovascular actions of neurotrophins. Physiol Rev 89: 279–308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caporali A, Pani E, Horrevoets AJ, Kraenkel N, Oikawa A, Sala-Newby GB, Meloni M, Cristofaro B, Graiani G, Leroyer AS, Boulanger CM, Spinetti G, Yoon SO, Madeddu P, Emanueli C. Neurotrophin p75 receptor (p75NTR) promotes endothelial cell apoptosis and inhibits angiogenesis: implications for diabetes-induced impaired neovascularization in ischemic limb muscles. Circ Res 103: e15–e26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299–309, 2003 [DOI] [PubMed] [Google Scholar]
- 12.Chun LL, Patterson PH. Role of nerve growth factor in the development of rat sympathetic neurons in vitro. I. Survival, growth, and differentiation of catecholamine production. J Cell Biol 75: 694–704, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costantini DL, Arruda EP, Agarwal P, Kim KH, Zhu Y, Zhu W, Lebel M, Cheng CW, Park CY, Pierce SA, Guerchicoff A, Pollevick GD, Chan TY, Kabir MG, Cheng SH, Husain M, Antzelevitch C, Srivastava D, Gross GJ, Hui CC, Backx PH, Bruneau BG. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123: 347–358, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowley C, Spencer SD, Nishimura MC, Chen KS, Pitts-Meek S, Armanini MP, Ling LH, McMahon SB, Shelton DL, Levinson AD, et al. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell 76: 1001–1011, 1994 [DOI] [PubMed] [Google Scholar]
- 15.Dhanoa NK, Krol KM, Jahed A, Crutcher KA, Kawaja MD. Null mutations for exon III and exon IV of the p75 neurotrophin receptor gene enhance sympathetic sprouting in response to elevated levels of nerve growth factor in transgenic mice. Exp Neurol 198: 416–426, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci 28: 191–222, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Glebova NO, Ginty DD. Heterogeneous requirement of NGF for sympathetic target innervation in vivo. J Neurosci 24: 743–751, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Habecker BA, Bilimoria P, Linick C, Gritman K, Lorentz CU, Woodward W, Birren SJ. Regulation of cardiac innervation and function via the p75 neurotrophin receptor. Auton Neurosci 140: 40–48, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannila SS, Lawrance GM, Ross GM, Kawaja MD. TrkA and mitogen-activated protein kinase phosphorylation are enhanced in sympathetic neurons lacking functional p75 neurotrophin receptor expression. Eur J Neurosci 19: 2903–2908, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Haverkamp W, Hindricks G, Gulker H. Antiarrhythmic properties of beta-blockers. J Cardiovasc Pharmacol 16, Suppl 5: S29–S32, 1990 [PubMed] [Google Scholar]
- 21.He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90: 739–751, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience 156: 129–142, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber AB, Weinmann O, Brosamle C, Oertle T, Schwab ME. Patterns of Nogo mRNA and protein expression in the developing and adult rat and after CNS lesions. J Neurosci 22: 3553–3567, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, Lee JK, Matsumura K, Tomita Y, Miyoshi S, Shimoda K, Makino S, Sano M, Kodama I, Ogawa S, Fukuda K. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med 13: 604–612, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Jahed A, Kawaja MD. The influences of p75 neurotrophin receptor and brain-derived neurotrophic factor in the sympathetic innervation of target tissues during murine postnatal development. Auton Neurosci 118: 32–42, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Jouven X, Empana JP, Schwartz PJ, Desnos M, Courbon D, Ducimetiere P. Heart-rate profile during exercise as a predictor of sudden death. N Engl J Med 352: 1951–1958, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Jung BC, Dave AS, Tan AY, Gholmieh G, Zhou S, Wang DC, Akingba AG, Fishbein GA, Montemagno C, Lin SF, Chen LS, Chen PS. Circadian variations of stellate ganglion nerve activity in ambulatory dogs. Heart Rhythm 3: 78–85, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Kaplan DR, Miller FD. Axon growth inhibition: signals from the p75 neurotrophin receptor. Nat Neurosci 6: 435–436, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kiriazis H, Du XJ, Feng X, Hotchkin E, Marshall T, Finch S, Gao XM, Lambert G, Choate JK, Kaye DM. Preserved left ventricular structure and function in mice with cardiac sympathetic hyperinnervation. Am J Physiol Heart Circ Physiol 289: H1359–H1365, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Kjekshus J. Arrhythmias and mortality in congestive heart failure. Am J Cardiol 65: 42I–48I, 1990 [DOI] [PubMed] [Google Scholar]
- 31.Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J Neurosci 19: 5393–5408, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolodkin AL, Levengood DV, Rowe EG, Tai YT, Giger RJ, Ginty DD. Neuropilin is a semaphorin III receptor. Cell 90: 753–762, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, Nguyen-Tran VT, Gu Y, Ikeda Y, Chu PH, Ross J, Giles WR, Chien KR. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell 107: 801–813, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell 118: 243–255, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Lee KF, Bachman K, Landis S, Jaenisch R. Dependence on p75 for innervation of some sympathetic targets. Science 263: 1447–1449, 1994 [DOI] [PubMed] [Google Scholar]
- 36.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69: 737–749, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol 286: H2229–H2236, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Long JB, Jay SM, Segal SS, Madri JA. VEGF-A and semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol 334: 119–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Sendon J, Swedberg K, McMurray J, Tamargo J, Maggioni AP, Dargie H, Tendera M, Waagstein F, Kjekshus J, Lechat P, Torp-Pedersen C. Expert consensus document on beta-adrenergic receptor blockers. Eur Heart J 25: 1341–1362, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Mann DL. Basic mechanisms of disease progression in the failing heart: the role of excessive adrenergic drive. Prog Cardiovasc Dis 41: 1–8, 1998 [DOI] [PubMed] [Google Scholar]
- 41.Max SR, Rohrer H, Otten U, Thoenen H. Nerve growth factor-mediated induction of tyrosine hydroxylase in rat superior cervical ganglia in vitro. J Biol Chem 253: 8013–8015, 1978 [PubMed] [Google Scholar]
- 42.Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH. Circadian variation in the frequency of sudden cardiac death. Circulation 75: 131–138, 1987 [DOI] [PubMed] [Google Scholar]
- 43.Nabauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation 93: 168–177, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Nykjaer A, Willnow TE, Petersen CM. p75NTR–live or let die. Curr Opin Neurobiol 15: 49–57, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Parrish DC, Alston EN, Rohrer H, Nkadi P, Woodward WR, Schutz G, Habecker BA. Infarction-induced cytokines cause local depletion of tyrosine hydroxylase in cardiac sympathetic nerves. Exp Physiol 95: 304–314, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parrish DC, Gritman K, Van Winkle DM, Woodward WR, Bader M, Habecker BA. Postinfarct sympathetic hyperactivity differentially stimulates expression of tyrosine hydroxylase and norepinephrine transporter. Am J Physiol Heart Circ Physiol 294: H99–H106, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Randall WC, Szentivanyi M, Pace JB, Wechsler JS, Kaye MP. Patterns of sympathetic nerve projections onto the canine heart. Circ Res 22: 315–323, 1968 [DOI] [PubMed] [Google Scholar]
- 48.Rubart M, Zipes DP. Mechanisms of sudden cardiac death. J Clin Invest 115: 2305–2315, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci 11: 649–658, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368: 246–249, 1994 [DOI] [PubMed] [Google Scholar]
- 51.Stevens MJ, Raffel DM, Allman KC, Dayanikli F, Ficaro E, Sandford T, Wieland DM, Pfeifer MA, Schwaiger M. Cardiac sympathetic dysinnervation in diabetes: implications for enhanced cardiovascular risk. Circulation 98: 961–968, 1998 [DOI] [PubMed] [Google Scholar]
- 52.Thoenen H, Angeletti PU, Levi-Montalcini R, Kettler R. Selective induction by nerve growth factor of tyrosine hydroxylase and dopamine- -hydroxylase in the rat superior cervical ganglia. Proc Natl Acad Sci USA 68: 1598–1602, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas D, Kiehn J, Katus HA, Karle CA. Adrenergic regulation of the rapid component of the cardiac delayed rectifier potassium current, IKr, and the underlying hERG ion channel. Basic Res Cardiol 99: 279–287, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Tsuji H, Larson MG, Venditti FJ, Jr, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation 94: 2850–2855, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Wallukat G. The beta-adrenergic receptors. Herz 27: 683–690, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Cheng J, Joyner RW, Wagner MB, Hill JA. Remodeling of early-phase repolarization: a mechanism of abnormal impulse conduction in heart failure. Circulation 113: 1849–1856, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Welcher AA, Bitler CM, Radeke MJ, Shooter EM. Nerve growth factor binding domain of the nerve growth factor receptor. Proc Natl Acad Sci USA 88: 159–163, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan H, Chao MV. Disruption of cysteine-rich repeats of the p75 nerve growth factor receptor leads to loss of ligand binding. J Biol Chem 266: 12099–12104, 1991 [PubMed] [Google Scholar]
- 59.Yaron A, Huang PH, Cheng HJ, Tessier-Lavigne M. Differential requirement for plexin-A3 and -A4 in mediating responses of sensory and sympathetic neurons to distinct class 3 semaphorins. Neuron 45: 513–523, 2005 [DOI] [PubMed] [Google Scholar]
- 60.Zampieri N, Chao MV. Mechanisms of neurotrophin receptor signalling. Biochem Soc Trans 34: 607–611, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Zhou S, Cao JM, Swissa M, Gonzalez-Gomez I, Chang CM, Chien K, Miyauchi Y, Fu KJ, Yi J, Asotra K, Karagueuzian HS, Fishbein MC, Chen PS, Chen LS. Low-affinity nerve growth factor receptor p75NTR immunoreactivity in the myocardium with sympathetic hyperinnervation. J Cardiovasc Electrophysiol 15: 430–437, 2004 [DOI] [PubMed] [Google Scholar]
- 62.Zhou S, Chen LS, Miyauchi Y, Miyauchi M, Kar S, Kangavari S, Fishbein MC, Sharifi B, Chen PS. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res 95: 76–83, 2004 [DOI] [PubMed] [Google Scholar]
- 63.Zuurbier CJ, Emons VM, Ince C. Hemodynamics of anesthetized ventilated mouse models: aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol 282: H2099–H2105, 2002 [DOI] [PubMed] [Google Scholar]